Recovery of Calcium from Reaction Fly Ash
Abstract
1. Introduction
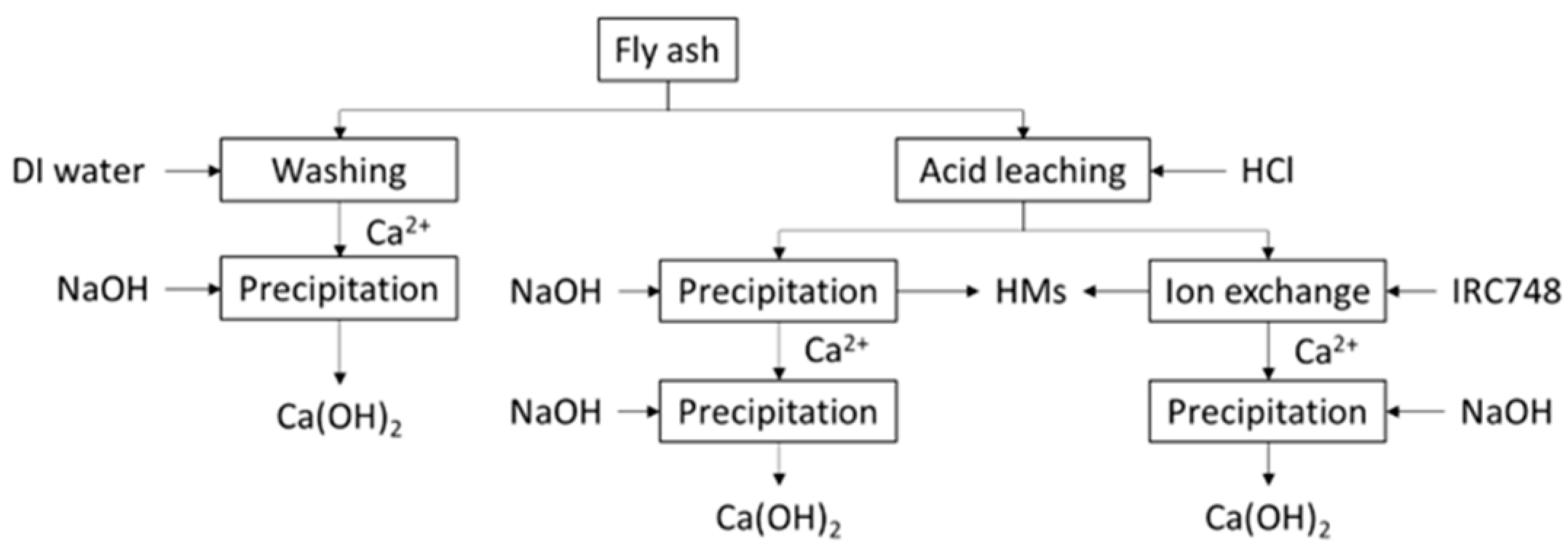
2. Materials and Methods
2.1. Materials
2.2. Fly Ash Washing
2.3. Resin and Column Pretreatment
2.4. Analyst
2.5. Separation of Ion Exchange and Chemical Precipitation
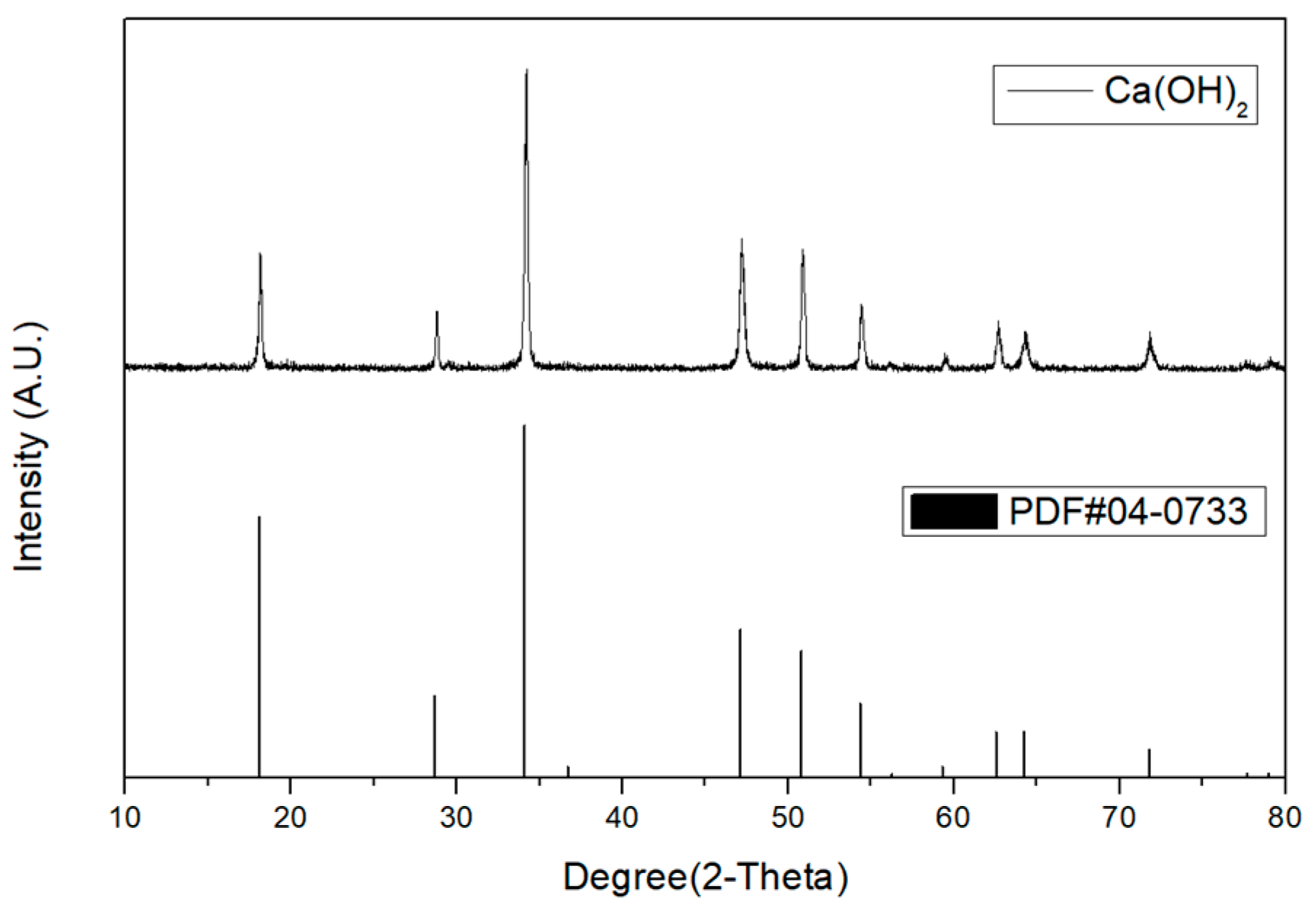
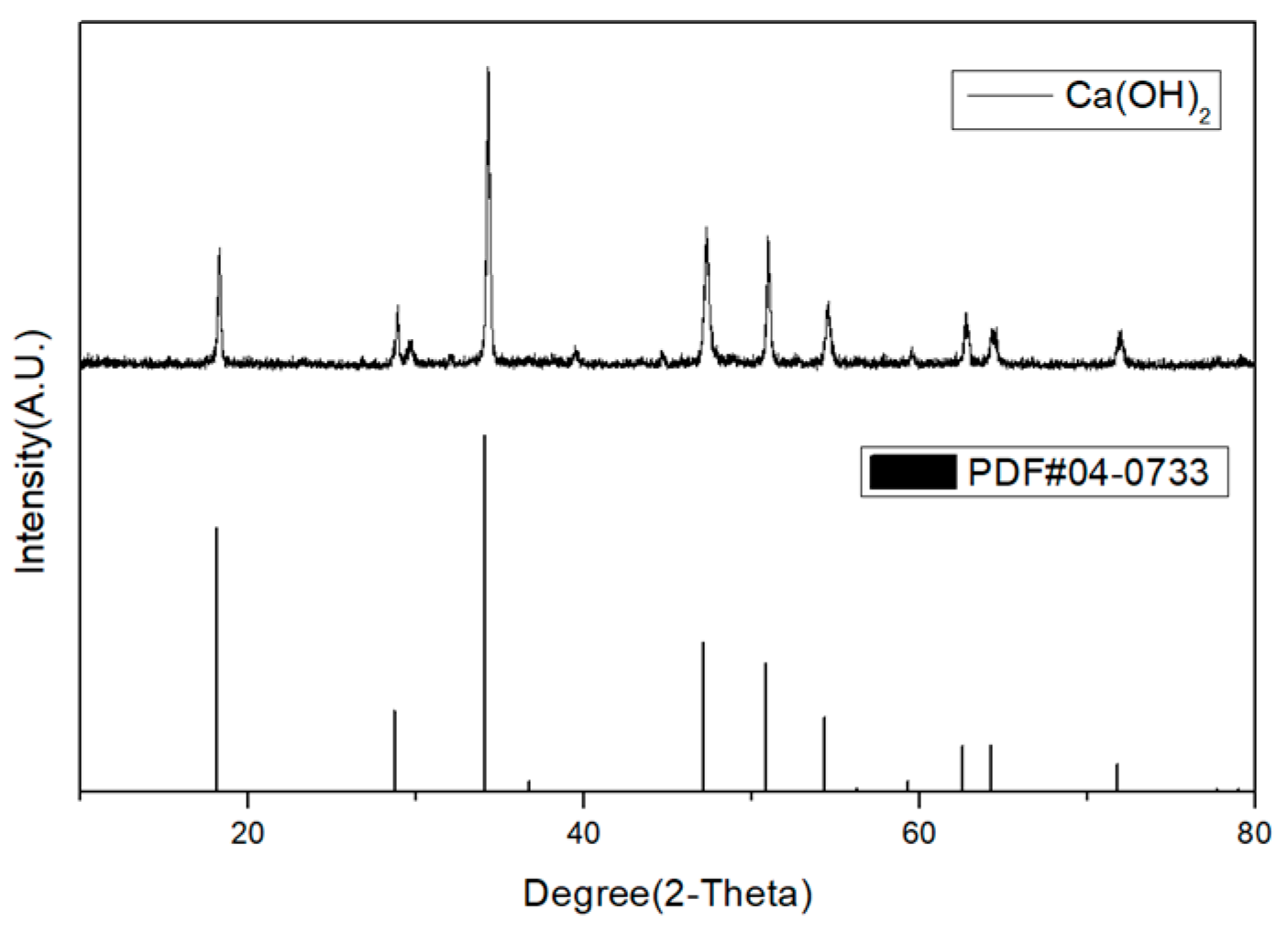
2.6. Analysis of Purity
3. Results and Discussion
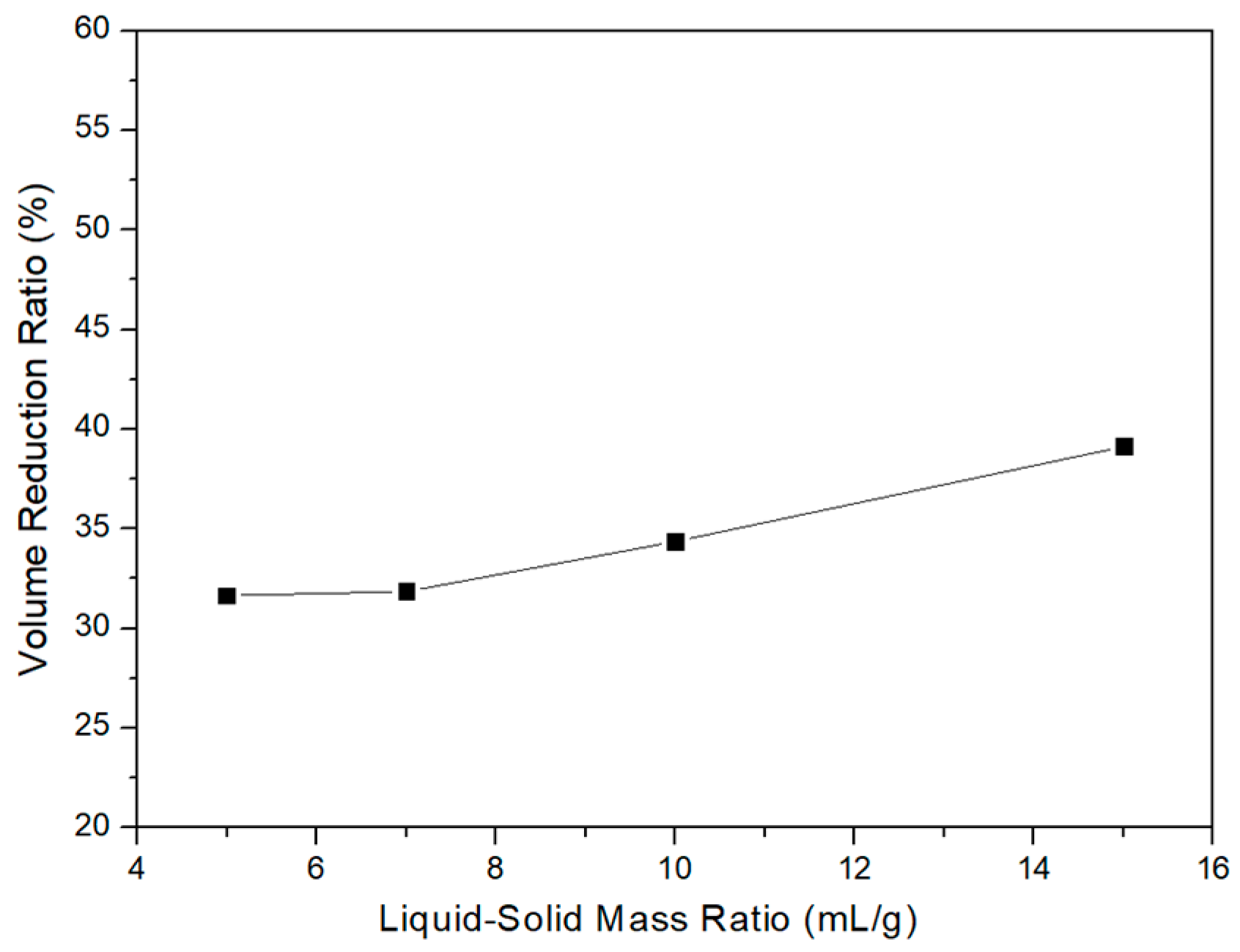
3.1. Washing with Deionized Water
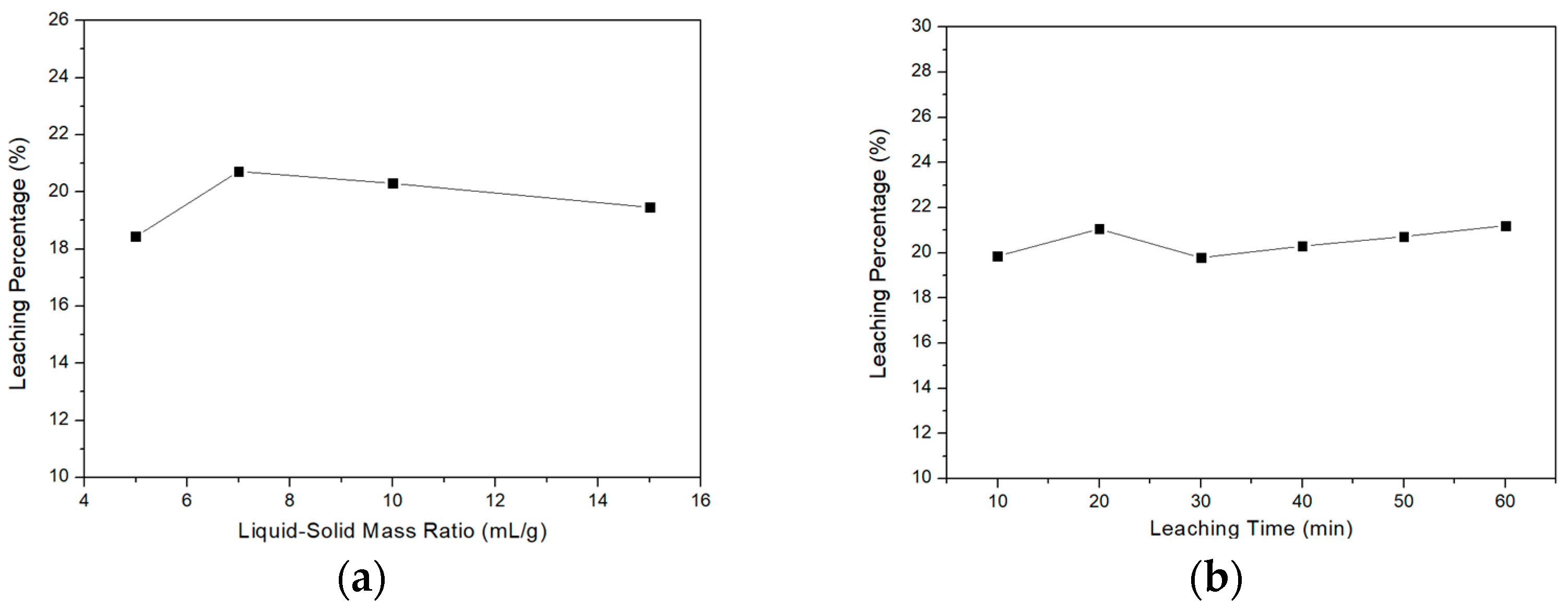
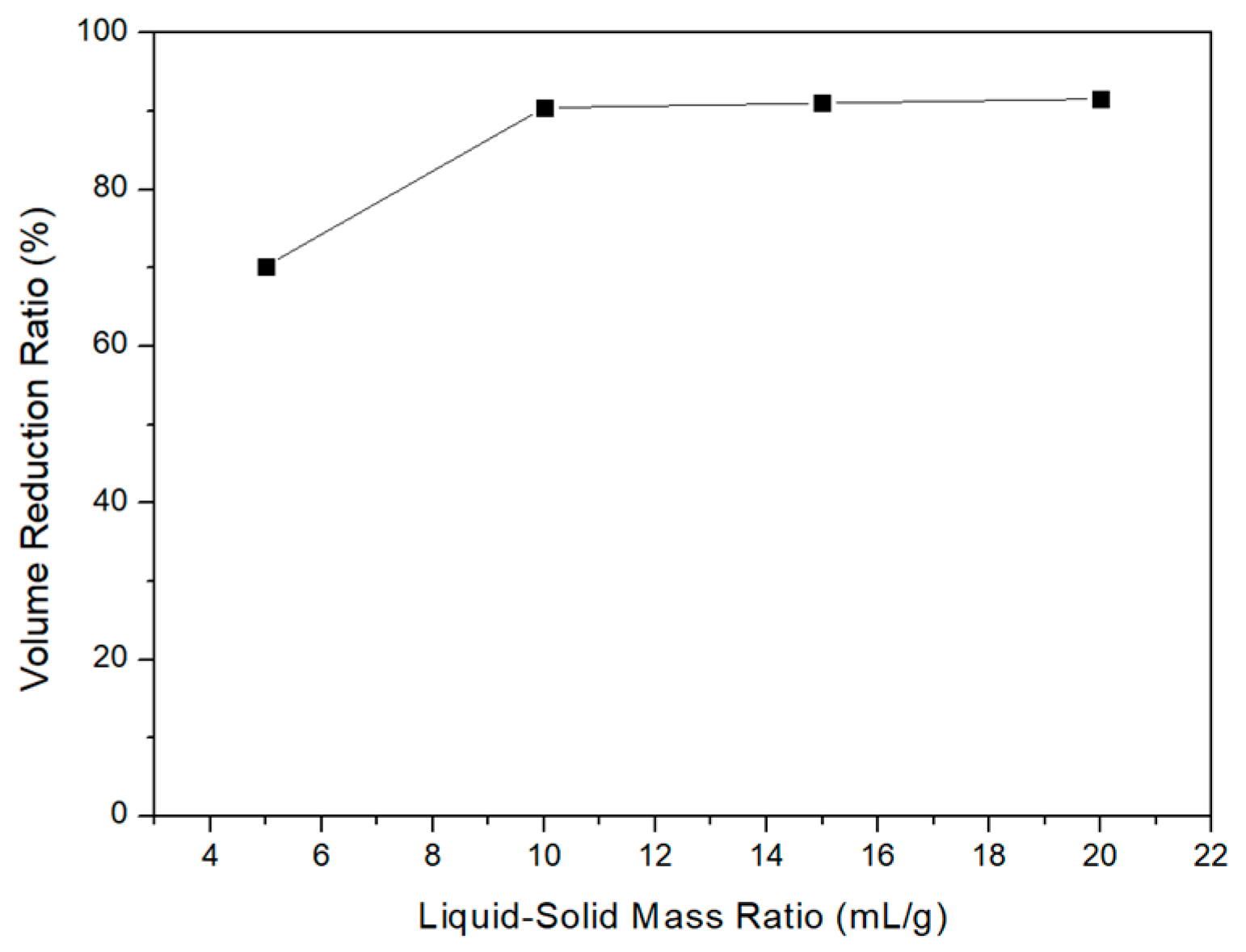
3.2. Acid Leaching
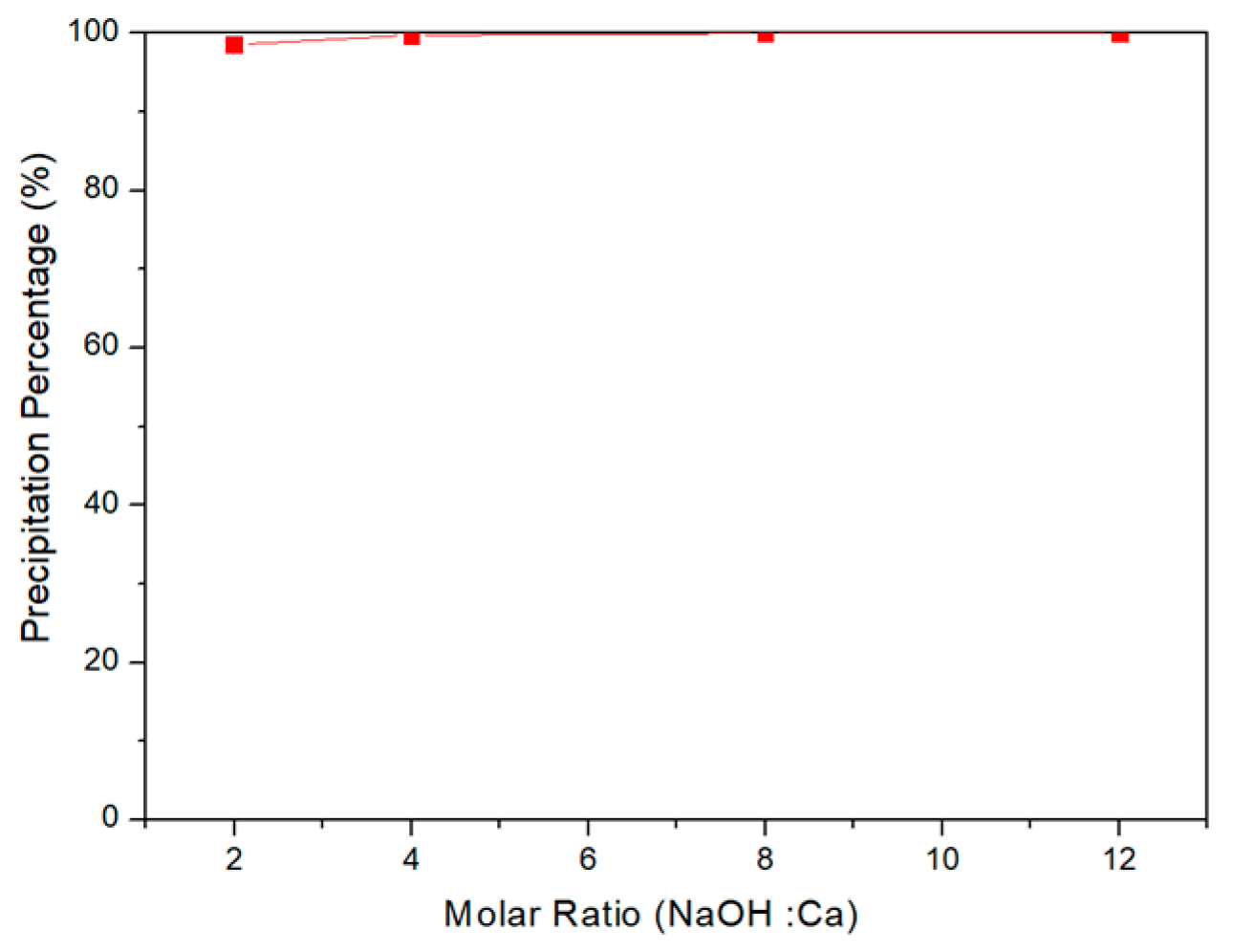
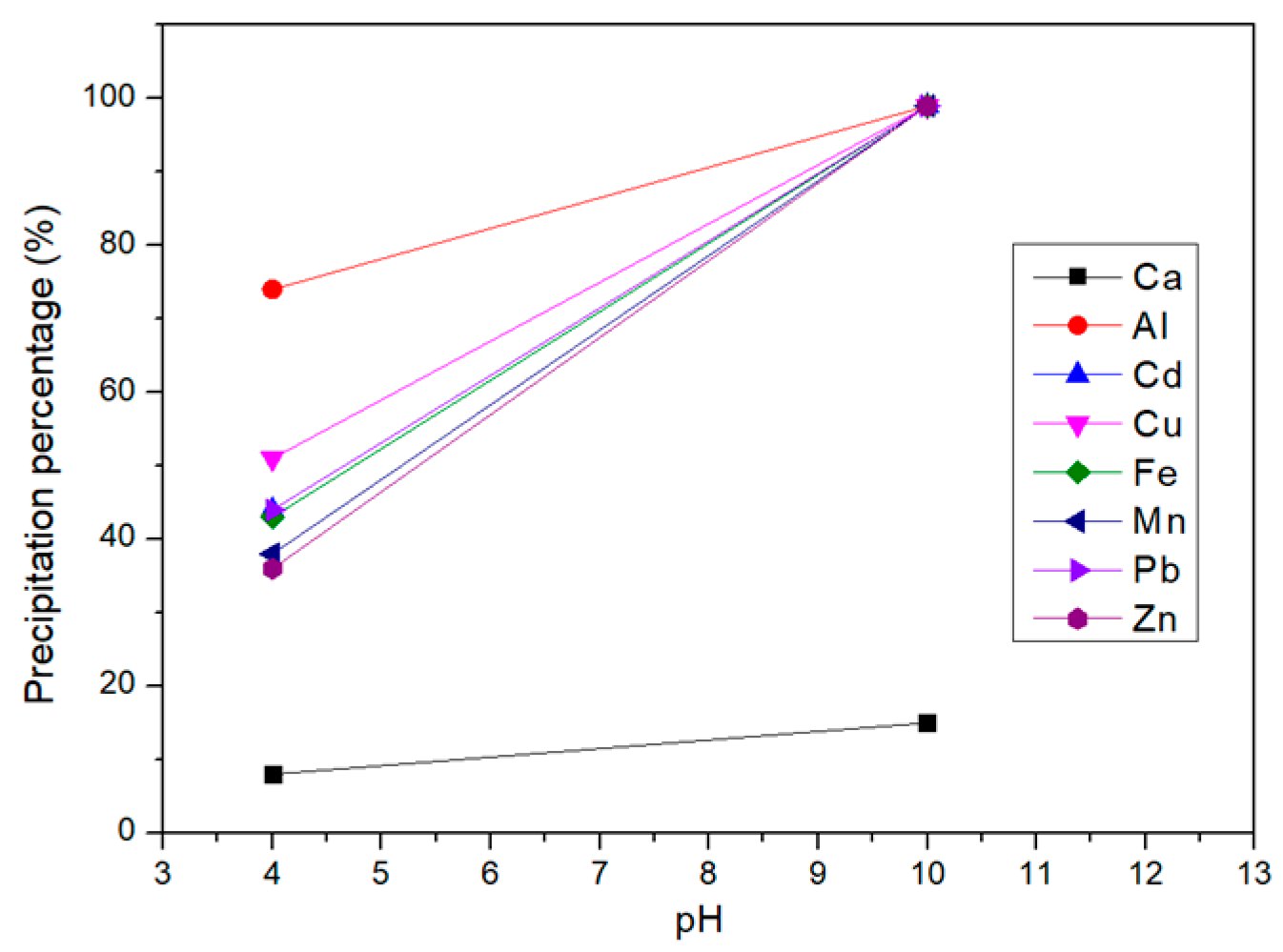
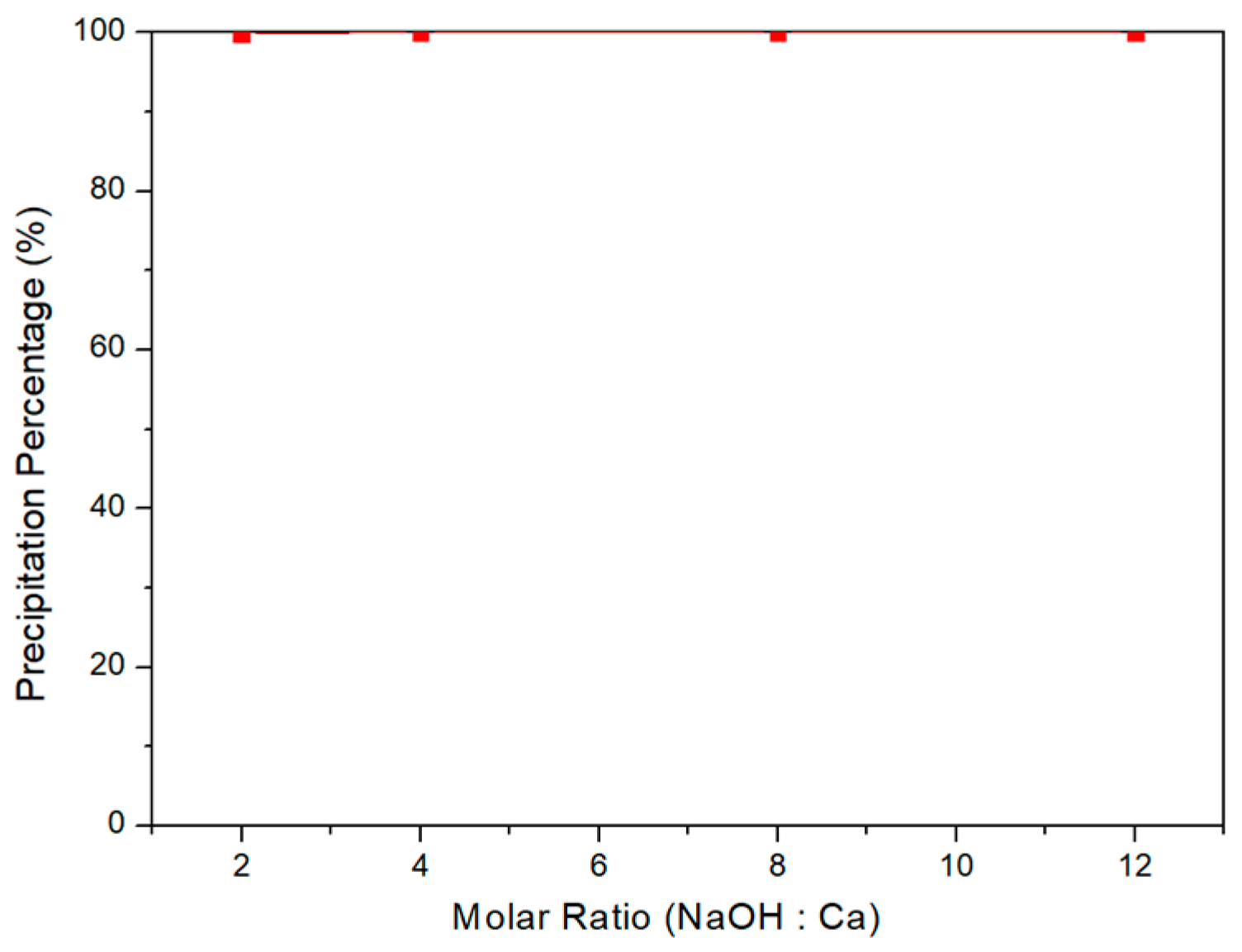
3.3. Selective Chemical Precipitation of Water Washing
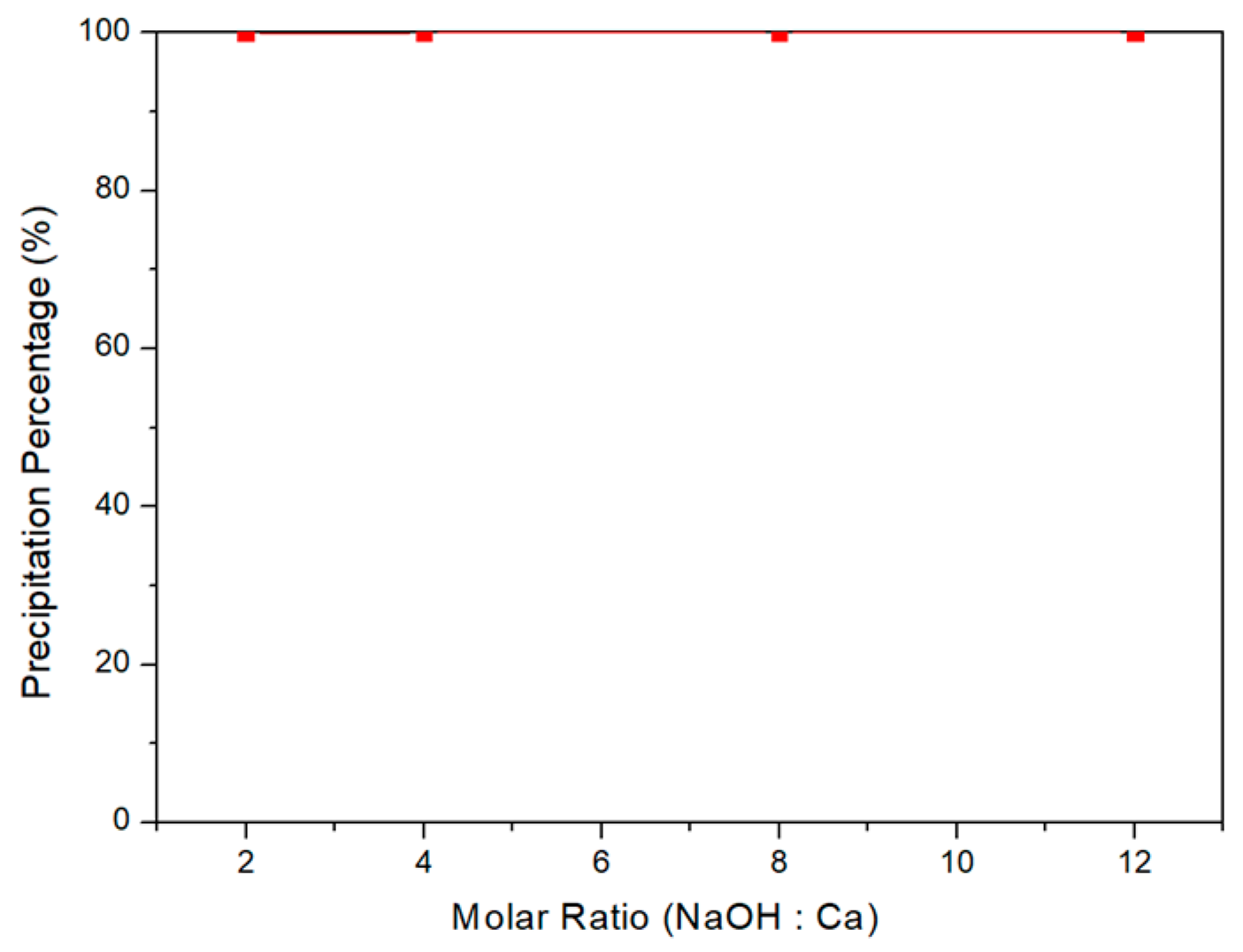
3.4. Selective Chemical Precipitation of Acid Leaching
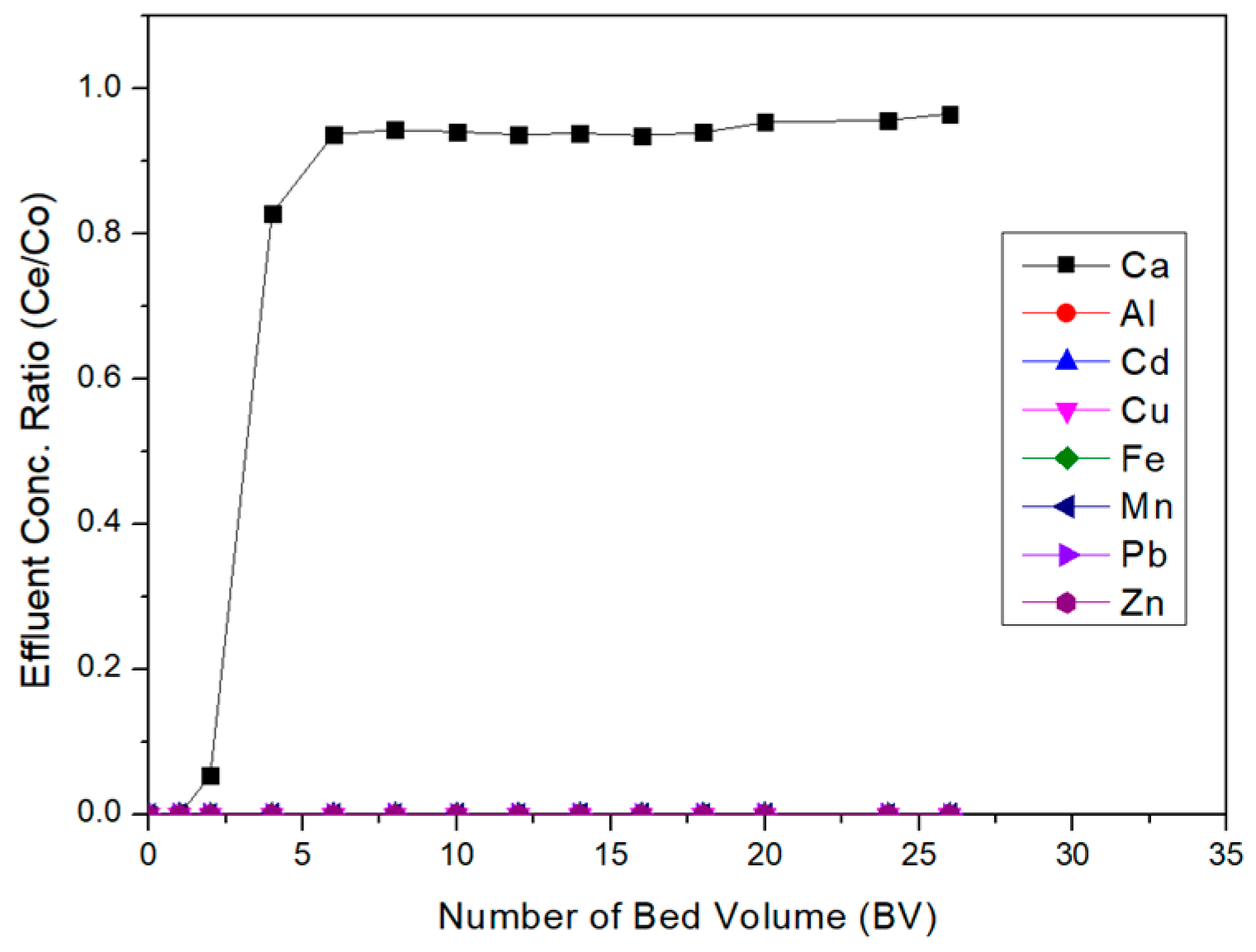
3.5. Ion Exchange for Acid Leaching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lv, J.; Gu, F.; Yang, J.; Guo, J. Environmental and economic performance of an integrated municipal solid waste treatment: A Chinese case study. Sci. Total Environ. 2020, 709, 136096. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.H.; Rechberger, H. Waste to energy—Key element for sustainable waste management. Waste Manag. 2015, 37, 3–12. [Google Scholar] [CrossRef]
- Eghbali, H.; Arkat, J.; Tavakkoli-Moghaddam, R. Sustainable supply chain network design for municipal solid waste management: A case study. J. Clean. Prod. 2022, 381, 135211. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Granular Material Development Applied in an Experimental Section for Civil Engineering Purposes. Appl. Sci. 2020, 10, 6782. [Google Scholar] [CrossRef]
- Kirkelund, G.M.; Skevi, L.; Ottosen, L.M. Electrodialytically treated MSWI fly ash use in clay bricks. Constr. Build. Mater. 2020, 254, 119286. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Zhao, X.; Zhu, B.; Zhang, G. A Review on the Management of Municipal Solid Waste Fly Ash in American. Procedia Environ. Sci. 2016, 31, 535–540. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.C.; Quinta-Ferreira, R.M. Treatment and use of air pollution control residues from MSW incineration: An overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef]
- Chang, Y.M.; Fan, W.P.; Dai, W.C.; Hsi, H.C.; Wu, C.H.; Chen, C.H. Characteristics of PCDD/F content in fly ash discharged from municipal solid waste incinerators. J. Hazard. Mater. 2011, 192, 521–529. [Google Scholar] [CrossRef]
- Loginova, E.; Proskurnin, M.; Brouwers, H.J.H. Municipal solid waste incineration (MSWI) fly ash composition analysis: A case study of combined chelatant-based washing treatment efficiency. J. Environ. Manag. 2019, 235, 480–488. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Shu, C.M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.A.D.; Calmano, W.; Ecke, H. Wet extraction of heavy metals and chloride from MSWI and straw combustion fly ashes. Waste Manag. 2009, 29, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Comans, R.N.J.; Meima, J.A.; Geelhoed, P.A. Reduction of contaminant leaching from MSWI bottom ash by addition of sorbing components. Waste Manag. 2000, 20, 125–133. [Google Scholar] [CrossRef]
- Dontriros, S.; Likitlersuang, S.; Janjaroen, D. Mechanisms of chloride and sulfate removal from municipal-solid-waste-incineration fly ash (MSWI FA): Effect of acid-base solutions. Waste Manag. 2020, 101, 44–53. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Y.; He, D.; Yang, E.H. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef]
- Meima, J.A.; Comans, R.N.J. Overview of Geochemical Processes Controlling Leaching Characteristics of MSWI Bottom Ash. In Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1997; Volume 71, pp. 447–457. [Google Scholar] [CrossRef]
- Nithiya, A.; Saffarzadeh, A.; Shimaoka, T. Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (MSWI) bottom ash. Waste Manag. 2018, 73, 342–350. [Google Scholar] [CrossRef]
- Santos, R.M.; Mertens, G.; Salman, M.; Cizer, O.; Gerven, T.V. Comparative study of ageing, heat treatment and accelerated carbonation for stabilization of municipal solid waste incineration bottom ash in view of reducing regulated heavy metal/metalloid leaching. J. Environ. Manag. 2013, 128, 807–821. [Google Scholar] [CrossRef]
- Ye, Z.L.; Xie, X.; Dai, L.; Wang, Z.; Wu, W.; Zhao, F.; Xie, X.; Huang, S.; Liu, M.; Chen, S. Full-scale blending treatment of fresh MSWI leachate with municipal wastewater in a wastewater treatment plant. Waste Manag. 2014, 34, 2305–2311. [Google Scholar] [CrossRef]
- Yen, C.P.; Zhou, S.Y.; Shen, Y.H. The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash. Sustainability 2020, 12, 9086. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. Percolation and batch leaching tests to assess release of inorganic pollutants from municipal solid waste incinerator residues. Waste Manag. 2011, 31, 236–245. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Phoungthong, K.; Shi, D.X.; Shen, W.H.; Shao, L.M.; He, P.J. Leaching characteristics of calcium-based compounds in MSWI Residues: From the viewpoint of clogging risk. Waste Manag. 2015, 42, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Weibel, G.; Eggenberger, U.; Kulik, D.A.; Hummel, W.; Schlumberger, S.; Klimk, W.; Fisch, M.; Mader, U.K. Extraction of heavy metals from MSWI fly ash using hydrochloric acid and sodium chloride solution. Waste Manag. 2018, 76, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Karlfeldt, K.; Steenari, B.M. Assessment of metal mobility in MSW incineration ashes using water as the reagent. Fuel 2007, 86, 1983–1993. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Sun, Y.; Fang, G.; Li, Y. Release of soluble ions and heavy metal during fly ash washing by deionized water and sodium carbonate solution. Chemosphere 2022, 307, 135860. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xi, B.; Li, X.; Zhang, L.; Wei, Z. Effect of water-extraction on characteristics of melting and solidification of fly ash from municipal solid waste incinerator. J. Hazard. Mater. 2009, 161, 871–877. [Google Scholar] [CrossRef]
- Wang, K.S.; Chiang, K.Y.; Lin, K.L.; Sun, C.J. Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 2001, 62, 73–81. [Google Scholar] [CrossRef]
- Cao, Y.N.; Luo, J.J.; Sun, S.Q. Characteristics of MSWI fly ash with acid leaching treatment. J. Fuel Chem. Technol. 2021, 49, 1208–1218. [Google Scholar] [CrossRef]
- Tang, J.; Dong, S.; Feng, Q.; Su, M.; Wei, Y.; Liang, J.; Zhang, H.; Huang, L.; Kong, L.; Wang, N.; et al. Optimizing critical metals recovery and correlative decontamination from MSWI fly ash: Evaluation of an integrating two-step leaching hydrometallurgical process. J. Clean. Prod. 2022, 368, 133017. [Google Scholar] [CrossRef]
- Nagib, S.; Inoue, K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy 2000, 56, 269–292. [Google Scholar] [CrossRef]
- Monhemius, J. Precipitation diagrams for metal hydroxides, sulphides, arsenates and phosphates. Trans. Inst. Min. Metall. 1977, 86, C202–C206. Available online: https://www.researchgate.net/publication/266137129_Precipitation_diagrams_for_metal_hydroxides_sulphides_arsenates_and_phosphates (accessed on 20 December 2022).
- Mendes, F.D.; Martins, A.H. Selective sorption of nickel and cobalt from sulphate solutions using chelating resins. Int. J. Miner. Process. 2004, 74, 359–371. [Google Scholar] [CrossRef]
- Jang, K.; Choi, W.Y.; Lee, D.; Park, J.; Yoo, Y. Purification of landfill gas by extracted calcium ions from municipal solid waste incineration fly ash. Sci. Total Environ. 2022, 807, 150729. [Google Scholar] [CrossRef] [PubMed]


| Element | Ca | Al | Cd | Cu | Fe | Mn | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| weight percentage (%) | 48.11 | 0.602 | 0.014 | 0.051 | 0.633 | 0.022 | 0.139 | 0.775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-Z.; Lin, H.-H.; Tang, Y.-C.; Shen, Y.-H. Recovery of Calcium from Reaction Fly Ash. Sustainability 2023, 15, 2428. https://doi.org/10.3390/su15032428
Wang J-Z, Lin H-H, Tang Y-C, Shen Y-H. Recovery of Calcium from Reaction Fly Ash. Sustainability. 2023; 15(3):2428. https://doi.org/10.3390/su15032428
Chicago/Turabian StyleWang, Jian-Zhi, Hsiao-Han Lin, Yi-Chin Tang, and Yun-Hwei Shen. 2023. "Recovery of Calcium from Reaction Fly Ash" Sustainability 15, no. 3: 2428. https://doi.org/10.3390/su15032428
APA StyleWang, J.-Z., Lin, H.-H., Tang, Y.-C., & Shen, Y.-H. (2023). Recovery of Calcium from Reaction Fly Ash. Sustainability, 15(3), 2428. https://doi.org/10.3390/su15032428







