Isotopic and Hydrochemical Characteristics of the Changqing-Xiaolipu Water Resource, Jinan, Eastern China: Implications for Water Resources in the Yellow River Basin
Abstract
1. Introduction
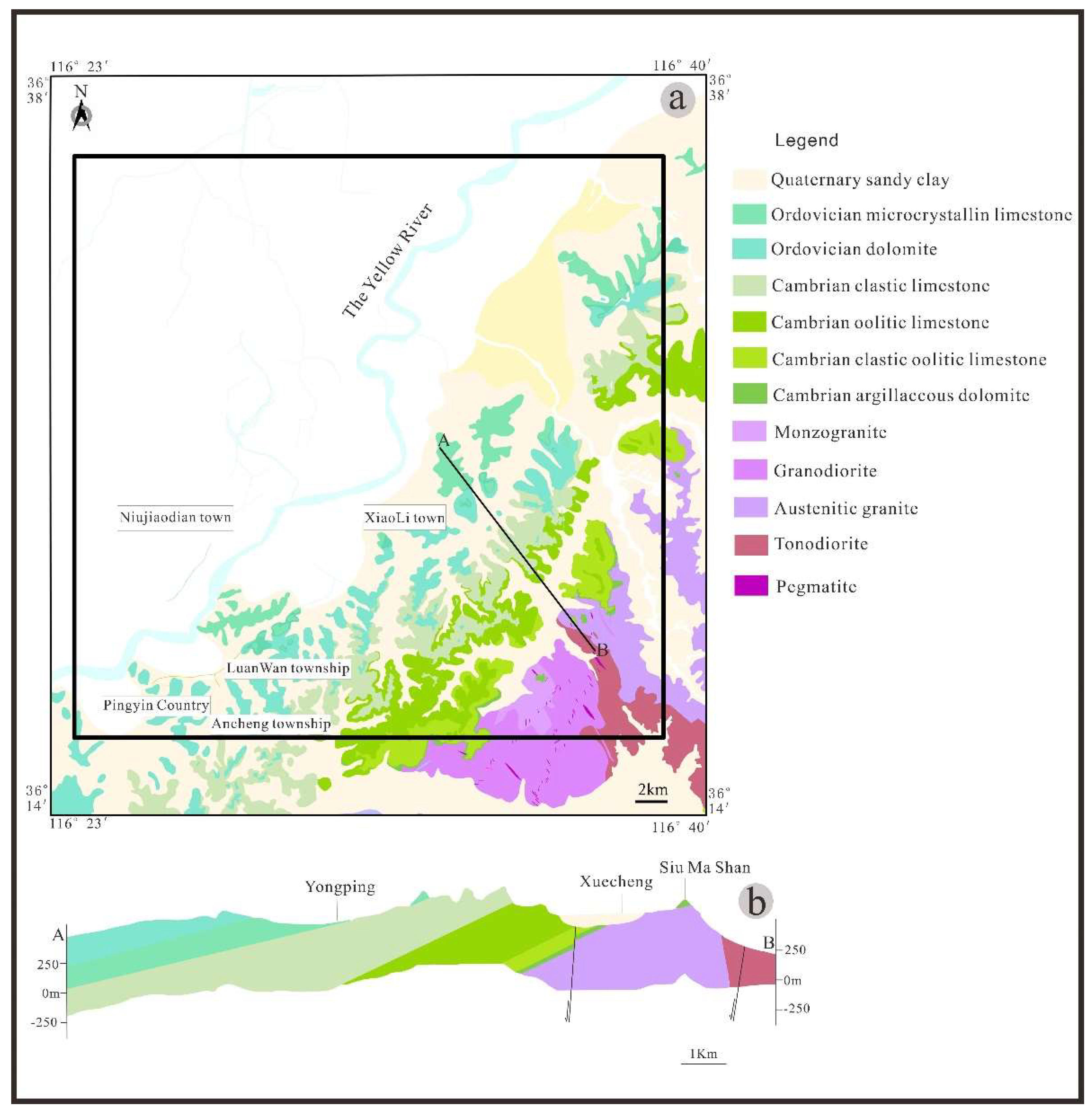
2. Geological and Hydrogeological Setting
3. Method and Analyses of Samples
4. Results
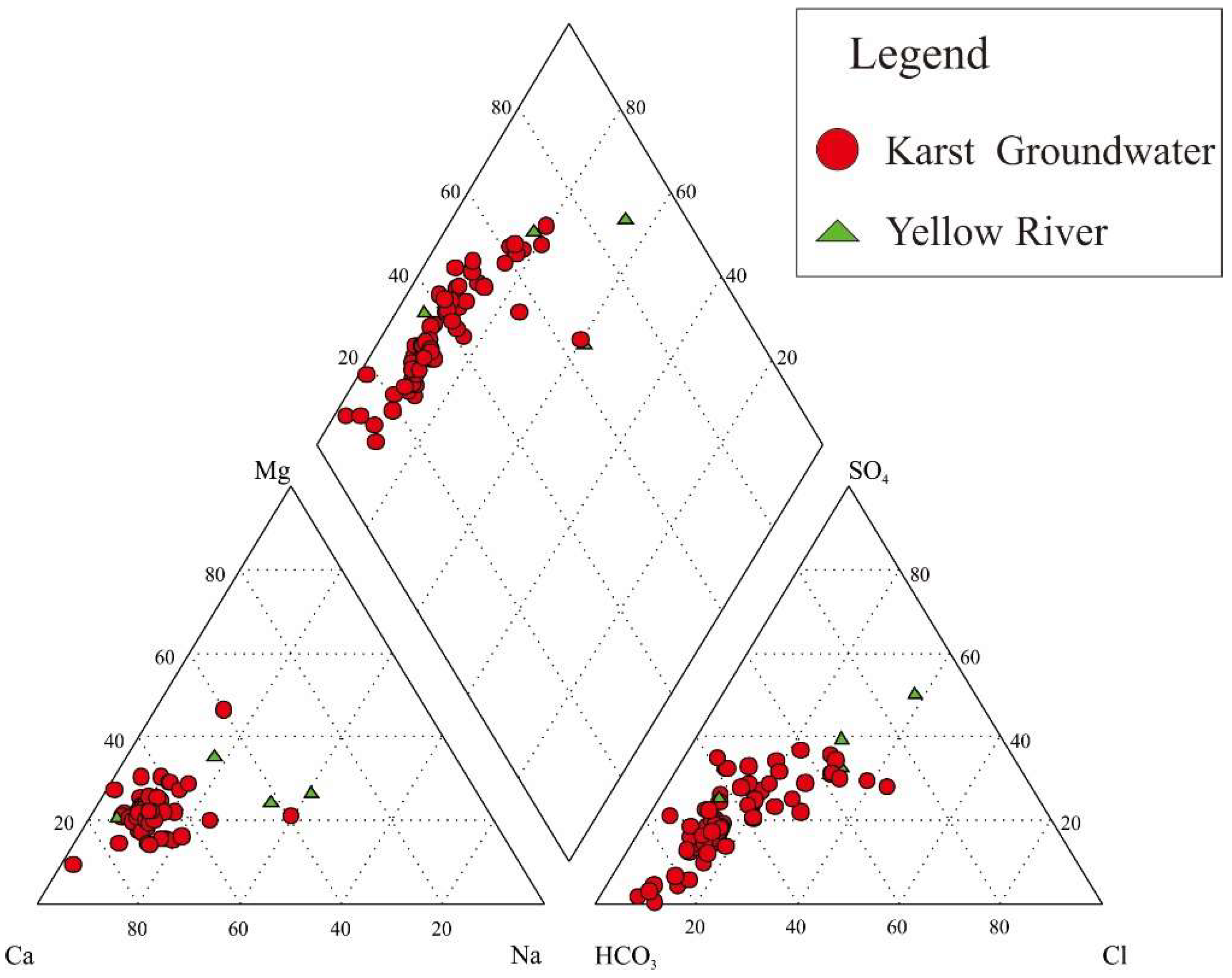
4.1. Groundwater Chemical Characteristics
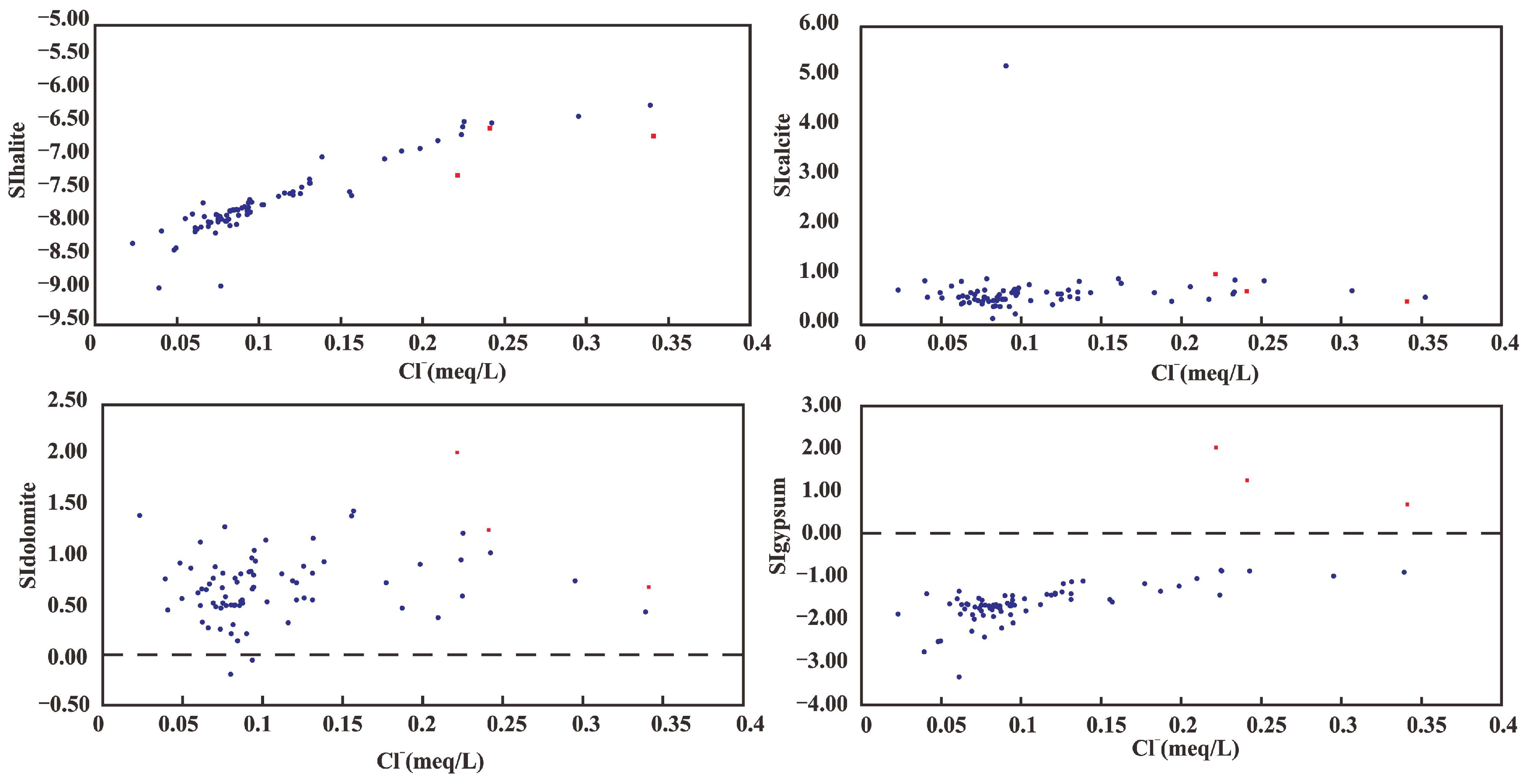
4.2. Saturation Indices
4.3. Isotopic Characteristics of Water in the Changqing-Xiaolipu Area
5. Discussion
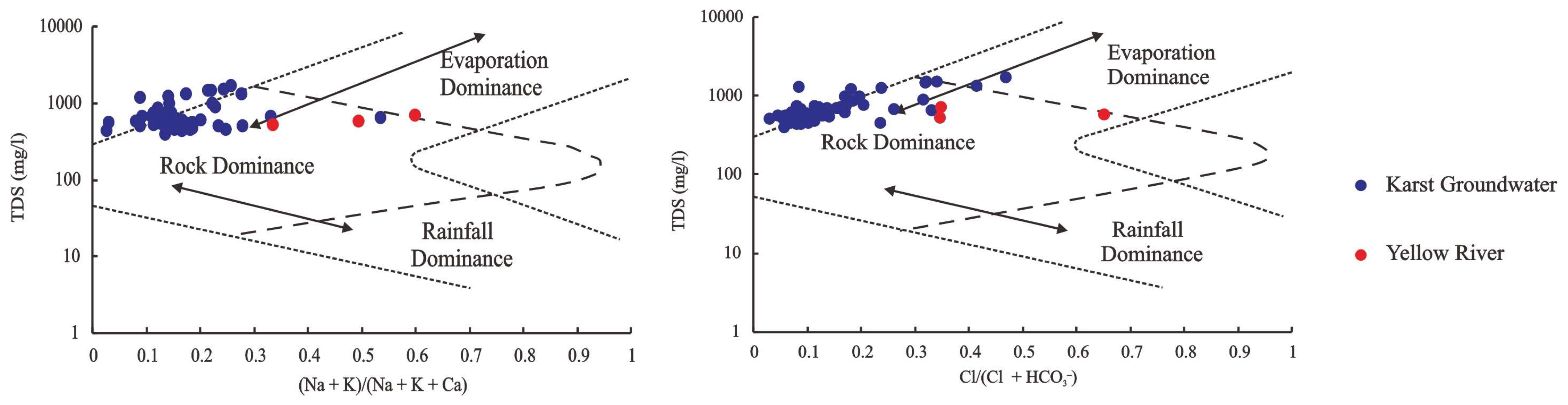
5.1. Origin and Recharge of Groundwater
5.2. Hydrochemical Evidence for Mixing between Yellow River Water and Karst Groundwater and Its Quantification
5.3. Water Quality Assessment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balek, J. Groundwater recharge concepts. In Estimation of Natural Groundwater Recharge; Springer: Dordrecht, The Netherlands, 1988; pp. 3–9. [Google Scholar]
- Lerner, D.N.; Issar, A.S.; Simmers, I. Groundwater Recharge: A Guide to Understanding and Estimating Natural Recharge; IAH International Contributions to Hydrogeology No. 8; Heinz Heise: Hannover, Germany, 1990. [Google Scholar]
- Simmers, I.; Hendrickx, J.M.H.; Kruseman, G.P.; Rushton, K.R. Recharge of Phreatic Aquifers in (Semi-)Arid Areas; IAH International Contributions to Hydrogeology 19; Routledge: Oxfordshire, UK, 1997; Volume 49, pp. e34–e35. [Google Scholar]
- Han, D.; Liang, X.; Currell, M.J.; Song, X.; Chen, Z.; Jin, M.; Liu, C.; Han, Y. Environmental isotopic and hydrochemical characteristics of groundwater systems in Daying and Qicun geothermal fields, Xinzhou Basin, Shanxi, China. Hydrol. Process. 2010, 24, 3157–3176. [Google Scholar] [CrossRef]
- El Osta, M.; Masoud, M.; Alqarawy, A.; Elsayed, S.; Gad, M. Groundwater Suitability for Drinking and Irrigation Using Water Quality Indices and Multivariate Modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 2022, 14, 483. [Google Scholar] [CrossRef]
- Masoud, M.; El Osta, M.; Alqarawy, A.; Elsayed, S.; Gad, M. Evaluation of groundwater quality for agricultural under different conditions using water quality indices, partial least squares regression models, and GIS approaches. Appl. Water Sci. 2022, 12, 244. [Google Scholar] [CrossRef]
- Shakeri, A.; Ghoreyshinia, S.; Mehrabi, B. Surface and groundwater quality in Taftan geothermal field, SE Iran. Water Qual. Expo. Health 2015, 7, 205–218. [Google Scholar] [CrossRef]
- Gad, M.; Saad, A. Hydrogeochemical evaluation of fractured Limestone aquifer by applying a geochemical model in eastern Nile Valley, Egypt. Environ. Earth Sci. 2017, 76, 641. [Google Scholar] [CrossRef]
- Gad, M.; Dahab, K.; Ibrahim, H. Applying of a geochemical model on the Nubian sandstone aquifer in Siwa Oasis, Western Desert, Egypt. Environ. Earth Sci. 2018, 77, 401. [Google Scholar] [CrossRef]
- Gad, M.; El-Hendawy, S.; Al-Suhaibani, N.; Tahir, M.U.; Mubushar, M.; Elsayed, S. Combining hydrogeochemical characterization and a hyperspectral reflectance tool for assessing quality and suitability of two groundwater resources for irrigation in Egypt. Water 2020, 12, 2169. [Google Scholar] [CrossRef]
- Hussein, H.A.; Ricka, A.; Kuchovsky, T.; El Osta, M.M. Groundwater hydrochemistry and origin in the south-eastern part of WadiEl Natrun, Egypt. Arab. Geosci. 2017, 10, 170–184. [Google Scholar] [CrossRef]
- Egbueri, J.C. Assessment of the quality of groundwaters proximal to dumpsites in Awka and Nnewi metropolises: A comparative approach. Int. J. Energy Water Resour. 2018, 2, 33–48. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.X.; Wang, P.; Chen, J.; Yang, L.; Xiao, K.; Zhang, X. Hydrogeochemical evolution and heavy metal contamination in groundwater of a reclaimed land on Zhoushan Island. Water 2018, 10, 316. [Google Scholar] [CrossRef]
- Mgbenu, C.N.; Egbueri, J.C. The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl. Water Sci. 2019, 9, 22. [Google Scholar] [CrossRef]
- Kumar, S.K.; Logeshkumaran, A.; Magesh, N.S.; Godson, P.S.; Chandrasekar, N. Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl. Water Sci. 2015, 5, 335–343. [Google Scholar] [CrossRef]
- Mirzavand, M.; Sadeghi, S.; Bagheri, R. Groundwater and soil salinization and geochemical evolution of Femenin-Ghahavand plain, Iran. Environ. Sci. Pollut. Res. 2020, 27, 43056–43066. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, S.; Lian, E.G.; Li, C.; Yang, C.f.; Liu, P.F.; Deng, K. Hydrogen and Oxygen Isotopes in Yangtze River Water and Its Application in Tracing Basin-Scale Water Cycle. J. Tongji Univ. 2021, 49, 1353–1362. [Google Scholar]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Gibson, J.J.; Aggarwal, P.; Hogan, J.; Kendall, C.; Martinelli, L.A.; Stichler, W.; Rank, D.; Goni, I.; Choudhry, M.; Gat, J.; et al. Isotope studies in large river basins: A new global research focus. Eos Trans. Am. Geophys. Union 2002, 83, 613–617. [Google Scholar] [CrossRef]
- Xu, J.X.; Xing, L.T.; Tong, G.Y.; Fan, L.Q. Groundwater environment evolution and its conservation in Jinan spring catchment. Eng. Geol. 2004, 31, 69–73. [Google Scholar]
- Xing, L.; Lu, M.; Hu, L. Present situation and protection strategies for environmental problems of karst groundwater in Jinan spring region. J. Jinan Univ. Sci. Technol. Ed. 2006, 20, 345. [Google Scholar]
- Li, T.X.; Li, L.; Liu, Y.J. Systematic Analysis of Characteristics and Effected Elements of Spring in Jinan City. Land Resour. Shandong Prov. 2003, 19, 48–51. [Google Scholar]
- Bo, K.T.; Cai, Y.X. Calculation of Karst Groundwater Resources and Analysis on Exploitation Potentiality of Changqing-Xiaolipu Hydrogeologic Unit. ShangDong Land Resour. 2015, 31, 37–42. [Google Scholar]
- Fan, X.M.; Shu, L.C.; Liu, G.H.; Schwanenberg, D.; Ongor, B.T.I. Coupled SOBEK and Visual MODFLOW Model for Yellow River Delta Water Resources Interaction Analysis; Yellow River Conservancy Press: Zhenzhou, China, 2007; pp. 220–226. [Google Scholar]
- Lou, G.Y.; Li, R.; Zhang, S.F.; Ge, L.; Fan, X.M. Study on Ground Water Level & Water Balance Influence under Different Water Compensation Schemes of the Yellow River Delta. In Proceedings of the 3rd International Yellow River Forum, Dongying, China, 16–19 October 2007; pp. 303–313. [Google Scholar]
- Gonçalves, J.M.; Pereira, L.S.; Fang, S.X.; Dong, B. Modelling and multicriteria analysis of water saving scenarios for an irrigation district in the upper Yellow River Basin. Water 2007, 94, 93–108. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Li, J.; Luo, B.; Huang, C. Geochemical and isotopic evidence of shallow groundwater salinization in a reclaimed coastal zone: The Yellow River Delta, China. Environ. Earth Sci. 2016, 75, 1107. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Ground Water; Preatice-Hall Inc.: Hoboken, NJ, USA, 1979. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Berg, M.; Pernet-Coudrier, B.; Qi, W.; Liu, H. The geochemistry of the Yangtze River: Seasonality of concentrations and temporal trends of chemical loads. Glob. Biogeochem. Cycles 2012, 26, GB2028-1–GB2028-14. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Li, J.; Liu, X.; Chetelat, B.; Wang, B.; Wang, F. Assessment of the sources of nitrate in the Changjiang River, China using a nitrogen and oxygen isotopic approach. Environ. Sci. Technol. 2010, 44, 1573–1578. [Google Scholar] [CrossRef]
- Lambs, L.; Muller, E.; Fromard, F. The Guianese paradox: How can the freshwater outflow from the Amazon increase the salinity of the Guianan shore? J. Hydrol. 2007, 342, 88–96. [Google Scholar] [CrossRef]
- Faure, G.; Mensing, T.M. Isotope Principle and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Edmunds, W.M.; Smedley, P.L. Residence time indicators in groundwater: The East Midlands Triassic sandstone aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Eugster, H.P.; Jones, B.F. Behavior of major solutes during closed-basin brine evolution. Science 1979, 279, 609–631. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 216, p. 303. [Google Scholar]

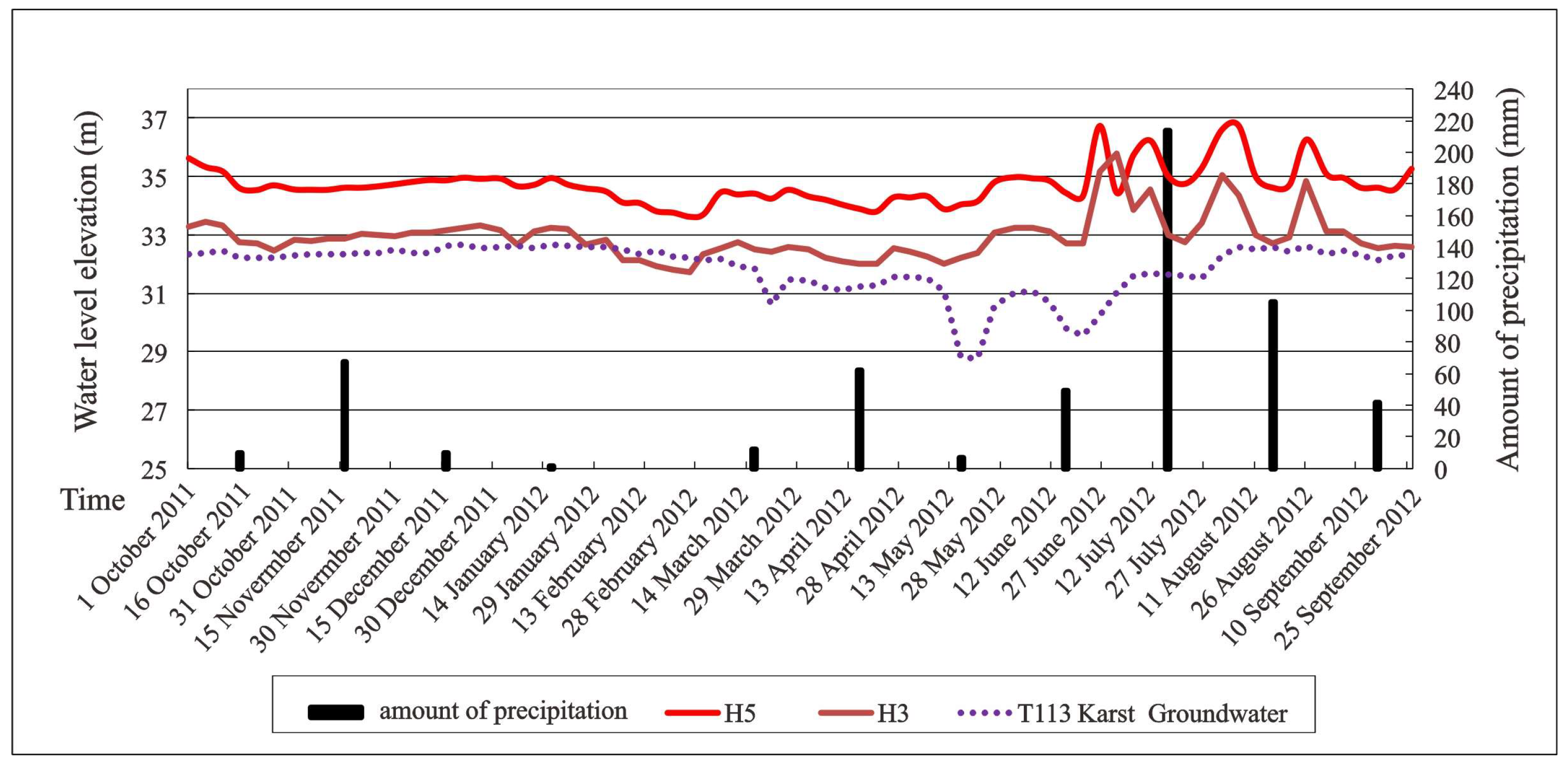

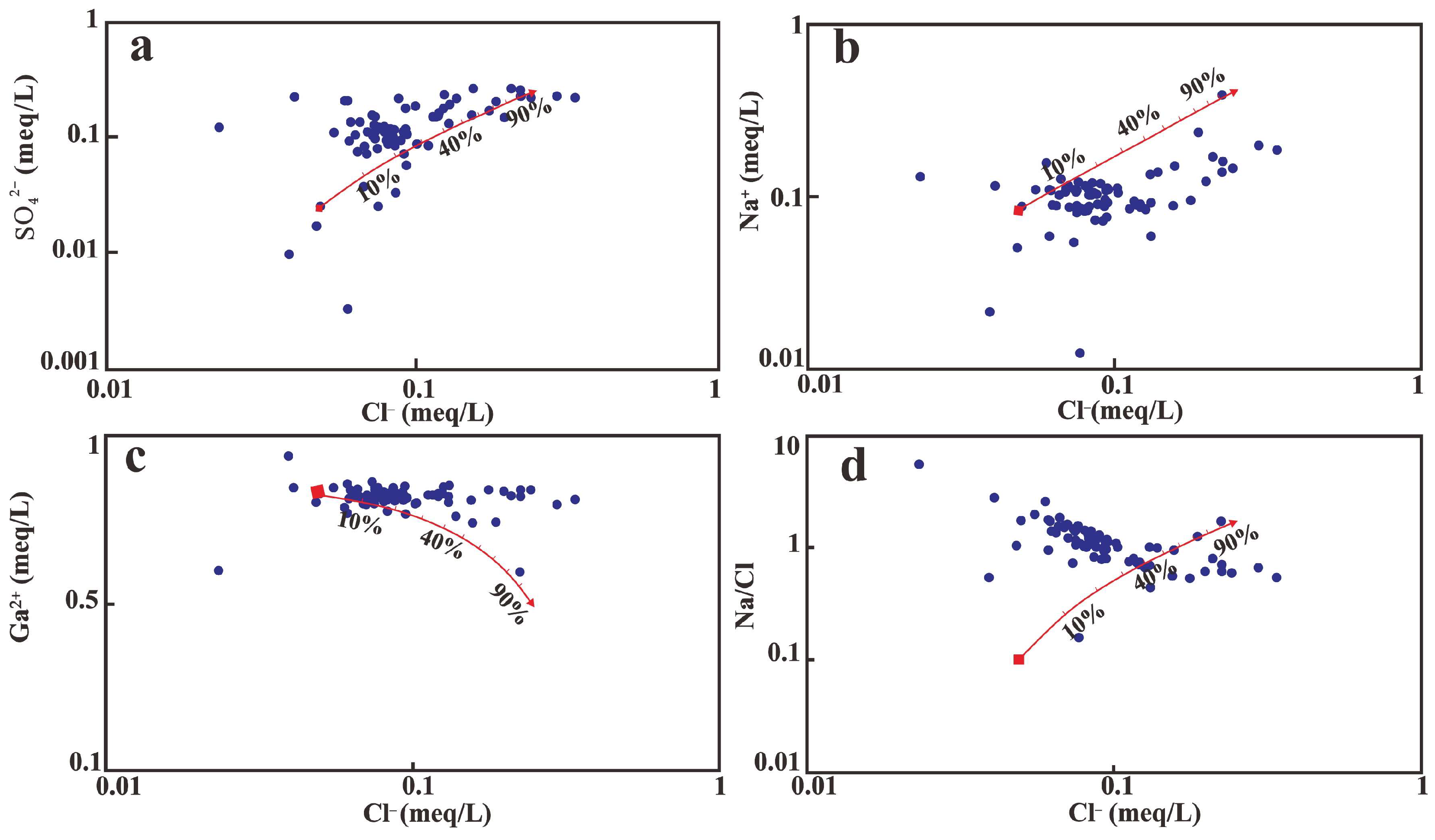
| Group | Water Number | Water Temperature (℃) | pH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | TDS (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karst Groundwater | CC6-1 | 16 | 7.7 | 0.44 | 15.0 | 103 | 18.8 | 28.5 | 57.4 | 268 | 61.3 | 569 |
| CC6-2 | 16 | 7.6 | 0.50 | 14.3 | 100 | 19.8 | 29.5 | 55.2 | 257 | 61.4 | 555 | |
| CC6-3 | 16 | 7.7 | 0.50 | 18.6 | 101 | 23.6 | 33.4 | 44.2 | 276 | 62.3 | 575 | |
| CC6-4 | 16 | 7.7 | 0.60 | 18.6 | 105 | 22.0 | 31.9 | 61.9 | 268 | 64.1 | 588 | |
| CC8-1 | 16 | 7.7 | 0.56 | 15.0 | 101 | 22.5 | 29.8 | 64.1 | 268 | 77.1 | 594 | |
| CC8-2 | 16 | 7.6 | 0.50 | 14.3 | 99.53 | 22.0 | 31.1 | 48.6 | 273 | 59.0 | 566 | |
| CC8-3 | 16 | 7.7 | 0.50 | 18.6 | 97.72 | 23.6 | 39.8 | 46.4 | 273 | 51.6 | 566 | |
| CC8-4 | 16 | 7.8 | 0.50 | 18.6 | 104 | 22.5 | 33.4 | 64.1 | 276 | 55.3 | 591 | |
| CC11-1 | 16 | 7.4 | 0.44 | 13.3 | 94.2 | 20.7 | 30.8 | 64.0 | 268 | 48.6 | 547 | |
| CC11-2 | 16 | 7.7 | 0.50 | 14.3 | 95.9 | 20.3 | 27.9 | 59.7 | 279 | 49.6 | 527 | |
| CC11-3 | 16 | 7.7 | 0.70 | 18.6 | 95.9 | 20.9 | 27.9 | 53.0 | 270 | 45.5 | 549 | |
| CC11-4 | 16 | 7.7 | 0.50 | 18.6 | 102 | 22.0 | 34.2 | 53.0 | 281 | 49.3 | 577 | |
| CC12-1 | 16 | 7.6 | 0.44 | 10.0 | 118 | 20.1 | 28.3 | 81.8 | 257 | 56.5 | 579 | |
| CC12-2 | 16 | 7.8 | 0.40 | 14.3 | 92.3 | 20.9 | 23.9 | 53.0 | 267 | 43.5 | 531 | |
| CC12-3 | 16 | 7.8 | 0.50 | 17.1 | 88.7 | 22.0 | 29.5 | 68.5 | 273 | 38.2 | 553 | |
| CC12-4 | 16 | 7.7 | 0.40 | 18.6 | 95.0 | 20.9 | 32.7 | 59.7 | 278 | 38.5 | 560 | |
| CX5-1 | 17 | 7.6 | 1.60 | 48.0 | 235 | 37.9 | 32.7 | 59.7 | 350 | 368 | 1308 | |
| CX5-2 | 15 | 7.5 | 1.30 | 28.6 | 179 | 39.1 | 67.9 | 92.8 | 332 | 223 | 980 | |
| CX5-3 | 16 | 7.5 | 1.60 | 37.1 | 236 | 41.7 | 111 | 156 | 355 | 301 | 1238 | |
| CX7-1 | 15 | 7.7 | 0.56 | 14.4 | 94.4 | 18.7 | 35.5 | 18.2 | 310 | 32.7 | 548 | |
| CX7-2 | 15 | 7.9 | 0.75 | 20.0 | 112 | 17.0 | 22.8 | 61.9 | 300 | 70.7 | 619 | |
| CX7-3 | 15 | 7.4 | 0.70 | 22.9 | 136 | 22.5 | 35.8 | 55.2 | 406 | 53.4 | 750 | |
| CX36 | 16 | 7.6 | 0.90 | 48.0 | 165 | 23.6 | 122 | 210 | 265 | 44.1 | 893 | |
| CX39 | 15 | 7.8 | 0.60 | 15.7 | 78.7 | 17.6 | 25.5 | 42.0 | 271 | 10.2 | 476 | |
| CX40 | 15 | 7.9 | 0.50 | 10.0 | 67.0 | 13.7 | 15.9 | 11.1 | 256 | 5.50 | 395 | |
| CX46 | 16 | 7.9 | 0.70 | 14.3 | 75.1 | 15.9 | 21.5 | 44.2 | 255 | 9.54 | 451 | |
| CX58 | 16 | 7.7 | 0.56 | 14.4 | 67.4 | 20.1 | 22.8 | 1.67 | 304 | 4.21 | 453 | |
| CX58 | 16 | 7.9 | 0.75 | 12.0 | 71.1 | 21.9 | 29.2 | 42.0 | 255 | 5.59 | 448 | |
| CX58 | 16 | 8.0 | 0.60 | 15.7 | 71.6 | 21.4 | 35.7 | 29.3 | 278 | 8.01 | 475 | |
| C7-1 | 15 | 7.8 | 0.66 | 16.6 | 80.5 | 18.1 | 30.4 | 63.9 | 262 | 7.99 | 502 | |
| C7-2 | 15 | 8.0 | 0.88 | 16.0 | 76.1 | 18.2 | 24.1 | 33.1 | 255 | 11.7 | 448 | |
| C7-3 | 15 | 8.2 | 0.70 | 17.1 | 80.5 | 15.9 | 27.9 | 39.8 | 268 | 12.6 | 478 | |
| C7-4 | 15 | 7.8 | 0.60 | 1.56 | 77.8 | 18.2 | 27.4 | 12.2 | 278 | 11.1 | 439 | |
| C19-1 | 16 | 7.9 | 1.11 | 24.7 | 84.2 | 18.2 | 21.6 | 102 | 229 | 22.8 | 515 | |
| C19-2 | 16 | 8.1 | 0.80 | 19.3 | 94.6 | 22.5 | 38.1 | 95.2 | 231 | 28.9 | 542 | |
| C28-1 | 16 | 8.1 | 0.60 | 20.0 | 53.0 | 37.5 | 9.52 | 68.3 | 304 | 5.06 | 515 | |
| C28-2 | 16 | 8.0 | 1.11 | 12.0 | 128 | 22.5 | 25.4 | 117 | 263 | 83.3 | 664 | |
| C29 | 16 | 7.7 | 2.32 | 20.0 | 97.2 | 18.23 | 33.0 | 108 | 221 | 50.2 | 566 | |
| C31 | 16 | 7.9 | 1.11 | 14.0 | 106 | 18.8 | 29.2 | 79.5 | 261 | 43.9 | 566 | |
| C230 | 16 | 7.7 | 0.63 | 22.4 | 235 | 43.7 | 79.9 | 159 | 357 | 285 | 1201 | |
| CK1-1 | 15 | 7.7 | 0.56 | 16.6 | 133 | 31.0 | 43.1 | 60.9 | 334 | 109 | 750 | |
| CK1-2 | 15 | 7.9 | 0.75 | 20.0 | 104 | 21.9 | 40.6 | 61.9 | 295 | 38.2 | 595 | |
| CK1-3 | 16 | 7.7 | 0.70 | 20.0 | 123 | 28.0 | 45.4 | 77.3 | 329 | 73.5 | 712 | |
| CK1-4 | 15 | 7.7 | 0.30 | 18.6 | 127 | 27.3 | 54.8 | 56.1 | 342 | 57.0 | 696 | |
| T69 | 16 | 7.7 | 0.70 | 14.3 | 96.8 | 17.6 | 22.3 | 66.3 | 248 | 39.2 | 519 | |
| T80 | 16 | 7.9 | 0.67 | 16.0 | 93.5 | 21.9 | 37.2 | 39.1 | 291 | 16.4 | 531 | |
| T85 | 16 | 7.8 | 0.88 | 20.0 | 106 | 15.8 | 15.2 | 113 | 238 | 45.1 | 566 | |
| T94-1 | 16 | 7.4 | 13.1 | 65.0 | 284 | 35.7 | 190 | 263 | 403 | 191 | 1462 | |
| T94-2 | 25 | 7.5 | 12.6 | 75.0 | 271 | 38.6 | 201 | 278 | 424 | 194 | 1512 | |
| T94-3 | 17 | 7.6 | 12.8 | 70.0 | 291 | 35.7 | 202 | 252 | 389 | 238 | 1491 | |
| T96 | 17 | 8.1 | 0.80 | 20.0 | 128 | 30.7 | 61.9 | 84.0 | 239 | 193.8 | 758 | |
| T97 | 16 | 7.9 | 0.50 | 14.3 | 74.2 | 18.1 | 23.8 | 17.9 | 268 | 9.43 | 441 | |
| T99 | 16 | 7.8 | 0.44 | 7.5 | 82.4 | 23.8 | 20.2 | 9.76 | 340 | 6.18 | 505 | |
| T101 | 17 | 7.6 | 0.40 | 22.9 | 167 | 28.5 | 64.5 | 163 | 284 | 128 | 876 | |
| T105 | 16 | 7.4 | 5.43 | 100 | 304 | 43.4 | 275 | 243 | 310 | 400 | 1701 | |
| T105 | 17 | 7.6 | 5.00 | 86.7 | 239 | 37.3 | 207 | 217 | 292 | 258 | 1342 | |
| T112 | 16 | 7.6 | 1.68 | 44.0 | 159 | 45.8 | 93.2 | 198 | 378 | 58.3 | 978 | |
| T112V | 16 | 7.7 | 0.89 | 53.3 | 109 | 24.1 | 90.8 | 134 | 257 | 12.4 | 682 | |
| T112b | 16 | 7.8 | 0.78 | 26.8 | 110 | 23.2 | 59.9 | 82.6 | 293 | 13.6 | 611 | |
| T113 | 17 | 8.1 | 1.10 | 86.7 | 76.0 | 24.7 | 96.4 | 150 | 194 | 8.57 | 652 | |
| PT1-1 | 16 | 7.6 | 0.85 | 20.7 | 132 | 29.7 | 58.9 | 107 | 303 | 66.5 | 718 | |
| PT1-2 | 16 | 7.7 | 0.80 | 19.3 | 131 | 27.0 | 56.7 | 97.0 | 296 | 64.8 | 693 | |
| PT1-3 | 16 | 7.7 | 0.81 | 19.8 | 129 | 27.5 | 56.7 | 98.3 | 303 | 65.0 | 700 | |
| PT1-4 | 16 | 7.5 | 0.76 | 20.8 | 129 | 26.9 | 55.7 | 98.9 | 306 | 63.0 | 701 | |
| 165 | 16 | 7.8 | 0.70 | 20.0 | 138 | 28.5 | 57.1 | 110 | 276 | 92.8 | 737 | |
| SJ | 16 | 8.0 | 0.38 | 3.64 | 130 | 8.51 | 11.4 | 3.84 | 241 | 47.8 | 555 | |
| QL1 | 16 | 7.8 | 0.20 | 15.7 | 128 | 22.5 | 36.9 | 95.2 | 247 | 110.3 | 671 | |
| G2-1 | 16 | 7.8 | 0.69 | 17.9 | 95.8 | 19.1 | 37.6 | 61.5 | 277 | 29.5 | 553 | |
| G2-2 | 16 | 7.4 | 0.83 | 20.0 | 103 | 21.3 | 40.1 | 64.4 | 296 | 29.6 | 593 | |
| G2-3 | 16 | 7.9 | 0.72 | 14.2 | 96.4 | 18.8 | 36.5 | 60.0 | 272 | 28.2 | 542 | |
| G2-4 | 16 | 7.6 | 0.72 | 18.6 | 91.7 | 15.8 | 32.9 | 60.6 | 272 | 25.9 | 531 | |
| G2-5 | 16 | 7.9 | 0.95 | 11.3 | 95.4 | 17.6 | 33.5 | 61.2 | 269 | 25.9 | 527 | |
| G2-6 | 16 | 7.8 | 0.76 | 19.3 | 88.2 | 16.2 | 25.1 | 69.8 | 260 | 27.9 | 522 | |
| G2-7 | 16 | 7.8 | 0.91 | 16.6 | 90.2 | 16.2 | 26.7 | 47.2 | 256 | 20.6 | 488 | |
| XTS | 25 | 8.3 | 1.30 | 22.9 | 73.3 | 23.1 | 45.4 | 104 | 146 | 28.6 | 454 | |
| HHS | 16 | 7.7 | 0.50 | 18.6 | 102 | 20.9 | 33.4 | 55.2 | 276 | 54.1 | 577 | |
| Yellow River | H5 | 17 | 8.2 | 4.00 | 93.3 | 65.2 | 31.8 | 114 | 155 | 213 | 18.7 | 707 |
| H6 | 26 | 8.6 | 6.57 | 31.4 | 75.1 | 33.5 | 66.1 | 122 | 124 | 54.5 | 531 | |
| H11 | 15 | 8.5 | 3.71 | 70.0 | 75.1 | 26.3 | 118 | 210 | 63.1 | 1.29 | 577 |
| Group | Sample Number | 18O (‰) | D (‰) | 14C (pMC) |
|---|---|---|---|---|
| Karst Groundwater | CL1 | −8.41 | −63.67 | 84.2 |
| CX4 | −7.07 | −52.70 | 95.3 | |
| G2 | −8.65 | −63.04 | ||
| T84 | −7.96 | −58.09 | ||
| T89 | −8.57 | −60.39 | ||
| T93 | −8.27 | −59.85 | ||
| T105 | −7.70 | −56.35 | 96.7 | |
| X5 | −8.93 | −64.66 | ||
| Yellow River | H5 | −8.83 | −65.00 | 90.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Yu, J.; Wu, D.; Han, Y.; Sun, B.; Zheng, L.; Chen, H.; Liu, R. Isotopic and Hydrochemical Characteristics of the Changqing-Xiaolipu Water Resource, Jinan, Eastern China: Implications for Water Resources in the Yellow River Basin. Sustainability 2023, 15, 2439. https://doi.org/10.3390/su15032439
Yu D, Yu J, Wu D, Han Y, Sun B, Zheng L, Chen H, Liu R. Isotopic and Hydrochemical Characteristics of the Changqing-Xiaolipu Water Resource, Jinan, Eastern China: Implications for Water Resources in the Yellow River Basin. Sustainability. 2023; 15(3):2439. https://doi.org/10.3390/su15032439
Chicago/Turabian StyleYu, Dalu, Jieqing Yu, Di Wu, Yu Han, Bin Sun, Lishuang Zheng, Huanliang Chen, and Rui Liu. 2023. "Isotopic and Hydrochemical Characteristics of the Changqing-Xiaolipu Water Resource, Jinan, Eastern China: Implications for Water Resources in the Yellow River Basin" Sustainability 15, no. 3: 2439. https://doi.org/10.3390/su15032439
APA StyleYu, D., Yu, J., Wu, D., Han, Y., Sun, B., Zheng, L., Chen, H., & Liu, R. (2023). Isotopic and Hydrochemical Characteristics of the Changqing-Xiaolipu Water Resource, Jinan, Eastern China: Implications for Water Resources in the Yellow River Basin. Sustainability, 15(3), 2439. https://doi.org/10.3390/su15032439






