Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass
Abstract
1. Introduction
2. Lignocellulosic Biomass (LCB)
3. Thermophilic Anaerobic Digestion

4. Impact of Thermophilic Inoculum on AD
| Stages of TAD | Microbes Reported | Function |
|---|---|---|
| Hydrolysis (bacteria and fungi) Microbial domain (genera -> species) | 1Defluviitoga tunisiensis L3 [64] 2 Caldicellulosiruptor bescii [68] 3 Gracilibacter thermotolerans JW/YJL-S1 [69] | 1,2 Cellulolytic, 3 polysaccharides |
| Proteiniborus indolifex [70] | Protein-utilizing | |
| Acidaminococcus intestini | Amino acid-utilizing, mainly glutamic acid | |
| Thermoanaerobacterium thermosaccharolyticum [71], Caldanaerobacter subterraneus [71], Thermoanaerobacter pseudethanolicus [71], Clostridium cellulolyticum [71] | Thermophilic cellulolytic | |
| Acidogenesis (fermentation bacteria) | G. thermotolerans [69] | Glucose-degrading |
| Acetogenesis (bacteria) | 1Syntrophaceticus sp. [54] 2 Thermogymnomonas acidicola [54] 3 Gelria glutamica [54] | 1 Syntrophic acetate-oxidizing bacterium 2 Thermophilic acetogen 3 Syntrophic glutamate-degrading |
| Methanogenesis (archaea) | Methanoculleus, Methanobacterium Thermogymnomonas, Thermoplasmata, Methanospirillum, Thermoprotei Methanobrevibacter, Methanolinea, Methanosaeta, Methanimicrococcus [54] | Methane-forming archaea |
5. Factors Affecting the Performance of Thermophilic Inoculum
5.1. Ammonia Inhibition
5.2. Organic Acid Accumulation
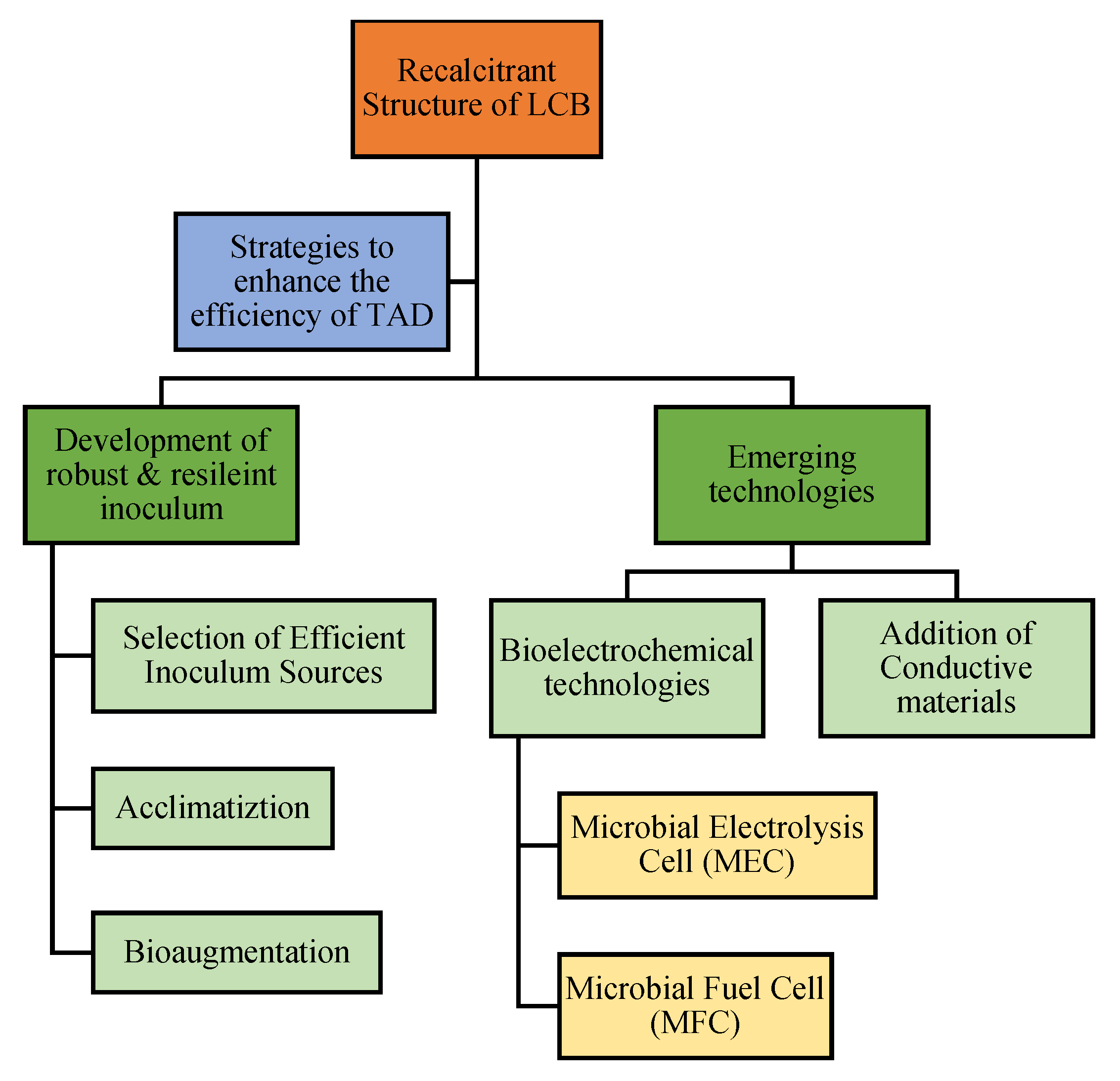
6. Strategies to Enhance the Efficiency of TAD
6.1. Development of Robust Microbiome: Selection, Acclimatization, and Bioaugmentation of Inoculum
6.2. Adoption of Emerging Technologies
6.2.1. Bioelectrochemical Technologies
6.2.2. Addition of Conductive Materials
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAD | Thermophilic anaerobic digestion |
| MAD | Mesophilic anaerobic digestion |
| AD | Anaerobic digestion |
| LCB | Lignocellulosic biomass |
| HRT | Hydraulic retention time |
| IPCC | Intergovernmental Panel on Climate Change |
| EJ | Exajoule |
| OLR | Organic loading rate |
| DGGE | Denaturing gradient gel electrophoresis |
| CAZymes | Carbohydrate-active enzymes |
| GH | Glycoside hydrolase |
| S/I | Substrate to inoculum ratio |
| SS-AD | Solid-state anaerobic digestion |
| TAN | Total ammonia nitrogen |
| FAN | Free ammonia nitrogen |
| VFA | Volatile fatty acids |
| SSCP | Single-strand conformation polymorphism |
| FISH | Fluorescence in situ hybridization |
| CMs | Conductive materials |
| DIET | Direct interspecies electron transfer |
| GAC | Granular activated carbon |
| IHT | Interspecies hydrogen transfer |
| FBR | Fixed bed reactor |
| CSTR | Continuous stirred tank reactor |
| SMDC | Submersible microbial desalination cell |
| MEC | Microbial electrolysis cell |
| MFC | Microbial fuel cell |
| CEM | Cation exchange membrane |
| WWTP | Wastewater treatment plant |
| CFU | Colony-forming units |
References
- Othman, M.N.; Lim; J.S.; Theo; W.L.; Hashim; H.; Ho, W.S. Optimisation and targeting of supply-demand of biogas system through gas system cascade analysis (GASCA) framework. J. Clean. Prod. 2016, 146, 101–115. [Google Scholar] [CrossRef]
- Shukla, P.; Skea, J.; Buendia, E.C.; Masson-Delmot, V. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. Available online: https://www.ipcc.ch/srccl/ (accessed on 10 October 2022).
- Frank, S.; Gusti, M.; Havlík, P.; Lauri, P.; Di Fulvio, F.; Forsell, N.; Hasegawa, T.; Krisztin, T.; Palazzo, A.; Valin, H. Land-based climate change mitigation potentials within the agenda for sustainable development. Environ. Res. Lett. 2021, 16, 024006. [Google Scholar] [CrossRef]
- Wu, W.; Hasegawa, T.; Ohashi, H.; Hanasaki, N.; Liu, J.; Matsui, T.; Fujimori, S.; Masui, T.; Takahashi, K. Global advanced bioenergy potential under environmental protection policies and societal transformation measures. GCB Bioenergy 2019, 11, 1041–1055. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Meyer, L.A. Change, L.C. 2014 Synthesis Report; CDP: Geneva, Switzerland, 2014. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane production in two-stage anaerobic digestion system. Int. J. Hydrogen Energy 2019, 44, 17363–17380. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S. A review on biomethane potential of paddy straw and diverse prospects to enhance its biodigestibility. J. Clean. Prod. 2019, 217, 295–307. [Google Scholar] [CrossRef]
- Singh, R.; Meenu, H.; Kumar, S.; Yadav, Y.K. Potential feedstock for sustainable biogas production and its supply chain management. In Biogas Production; Balagurusamy, N., Chandel, A.K., Eds.; Springer Nature Switzerland AG: Berlin/Heidelberg, Germany, 2020; pp. 147–164. [Google Scholar]
- Dahmen, N.; Lewandowski, I.; ZibekM, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Sidana, A.; Yadav, S.K. Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process. Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Lanko, I.; Hejnic, J.; Říhová-Ambrožová, J.; Ferrer, I.; Jenicek, P. Digested sludge quality in mesophilic, thermophilic and temperature-phased anaerobic digestion systems. Water 2021, 13, 2839. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zeeman, G.; Van Loon, W.K.P.; Bot, G.P.A.; Lettinga, G. Effect of temperature and temperature fluctuation on thermophilic anaerobic digestion of cattle manure. Bioresour. Technol. 2004, 95, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Man-chang, W.; Ke-wei, S.; Yong, Z. Influence of temperature fluctuation on thermophilic anaerobic digestion of municipal organic solid waste. J. Zhejiang Univ. Sci. B 2006, 7, 180–185. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Murphy, J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem. Eng. J. 2018, 350, 681–691. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Govil, T.; Tripathi, A.K.; McGeary, J.; Farrar, K.; Sani, R.K. Thermophilic anaerobic digestion: Enhanced and sustainable methane production from co-digestion of food and lignocellulosic wastes. Energies 2018, 11, 2058. [Google Scholar] [CrossRef]
- Lloret, E.; Pastor, L.; Pradas, P.; Pascual, J.A. Semi full-scale thermophilic anaerobic digestion (TAnD) for advanced treatment of sewage sludge: Stabilization process and pathogen reduction. Chem. Eng. J. 2013, 232, 42–50. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters. In Wastewater Microbiology Series 15; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Suryawanshi, P.C.; Chaudhari, A.B.; Kothari, R.M. Thermophilic anaerobic digestion: The best option for waste treatment. Crit. Rev. Biotechnol. 2010, 30, 31–40. [Google Scholar] [CrossRef]
- Som Gupta, A. Feasibility Study for Production of Biogas from Wastewater and Sewage Sludge-Development of a Sustainability Assessment Framework and its Application. 2020, p. 139. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2:1435267 (accessed on 31 December 2022).
- Tchobanoglous, G.; Burton, F.L.; Stensel, D.H. Wastewater Engineering: Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2014; p. 421. [Google Scholar]
- Kalogo, Y.; Monteith, H.; Water, G. Energy and Resource from Sludge; Water Environment Research Foundation: Alexandria, VA, USA, 2013. [Google Scholar]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Domínguez-Bocanegra, A.R.; Torres-Muñoz, J.A.; López, R.A. Production of Bioethanol from agro-industrial wastes. Fuel 2015, 149, 85–89. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Hutňan, M. Maize Silage as Substrate for Biogas Production. Int. J. Innov. Approaches Agric. Res. 2016, 5, 230–240. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Ramirez-Contreras, N.E.; Rodriguez, D.T.; Silva-Lora, E.; Frear, C.S.; Stockle, C.; Garcia-Perez, M. Evolution of palm oil mills into bio-refineries: Literature review on current and potential uses of residual biomass and effluents. Resour. Conserv. Recycl. 2016, 110, 99–114. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Feasibility of biogas production from napier grass. Energy Procedia. 2014, 61, 1229–1233. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Lizasoain, J.; Trulea, A.; Gittinger, J.; Kral, I.; Piringer, G.; Schedl, A.; Nilsen, P.J.; Potthast, A.; Gronauer, A.; Bauer, A. Corn stover for biogas production: Effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 2017, 244, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Improvement of gaseous energy recovery from sugarcane bagasse by dark fermentation followed by biomethanation process. Bioresour. Technol. 2015, 194, 354–363. [Google Scholar] [CrossRef]
- Janke, L.; Leite, A.; Nikolausz, M.; Schmidt, T.; Liebetrau, J.; Nelles, M.; Stinner, W. Biogas Production from Sugarcane Waste: Assessment on Kinetic Challenges for Process Designing. Int. J. Mol. Sci. 2015, 16, 20685–20703. [Google Scholar] [CrossRef]
- Battista, F.; Fino, D.; Mancini, G. Optimization of biogas production from coffee production waste. Bioresour. Technol. 2016, 200, 884–890. [Google Scholar] [CrossRef]
- Ulsido, M.D.; Li, M. Solid waste management practices in wet coffee processing industries of Gidabo watershed, Ethiopia. Waste Manag. Res. 2016, 34, 638–645. [Google Scholar] [CrossRef]
- Kalia, V.C.; Sonakya, V.; Raizada, N. Anaerobic digestion of banana stem waste. Bioresour. Technol. 2000, 73, 191–193. [Google Scholar] [CrossRef]
- Teghammar, A.; Forgács, G.; Horváth, I.S.; Taherzadeh, M.J. Techno-economic study of NMMO pretreatment and biogas production from forest residues. Appl. Energy 2014, 116, 125–133. [Google Scholar] [CrossRef]
- FNR. Leitfaden Biogas. Gulzow Fachagentur Nachwachsende Rohstoffe. 2016. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/l/e/leitfadenbiogas2013_web_komp.pdf (accessed on 31 December 2022).
- Paterson, M. Implementation Guide for Small-Scale Biogas Plants; Farmers Handbook: Carrick-on-Suir, Ireland, 2015; pp. 1–122. [Google Scholar]
- Van Fan, Y.; Klemeš, J.J.; Perry, S.; Lee, C.T. Anaerobic digestion of lignocellulosic waste: Environmental impact and economic assessment. J. Environ. Manag. 2019, 231, 352–363. [Google Scholar] [CrossRef]
- Moerland, M.J.; Pérez, L.C.; Sobrino, M.E.R.V.; Chatzopoulos, P.; Meulman, B.; de Wilde, V.; Zeeman, G.; Buisman, C.J.; van Eekert, M.H. Thermophilic (55 °C) and hyper-thermophilic (70 °C) anaerobic digestion as novel treatment technologies for concentrated black water. Bioresour. Technol. 2021, 340, 125705. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hidaka, T.; Tsuno, H. Effect of temperature on performance and microbial diversity in hyperthermophilic digester system fed with kitchen garbage. Bioresour. Technol. 2008, 99, 6852–6860. [Google Scholar] [CrossRef]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef]
- Kabaivanova, L.; Petrova, P.; Hubenov, V.; Simeonov, I. Biogas Production Potential of Thermophilic Anaerobic Biodegradation of Organic Waste by a Microbial Consortium Identified with Metagenomics. Life 2022, 12, 702. [Google Scholar] [CrossRef]
- Moset, V.; Poulsen, M.; Wahid, R.; Højberg, O.; Møller, H. Mesophilic versus thermophilic anaerobic digestion of cattle manure: Methane productivity and microbial ecology. Microb. Biotechnol. 2015, 8, 787–800. [Google Scholar] [CrossRef]
- Westerholm, M.; Schnürer, A. Microbial Responses to Different Operating Practices for Biogas Production Systems. In Microbial Responses to Different Operating Practices for Biogas Production Systems; IntechOpen: London, UK, 2012; p. 13. [Google Scholar] [CrossRef]
- Hamzah, M.A.F.; Jahim, J.; Abdul, P.M. Comparative start-up between mesophilic and thermophilic for acidified palm oil mill effluent treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012028. [Google Scholar] [CrossRef]
- İnce, E.; İnce, M.; Engin, G.Ö. Comparison of thermophilic and mesophilic anaerobic treatments for potato processing wastewater using a contact reactor. Glob. Nest J. 2017, 19, 318–326. [Google Scholar] [CrossRef]
- Samaras, V.G.; Mathiopoulou, A.I.; Sirigou, I.E.; Stasinakis, A.S.; Lekkas, T.D. Comparison of Mesophilic and Thermophilic Sludge Anaerobic Digestion: Role of Sludge Retention Time, Reactor’s Configuration and Sonolysis Pre-Treatment on Process Performance. Proceeding of the 3rd International Conference on Industrial and Hazardous Waste Management, Chania, Greece, 12–14 September 2012; pp. 1–8. [Google Scholar]
- Zupančič, G.D.; Roš, M. Heat and energy requirements in thermophilic anaerobic sludge digestion. Renew. Energy 2003, 28, 2255–2267. [Google Scholar] [CrossRef]
- Kushkevych, I.; Cejnar, J.; Vítězová, M.; Vítěz, T.; Dordević, D.; Bomble, Y. Occurrence of thermophilic microorganisms in different full scale biogas plants. Int. J. Mol. Sci. 2020, 21, 283. [Google Scholar] [CrossRef]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Campanaro, S.; Treu, L.; Kougias, P.G.; Zhu, X.; Angelidaki, I. Taxonomy of anaerobic digestion microbiome reveals biases associated with the applied high throughput sequencing strategies. Sci. Rep. 2018, 8, 1926. [Google Scholar] [CrossRef]
- Sikora, A.; Detman, A.; Chojnacka, A.; Blaszczyk, M.K. Anaerobic Digestion: I. A Common Process Ensuring Energy Flow and the Circulation of Matter in Ecosystems. II. A Tool for the Production of Gaseous Biofuels. In Fermentation Processes; IntechOpen: London, UK, 2017; p. 33. [Google Scholar] [CrossRef]
- Gómez-Quiroga, X.; Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Successful and stable operation of anaerobic thermophilic co-digestion of sun-dried sugar beet pulp and cow manure under short hydraulic retention time. Chemosphere 2022, 293, 133484. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.-K.; Lu, H.; Jia, Y.; Lee, P.K.H. Metagenomic reconstruction of key anaerobic digestion pathways in municipal sludge and industrial wastewater biogas-producing systems. Front. Microbiol. 2016, 7, 778. [Google Scholar] [CrossRef]
- Blumer-Schuette, S.; Brown, S.; Sander, K.; Bayer, E.A.; Kataeva, I.; Zurawski, J.V.; Conway, J.M.; Adams, M.W.W.; Kelly, R.M. Thermophilic lignocellulose deconstruction. FEMS Microbiol. Rev. 2014, 38, 393–448. [Google Scholar] [CrossRef]
- Silva, I.M.O.; Dionisi, D. Anaerobic digestion of wheatgrass under mesophilic and thermophilic conditions and different inoculum sources. Chem. Eng. Trans. 2016, 50, 19–24. [Google Scholar] [CrossRef]
- Shikata, A.; Sermsathanaswadi, J.; Thianheng, P.; Baramee, S.; Tachaapaikoon, C.; Waeonukul, R.; Pason, P.; Ratanakhanokchai, K.; Kosugi, A. Characterization of an Anaerobic, Thermophilic, Alkaliphilic, High Lignocellulosic Biomass-Degrading Bacterial Community, ISHI-3, Isolated from Biocompost. Enzyme Microb. Technol. 2018, 118, 66–75. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.; Su, X.; Wang, X.; Zhao, S.; Liu, L.; Luo, Y.; Liu, D.; Zheng, H.; et al. Metatranscriptomic Analyses of Plant Cell Wall Polysaccharide Degradation by Microorganisms in the Cow Rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Maus, I.; Cibis, K.G.; Bremges, A.; Stolze, Y.; Wibberg, D.; Tomazetto, G.; Blom, J.; Sczyrba, A.; König, H.; Pühler, A.; et al. Genomic characterization of Defluviitoga tunisiensis L3, a key hydrolytic bacterium in a thermophilic biogas plant and its abundance as determined by metagenome fragment recruitment. J. Biotechnol. 2016, 232, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Whitham, J.M.; Holwerda, E.K.; Shao, X.; Tian, L.; Wu, Y.-W.; Lombard, V.; Henrissat, B.; Klingeman, D.M.; Yang, Z.K.; et al. Development and characterization of stable anaerobic thermophilic methanogenic microbiomes fermenting switchgrass at decreasing residence times. Biotechnol. Biofuels 2018, 11, 243. [Google Scholar] [CrossRef]
- Wongwilaiwalin, S.; Rattanachomsri, U.; Laothanachareon, T.; Eurwilaichitr, L.; Igarashi, Y.; Champreda, V. Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzyme Microb. Technol. 2010, 47, 283–290. [Google Scholar] [CrossRef]
- Lin, L.; Li, Y. Sequential batch thermophilic solid-state anaerobic digestion of lignocellulosic biomass via recirculating digestate as inoculum–Part I: Reactor performance. Bioresour. Technol. 2017, 236, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mulat, D.G.; Huerta, S.G.; Kalyani, D.; Horn, S.J. Enhancing methane production from lignocellulosic biomass by combined steam-explosion pretreatment and bioaugmentation with cellulolytic bacterium Caldicellulosiruptor bescii. Biotechnol. Biofuels 2018, 1–15. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Romanek, C.S.; Mills, G.L.; Davis, R.C.; Whitman, W.; Wiegel, J. Gracilibacter thermotolerans gen. nov., sp. nov., an anaerobic, thermotolerant bacterium from a constructed wetland receiving acid sulfate water. Int. J. Syst. Evol. Microbiol. 2006, 56, 2089–2093. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Klocke, M. Proteiniborus indolifex sp. nov., isolated from a thermophilic industrial-scale biogas plant. Int. J. Syst. Evol. Microbiol. 2018, 68, 824–828. [Google Scholar] [CrossRef]
- Strang, O.; Ács, N.; Wirth, R.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Bioaugmentation of the thermophilic anaerobic biodegradation of cellulose and corn stover. Anaerobe 2017, 46, 104–113. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: Effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. [Google Scholar] [CrossRef]
- Krakat, N.; Anjum, R.; Dietz, D.; Demirel, B. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Liu, T. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 2003, 53, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A critical review of conventional and emerging methods for improving process stability in thermophilic anaerobic digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Angelidaki, I. Different cultivation methods to acclimatise ammonia-tolerant methanogenic consortia. Bioresour. Technol. 2017, 232, 1–9. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef]
- Liu, Y.; Ngo, H.H.; Guo, W.; Peng, L.; Wang, D.; Ni, B. The roles of free ammonia (FA) in biological wastewater treatment processes: A review. Environ. Int. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Nakakubo, R.; Møller, H.B.; Nielsen, A.M.; Matsuda, J. Ammonia inhibition of methanogenesis and identification of process indicators during anaerobic digestion. Environ. Eng. Sci. 2008, 25, 1487–1496. [Google Scholar] [CrossRef]
- Kayhanian, M. Performance of a high-solids anaerobic digestion process under various ammonia concentrations. J. Chem. Technol. Biotechnol. 1994, 59, 349–352. [Google Scholar] [CrossRef]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Hadj, B.E.; Astals, S.; Galí, A.; Mace, S.; Mata-Áivarez, J. Ammonia influence in anaerobic digestion of OFMSW. Water Sci. Technol. 2009, 59, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, M.; Praeg, N.; Illmer, P. Soil-Derived Inocula Enhance Methane Production and Counteract Common Process Failures During Anaerobic Digestion. Front. Microbiol. 2020, 11, 572759. [Google Scholar] [CrossRef]
- Levén, L.; Eriksson, A.R.B.; Schnürer, A. Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste. FEMS Microbiol. Ecol. 2007, 59, 683–693. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Chen, C.; Luo, C.; Li, J.; Shen, J.; Wang, L. Acidification of Waste Activated Sludge During Thermophilic Anaerobic Digestion. Procedia Environ. Sci. 2012, 16, 391–400. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Kim, M.; Liu, C.; Noh, J.-W.; Yang, Y.; Oh, S.; Shimizu, K.; Lee, D.-Y.; Zhang, Z. Hydrogen and methane production from untreated rice straw and raw sewage sludge under thermophilic anaerobic conditions. Int. J. Hydrogen Energy 2013, 38, 8648–8656. [Google Scholar] [CrossRef]
- Hori, T.; Haruta, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Dynamic transition of a methanogenic population in response to the concentration of volatile fatty acids in a thermophilic anaerobic digester. Appl. Environ. Microbiol. 2006, 72, 1623–1630. [Google Scholar] [CrossRef]
- Cord-ruwisch, R.; Mercz, T.I.; Hoh, C.; Strong, G.E. Dissolved Hydrogen Concentration as an On-Line Control Parameter for the Automated Operation and Optimization of Anaerobic Digesters. Biotechnol. Bioeng. 1997, 56, 626–634. [Google Scholar] [CrossRef]
- Jang, H.M.; Choi, Y.K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef]
- Fdéz, L.A.; Álvarez-Gallego, C.; Márquez, D.S.; García, L.I.R. Start-up of thermophilic-dry anaerobic digestion of OFMSW using adapted modified SEBAC inoculum. Bioresour. Technol. 2010, 101, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Mladenovska, Z.; Iranpour, R.; Westermann, P. State of the art and future perspectives of thermophilic anaerobic digestion. Water Sci. Technol. 2002, 45, 293–298. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battistoni, P.; Mata-Alvarez, J.; Cecchi, F. Anaerobic digestion of organic solid waste: Process behaviour in transient conditions. Water Sci. Technol. 2003, 48, 1–8. [Google Scholar] [CrossRef]
- Palatsi, J.; Gimenez-Lorang, A.; Ferrer, I.; Flotats, X. Start-up strategies of thermophilic anaerobic digestion of sewage sludge. Water Sci. Technol. 2009, 59, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, Y.; Li, Y.; Chi, Y.; Yang, M. Rapid establishment of thermophilic anaerobic microbial community during the one-step startup of thermophilic anaerobic digestion from a mesophilic digester. Water Res. 2015, 69, 9–19. [Google Scholar] [CrossRef]
- Ghanimeh, S.; El-Fadel, M.; Saikaly, P.E. Performance of thermophilic anaerobic digesters using inoculum mixes with enhanced methanogenic diversity. J. Chem. Technol. Biotechnol. 2018, 93, 207–214. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Overcoming organic and nitrogen overload in thermophilic anaerobic digestion of pig slurry by coupling a microbial electrolysis cell. Bioresour. Technol. 2016, 216, 362–372. [Google Scholar] [CrossRef]
- Tremouli; Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G. Exploitation of Digestate from Thermophilic and Mesophilic Anaerobic Digesters Fed with Fermentable Food Waste Using the MFC Technology. Waste Biomass Valorization 2021, 12, 5361–5370. [Google Scholar] [CrossRef]
- Carrillo-Peña, D.; Escapa, A.; Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R.; Mateos, R. Bioelectrochemical enhancement of methane production from exhausted vine shoot fermentation broth by integration of MEC with anaerobic digestion. Biomass Convers. Biorefinery 2022, 1–10. [Google Scholar] [CrossRef]
- Park, J.-G.; Lee, B.; Lee, U.-J.; Jun, H.-B. An anaerobic digester with microbial electrolysis cell enhances relative abundance of methylotrophic methanogens in bulk solution. Environ. Eng. Res. 2021, 27, 210666. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Chung, T.; Hai, F.I.; Haile, T.; Al-Mamun, A.; Dhar, B.R. Microbial electrolysis followed by chemical precipitation for effective nutrients recovery from digested sludge centrate in WWTPs. Chem. Eng. J. 2019, 361, 256–265. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Al-Mamun, A.; Dhar, B.R. Anodic performance of microbial electrolysis cells in response to ammonia nitrogen. J. Environ. Eng. Sci. 2018, 14, 37–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Submersible microbial desalination cell for simultaneous ammonia recovery and electricity production from anaerobic reactors containing high levels of ammonia. Bioresour. Technol. 2015, 177, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A. Bioresource Technology A review on the applications of microbial electrolysis cells in anaerobic digestion. ScienceDirect 2017, 255, 340–348. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Wang, T.; Zeng, L.; Bai, C.; Wu, R.; Xing, Z.; Xiao, G.; Shi, X. Enhanced sludge thermophilic anaerobic digestion performance by single-chambered microbial electrolysis cells under ammonia inhibition. J. Environ. Chem. Eng. 2022, 10, 107802. [Google Scholar] [CrossRef]
- Viggi, C.C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef]
- Dubé, C.-D.; Guiot, S.R. Direct Interspecies Electron Transfer in Anaerobic Digestion: A Review. In Biogas Science and Technology, 151st ed.; Gübitz, G., Bauer, A., Bochmann, G., Gronauer, A., Weiss, S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–200. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef]
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Dubey, M.; Ahmed, B.; Gahlot, P.; Khan, A.A.; Rajpal, A.; Kazmi, A.; Tyagi, V.K. Carbon-based conductive materials facilitated anaerobic co-digestion of agro waste under thermophilic conditions. Waste Manag. 2021, 124, 17–25. [Google Scholar] [CrossRef]
- Shen, Y.; Forrester, S.; Koval, J.; Urgun-Demirtas, M. Yearlong semi-continuous operation of thermophilic two-stage anaerobic digesters amended with biochar for enhanced biomethane production. J. Clean. Prod. 2017, 167, 863–874. [Google Scholar] [CrossRef]
- Lim, E.Y.; Tian, H.; Chen, Y.; Ni, K.; Zhang, J.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.Q.; Woodard, T.L.; Zhang, Y.B.; Sun, D.Z.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
| Residue | C:H:L | TS% | VS% | Methane Yield (m3/kg-VS) | References |
|---|---|---|---|---|---|
| Silage maize | 16:11:38 | 30.8 | 94.1 | 0.259 | [28] |
| Grass silage | 31:29:10 | 50 | 92 | 0.344–0.383 | [29,30] |
| Paddy straw | 38:23:13 | 93 | 80 | 0.202 | [7] |
| Wheat straw | 33:22:19 | 93.1 | 76.8 | 0.282 | [31,32] |
| Corn stover | 39:26.6:19 | 86 | 94.3 | 0.296 | [33,34] |
| Sugarcane bagasse | 42:22:18 | 94 | 97 | 0.122–0.236 | [35,36] |
| Coffee pulp | 31:11:23 | 55 | 91 | 0.131 | [37,38] |
| Pulp and paper sludge | na | 24.2 | 77 | 0.432 | [39] |
| Forestry residues | 42:na:44 | 50 | 64 | 0.214 | [40] |
| Banana stalks (sun-dried) | 56:8:18 | 92 | 83 | 0.236 | [39] |
| Chicken manure | 12:20:2 | 40 | 75 | 0.309 * | [41,42] |
| Cattle manure | 27:12:13 | 25 | 76 | 0.236 * | [41,42] |
| Parameters | MAD | TAD |
|---|---|---|
| Start-up period | Long | Short |
| Hydrolysis rate | Low | High |
| Biogas production | Low | High |
| Methane content | Low | High |
| Retention time | Long | Short |
| Pathogen reduction | Low | High |
| Reactor volume | Large | Small |
| Process stability | High | Low |
| Energy—consumption and recovery | Low; Low | High; High |
| Digestate quality | Low | High |
| Substrate | Temperature (°C) | TAN Conc. | FAN Critical Conc. | Major Findings/Impacts | References |
|---|---|---|---|---|---|
| Pig manure | 51 | 4.6–11.0 g-N/L | 1450 mg-N/L | 50% inhibition of methanogenesis at 11.0 g NH4-N/L | [81] |
| Paper and yard waste | 54–60 | 0.75–2.5 g-N/kg | - | 50% reduction in CH4 at 1.5 g-N/kg Complete failure of digester at 2.5 g-N/kg | [82] |
| OFMSW | 55 | 1.75–3 g-N/L | 660 mg-N/L | Methane yield decreased by 63% at 432 mg-N/L of FAN | [83] |
| OFMSW | 55 | 1.7–5.6 g/L | 468 mg/L | 50% methane inhibition at specified TAN and FAN levels | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. https://doi.org/10.3390/su15031859
Singh R, Hans M, Kumar S, Yadav YK. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability. 2023; 15(3):1859. https://doi.org/10.3390/su15031859
Chicago/Turabian StyleSingh, Richa, Meenu Hans, Sachin Kumar, and Yogender Kumar Yadav. 2023. "Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass" Sustainability 15, no. 3: 1859. https://doi.org/10.3390/su15031859
APA StyleSingh, R., Hans, M., Kumar, S., & Yadav, Y. K. (2023). Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability, 15(3), 1859. https://doi.org/10.3390/su15031859








