Size Distribution and Secondary Formation of Particulate Organic Nitrates in Winter in a Coastal Area
Abstract
:1. Introduction
2. Materials and Methods
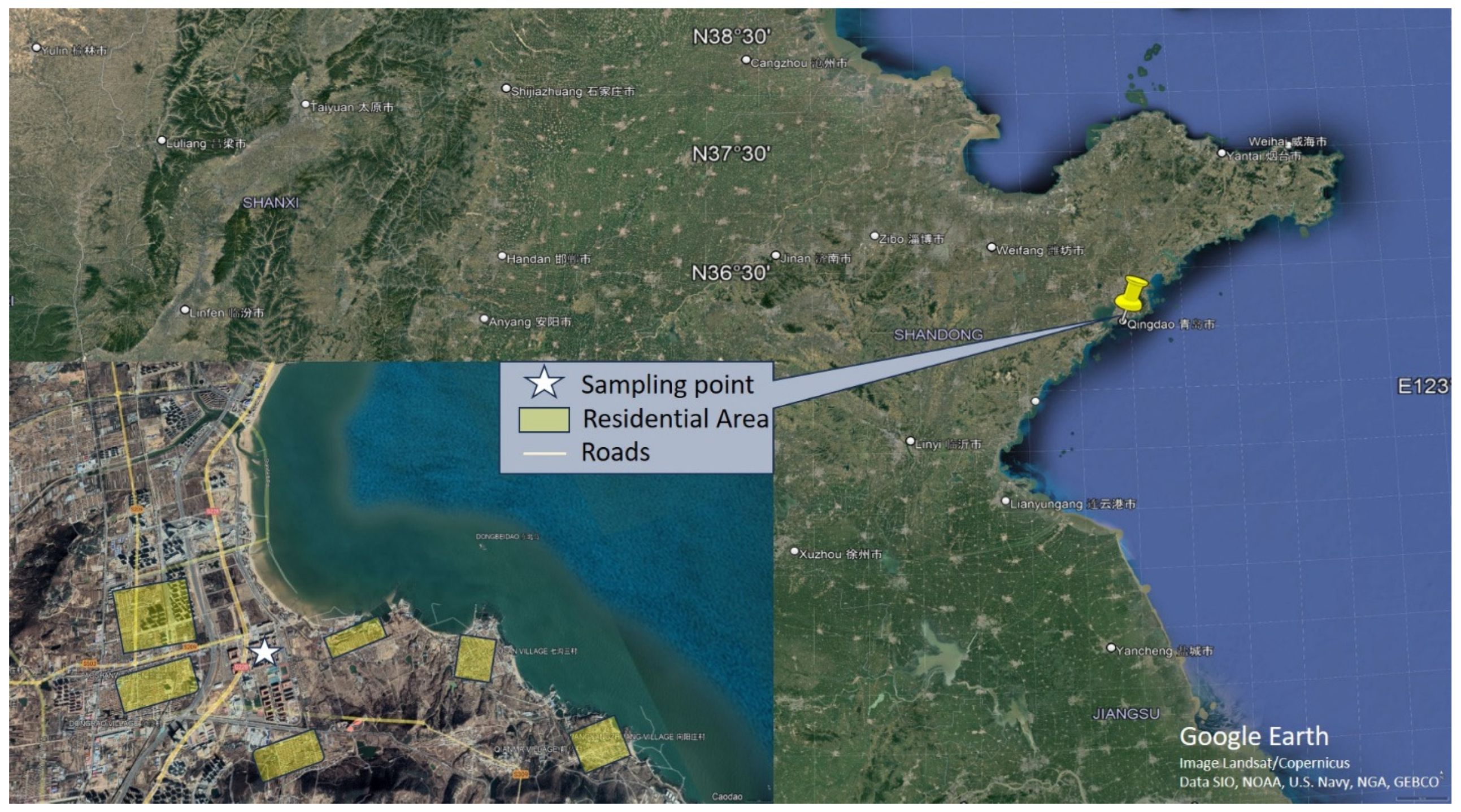
2.1. Study Area
2.2. Sample Collection and Auxiliary Measurements
2.3. Organic Nitrate Determination and Chemical Analyses
2.4. Quality Assurance and Quality Control
2.5. Data Processing
3. Results
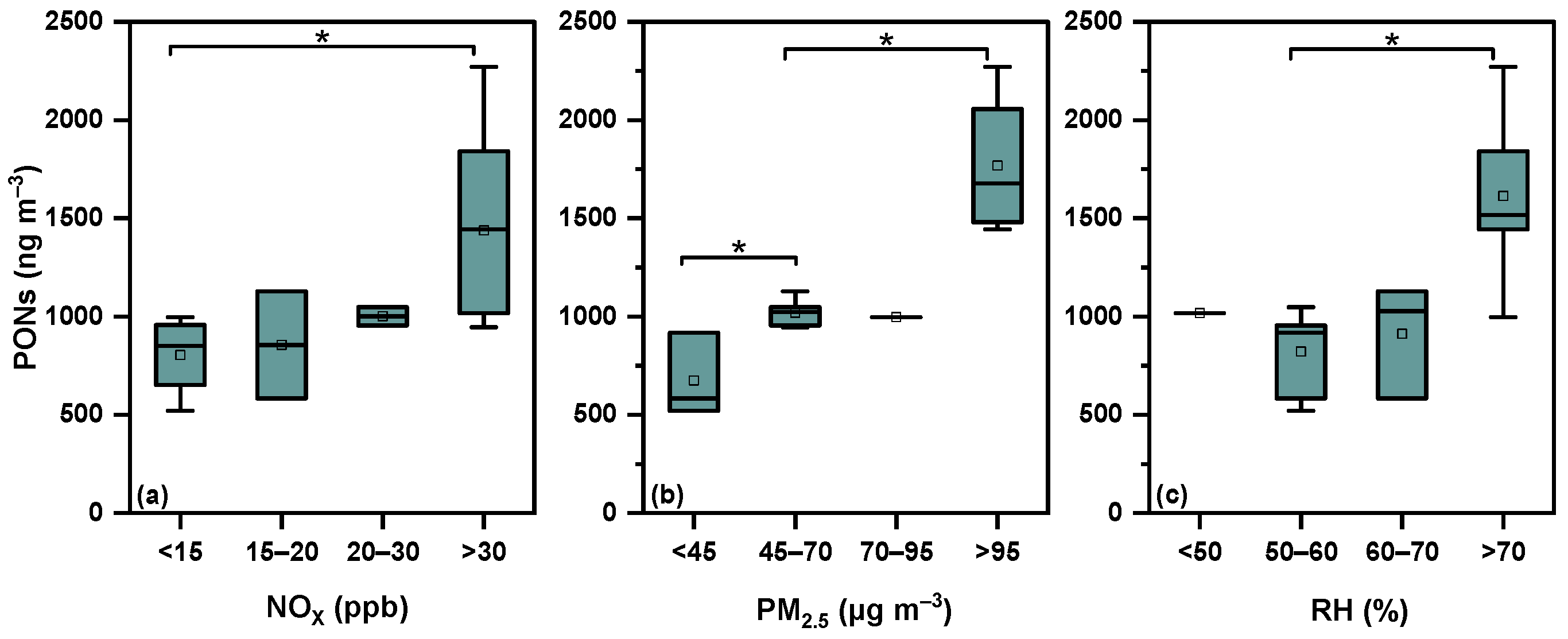
3.1. Concentrations and Variation Patterns of PONs
3.2. Size Distributions and Chemical Compositions
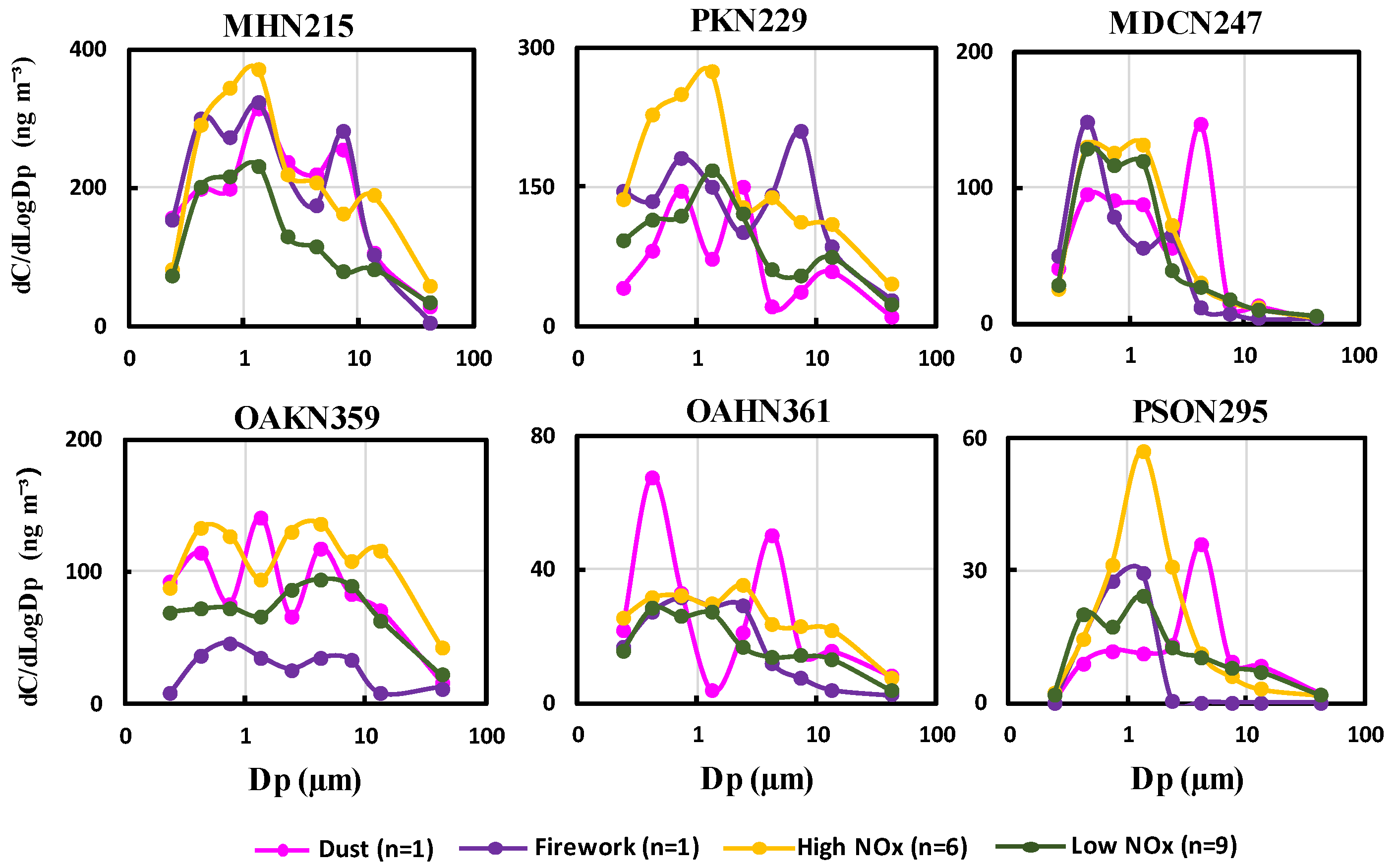
3.3. Effects of Primary Emissions on the Size Distribution Characteristics
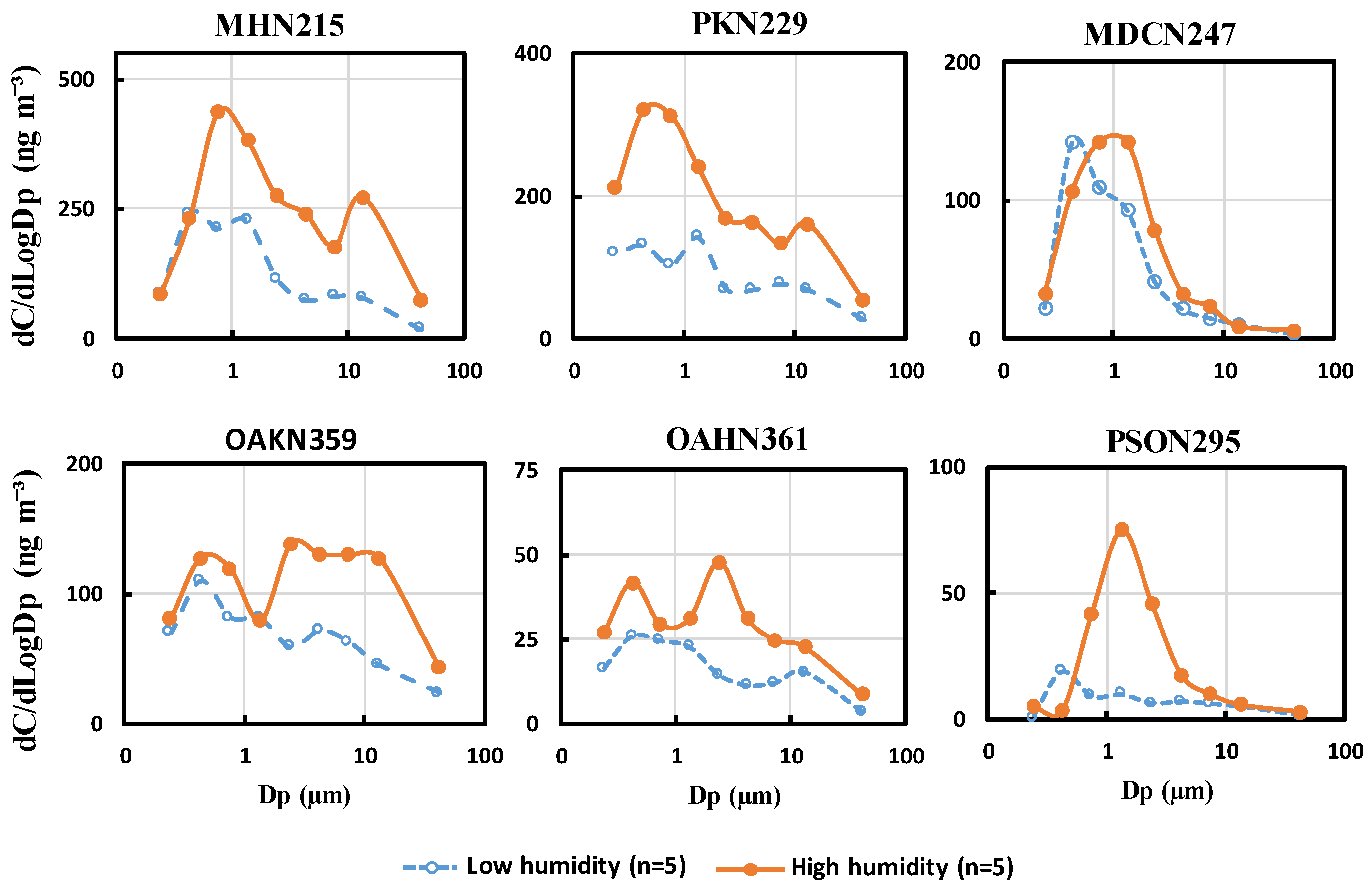
3.4. Changes in Size Distribution Caused by Moisture and Aqueous Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayres, B.R.; Allen, H.M.; Draper, D.C.; Brown, S.S.; Wild, R.J.; Jimenez, J.L.; Day, D.A.; Campuzano-Jost, P.; Hu, W.; de Gouw, J.; et al. Organic nitrate aerosol formation via NO3 + biogenic volatile organic compounds in the southeastern United States. Atmos. Chem. Phys. 2015, 15, 13377–13392. [Google Scholar] [CrossRef]
- Jia, N.; Li, Y.; Chen, R.; Yang, H. A Review of Global PM2.5 Exposure Research Trends from 1992 to 2022. Sustainability 2023, 15, 10509. [Google Scholar] [CrossRef]
- Lee, A.K.Y.; Adam, M.G.; Liggio, J.; Li, S.M.; Li, K.; Willis, M.D.; Abbatt, J.P.D.; Tokarek, T.W.; Odame-Ankrah, C.A.; Osthoff, H.D.; et al. A large contribution of anthropogenic organo-nitrates to secondary organic aerosol in the Alberta oil sands. Atmos. Chem. Phys. 2019, 19, 12209–12219. [Google Scholar] [CrossRef]
- Ng, N.L.; Brown, S.S.; Archibald, A.T.; Atlas, E.; Cohen, R.C.; Crowley, J.N.; Day, D.A.; Donahue, N.M.; Fry, J.L.; Fuchs, H.; et al. Nitrate radicals and biogenic volatile organic compounds: Oxidation, mechanisms, and organic aerosol. Atmos. Chem. Phys. 2017, 17, 2103–2162. [Google Scholar] [CrossRef]
- Xu, L.; Suresh, S.; Guo, H.; Weber, R.J.; Ng, N.L. Aerosol characterization over the southeastern United States using high-resolution aerosol mass spectrometry: Spatial and seasonal variation of aerosol composition and sources with a focus on organic nitrates. Atmos. Chem. Phys. 2015, 15, 7307–7336. [Google Scholar] [CrossRef]
- Rollins, A.W.; Browne, E.C.; Min, K.E.; Pusede, S.E.; Wooldridge, P.J.; Gentner, D.R.; Goldstein, A.H.; Liu, S.; Day, D.A.; Russell, L.M.; et al. Evidence for NO(x) control over nighttime SOA formation. Science 2012, 337, 1210–1212. [Google Scholar] [CrossRef]
- Mao, J.; Paulot, F.; Jacob, D.J.; Cohen, R.C.; Crounse, J.D.; Wennberg, P.O.; Keller, C.A.; Hudman, R.C.; Barkley, M.P.; Horowitz, L.W. Ozone and organic nitrates over the eastern United States: Sensitivity to isoprene chemistry. J. Geophys. Res. Atmos. 2013, 118, 11256–11268. [Google Scholar] [CrossRef]
- Lee, B.H.; Mohr, C.; Lopez-Hilfiker, F.D.; Lutz, A.; Hallquist, M.; Lee, L.; Romer, P.; Cohen, R.C.; Iyer, S.; Kurtén, T.; et al. Highly functionalized organic nitrates in the southeast United States: Contribution to secondary organic aerosol and reactive nitrogen budgets. Proc. Natl. Acad. Sci. USA 2016, 113, 1516–1521. [Google Scholar] [CrossRef]
- Farmer, D.K.; Perring, A.E.; Wooldridge, P.J.; Blake, D.R.; Baker, A.; Meinardi, S.; Huey, L.G.; Tanner, D.; Vargas, O.; Cohen, R.C. Impact of organic nitrates on urban ozone production. Atmos. Chem. Phys. 2011, 11, 4085–4094. [Google Scholar] [CrossRef]
- Zare, A.; Romer, P.S.; Nguyen, T.; Keutsch, F.N.; Skog, K.; Cohen, R.C. A comprehensive organic nitrate chemistry: Insights into the lifetime of atmospheric organic nitrates. Atmos. Chem. Phys. 2018, 18, 15419–15436. [Google Scholar] [CrossRef]
- Tegen, I.; Schepanski, K. Climate Feedback on Aerosol Emission and Atmospheric Concentrations. Curr. Clim. Chang. Rep. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Xu, L.; Guo, H.; Boyd, C.M.; Klein, M.; Bougiatioti, A.; Cerully, K.M.; Hite, J.R.; Isaacman-VanWertz, G.; Kreisberg, N.M.; Knote, C.; et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proc. Natl. Acad. Sci. USA 2015, 112, 37–42. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Li, R.; Dong, S.; Chen, J.; Zhang, Y.; Zheng, P.; Li, M.; Chen, T.; Liu, Y.; et al. Significant impacts of anthropogenic activities on monoterpene and oleic acid-derived particulate organic nitrates in the North China Plain. Atmos. Res. 2021, 256, 105585. [Google Scholar] [CrossRef]
- Xu, L.; Du, L.; Tsona, N.T.; Ge, M. Anthropogenic Effects on Biogenic Secondary Organic Aerosol Formation. Adv. Atmos. Sci. 2021, 38, 1053–1084. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhang, J.; Li, M.; Li, H.; Liu, Z.; Bi, Y.; Wu, D.; Yin, X.; Gu, R.; et al. Particulate organic nitrates at Mount Tai in winter and spring: Variation characteristics and effects of mountain-valley breezes and elevated emission sources. Environ. Res. 2022, 212, 113182. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Li, R.; Dong, S.; Zhang, Y.; Zheng, P.; Li, M.; Chen, T.; Liu, Y.; Xue, L.; et al. Particulate organic nitrates in eastern China: Variation characteristics and effects of anthropogenic activities. Atmos. Chem. Phys. Discuss. 2019, 19, 1–22. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Li, K.; Chen, Y.; Zhang, H.; Zhang, X.; Wu, Z.; Liu, Y.; Wang, X.; Wang, W.; et al. Enhanced secondary organic aerosol formation from the photo-oxidation of mixed anthropogenic volatile organic compounds. Atmos. Chem. Phys. 2021, 21, 7773–7789. [Google Scholar] [CrossRef]
- Xu, Y.; Miyazaki, Y.; Tachibana, E.; Sato, K.; Ramasamy, S.; Mochizuki, T.; Sadanaga, Y.; Nakashima, Y.; Sakamoto, Y.; Matsuda, K.; et al. Aerosol Liquid Water Promotes the Formation of Water-Soluble Organic Nitrogen in Submicrometer Aerosols in a Suburban Forest. Environ. Sci. Technol. 2020, 54, 1406–1414. [Google Scholar] [CrossRef]
- Riedel, T.; Lin, Y.-H.; Budisulistiorini, S.; Gaston, C.; Thornton, J.; Zhang, Z.; Vizuete, W.; Gold, A.; Surratt, J. Heterogeneous Reactions of Isoprene-Derived Epoxides: Reaction Probabilities and Molar Secondary Organic Aerosol Yield Estimates. Environ. Sci. Technol. Lett. 2015, 2, 38–42. [Google Scholar] [CrossRef]
- Northcross, A.L.; Jang, M. Heterogeneous SOA yield from ozonolysis of monoterpenes in the presence of inorganic acid. Atmos. Environ. 2007, 41, 1483–1493. [Google Scholar] [CrossRef]
- Rindelaub, J.D.; Borca, C.H.; Hostetler, M.A.; Slade, J.H.; Lipton, M.A.; Slipchenko, L.V.; Shepson, P.B. The acid-catalyzed hydrolysis of an α-pinene-derived organic nitrate: Kinetics, products, reaction mechanisms, and atmospheric impact. Atmos. Chem. Phys. 2016, 16, 15425–15432. [Google Scholar] [CrossRef]
- Fry, J.L.; Brown, S.S.; Middlebrook, A.M.; Edwards, P.M.; Campuzano-Jost, P.; Day, D.A.; Jimenez, J.L.; Allen, H.M.; Ryerson, T.B.; Pollack, I.; et al. Secondary organic aerosol (SOA) yields from NO3 radical + isoprene based on nighttime aircraft power plant plume transects. Atmos. Chem. Phys. 2018, 18, 11663–11682. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.; Wang, Y.; Gao, W.; Li, H.; Zhang, Y.; Li, J.; Zhao, S.; Yan, Y.; Ji, D.; et al. Exploring the inorganic and organic nitrate aerosol formation regimes at a suburban site on the North China Plain. Sci. Total Environ. 2021, 768, 144538. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Luecken, D.J.; Xu, L.; Boyd, C.M.; Ng, N.L.; Baker, K.R.; Ayres, B.R.; Bash, J.O.; Baumann, K.; Carter, W.P.L.; et al. Modeling the Current and Future Roles of Particulate Organic Nitrates in the Southeastern United States. Environ. Sci. Technol. 2015, 49, 14195–14203. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Song, W.; Geng, F.; Wang, L. Seasonal and diurnal variations of particulate organosulfates in urban Shanghai, China. Atmos. Environ. 2014, 85, 152–160. [Google Scholar] [CrossRef]
- He, Q.-F.; Ding, X.; Wang, X.-M.; Yu, J.-Z.; Fu, X.-X.; Liu, T.-Y.; Zhang, Z.; Xue, J.; Chen, D.-H.; Zhong, L.-J.; et al. Organosulfates from Pinene and Isoprene over the Pearl River Delta, South China: Seasonal Variation and Implication in Formation Mechanisms. Environ. Sci. Technol. 2014, 48, 9236–9245. [Google Scholar] [CrossRef]
- Zhang, J.K.; Cheng, M.T.; Ji, D.S.; Liu, Z.R.; Hu, B.; Sun, Y.; Wang, Y.S. Characterization of submicron particles during biomass burning and coal combustion periods in Beijing, China. Sci. Total Environ. 2016, 562, 812–821. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, Z.; Zhu, M.; Wang, X. Molecular Characterization of Organosulfates in Arctic Ocean and Antarctic atmospheric aerosols. Atmos. Chem. Phys. Discuss. 2019, 19, 1–24. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Guo, S.; Wang, Y.; Zheng, J.; Yang, Y.; Zhu, W.; Tang, R.; Li, X.; Liu, Y.; et al. The secondary formation of organosulfates under interactions between biogenic emissions and anthropogenic pollutants in summer in Beijing. Atmos. Chem. Phys. 2018, 18, 10693–10713. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Zheng, P.; Chen, T.; Liu, Y.; Han, G.; Simpson, I.J.; Wang, X.; Blake, D.R.; Li, Z.; et al. Observations of C1–C5 alkyl nitrates in the Yellow River Delta, northern China: Effects of biomass burning and oil field emissions. Sci. Total Environ. 2019, 656, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yang, Y.; Li, Y.; Wang, H.; Yu, F.; Liu, J. Beijing Heavy-Duty Diesel Vehicle Battery Capacity Conversion and Emission Estimation in 2022. Sustainability 2023, 15, 11019. [Google Scholar] [CrossRef]
- Yang, X.; Deng, X.; Li, G.; Liu, Y.; Gao, Q. Human Activities Aggravate VOC Pollution in the Huangshui River of the Tibetan Plateau. Sustainability 2022, 14, 11983. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, X.; Dong, S.; Liu, Z.; Mu, J.; Lu, C.; Zhang, J.; Li, M.; Xue, L.; Wang, W. Size distributions of nitrated phenols in winter at a coastal site in north China and the impacts from primary sources and secondary formation. Chemosphere 2020, 250, 126256. [Google Scholar] [CrossRef]
- Cloud. IBM. 2019. Available online: https://www.wunderground.com (accessed on 25 February 2019).
- Li, R.; Wang, X.; Gu, R.; Lu, C.; Zhu, F.; Xue, L.; Xie, H.; Du, L.; Chen, J.; Wang, W. Identification and semi-quantification of biogenic organic nitrates in ambient particulate matters by UHPLC/ESI-MS. Atmos. Environ. 2018, 176, 140–147. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, Y.; Gu, R.; Xia, M.; Li, H.; Dong, S.; Xue, L.; Wu, Z.; Zhang, Y.; et al. Pollution characteristics and formation of particulate organic nitrates in winter in Beijing City. Geochimica 2020, 49, 252–261. [Google Scholar] [CrossRef]
- Miousse, I.R.; Hale, R.B.; Alsbrook, S.; Boysen, G.; Broadnax, T.; Murry, C.; Williams, C.; Park, C.H.; Richards, R.; Reedy, J.; et al. Climate Change and New Challenges for Rural Communities: Particulate Matter Matters. Sustainability 2023, 15, 16192. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, P.; Chen, Y. Analysis of the Social and Economic Factors Influencing PM2.5 Emissions at the City Level in China. Sustainability 2023, 15, 16335. [Google Scholar] [CrossRef]
- Shchekin, A.K.; Podguzova, T.S. The modified Thomson equation in the theory of heterogeneous vapor nucleation on charged solid particles. Atmos. Res. 2011, 101, 493–502. [Google Scholar] [CrossRef]
- Chim, M.M.; Cheng, C.T.; Davies, J.F.; Berkemeier, T.; Shiraiwa, M.; Zuend, A.; Chan, M.N. Compositional evolution of particle-phase reaction products and water in the heterogeneous OH oxidation of model aqueous organic aerosols. Atmos. Chem. Phys. 2017, 17, 14415–14431. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhou, B.; Cheng, C.; Cao, J.; Shen, Z.; An, Z. Chemical composition and size distribution of wintertime aerosols in the atmosphere of Mt. Hua in central China. Atmos. Environ. 2011, 45, 1251–1258. [Google Scholar] [CrossRef]
- Zhang, D.; Zang, J.; Shi, G.; Iwasaka, Y.; Matsuki, A.; Trochkine, D. Mixture state of individual Asian dust particles at a coastal site of Qingdao, China. Atmos. Environ. 2003, 37, 3895–3901. [Google Scholar] [CrossRef]
- Huang, X.-F.; Dai, J.; Zhu, Q.; Yu, K.; Du, K. Abundant Biogenic Oxygenated Organic Aerosol in Atmospheric Coarse Particles: Plausible Sources and Atmospheric Implications. Environ. Sci. Technol. 2020, 54, 1425–1430. [Google Scholar] [CrossRef]
- Hu, W.; Hu, M.; Hu, W.; Jimenez, J.L.; Yuan, B.; Chen, W.; Wang, M.; Wu, Y.; Chen, C.; Wang, Z.; et al. Chemical composition, sources, and aging process of submicron aerosols in Beijing: Contrast between summer and winter. J. Geophys. Res. Atmos. 2016, 121, 1955–1977. [Google Scholar] [CrossRef]
- Hu, J.; Wang, P.; Ying, Q.; Zhang, H.; Chen, J.; Ge, X.; Li, X.; Jiang, J.; Wang, S.; Zhang, J.; et al. Modeling biogenic and anthropogenic secondary organic aerosol in China. Atmos. Chem. Phys. 2017, 17, 77–92. [Google Scholar] [CrossRef]
- Wang, G.H.; Zhou, B.H.; Cheng, C.L.; Cao, J.J.; Li, J.J.; Meng, J.J.; Tao, J.; Zhang, R.J.; Fu, P.Q. Impact of Gobi desert dust on aerosol chemistry of Xi’an, inland China during spring 2009: Differences in composition and size distribution between the urban ground surface and the mountain atmosphere. Atmos. Chem. Phys. 2013, 13, 819–835. [Google Scholar] [CrossRef]
- Lv, S.; Wang, F.; Wu, C.; Chen, Y.; Liu, S.; Zhang, S.; Li, D.; Du, W.; Zhang, F.; Wang, H.; et al. Gas-to-Aerosol Phase Partitioning of Atmospheric Water-Soluble Organic Compounds at a Rural Site in China: An Enhancing Effect of NH3 on SOA Formation. Environ. Sci. Technol. 2022, 56, 3915–3924. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Xue, L.; Gao, X.; Nie, W.; Yu, Y.; Zhou, Y.; Yang, L.; Zhang, Q.; Wang, T. Size-resolved aerosol ionic composition and secondary formation at Mount Heng in South Central China. Front. Environ. Sci. Eng. 2013, 7, 815–826. [Google Scholar] [CrossRef]
- Kong, S.F.; Li, L.; Li, X.X.; Yin, Y.; Chen, K.; Liu, D.T.; Yuan, L.; Zhang, Y.J.; Shan, Y.P.; Ji, Y.Q. The impacts of firework burning at the Chinese Spring Festival on air quality: Insights of tracers, source evolution and aging processes. Atmos. Chem. Phys. 2015, 15, 2167–2184. [Google Scholar] [CrossRef]
- Singh, A.; Pant, P.; Pope, F.D. Air quality during and after festivals: Aerosol concentrations, composition and health effects. Atmos. Res. 2019, 227, 220–232. [Google Scholar] [CrossRef]
- Morales, A.C.; Jayarathne, T.; Slade, J.H.; Laskin, A.; Shepson, P.B. The production and hydrolysis of organic nitrates from OH radical oxidation of β-ocimene. Atmos. Chem. Phys. 2021, 21, 129–145. [Google Scholar] [CrossRef]
- Pullinen, I.; Schmitt, S.; Kang, S.; Sarrafzadeh, M.; Schlag, P.; Andres, S.; Kleist, E.; Mentel, T.F.; Rohrer, F.; Springer, M.; et al. Impact of NOx on secondary organic aerosol (SOA) formation from α-pinene and β-pinene photooxidation: The role of highly oxygenated organic nitrates. Atmos. Chem. Phys. 2020, 20, 10125–10147. [Google Scholar] [CrossRef]
- Fang, Y.; Ye, C.; Wang, J.; Wu, Y.; Hu, M.; Lin, W.; Xu, F.; Zhu, T. Relative humidity and O3 concentration as two prerequisites for sulfate formation. Atmos. Chem. Phys. 2019, 19, 12295–12307. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wu, Y.; Tao, J.; Xia, Y.; Wang, C.; Zhang, R. Effects of chemical compositions in fine particles and their identified sources on hygroscopic growth factor during dry season in urban Guangzhou of South China. Sci. Total Environ. 2021, 801, 149749. [Google Scholar] [CrossRef]
- Aljawhary, D.; Zhao, R.; Lee, A.K.Y.; Wang, C.; Abbatt, J.P.D. Kinetics, Mechanism, and Secondary Organic Aerosol Yield of Aqueous Phase Photo-Oxidation of α-Pinene Oxidation Products. J. Phys. Chem. A 2016, 120, 1395–1407. [Google Scholar] [CrossRef]



| Parameters | PM1 | PM1.8 | PM10 | TSP |
|---|---|---|---|---|
| MHN215 | 138 ± 85.5 | 215 ± 114.4 | 333 ± 180.4 | 397 ± 240.0 |
| PKN229 | 115 ± 92.5 | 164 ± 105.4 | 243 ± 138.7 | 294 ± 162.9 |
| MDCN247 | 67.2 ± 15.6 | 97.3 ± 26.2 | 123 ± 31.2 | 129 ± 31.0 |
| OAKN359 | 63.8 ± 31.1 | 84.5 ± 38.7 | 155.4 ± 67.3 | 197 ± 93.6 |
| OAHN361 | 19.6 ± 6.3 | 26.4 ± 7.8 | 42.7 ± 16.8 | 50.8 ± 21.4 |
| PSON295 | 9.7 ± 7.0 | 19.4 ± 16.2 | 29.2 ± 24.7 | 31.8 ± 25.7 |
| ∑PONs | 415 ± 238 | 606 ± 309 | 927 ± 459 | 1099 ± 577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakoor, A.; Wang, X.; Chen, J.; Liang, Y.; Kamal, S.; Ali, J.; Syed, J.H.; Xue, L.; Wang, W. Size Distribution and Secondary Formation of Particulate Organic Nitrates in Winter in a Coastal Area. Sustainability 2023, 15, 16776. https://doi.org/10.3390/su152416776
Shakoor A, Wang X, Chen J, Liang Y, Kamal S, Ali J, Syed JH, Xue L, Wang W. Size Distribution and Secondary Formation of Particulate Organic Nitrates in Winter in a Coastal Area. Sustainability. 2023; 15(24):16776. https://doi.org/10.3390/su152416776
Chicago/Turabian StyleShakoor, Ayesha, Xinfeng Wang, Jing Chen, Yiheng Liang, Sajid Kamal, Jawad Ali, Jabir Hussain Syed, Likun Xue, and Wenxing Wang. 2023. "Size Distribution and Secondary Formation of Particulate Organic Nitrates in Winter in a Coastal Area" Sustainability 15, no. 24: 16776. https://doi.org/10.3390/su152416776
APA StyleShakoor, A., Wang, X., Chen, J., Liang, Y., Kamal, S., Ali, J., Syed, J. H., Xue, L., & Wang, W. (2023). Size Distribution and Secondary Formation of Particulate Organic Nitrates in Winter in a Coastal Area. Sustainability, 15(24), 16776. https://doi.org/10.3390/su152416776








