Oxidative Stress Trajectories during Lifespan: The Possible Mediation Role of Hormones in Redox Imbalance and Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Epidemiological Sample
2.2. OS Biological Measurements
2.3. Confounding Factors
- -
- BMI: self-reported height and weight were used to calculate BMI ([Weight (kg)]/[(Height (m))2]) and the epidemiological sample was classified as Underweight, Normal, Overweight, or Obese according to the reference values provided by the OMS for the different age groups [35]. In the present study, we grouped the subjects as Overweight or Obese (OwO) and not overweight or obese (not OwO).
- -
- Smoking habit: subjects were asked to indicate whether they were exposed or not to tobacco smoke and, consequently, each subject was categorized as a non-smoker or an active smoker.
2.4. Statistical Analyses
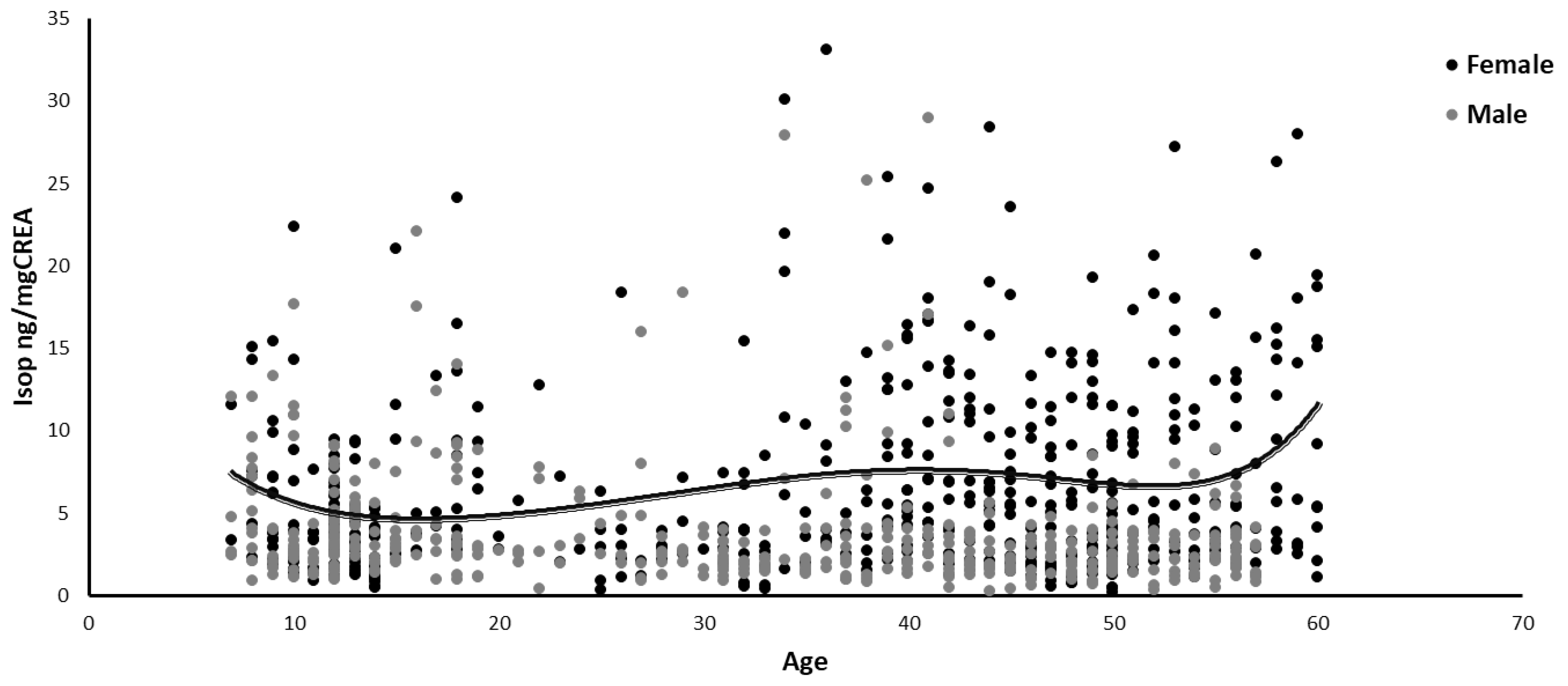
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative Stress-Mediated Aging during the Fetal and Perinatal Periods. Oxid. Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Juhasz, B.; Tosaki, A. Management of multicellular senescence and oxidative stress. J. Cell. Mol. Med. 2013, 17, 936–957. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Cleal, J.K.; Hanson, M.A. Review: Placenta, evolution and lifelong health. Placenta 2012, 33, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a ‘good’ look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Park, C.B.; Larsson, N.G. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef]
- Finley, L.W.S.; Haigis, M.C. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 2009, 8, 173–188. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Nicks, K.M.; Fowler, T.W.; Gaddy, D. Reproductive hormones and bone. Curr. Osteoporos. Rep. 2010, 8, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L. Modulatory influence of sex hormones on vascular aging. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H522–H526. [Google Scholar] [CrossRef] [PubMed]
- Tenkorang, M.A.; Snyder, B.; Cunningham, R.L. Sex-related differences in oxidative stress and neurodegeneration. Steroids 2018, 133, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Duong, P.; Tenkorang, M.A.A.; Trieu, J.; McCuiston, C.; Rybalchenko, N.; Cunningham, R.L. Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment. Biol. Sex Differ. 2020, 11, 12. [Google Scholar] [CrossRef]
- Holmes, S.; Singh, M.; Su, C.; Cunningham, R.L. Effects of Oxidative Stress and Testosterone on Pro-Inflammatory Signaling in a Female Rat Dopaminergic Neuronal Cell Line. Endocrinology 2016, 157, 2824–2835. [Google Scholar] [CrossRef]
- Snyder, B.; Duong, P.; Trieu, J.; Cunningham, R.L. Androgens modulate chronic intermittent hypoxia effects on brain and behavior. Horm. Behav. 2018, 106, 62–73. [Google Scholar] [CrossRef]
- Decaroli, M.C.; Rochira, V. Aging and sex hormones in males. Virulence 2017, 8, 545–570. [Google Scholar] [CrossRef]
- Auyeung, B.; Lombardo, M.V.; Baron-Cohen, S. Prenatal and postnatal hormone effects on the human brain and cognition. Pflugers Arch. 2013, 465, 557–571. [Google Scholar] [CrossRef]
- Rao, P.M.; Kelly, D.M.; Jones, T.H. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef]
- Doshi, S.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife. Health 2013, 4, 140. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Vural, P.; Canbaz, M.; Akgul, C. Effects of menopause and postmenopausal tibolone treatment on plasma TNFalpha, IL-4, IL-10, IL-12 cytokine pattern and some bone turnover markers. Pharmacol. Res. 2006, 53, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Neri, S.; Sciacchitano, S.; Di Pino, L.; Costa, M.P.; Marchese, G.; Celotta, G.; Cassibba, N.; Pennisi, G.; Caschetto, S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas 2006, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Capacci, F.; Cellai, F.; Sgarrella, C.; Bellisario, V.; Trucco, G.; Tofani, L.; Peluso, A.; Poli, C.; Arena, L.; et al. Wood dust and urinary 15-F2t isoprostane in Italian industry workers. Environ. Res. 2019, 173, 300–305. [Google Scholar] [CrossRef]
- Bellisario, V.; Piccioni, P.; Bugiani, M.; Squillacioti, G.; Levra, S.; Gulotta, C.; Mengozzi, G.; Perboni, A.; Grignani, E.; Bono, R. Tobacco smoke exposure, urban and environmental factors as respiratory disease predictors in Italian adolescents. Int. J. Environ. Res. Public Health 2019, 16, 4048. [Google Scholar] [CrossRef]
- Bellisario, V.; Mengozzi, G.; Grignani, E.; Bugiani, M.; Sapino, A.; Bussolati, G.; Bono, R. Towards a formalin-free hospital. Levels of 15-F2t-isoprostane and malondialdehyde to monitor exposure to formaldehyde in nurses from operating theatres. Toxicol. Res. 2016, 5, 1122–1129. [Google Scholar] [CrossRef]
- Bono, R.; Bellisario, V.; Romanazzi, V.; Pirro, V.; Piccioni, P.; Pazzi, M.; Bugiani, M.; Vincenti, M. Oxidative stress in adolescent passive smokers living in urban and rural environments. Int. J. Hyg. Environ. Health 2014, 217, 287–293. [Google Scholar] [CrossRef]
- Jacquemin, B.; Siroux, V.; Sanchez, M.; Carsin, A.-E.; Schikowski, T.; Adam, M.; Bellisario, V.; Buschka, A.; Bono, R.; Brunekreef, B.; et al. Ambient air pollution and adult asthma incidence in six european cohorts (Escape). Environ. Health Perspect. 2015, 123. [Google Scholar] [CrossRef]
- Hopf, N.B.; Bourgkard, E.; Demange, V.; Hulo, S.; Sauvain, J.J.; Levilly, R.; Jeandel, F.; Robert, A.; Guichard, Y.; Pralong, J.A.; et al. Early Effect Markers and Exposure Determinants of Metalworking Fluids Among Metal Industry Workers: Protocol for a Field Study. JMIR Res. Protoc. 2019, 8, e13744. [Google Scholar] [CrossRef]
- Romanazzi, V.; Pirro, V.; Bellisario, V.; Mengozzi, G.; Peluso, M.; Pazzi, M.; Bugiani, M.; Verlato, G.; Bono, R. 15-F2t isoprostane as biomarker of oxidative stress induced by tobacco smoke and occupational exposure to formaldehyde in workers of plastic laminates. Sci. Total Environ. 2013, 442, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P. Bisphenol a, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int. J. Environ. Res. Public Health 2019, 16, 2025. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, G.; Bellisario, V.; Grignani, E.; Mengozzi, G.; Bardaglio, G.; Dalmasso, P.; Bono, R. The Asti Study: The Induction of Oxidative Stress in A Population of Children According to Their Body Composition and Passive Tobacco Smoking Exposure. Int. J. Environ. Res. Public Health 2019, 16, 490. [Google Scholar] [CrossRef]
- WHO. WHO European Regional Obesity Report 2022; WHO: Geneva, Switzerland, 2022.
- Sacks, D. Age limits and adolescents. Paediatr. Child Health 2003, 8, 577. [Google Scholar] [CrossRef]
- Weismiller, D.G. Menopause. Prim. Care Clin. Off. Pract. 2009, 36, 199–226. [Google Scholar] [CrossRef]
- Abitudine al Fumo Dati Sorveglianza Passi. Available online: https://www.epicentro.iss.it/passi/dati/fumo#dati (accessed on 16 September 2022).
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress and male reproductive biology. Reprod. Fertil. Dev. 2004, 16, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Brennan, L.A.; Goldberg, M.; Chung, W.K.; Wei, Y.; Santella, R.M.; Terry, M.B. Influence of pubertal development on urinary oxidative stress biomarkers in adolescent girls in the New York LEGACY cohort. Free Radic. Res. 2020, 54, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, A.I.; Mesa, M.D.; Anguita-Ruiz, A.; González-Gil, E.M.; Vázquez-Cobela, R.; Moreno, L.A.; Gil, Á.; Gil-Campos, M.; Leis, R.; Bueno, G.; et al. Antioxidants and Oxidative Stress in Children: Influence of Puberty and Metabolically Unhealthy Status. Antioxidants 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Schoina, M.; Valsamakis, G.; Salakos, N.; Avloniti, A.; Chatzinikolaou, A.; Margeli, A.; Skevaki, C.; Papagianni, M.; Kanaka-Gantenbein, C.; et al. Interrelations among the adipocytokines leptin and adiponectin, oxidative stress and aseptic inflammation markers in pre- and early-pubertal normal-weight and obese boys. Endocrine 2017, 55, 925–933. [Google Scholar] [CrossRef]
- Elhadd, T.A.; Khan, F.; Kirk, G.; McLaren, M.; Newton, R.W.; Greene, S.A.; Belch, J.J.F. Influence of puberty on endothelial dysfunction and oxidative stress in young patients with type 1 diabetes. Diabetes Care 1998, 21, 1990–1996. [Google Scholar] [CrossRef]
- Amro, B.; Aristondo, M.E.R.; Alsuwaidi, S.; Almaamari, B.; Hakim, Z.; Tahlak, M.; Wattiez, A.; Koninckx, P.R. New Understanding of Diagnosis, Treatment and Prevention of Endometriosis. Int. J. Environ. Res. Public Health 2022, 19, 6725. [Google Scholar] [CrossRef]
- Barradas, V.; Antoniassi, M.P.; Intasqui, P.; Nichi, M.; Bertolla, R.P.; Spaine, D.M. Evaluation of oxidative stress in seminal plasma of adolescents with varicocele. Reprod. Fertil. 2021, 2, 141–150. [Google Scholar] [CrossRef]
- Wang, Z.; Chandrasena, E.R.; Yuan, Y.; Peng, K.W.; Van Breemen, R.B.; Thatcher, G.R.J.; Bolton, J.L. Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens. Chem. Res. Toxicol. 2010, 23, 1365–1373. [Google Scholar] [CrossRef]

| All sample (N = 815) | Puberty (N = 290) Pre-Puberty (N = 75) Puberty (N = 159) Post-Puberty (N = 56) | Menopause (N = 375) Pre-Menopause (N = 125) Early Menopause (N = 116) Late Menopause (N = 134) | |||
|---|---|---|---|---|---|
| Gender (%) | Male: 401 (49.2%) Female: 414 (50.8%) | Pre-puberty | Male: 43 (57.3%) Female: 32 (42.7%) | Pre-menopause | Male: 53 (42.4%) Female: 72 (57.6%) |
| Puberty | Male: 80 (50.3%) Female: 79 (49.7%) | Early menopause | Male: 45 (38.8%) Female: 71 (61.2%) | ||
| Post-puberty | Male: 33 (58.9%) Female: 23 (41.1%) | Late menopause | Male: 61 (45.5%) Female: 73(54.6%) | ||
| Age (years) [Mean ± S.D.] | 32.6 ± 0.6 Male: 30.8 ± 0.8 Female: 34.2 ± 0.8 | Pre-puberty | 8.9 ± 0.1 | Pre-menopause | 41.8 ± 0.1 |
| Puberty | 12.8 ± 0.08 | Early menopause | 47.7 ± 0.1 | ||
| Post-puberty | 17.7 ± 0.1 | Late menopause | 55.1 ± 0.2 | ||
| OwO (%) | 261 (32%) Male: 159 (39.6%) Female: 102 (24.6%) | Pre-puberty | Male: 8 (18.6%) Female: 6 (18.7%) | Pre-menopause | Male: 14 (26.4%) Female: 12 (16.6%) |
| Puberty | Male: 17 (21.2%) Female: 11 (13.9%) | Early menopause | Male: 11 (24.4%) Female: 26 (36.6%) | ||
| Post-puberty | Male: 8 (24.2%) Female: 5 (21.7%) | Late menopause | Male: 16 (26.2%) Female: 23 (31.5%) | ||
| Tobacco Smoke (%) | No: 654 (80.2%) Active: 161 (19.8%) [85 Male/76 Female] | Pre-puberty | No: 75 (100%) Active: 0 | Pre-menopause | No: 83 (66.4%) Active: 42 (33.6%) [21 Male/21 Female] |
| Puberty | No: 156 (98.1%) Active: 3 (1.9%) [2 Male/1 Female] | Early menopause | No: 90 (77.6%) Active: 26 (22.4%) [13 Male/13 Female] | ||
| Post-puberty | No: 43 (76.8%) Active: 13 (23.2%) [9 Male/4 Female] | Late menopause | No: 99 (73.9%) Active: 35 (26.1%) [18 Male/17 Female] | ||
| 15-F2t-Isop [ng/mgCREA] [mean ± S.D./ min-max] | 4.6 ± 5.2 [0.1–41.2] Male: 3.6 ± 4 [0.1–39.8] Female: 5.5 ± 5.9 [0.2–42.3] | Pre-puberty | 5.5 ± 0.9 Male: 3.9 ± 4 [0.9–17.7] Female: 6.3 ± 5.7 [1–22.4] | Pre-menopause | 5.8 ± 5.7 Male: 3.8 ± 4.9 [0.2–28.9] Female: 7.2 ± 5.8 [1.2–28.4] |
| Puberty | 4.8 ± 0.4 Male: 4.5 ± 2 [0.9–9.1] Female: 3.4 ± 1.9 [0.5–9.5] | Early menopause | 4.1 ± 3.2 Male: 2.5 ± 1.4 [0.6–8.4] Female: 5 ± 3.6 [0.2–14.7] | ||
| Post-puberty | 5.1 ± 0.8 Male: 4.8 ± 4.9 [0.9–22.1] Female: 6.7 ± 6.2 [1.1–24.1] | Late menopause | 5.2 ± 5 Male: 2.9 ± 1.9 [0.3–8.9] Female: 7.1 ± 5.9 [1.1–27.2] | ||
| Regression Model (All Sample) | |||||
|---|---|---|---|---|---|
| F2t-Isop [ng/mgCREA] | B Coef. | Std.Err. | z | p > |z| | C.I. [95%] |
| Sex | 1.2 | 0.2 | 6.1 | <0.001 | 0.81/1.6 |
| OwO | −0.3 | 0.2 | −1.4 | 0.16 | −0.7/0.11 |
| Smoke | 0.09 | 0.07 | 1.3 | 0.19 | −0.05/0.24 |
| Age | 0.02 | 0.005 | 4.9 | <0.001 | 0.02/0.04 |
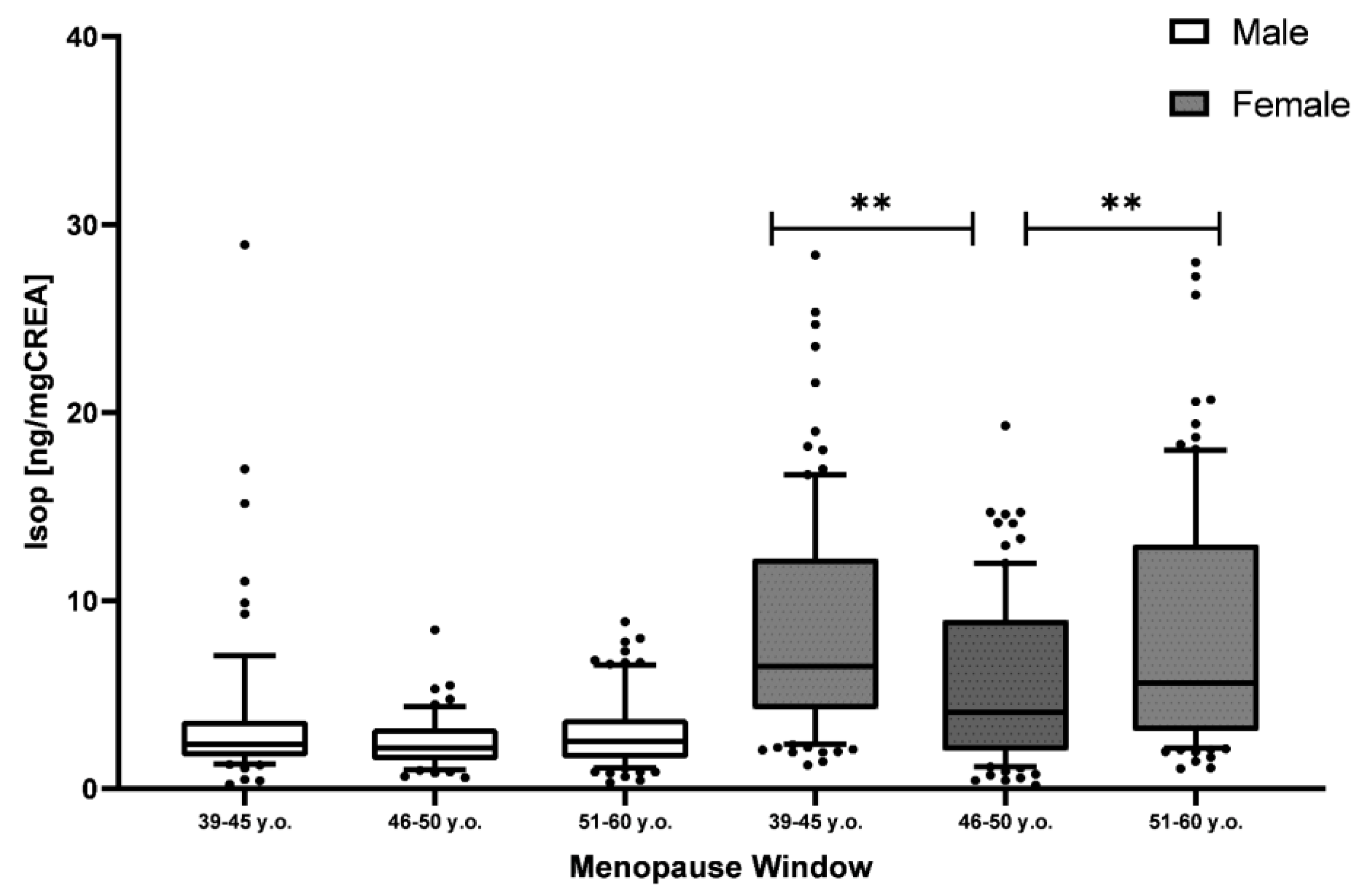
| Pairwise Comparisons of Means with Equal Variances | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||
| Puberty Classes | Tukey Contrast | Std.Err. | Tukey t | p > |t| | C.I. [95%] | Tukey Contrast | Std.Err. | Tukey t | p > |t| | C.I. [95%] |
| 2 vs. 1 | −1.8 | 0.6 | −2.8 | 0.2 | −3.4/0.3 | −2.8 | 0.7 | −3.9 | 0.001 | −3.1/−0.4 |
| 3 vs. 1 | 0.6 | 0.8 | 0.7 | 0.7 | −1.3/2.5 | −0.4 | 0.9 | −0.4 | 0.9 | −2.6/1.8 |
| 3 vs. 2 | 2.4 | 0.7 | 3.4 | 0.3 | −0.7/4.1 | 2.4 | 0.8 | 2.9 | 0.01 | 0.5/4.4 |
| Pairwise Comparisons of Means with Equal Variances | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||
| Puberty Classes | Tukey Contrast | Std.Err. | Tukey t | p > |t| | C.I. [95%] | Tukey Contrast | Std.Err. | Tukey t | p > |t| | C.I. [95%] |
| 5 vs. 4 | −1.9 | 1.1 | −1.8 | 0.17 | −4.6/0.6 | −1.5 | 0.9 | −1.5 | 0.03 | −3.8/−0.8 |
| 6 vs. 4 | 1.1 | 1.09 | 0.9 | 0.6 | −1.5/3.6 | −1.1 | 1.1 | −1 | 0.6 | −3.7/1.5 |
| 6 vs. 5 | 3.1 | 1.1 | 2.7 | 0.2 | −0.35/5.7 | 0.4 | 1.1 | 0.3 | 0.04 | 0.2/2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bono, R.; Squillacioti, G.; Ghelli, F.; Panizzolo, M.; Comoretto, R.I.; Dalmasso, P.; Bellisario, V. Oxidative Stress Trajectories during Lifespan: The Possible Mediation Role of Hormones in Redox Imbalance and Aging. Sustainability 2023, 15, 1814. https://doi.org/10.3390/su15031814
Bono R, Squillacioti G, Ghelli F, Panizzolo M, Comoretto RI, Dalmasso P, Bellisario V. Oxidative Stress Trajectories during Lifespan: The Possible Mediation Role of Hormones in Redox Imbalance and Aging. Sustainability. 2023; 15(3):1814. https://doi.org/10.3390/su15031814
Chicago/Turabian StyleBono, Roberto, Giulia Squillacioti, Federica Ghelli, Marco Panizzolo, Rosanna Irene Comoretto, Paola Dalmasso, and Valeria Bellisario. 2023. "Oxidative Stress Trajectories during Lifespan: The Possible Mediation Role of Hormones in Redox Imbalance and Aging" Sustainability 15, no. 3: 1814. https://doi.org/10.3390/su15031814
APA StyleBono, R., Squillacioti, G., Ghelli, F., Panizzolo, M., Comoretto, R. I., Dalmasso, P., & Bellisario, V. (2023). Oxidative Stress Trajectories during Lifespan: The Possible Mediation Role of Hormones in Redox Imbalance and Aging. Sustainability, 15(3), 1814. https://doi.org/10.3390/su15031814








