Improving the Heat Transfer of Phase Change Composites for Thermal Energy Storage by Adding Copper: Preparation and Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
Chemical and Microstructure Characterization
3. Results
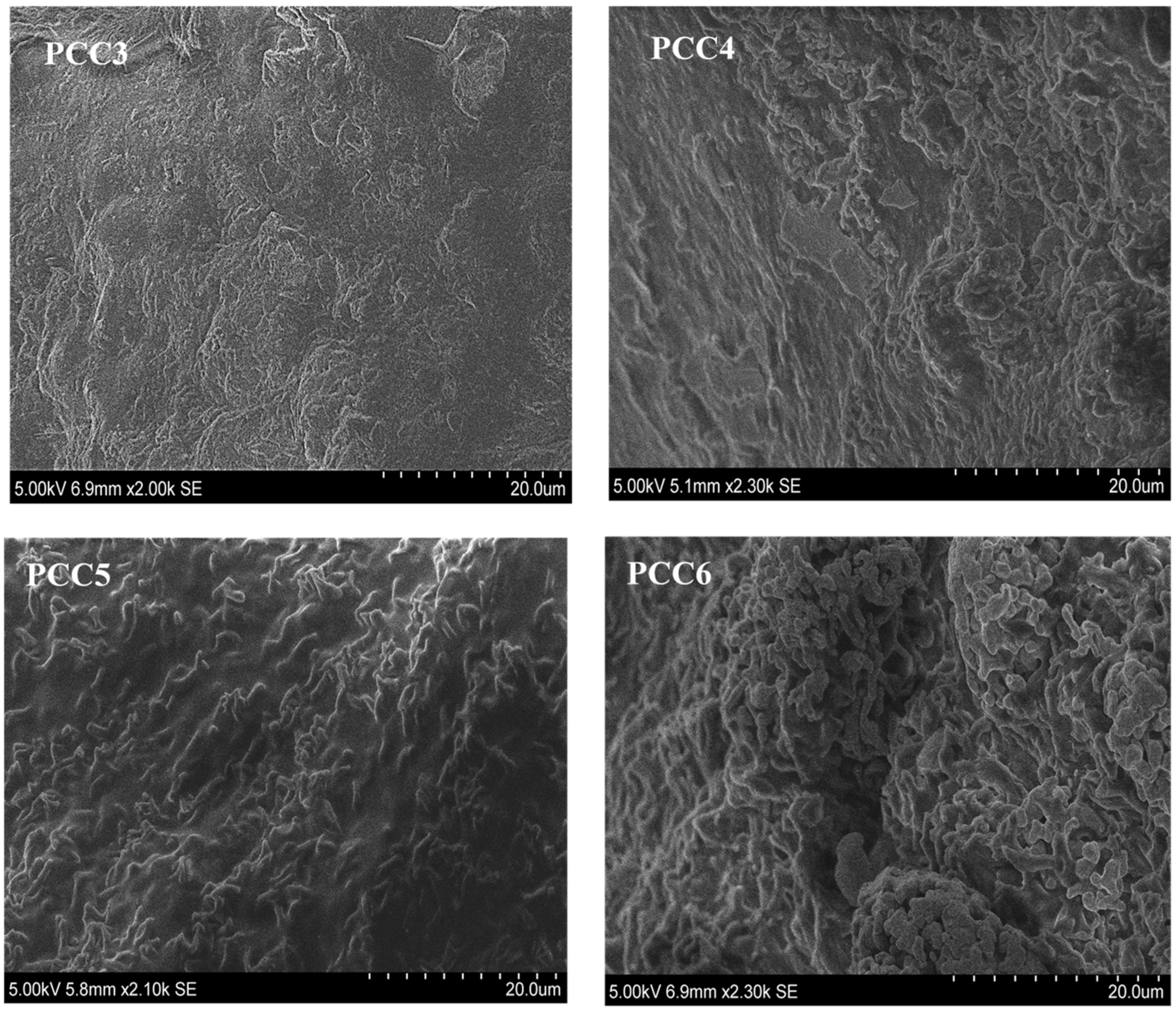
3.1. Morphologies and Microstructures of PCCs
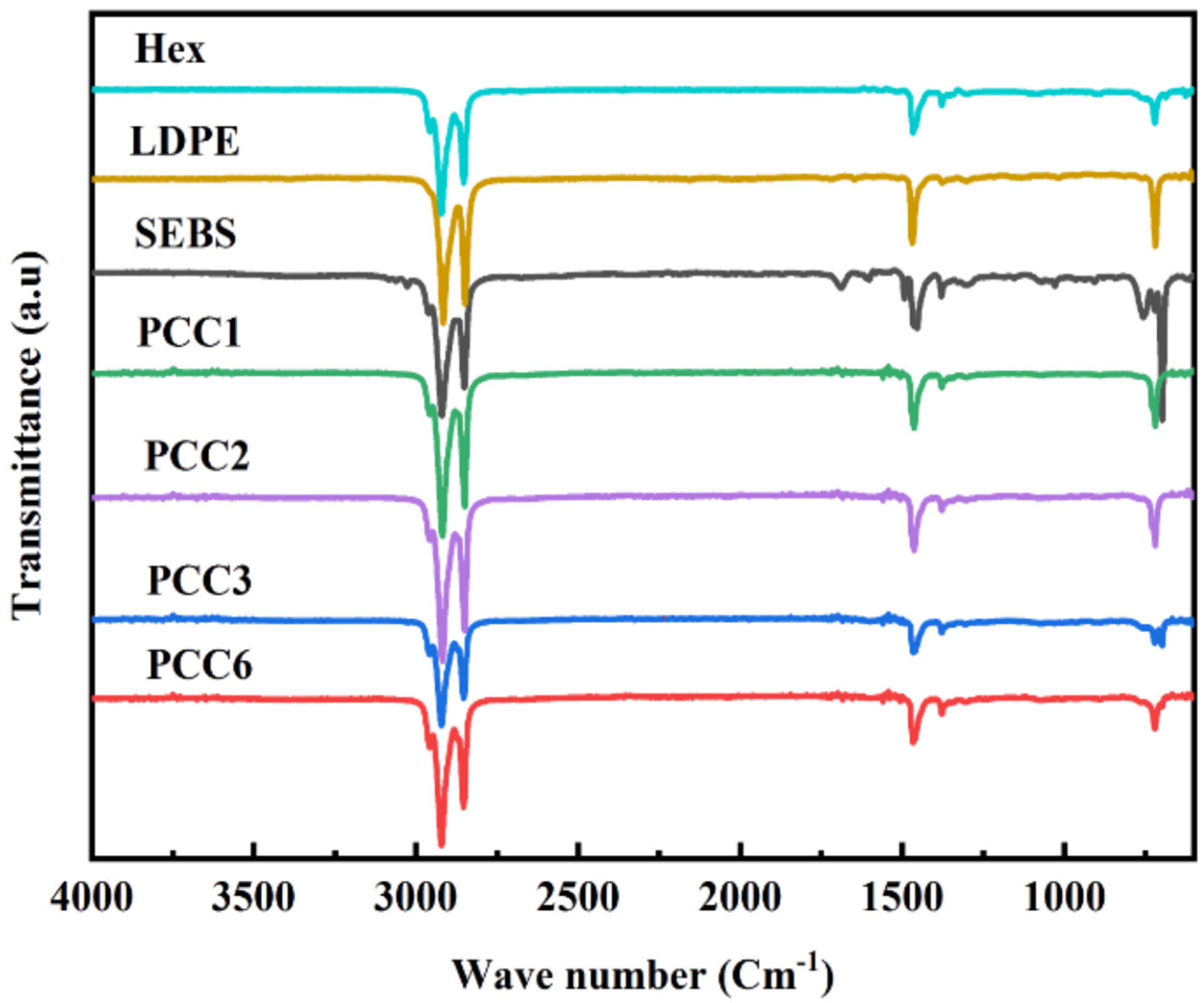
3.2. Chemical Compatibility of PCCs
3.3. Thermal Stability Analysis of PCCs
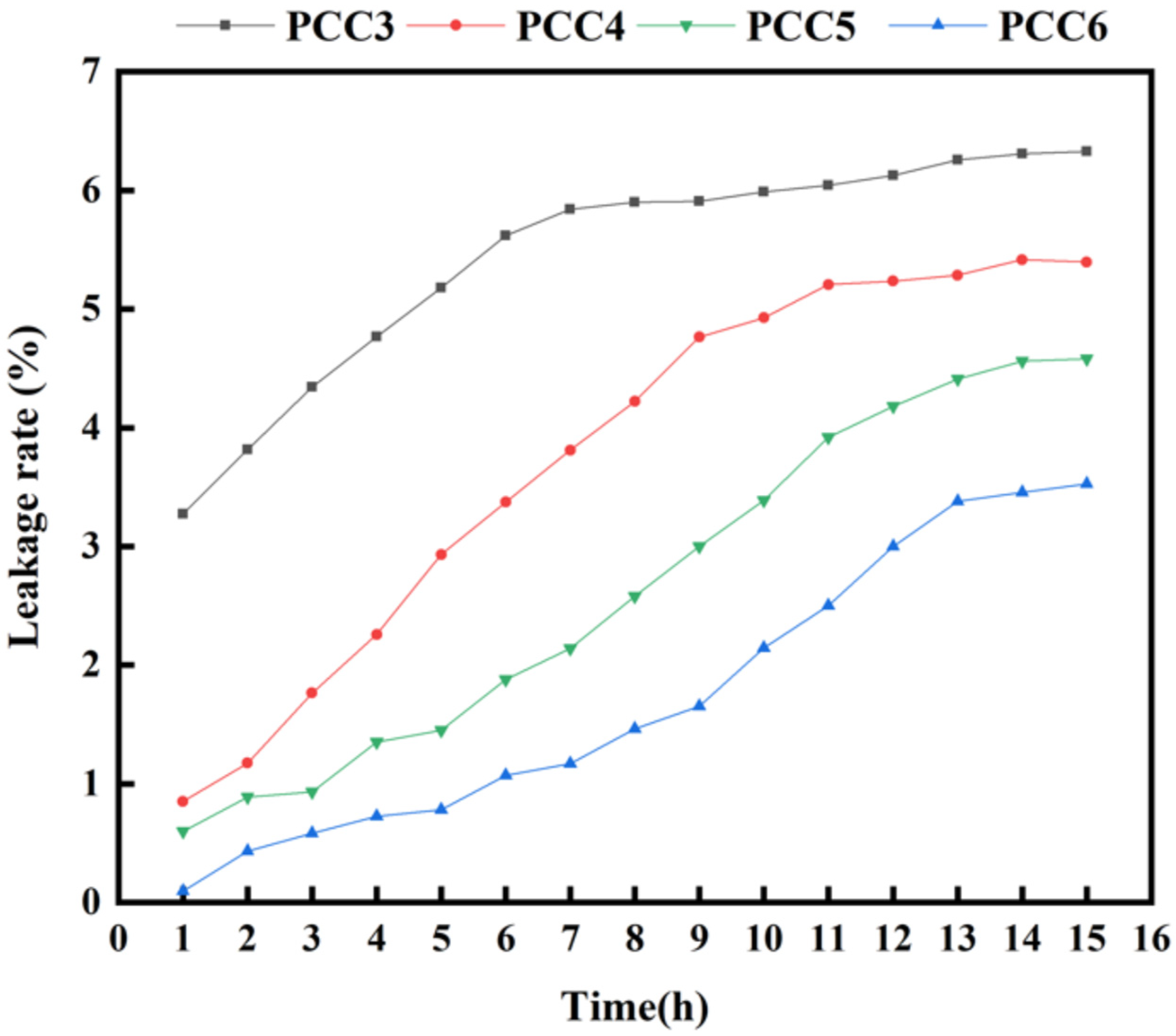
3.4. The Shape Stability of PCCs
3.5. TES Performance of PCCs
- Cp is the specific heat capacity at constant pressure in units of J/(g·K),
- ΔH is the latent heat of fusion in units of J/g.
- The operating temperature range of the storage is represented by T1 and T2.
- is the heat flow measured in W/g,
- is the rate of DSC heating measured in °C/s.
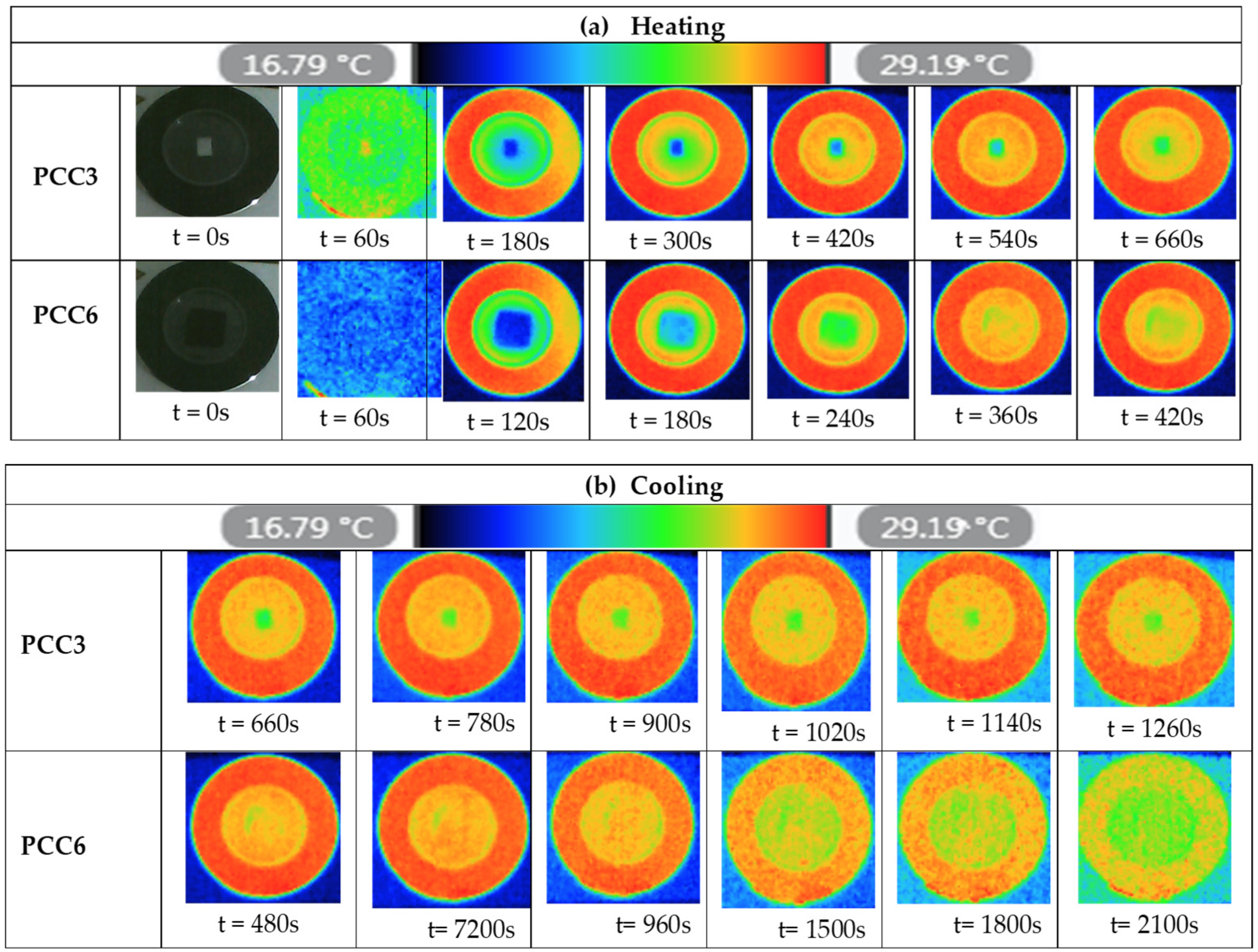
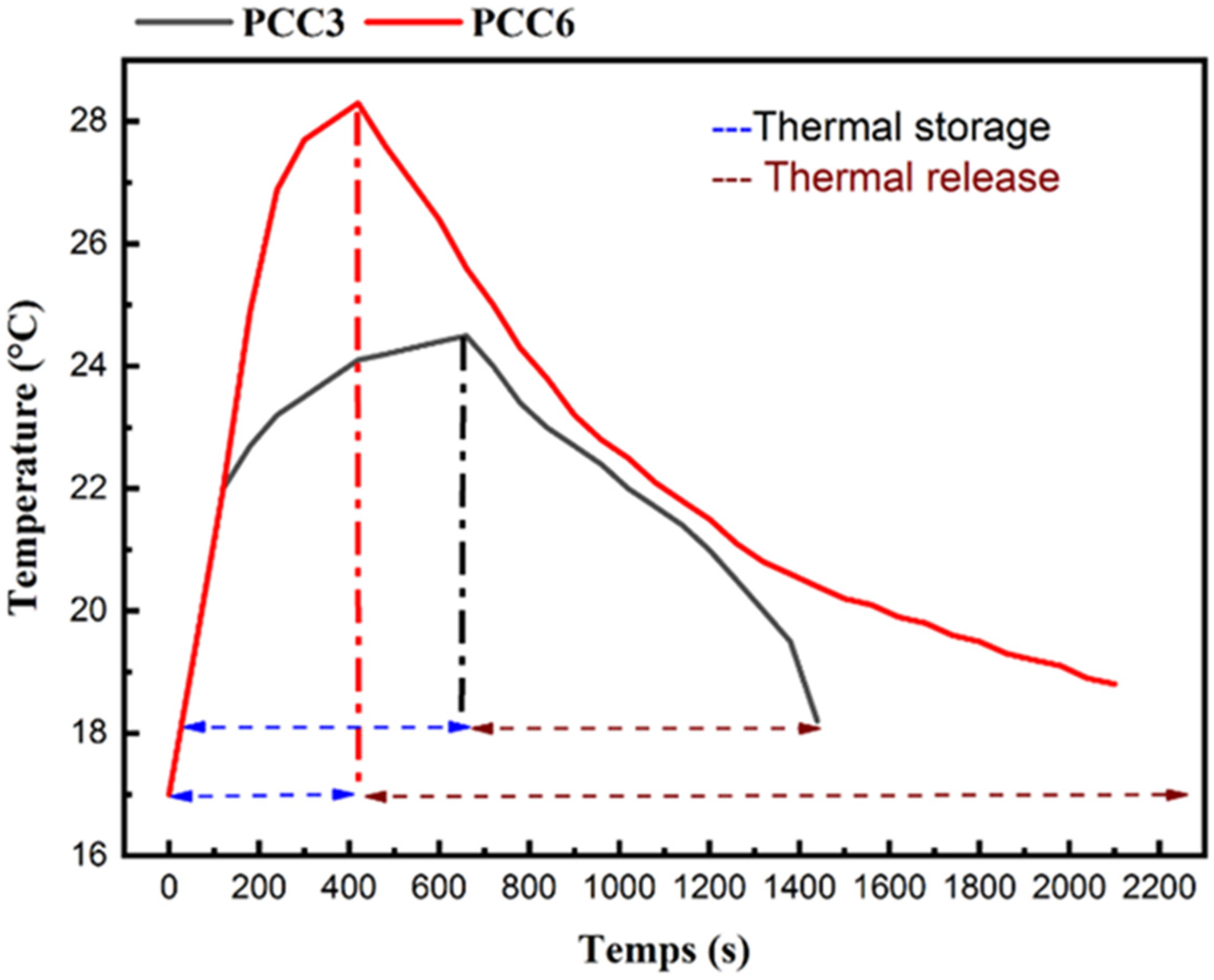
3.6. Infrared Thermography (IRT) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ge, X.; Chen, Y.; Liu, W.; Zhang, G.; Li, X.; Ge, J.; Li, C. Liquid cooling system for battery modules with boron nitride based thermal conductivity silicone grease. RSC Adv. 2022, 12, 4311–4321. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Tian, Y.; Zhou, D.; Ye, W.; Huang, Y.; Zhang, Y. Heat transfer enhancement in latent heat thermal energy storage using copper foams with varying porosity. Sol. Energy 2021, 221, 75–86. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Chirino, H.; Xu, B.; Xu, X.; Guo, P. Generalized diagrams of energy storage efficiency for latent heat thermal storage system in concentrated solar power plant. Appl. Therm. Eng. 2018, 129, 1595–1603. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Hughes, B.R.; Cheuk-Ming, M. A study of wind and buoyancy driven flows through commercial wind towers. Energy Build. 2011, 43, 1784–1791. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xu, X.; Zhang, S. Research progress of phase change cold storage materials used in cold chain transportation and their different cold storage packaging structures. J. Mol. Liq. 2020, 319, 114360. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Cai, Y.; Wang, R.; Li, T. Form-stable phase change composites: Preparation, performance, and applications for thermal energy conversion, storage and management. Energy Storage Mater. 2021, 42, 380–417. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Khodadadi, J. Melting and convection of phase change materials in different shape containers: A review. Renew. Sustain. Energy Rev. 2015, 43, 449–477. [Google Scholar] [CrossRef]
- Nejman, A.; Gromadzińska, E.; Kamińska, I.; Cieślak, M. Assessment of Thermal Performance of Textile Materials Modified with PCM Microcapsules Using Combination of DSC and Infrared Thermography Methods. Molecules 2019, 25, 122. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.C.; Katz, R.F.; Hewitt, I.J. Magmatic Intrusions Control Io’s Crustal Thickness. J. Geophys. Res. Planets 2020, 125, e2020JE006443. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Chen, L.; Gu, Y.; Guo, M.; Han, L.; Ben, X.; Yuan, H.; Lin, Z. Preparation and characterization of capric-stearic acid/montmorillonite/graphene composite phase change material for thermal energy storage in buildings. Constr. Build. Mater. 2021, 301, 124102. [Google Scholar] [CrossRef]
- Lu, X.; Liu, H.; Murugadoss, V.; Seok, I.; Huang, J.; Ryu, J.E.; Guo, Z. Polyethylene Glycol/Carbon Black Shape-Stable Phase Change Composites for Peak Load Regulating of Electric Power System and Corresponding Thermal Energy Storage. Eng. Sci. 2020, 9, 25–34. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H. Single-walled carbon nanotube for shape stabilization and enhanced phase change heat transfer of polyethylene glycol phase change material. Energy Convers. Manag. 2017, 143, 96–108. [Google Scholar] [CrossRef]
- Inaba, H.; Tu, P. Evaluation of thermophysical characteristics on shape-stabilized paraffin as a solid-liquid phase change material. Heat Mass Transf. 1997, 32, 307–312. [Google Scholar] [CrossRef]
- Trigui, A.; Karkri, M.; Boudaya, C.; Candau, Y.; Ibos, L.; Fois, M. Experimental investigation of a composite phase change material: Thermal-energy storage and release. J. Compos. Mater. 2012, 48, 49–62. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, Y.; Song, L.; Lu, H.; Chen, Z.; Fan, W. Preparation and characterizations of HDPE–EVA alloy/OMT nanocomposites/paraffin compounds as a shape stabilized phase change thermal energy storage material. Thermochim. Acta 2006, 451, 44–51. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J. Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid–liquid phase change materials. Appl. Energy 2009, 86, 170–174. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Trigui, A.; Karkri, M.; Krupa, I. Thermal conductivity and latent heat thermal energy storage properties of LDPE/wax as a shape-stabilized composite phase change material. Energy Convers. Manag. 2014, 77, 586–596. [Google Scholar] [CrossRef]
- Juarez, D.; Ferrand, S.; Fenollar, O.; Fombuena, V.; Balart, R. Improvement of thermal inertia of styrene–ethylene/butylene–styrene (SEBS) polymers by addition of microencapsulated phase change materials (PCMs). Eur. Polym. J. 2011, 47, 153–161. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, D.; Shi, J. Experimental and numerical study on melting of phase change materials in metal foams at pore scale. Int. J. Heat Mass Transf. 2014, 72, 646–655. [Google Scholar] [CrossRef]
- Vélez, C.; Khayet, M.; de Zárate, J.O. Temperature-dependent thermal properties of solid/liquid phase change even-numbered n-alkanes: N-Hexadecane, n-octadecane and n-eicosane. Appl. Energy 2015, 143, 383–394. [Google Scholar] [CrossRef]
- Tang, B.; Qiu, M.; Zhang, S. Thermal conductivity enhancement of PEG/SiO2 composite PCM by in situ Cu doping. Sol. Energy Mater. Sol. Cells 2012, 105, 242–248. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Ma, B.; Wen, R.; Zhang, M.; Huang, Y.; Fang, M.; Liu, Y.-G.; Wu, X. Polyethylene glycol/Cu/SiO 2 form stable composite phase change materials: Preparation, characterization, and thermal conductivity enhancement. RSC Adv. 2016, 6, 58740–58748. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar] [CrossRef]
- Zeng, J.L.; Cao, Z.; Yang, D.W.; Sun, L.X.; Zhang, L. Thermal conductivity enhancement of Ag nanowires on an organic phase change material. J. Therm. Anal. Calorim. 2009, 101, 385–389. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Li, Y.; Yin, X. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Tang, B.; Wu, C.; Qiu, M.; Zhang, X.; Zhang, S. PEG/SiO2–Al2O3 hybrid form-stable phase change materials with enhanced thermal conductivity. Mater. Chem. Phys. 2014, 144, 162–167. [Google Scholar] [CrossRef]
- Sharma, R.; Ganesan, P.; Tyagi, V.; Metselaar, H.; Sandaran, S. Thermal properties and heat storage analysis of palmitic acid-TiO 2 composite as nano-enhanced organic phase change material (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Guo, Z.; Guan, L.; Li, Y. Improved thermal properties of paraffin wax by the addition of TiO2 nanoparticles. Appl. Therm. Eng. 2014, 73, 1541–1547. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Wen, X.; Yin, H.; Liu, J. A novel CNT encapsulated phase change material with enhanced thermal conductivity and photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2018, 184, 82–90. [Google Scholar] [CrossRef]
- Ye, F.; Ge, Z.; Ding, Y.; Yang, J. Multi-walled carbon nanotubes added to Na2CO3/MgO composites for thermal energy storage. Particuology 2014, 15, 56–60. [Google Scholar] [CrossRef]
- Shi, J.-N.; Ger, M.-D.; Liu, Y.-M.; Fan, Y.-C.; Wen, N.-T.; Lin, C.-K.; Pu, N.-W. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives. Carbon 2012, 51, 365–372. [Google Scholar] [CrossRef]
- Lopez, J.; Acem, Z.; Del Barrio, E.P. KNO3/NaNO3– Graphite materials for thermal energy storage at high temperature: Part II. –Phase transition properties. Appl. Therm. Eng. 2010, 30, 1586–1593. [Google Scholar] [CrossRef]
- Li, M. A nano-graphite/paraffin phase change material with high thermal conductivity. Appl. Energy 2013, 106, 25–30. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Wang, T.; Li, W.; Chen, L.; Zou, R.; Zheng, J.; Li, X. Enhancing the thermal conductivity of n-eicosane/silica phase change materials by reduced graphene oxide. Mater. Chem. Phys. 2014, 147, 701–706. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Preparation and properties of highly conductive palmitic acid/graphene oxide composites as thermal energy storage materials. Energy 2013, 58, 628–634. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Tang, B.; Xu, S.; Shufen, Z. Novel light–driven CF/PEG/SiO2 composite phase change materials with high thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Liu, L.; Fang, G. Microstructure and thermal properties of cetyl alcohol/high density polyethylene composite phase change materials with carbon fiber as shape-stabilized thermal storage materials. Appl. Energy 2017, 200, 19–27. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.X.; Yan, T.; Dai, Y.J.; Wang, R.Z. High performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage. Int. J. Heat Mass Transf. 2016, 102, 733–744. [Google Scholar] [CrossRef]
- Chriaa, I.; Karkri, M.; Trigui, A.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. The performances of expanded graphite on the phase change materials composites for thermal energy storage. Polymer 2020, 212, 123128. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, M.; Huang, F.; Lin, T.; Wan, D. Effect of graphene aerogel on thermal behavior of phase change materials for thermal management. Sol. Energy Mater. Sol. Cells 2013, 113, 195–200. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, Q.; Hu, D.; Feng, J. Core–shell-like structured graphene aerogel encapsulating paraffin: Shape-stable phase change material for thermal energy storage. J. Mater. Chem. A 2014, 3, 4018–4025. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem. Eng. J. 2017, 315, 481–490. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Z.; Wang, C.; Chen, K.; Cai, Z.; Wang, T. Preparation and Thermal Properties of Stearic Acid/n-Octadecane Binary Eutectic Mixture as Phase Change Materials for Energy Storage. Chemistryselect 2019, 4, 4125–4130. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using β-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Zhou, W.; Wang, J.; Wang, Y. Characterization of polymethyl methacrylate/polyethylene glycol/aluminum nitride composite as form-stable phase change material prepared by in situ polymerization method. Thermochim. Acta 2011, 524, 128–134. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, Y.; Sun, L.; Cao, X.; Yang, X. Experimental studies on the supercooling and melting/freezing characteristics of nano-copper/sodium acetate trihydrate composite phase change materials. Renew. Energy 2016, 99, 1029–1037. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, Y.; Sun, L.; Cao, X.; Yang, X. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2019, 25, 251–295. [Google Scholar] [CrossRef]
- Al-Mahmodi, A.F.; Afolabi, L.O.; Awadh, M.G.; Batcha, M.F.M.; Zamani, N.; Isa, N.M.; Didane, D.H. Thermal Behaviour of Nanocomposite Phase Change Material for Solar Thermal Applications. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 88, 133–146. [Google Scholar] [CrossRef]
- Lokesh, S.; Murugan, P.; Sathishkumar, A.; Kumaresan, V.; Velraj, R. Melting/solidification characteristics of paraffin based nanocomposite for thermal energy storage applications. Therm. Sci. 2017, 21, 2517–2524. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, D.; Zhang, X.; Huang, J. Preparation and Melting/Freezing Characteristics of Cu/Paraffin Nanofluid as Phase-Change Material (PCM). Energy Fuels 2010, 24, 1894–1898. [Google Scholar] [CrossRef]
- Jesumathy, S.; Udayakumar, M.; Suresh, S. Experimental study of enhanced heat transfer by addition of CuO nanoparticle. Heat Mass Transf. 2011, 48, 965–978. [Google Scholar] [CrossRef]
- Nourani, M.; Hamdami, N.; Keramat, J.; Moheb, A.; Shahedi, M. Thermal behavior of paraffin-nano-Al2O3 stabilized by sodium stearoyl lactylate as a stable phase change material with high thermal conductivity. Renew. Energy 2016, 88, 474–482. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Yang, X.; Yu, X.; Duan, F.; Jin, L.; Meng, X. Experimental Investigation of a Spiral Tube Embedded Latent Thermal Energy Storage Tank Using Paraffin as PCM. Energy Procedia 2017, 105, 4543–4548. [Google Scholar] [CrossRef]
- Krupa, I.; Luyt, A. Thermal and mechanical properties of extruded LLDPE/wax blends. Polym. Degrad. Stab. 2001, 73, 157–161. [Google Scholar] [CrossRef]
- Utracki, L.A. Polyethylenes and Their Blends. In Polymer Blends Handbook; Springer: Dordrecht, The Netherlands, 2014; pp. 1559–1732. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Zhang, R.-M.; Xie, K.; Liu, N.; Wang, J. Heat conduction enhanced shape-stabilized paraffin/HDPE composite PCMs by graphite addition: Preparation and thermal properties. Sol. Energy Mater. Sol. Cells 2010, 94, 1636–1642. [Google Scholar] [CrossRef]
- Krupa, I.; Nógellová, Z.; Špitalský, Z.; Malíková, M.; Sobolčiak, P.; Abdelrazeq, H.W.; Ouederni, M.; Karkri, M.; Janigová, I.; A Al-Maadeed, M.A.S. Positive influence of expanded graphite on the physical behavior of phase change materials based on linear low-density polyethylene and paraffin wax. Thermochim. Acta 2015, 614, 218–225. [Google Scholar] [CrossRef]
- Chriaa, I.; Trigui, A.; Karkri, M.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. Thermal properties of shape-stabilized phase change materials based on Low Density Polyethylene, Hexadecane and SEBS for thermal energy storage. Appl. Therm. Eng. 2020, 171, 115072. [Google Scholar] [CrossRef]
- Avram, G.D.M.M. IR spectroscopy. In Application in Organic Chemistry; R. E. Krieger Publishing Company: Bucharest, Romania, 1966; 527p, ISBN 9780471038450. [Google Scholar]
- Masson, J.-F.; Pelletier, L.; Collins, P. Rapid FTIR method for quantification of styrene-butadiene type copolymers in bitumen. J. Appl. Polym. Sci. 2021, 79, 1034–1041. [Google Scholar] [CrossRef]
- Guilment, J.; Bokobza, L. Determination of polybutadiene microstructures and styrene–butadiene copolymers composition by vibrational techniques combined with chemometric treatment. Vib. Spectrosc. 2001, 26, 133–149. [Google Scholar] [CrossRef]
- Trigui, A.; Karkri, M.; Boudaya, C.; Candau, Y.; Ibos, L. Development and characterization of composite phase change material: Thermal conductivity and latent heat thermal energy storage. Compos. Part B Eng. 2013, 49, 22–35. [Google Scholar] [CrossRef]
- Molefi, J.; Luyt, A.; Krupa, I. Comparison of LDPE, LLDPE and HDPE as matrices for phase change materials based on a soft Fischer–Tropsch paraffin wax. Thermochim. Acta 2010, 500, 88–92. [Google Scholar] [CrossRef]
- Akishino, J.; Cerqueira, D.; Silva, G.; Swinka-Filho, V.; Munaro, M. Morphological and thermal evaluation of blends of polyethylene wax and paraffin. Thermochim. Acta 2016, 626, 9–12. [Google Scholar] [CrossRef]
- Mastral, F.; Esperanza, E.; Berrueco, C.; Juste, M.; Ceamanos, J. Fluidized bed thermal degradation products of HDPE in an inert atmosphere and in air–nitrogen mixtures. J. Anal. Appl. Pyrolysis 2003, 70, 1–17. [Google Scholar] [CrossRef]
- Zhu, N.; Li, S.; Hu, P.; Wei, S.; Deng, R.; Lei, F. A review on applications of shape-stabilized phase change materials embedded in building enclosure in recent ten years. Sustain. Cities Soc. 2018, 43, 251–264. [Google Scholar] [CrossRef]
- Moulahi, C.; Trigui, A.; Boudaya, C.; Karkri, M. Smart macroencapsulated resin/wax composite for energy conservation in the built environment. J. Thermoplast. Compos. Mater. 2015, 30, 887–914. [Google Scholar] [CrossRef]
- Trigui, A.; Karkri, M.; Peña, L.; Boudaya, C.; Candau, Y.; Bouffi, S.; Vilaseca, F. Thermal and mechanical properties of maize fibres–high density polyethylene biocomposites. J. Compos. Mater. 2012, 47, 1387–1397. [Google Scholar] [CrossRef]
- Azzouz, K.; Leducq, D.; Gobin, D. Enhancing the performance of household refrigerators with latent heat storage: An experimental investigation. Int. J. Refrig. 2009, 32, 1634–1644. [Google Scholar] [CrossRef]
- Moulahi, C.; Trigui, A.; Karkri, M.; Boudaya, C. Thermal performance of latent heat storage: Phase change material melting in horizontal tube applied to lightweight building envelopes. Compos. Struct. 2016, 149, 69–78. [Google Scholar] [CrossRef]
- Chen, P.; Gao, X.; Wang, Y.; Xu, T.; Fang, Y.; Zhang, Z. Metal foam embedded in SEBS/paraffin/HDPE form-stable PCMs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 149, 60–65. [Google Scholar] [CrossRef]
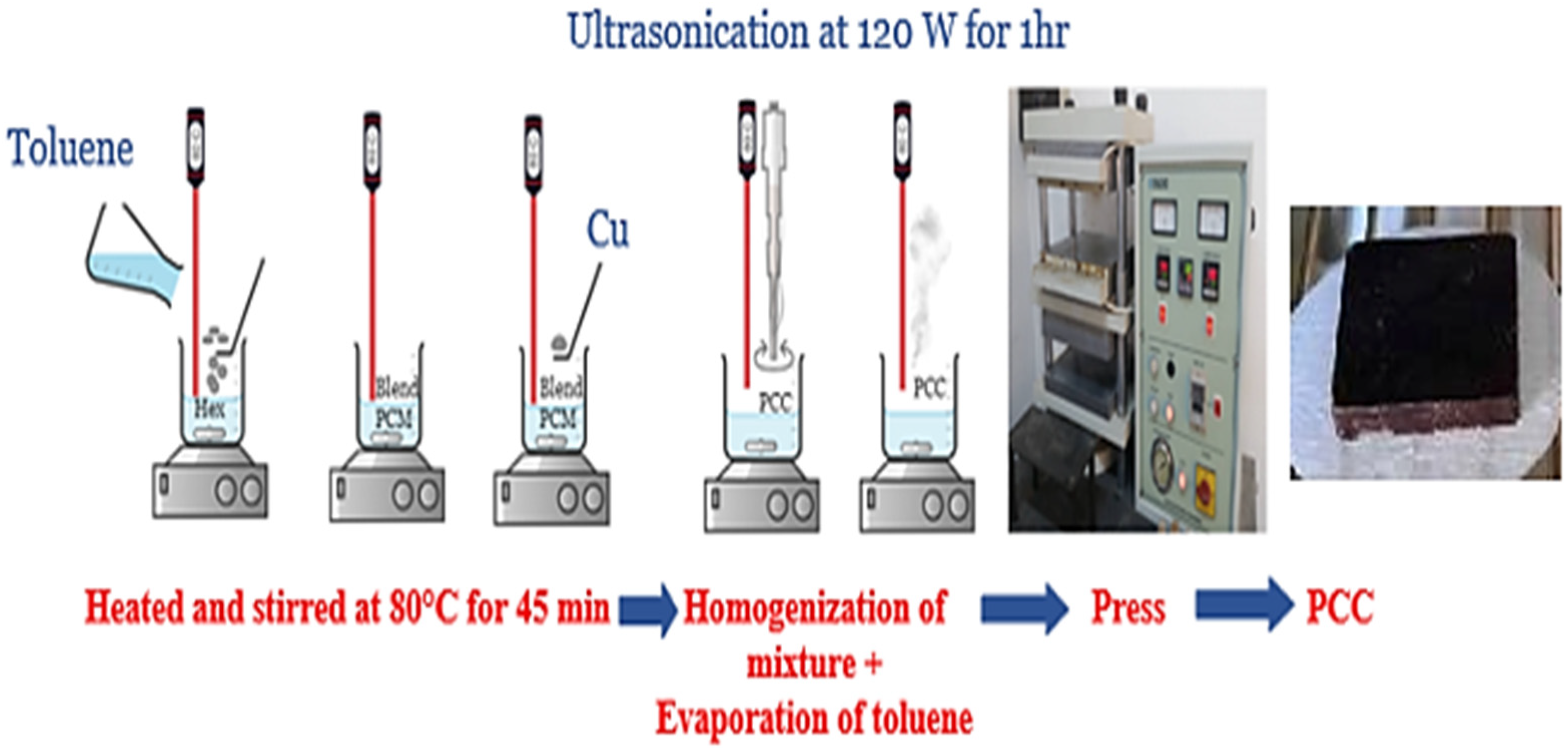







| PCCs | SEBS | Hex | LDPE | Cu |
|---|---|---|---|---|
| PCC1 | 0 | 75 | 25 | 0 |
| PCC2 | 25 | 75 | 0 | 0 |
| PCC3 | 20 | 75 | 5 | 0 |
| PCC4 | 15 | 75 | 5 | 5 |
| PCC5 | 10 | 75 | 5 | 10 |
| PCC6 | 5 | 75 | 5 | 15 |
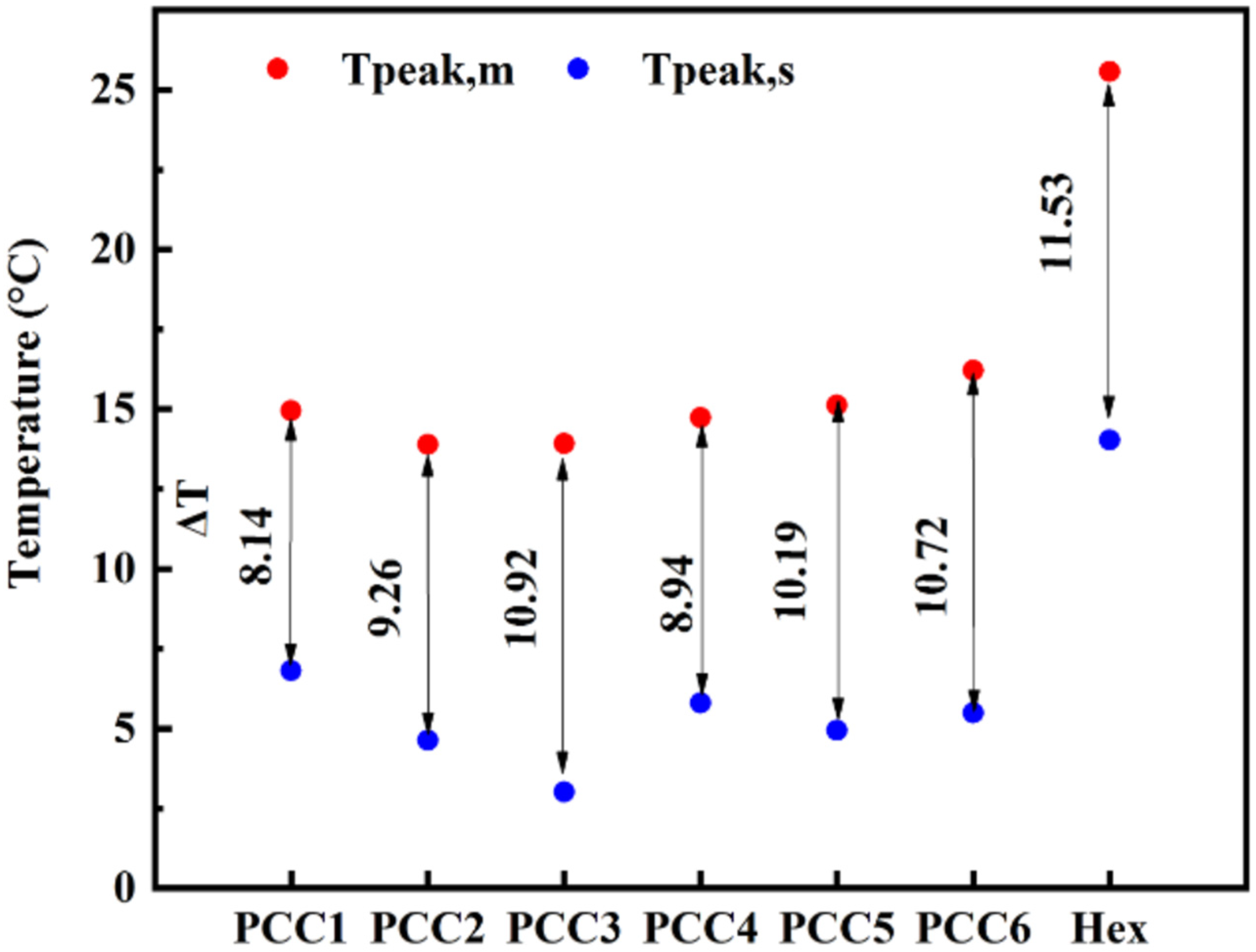
| Sample | Onset (°C) | Peak (°C) | Endset (°C) | Supercooling | ||||
|---|---|---|---|---|---|---|---|---|
| To,m | To,s | Tp,m | Tp,s | Te,m | Te,s | ΔT = Tp,m−Tp,s | ||
| PCM | Hex | 21.15 | 16.96 | 25.57 | 14.04 | 13.5 | 28.38 | 11.53 |
| PCCs | PCC1 | 7.71 | 8.7 | 14.95 | 6.81 | 16.88 | −2.4 | 8.14 |
| PCC2 | 7.37 | 6.44 | 13.89 | 4.63 | 15.83 | −2.48 | 9.26 | |
| PCC3 | 6.61 | 5.91 | 13.93 | 3.01 | 16.3 | −3.07 | 10.92 | |
| PCC4 | 10.63 | 7.85 | 14.74 | 5.80 | 17.89 | 2.81 | 8.94 | |
| PCC5 | 9.21 | 7.5 | 15.13 | 4.94 | 16 | 1.76 | 10.19 | |
| PCC6 | 8.44 | 7.5 | 16.21 | 5.49 | 16.44 | 0.92 | 10.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trigui, A.; Abdelmouleh, M. Improving the Heat Transfer of Phase Change Composites for Thermal Energy Storage by Adding Copper: Preparation and Thermal Properties. Sustainability 2023, 15, 1957. https://doi.org/10.3390/su15031957
Trigui A, Abdelmouleh M. Improving the Heat Transfer of Phase Change Composites for Thermal Energy Storage by Adding Copper: Preparation and Thermal Properties. Sustainability. 2023; 15(3):1957. https://doi.org/10.3390/su15031957
Chicago/Turabian StyleTrigui, Abdelwaheb, and Makki Abdelmouleh. 2023. "Improving the Heat Transfer of Phase Change Composites for Thermal Energy Storage by Adding Copper: Preparation and Thermal Properties" Sustainability 15, no. 3: 1957. https://doi.org/10.3390/su15031957
APA StyleTrigui, A., & Abdelmouleh, M. (2023). Improving the Heat Transfer of Phase Change Composites for Thermal Energy Storage by Adding Copper: Preparation and Thermal Properties. Sustainability, 15(3), 1957. https://doi.org/10.3390/su15031957







