Enrichment of a Mixed Culture of Purple Non-Sulfur Bacteria for Hydrogen Production from Organic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of the Purple Mixed Microbial Culture: Enrichment Phases
2.2. Hydrogen Production Phase: H2 Production by the Enriched Purple Inoculum (EPI)
2.3. Analytical Methods
2.4. Microbial Diversity Analyses
2.5. Data Analysis and Statistical Tools
3. Results
3.1. Enrichment and Hydrogen Production Phase of the Purple Mixed Microbial Culture
3.2. Microbial Community Structure of Purple Mixed Microbial Culture
3.3. Influence of Enrichment Operating Conditions on Microbial Community Structure
4. Discussion
4.1. Operational Enrichment Conditions
4.2. Microbial Community Structure in the Consortia
4.3. Hydrogen Production of the Consortia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Kumar, A.; Pal, A. An overview of conventional and non-conventional hydrogen production methods. Mater. Today Proc. 2021, 46, 5353–5359. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Marone, A. Biohydrogen production by dark fermentation: Scaling-up and technologies integration for a sustainable system. Rev. Environ. Sci. Bio/Technol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Verma, D.; Ram Kumar, N.; Subudhi, S. Isolation and characterization of a novel photoheterotrophic strain ‘Rubrivivax benzoatilyticus TERI-CHL1’: Photo fermentative hydrogen production from spent effluent. Int. J. Hydrogen Energy 2020, 45, 14245–14254. [Google Scholar] [CrossRef]
- Moscoviz, R.; Trably, E.; Bernet, N.; Carrere, H. The environmental biorefinery: State-of-the-art on the production of hydrogen and value-added biomolecules in mixed-culture fermentation. Green. Chem. 2018, 20, 3159–3179. [Google Scholar] [CrossRef]
- Anam, K.; Habibi, M.S.; Harwati, T.U.; Susilaningsih, D. Photofermentative hydrogen production using Rhodobium marinum from bagasse and soy sauce wastewater. Int. J. Hydrogen Energy 2012, 37, 15436–15442. [Google Scholar] [CrossRef]
- Ghimire, A.; Trably, E.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G.; Escudié, R. Effect of total solids content on biohydrogen production and lactic acid accumulation during dark fermentation of organic waste biomass. Bioresour. Technol. 2018, 248, 180–186. [Google Scholar] [CrossRef]
- Uyar, B.; Eroglu, I.; Yücel, M.; Gündüz, U. Photofermentative hydrogen production from volatile fatty acids present in dark fermentation effluents. Int. J. Hydrogen Energy 2009, 34, 4517–4523. [Google Scholar] [CrossRef]
- Nath, K.; Muthukumar, M.; Kumar, A.; Das, D. Kinetics of two-stage fermentation process for the production of hydrogen. Int. J. Hydrogen Energy 2008, 33, 1195–1203. [Google Scholar] [CrossRef]
- Laurinavichene, T.V.; Belokopytov, B.F.; Laurinavichius, K.S.; Khusnutdinova, A.N.; Seibert, M.; Tsygankov, A.A. Towards the integration of dark- and photo-fermentative waste treatment. 4. Repeated batch sequential dark- and photofermentation using starch as substrate. Int. J. Hydrogen Energy 2012, 37, 8800–8810. [Google Scholar] [CrossRef]
- Singh, L.; Wahid, Z.A. Methods for enhancing bio-hydrogen production from biological process: A review. J. Ind. Eng. Chem. 2015, 21, 70–80. [Google Scholar] [CrossRef]
- Monroy, I.; Buitrón, G. Production of polyhydroxybutyrate by pure and mixed cultures of purple non-sulfur bacteria: A review. J. Biotechnol. 2020, 317, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, C.Z.; Vich, D.V.; Hirasawa, J.S.; Varesche, M.B.A. Hydrogen production and consumption of organic acids by a phototrophic microbial consortium. Int. J. Hydrogen Energy 2012, 37, 11691–11700. [Google Scholar] [CrossRef]
- Tawfik, A.; El-Bery, H.; Kumari, S.; Bux, F. Use of mixed culture bacteria for photofermentive hydrogen of dark fermentation effluent. Bioresour. Technol. 2014, 168, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Cardeña, R.; Moreno, G.; Valdez-Vazquez, I.; Buitrón, G. Optimization of volatile fatty acids concentration for photofermentative hydrogen production by a consortium. Int. J. Hydrogen Energy 2015, 40, 17212–17223. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Argun, H.; Kargi, F. Photo-fermentative hydrogen gas production from dark fermentation effluent of ground wheat solution: Effects of light source and light intensity. Int. J. Hydrogen Energy 2010, 35, 1595–1603. [Google Scholar] [CrossRef]
- Adessi, A.; De Philippis, R. Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: A review. Int. J. Hydrogen Energy 2014, 39, 3127–3141. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Shanmugam, S.; Sekar, M.; Mathimani, T.; Incharoensakdi, A.; Kim, S.; Parthiban, A.; Geo, V.; Brindhadevi, K.; Pugazhendhi, A. Insights on biological hydrogen production routes and potential microorganisms for high hydrogen yield. Fuel 2021, 291, 120136. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from food processing wastes via photofermentation using Purple Non-sulfur Bacteria (PNSB)—A review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Ji, B.; Liu, Y. Towards mainstream deammonification of municipal wastewater: Partial nitrification-anammox versus partial denitrification-anammox. Sci. Total Environ. 2019, 692, 393–401. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Effect of light intensity and initial pH during hydrogen production by an integrated dark and photofermentation process. Int. J. Hydrogen Energy 2009, 34, 7497–7501. [Google Scholar] [CrossRef]
- Dong, L.; Zhenhong, Y.; Yongming, S.; Xiaoying, K.; Yu, Z. Hydrogen production characteristics of the organic fraction of municipal solid wastes by anaerobic mixed culture fermentation. Int. J. Hydrogen Energy 2009, 34, 812–820. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Revah, S.; Morales, M. Hydrogen production by an enriched photoheterotrophic culture using dark fermentation effluent as substrate: Effect of flushing method, bicarbonate addition, and outdoor–indoor conditions. Int. J. Hydrogen Energy 2015, 40, 9096–9105. [Google Scholar] [CrossRef]
- Silva-Illanes, F.; Tapia-Venegas, E.; Schiappacasse, M.; Trably, E.; Ruiz-Filippi, G. Impact of hydraulic retention time (HRT) and pH on dark fermentative hydrogen production from glycerol. Energy 2017, 141, 358–367. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H.; Zhang, T. Phototrophic hydrogen production from acetate and butyrate in wastewater. Int. J. Hydrogen Energy 2005, 30, 785–793. [Google Scholar] [CrossRef]
- Vesga-Baron, A.; Etchebehere, C.; Schiappacasse, M.C.; Chamy, R.; Tapia-Venegas, E. Controlled oxidation-reduction potential on dark fermentative hydrogen production from glycerol: Impacts on metabolic pathways and microbial diversity of an acidogenic sludge. Int. J. Hydrogen Energy 2021, 46, 5074–5084. [Google Scholar] [CrossRef]
- Tapia-Venegas, E.; Cabrol, L.; Brandhoff, B.; Hamelin, J.; Trably, E.; Steyer, J.P.; Ruiz-Filippi, G. Adaptation of acidogenic sludge to increasing glycerol concentrations for biohydrogen production. Appl. Microbiol. Biotechnol. 2015, 99, 8295–8308. [Google Scholar] [CrossRef]
- Ahmad, H.; Ramli, R.; Ismail, N.N.; Aidit, S.N.; Yusoff, N.; Samion, M.Z. Passively mode locked thulium and thulium/holmium doped fiber lasers using MXene Nb2C coated microfiber. Sci. Rep. 2021, 11, 11652. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.R.P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 2001, 4, 1–9. Available online: http://www.sciepub.com/reference/139944 (accessed on 8 January 2023).
- Legendre, P.; Legendre, L.F.J.; Numerical Ecology. Elsevier Science. 1998. Available online: https://books.google.fr/books?id=KBoHuoNRO5MC (accessed on 8 January 2023).
- Okubo, Y.; Futamata, H.; Hiraishi, A. Characterization of Phototrophic Purple Nonsulfur Bacteria Forming Colored Microbial Mats in a Swine Wastewater Ditch. Appl. Environ. Microbiol. 2006, 72, 6225–6233. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Batstone, D.J.; Grassino, M.; Vlaeminck, S.E.; Puyol, D.; Verstraete, W.; Hülsen, T. Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotechnol. Adv. 2020, 43, 107567. [Google Scholar] [CrossRef]
- Tiang, M.F.; Hanipa, M.A.F.; Abdul, P.M.; Jahim, J.M.; Mahmod, S.S.; Takriff, M.S.; Lay, C.-H.; Reungsang, A.; Wu, S.-Y. Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulphur bacteria: An overview. Int. J. Hydrogen Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Li, S.; Tabatabaei, M.; Li, F.; Ho, S.H. A review of green biohydrogen production using anoxygenic photosynthetic bacteria for hydrogen economy: Challenges and opportunities. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Cerruti, M.; Stevens, B.; Ebrahimi, S.; Alloul, A.; Vlaeminck, S.E.; Weissbrodt, D.G. Enrichment and Aggregation of Purple Non-sulfur Bacteria in a Mixed-Culture Sequencing-Batch Photobioreactor for Biological Nutrient Removal from Wastewater. Front. Bioeng. Biotechnol. 2020, 8, 557234. [Google Scholar] [CrossRef]
- Hülsen, T.; Barry, E.M.; Lu, Y.; Puyol, D.; Keller, J.; Batstone, D.J. Domestic wastewater treatment with purple phototrophic bacteria using a novel continuous photo anaerobic membrane bioreactor. Water Res. 2016, 100, 486–495. [Google Scholar] [CrossRef]
- Ren, N.Q.; Liu, B.F.; Ding, J.; Xie, G.J. Hydrogen production with R. faecalis RLD-53 isolated from freshwater pond sludge. Bioresour. Technol. 2009, 100, 484–487. [Google Scholar] [CrossRef]
- Guevara-Lopez, E.; Buitrón, G. Evaluation of different support materials used with a photo-fermentative consortium for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 17231–17238. [Google Scholar] [CrossRef]
- Brown, B.; Wilkins, M.; Saha, R. Rhodopseudomonas palustris: A biotechnology chassis. Biotechnol. Adv. 2022, 60, 108001. [Google Scholar] [CrossRef]
- Munjam, S.; Rani, K. Enhanced bioproduction of hydrogen by alginate immobilized cells of a purple non-sulphur bacterum Rhodocyclus tenuis KU 017. Int. J. Pharma Bio Sci. 2014, 5, B887–B897. [Google Scholar]
- Thapa, S.; Kim, T.; Pandit, S.; Song, Y.; Afsharian, Y.; Rahimnejad, M.; Kim, J.; Oh, S. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef] [PubMed]
- Paquete, C.M. Electroactivity across the cell wall of Gram-positive bacteria. Comput Struct Biotechnol. J. 2020, 18, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Khanthong, K.; Purnomo, C.W.; Daosud, W.; Laoong-u-thai, Y. Microbial diversity of marine shrimp pond sediment and its variability due to the effect of immobilized media in biohydrogen and biohythane production. J. Environ. Chem. Eng. 2021, 9, 106166. [Google Scholar] [CrossRef]
- Fischer-Romero, C.; Tindall, B.J.; Jüttner, F. Tolumonas auensis gen. nov., sp. nov., a toluene-producing bacterium from anoxic sediments of a freshwater lake. Int. J. Syst. Bacteriol. 1996, 46, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Youssef, M.K. MICROBIOLOGICAL SAFETY OF MEAT|Emerging Pathogens. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 340–344. [Google Scholar] [CrossRef]
- Kruse, S.; Goris, T.; Westermann, M.; Adrian, L.; Diekert, G. Hydrogen production by Sulfurospirillum species enables syntrophic interactions of Epsilonproteobacteria. Nat. Commun. 2018, 9, 4872. [Google Scholar] [CrossRef]
- Luan, J.; Xu, Y. The bio-hydrogen production by klebsiella, bacteroides and ruminococcus from wastewater which contained coconut oil, hydraulic oil or peanut oil. J. Biotechnol. 2014, 185, S124. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, H.; Varrone, C.; Shyryn, A.; Defemur, Z.; Wang, S.; Liu, W.; Yue, X. New insight into waste activated sludge acetogenesis triggered by coupling sulfite/ferrate oxidation with sulfate reduction-mediated syntrophic consortia. Chem. Eng. J. 2020, 400, 125885. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- McLellan, S.L.; Roguet, A. The unexpected habitat in sewer pipes for the propagation of microbial communities and their imprint on urban waters. Curr. Opin. Biotechnol. 2019, 57, 34–41. [Google Scholar] [CrossRef]
- Roalkvam, I.; Drønen, K.; Stokke, R.; Daae, F.L.; Dahle, H.; Steen, I.H. Physiological and genomic characterization of Arcobacter anaerophilus IR-1 reveals new metabolic features in Epsilonproteobacteria. Front. Microbiol. 2015, 6, 987. [Google Scholar] [CrossRef]
- Hamann, E.; Gruber-Vodicka, H.; Kleiner, M.; Tegetmeyer, H.E.; Riedel, D.; Littmann, S.; Strous, M. Environmental Breviatea harbour mutualistic Arcobacter epibionts. Nature 2016, 534, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, J.; Xu, Z.; Cai, S.; Chen, L.; Cai, T.; Ji, X.M. Bioconversion of waste activated sludge hydrolysate into polyhydroxyalkanoates using Paracoccus sp. TOH: Volatile fatty acids generation and fermentation strategy. Bioresour. Technol. 2022, 363, 127939. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, L.; Gabrielyan, L.; Trchounian, A. Bio-hydrogen production and the F0F1-ATPase activity of Rhodobacter sphaeroides: Effects of various heavy metal ions. Int. J. Hydrogen Energy 2012, 37, 17794–17800. [Google Scholar] [CrossRef]
- Hustede, E.; Steinbüchel, A.; Schlegel, H.G. Relationship between the photoproduction of hydrogen and the accumulation of PHB in non-sulphur purple bacteria. Appl. Microbiol. Biotechnol. 1993, 39, 87–93. [Google Scholar] [CrossRef]
- Sun, M.; Lv, Y.; Liu, Y. A New Hydrogen-Producing Strain and Its Characterization of Hydrogen Production. Appl. Biochem. Biotechnol. 2015, 177, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
- Zagrodnik, R.; Łaniecki, M. Hydrogen production from starch by co-culture of Clostridium acetobutylicum and Rhodobacter sphaeroides in one step hybrid dark- and photofermentation in repeated fed-batch reactor. Bioresour. Technol. 2017, 224, 298–306. [Google Scholar] [CrossRef]
- Moreira, F.S.; Rodrigues, M.S.; Sousa, L.M.; Batista, F.R.X.; Ferreira, J.S.; Cardoso, V.L. Single-stage repeated batch cycles using co-culture of Enterobacter cloacae and purple non-sulfur bacteria for hydrogen production. Energy 2022, 239, 122465. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from organic waste streams using purple non-sulfur bacteria. Bioresour. Technol. 2021, 323, 124610. [Google Scholar] [CrossRef]

| Operational Conditions | N° Experiment | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Agitation mode | On/Off | On/Off | On/Off | On/Off | Continuous | Continuous | Continuous | Continuous |
| Irradiance (μmolm−2s−1) | 32 | 32 | 16 | 16 | 32 | 32 | 16 | 16 |
| Carbon source | Malic acid | VFAs | Malic acid | VFAs | Malic acid | VFAs | Malic acid | VFAs |
| N° Experiment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Phase I: Enrichment | Purple coloring * | + | + | − | + | + | − | − | − |
| Accumulated biogas ** | + | − | − | − | − | − | − | − | |
| % CH4 *** | + | − | − | − | − | − | − | − | |
| % H2 *** | − | − | − | − | − | − | − | − | |
| Phase II: Enrichment | Accumulated biogas ** | 12.4 ± 0.5 | − | NE | − | − | NE | NE | NE |
| %H2 *** | 73.2 ± 2.0 | − | NE | − | − | NE | NE | NE | |
| H2 yield (mmol H2 g−1 COD) | 0.23 ± 0.3 | − | NE | − | − | NE | NE | NE | |
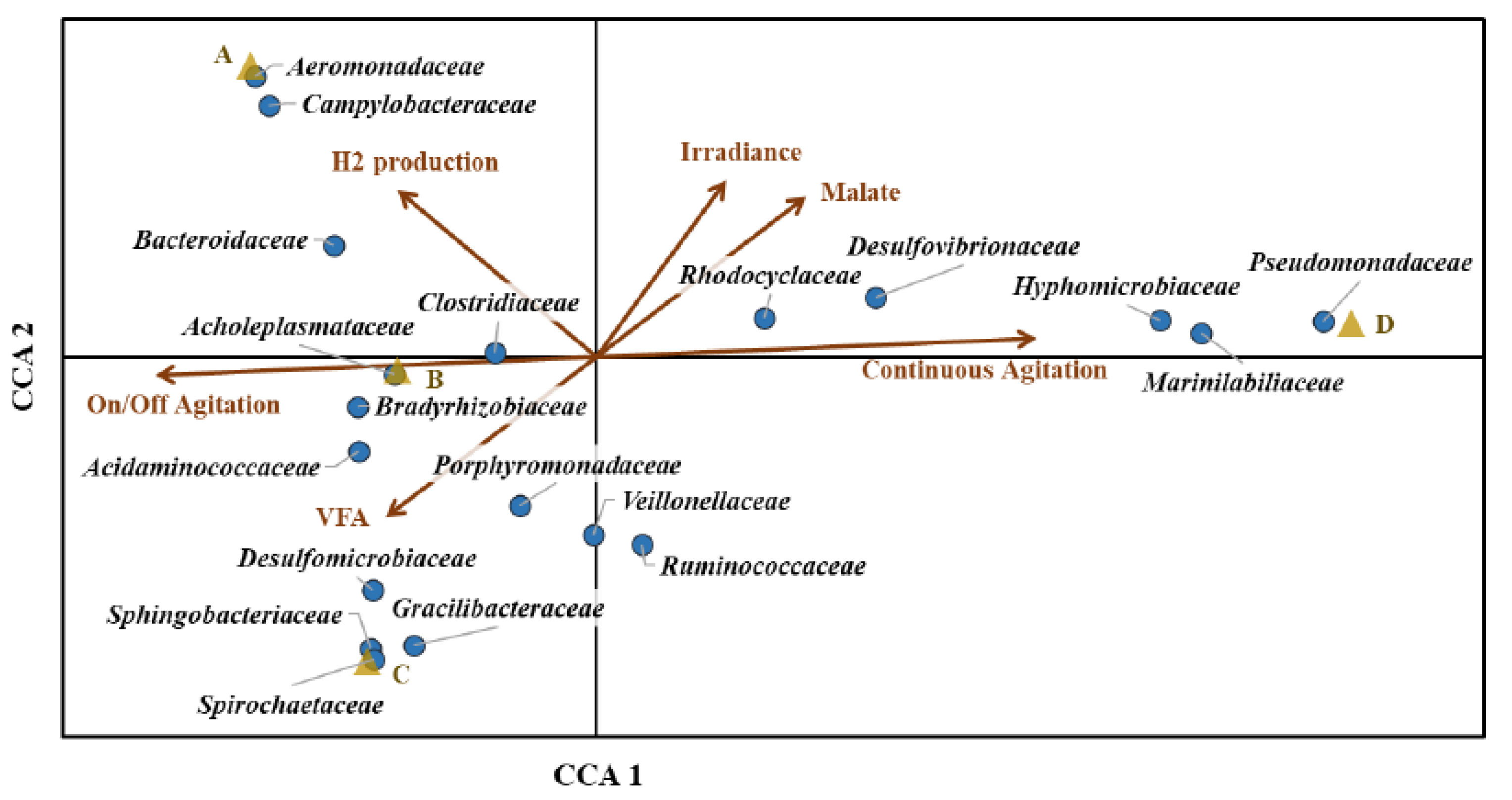
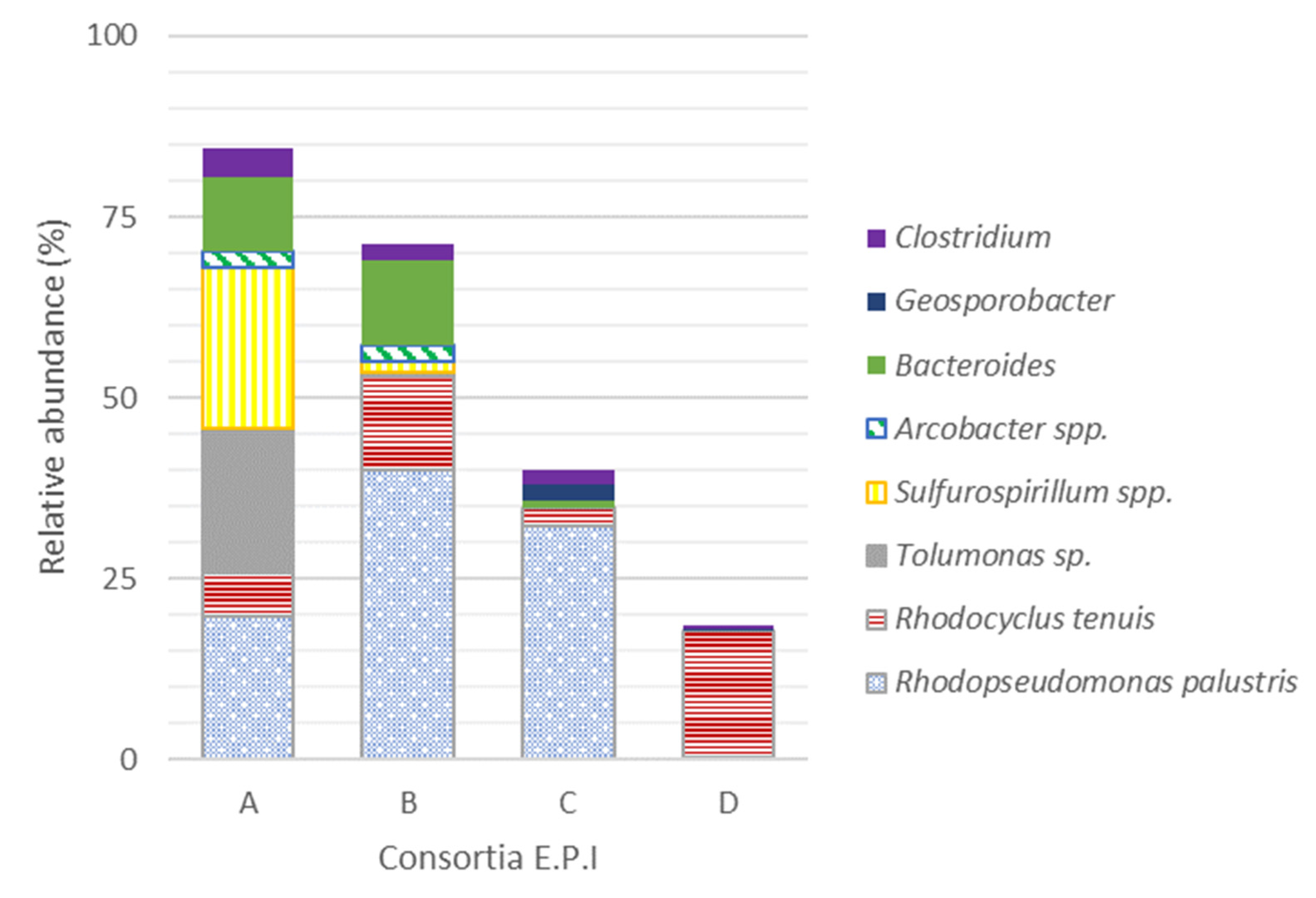
| Enriched Purple Inocula (EPI) name | A | B | − | C | D | − | − | − | |
| Hydrogen production phase | H2 yield (mmol H2 g−1 COD) | 9.37 ± 1.0 | - | NE | - | - | NE | NE | NE |
| Family | Genus | AI * (%) | EPI (%) | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| Pseudomonadaceae | Pseudomonas | 0.6 | 0.7 | 0.1 | 49.0 | |
| Other genus | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Bradyrhizobiaceae | Rhodopseudomonas | 19.7 | 39.9 | 32.2 | 0.2 | |
| Other genus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Campylobacteraceae | Sulfurospirillum | 22.2 | 1.3 | 0.0 | 0.0 | |
| Arcobacter | 2.2 | 2.4 | 0.0 | 0.0 | ||
| Other genus | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Aeromonadaceae | Tolumonas | 20.1 | 0.7 | 0.0 | 0.0 | |
| Other genus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Rhodocyclaceae | Rhodocyclus | 6.0 | 13.0 | 2.6 | 17.5 | |
| Other genus | 0.2 | 1.6 | 1.6 | 0.5 | 0.4 | |
| Porphyromonadaceae | Parabacteroides | 1.5 | 4.8 | 11.4 | 0.0 | |
| Other genus | 0.2 | 0.1 | 0.2 | 1.7 | 3.6 | |
| Veillonellaceae | Sporomusa | 0.0 | 0.0 | 7.9 | 2.6 | |
| Other genus | 0.2 | 0.9 | 1.4 | 5.1 | 2.1 | |
| Bacteroidaceae | Bacteroides | 10.3 | 11.7 | 1.0 | 0.1 | |
| Other genus | 10.3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Marinilabiliaceae | Alkaliflexus | 0.0 | 0.0 | 0.0 | 8.4 | |
| Other genus | 0.2 | 0.4 | 0.8 | 0.6 | 1.5 | |
| Desulfomicrobiaceae | Desulfomicrobium | 0.3 | 2.3 | 9.0 | 0.0 | |
| Other genus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Sphingobacteriaceae | Solitalea | 0.0 | 0.0 | 6.4 | 0.0 | |
| Other genus | 0.1 | 0.0 | 0.3 | 0.0 | 0.0 | |
| Spirochaetaceae | Treponema | 0.0 | 0.0 | 5.6 | 0.0 | |
| Other genus | 2.9 | 0.0 | 0.0 | 0.2 | 0.0 | |
| Acholeplasmataceae | Acholeplasma | 0.1 | 5.8 | 0.2 | 0.0 | |
| Other genus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Desulfovibrionaceae | Desulfovibrio | 1.8 | 3.3 | 0.1 | 5.8 | |
| Other genus | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Clostridiaceae | Geosporobacter | 0.0 | 0.0 | 2.3 | 0.1 | |
| Clostridium | 4.0 | 2.2 | 1.9 | 0.7 | ||
| Other genus | 1.8 | 0.5 | 1.8 | 0.0 | 1.6 | |
| Ruminococcaceae | Ruminococcus | 0.0 | 0.0 | 2.1 | 0.4 | |
| Other genus | 0.37 | 0.0 | 0.3 | 0.9 | 0.9 | |
| Gracilibacteraceae | Gracilibacter | 0.0 | 0.0 | 2.3 | 0.1 | |
| Other genus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Acidaminococcaceae | Phascolarctobacterium | 0.0 | 0.0 | 2.2 | 0.1 | |
| Succinispira | 1.1 | 0.0 | 0.0 | 0.0 | ||
| Other genus | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | |
| Hyphomicrobiaceae | Blastochloris | 0.1 | 0.4 | 0.0 | 1.7 | |
| Other genus | 0.1 | 0.0 | 0.0 | 0.0 | 0.3 | |
| Others families < 2.0% | 84.3 | 6.4 | 4.5 | 3.6 | 2.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.C.; Toledo-Alarcón, J.; Schiappacasse, M.C.; Tapia-Venegas, E. Enrichment of a Mixed Culture of Purple Non-Sulfur Bacteria for Hydrogen Production from Organic Acids. Sustainability 2023, 15, 16607. https://doi.org/10.3390/su152416607
Smith SC, Toledo-Alarcón J, Schiappacasse MC, Tapia-Venegas E. Enrichment of a Mixed Culture of Purple Non-Sulfur Bacteria for Hydrogen Production from Organic Acids. Sustainability. 2023; 15(24):16607. https://doi.org/10.3390/su152416607
Chicago/Turabian StyleSmith, Sean C., Javiera Toledo-Alarcón, María Cristina Schiappacasse, and Estela Tapia-Venegas. 2023. "Enrichment of a Mixed Culture of Purple Non-Sulfur Bacteria for Hydrogen Production from Organic Acids" Sustainability 15, no. 24: 16607. https://doi.org/10.3390/su152416607
APA StyleSmith, S. C., Toledo-Alarcón, J., Schiappacasse, M. C., & Tapia-Venegas, E. (2023). Enrichment of a Mixed Culture of Purple Non-Sulfur Bacteria for Hydrogen Production from Organic Acids. Sustainability, 15(24), 16607. https://doi.org/10.3390/su152416607








