Abstract
The numerous socioeconomic and ecological challenges that coral reef degradation poses in the Greater Caribbean have led to a surge in restoration efforts. In this context, outplanting nursery-reared coral colonies has emerged as one of the most common strategies used to rejuvenate degraded reefs and reinstate critical ecosystem processes such as coral recruitment. However, the extent to which coral outplanting promotes the recruitment of coral species remains a subject of ongoing debate. This study tested the hypothesis that reintroducing the threatened coral Acropora cervicornis to a degraded coral reef promotes coral recruitment. To test our hypothesis, a series of recruitment quadrats were established in an area populated with A. cervicornis outplants and in a reference location devoid of the coral. To further investigate the relationship between A. cervicornis and coral recruitment, an experiment was implemented in which half of the quadrats in the restored area received a coral outplant, while the other half were left undisturbed. After one year, all coral recruits located within the quadrats were counted and identified. It was found that in the restored area the mean recruit density exceeded that of the reference location by a factor of 2.15. Results also unveiled a positive association between coral recruitment and the presence of A. cervicornis. Specifically, the mean recruit density in quadrats that received an A. cervicornis colony was 2.21 to 4.65-times higher than in the quadrats without coral outplants. This intriguing observation underscores the pivotal role of A. cervicornis in shaping the recruitment dynamics of corals within degraded reef areas, highlighting the potential of active coral outplanting to enhance the resilience of deteriorating coral reef ecosystems.
1. Introduction
Coral reefs across the Greater Caribbean have faced severe degradation, primarily due to a combination of human activities such as unplanned shoreline development, biological factors like diseases, and physical disturbances, including hurricanes [1,2]. This widespread deterioration poses a significant threat to the socioeconomic stability of coastal communities reliant on healthy coral reefs for their sustenance [3]. The consequences of degraded coral reefs extend beyond food insecurity; they also lead to reduced income from tourism and small-scale fisheries, hinder the coastline’s capacity to mitigate storm surges, and impede coastal resilience to climate change [3,4]. To address the ongoing degradation of coral reefs, conservationists have turned to human-assisted coral propagation. This approach leverages corals’ ability to reproduce sexually by spawning gametes or brooding propagules, as well as asexually through fragmentation. In the context of sexual reproduction, laboratory protocols have been developed to facilitate coral egg fertilization, induce larval settlement on suitable substrates, and improve the chances of the settled larvae metamorphosizing and developing into a coral colony [5]. Subsequently, conservationists translocate the lab-reared coral colonies to selected reefs [5]. On the other hand, human-assisted asexual propagation involves cultivating coral fragments in ocean or land-based propagation units until they reach ecologically relevant sizes, after which they are reintroduced to the reefs. These “nursery” phases are vital as they significantly improve the survival and growth during the crucial and highly vulnerable early developmental stages of coral colonies [6,7,8]. The practice of outplanting nursery-reared coral colonies into degraded coral reefs is particularly prominent in the Caribbean, where 64% of restoration programs employ such an approach to increase the abundance of the major reef-forming species Acropora cervicornis, A. palmata, and Orbicella spp. [9].
Efforts to enhance the health of coral reefs through coral outplanting should ensure long-term population stability [10,11]; however, the success of these programs should not be solely determined by the population performance of the selected species [12]. In 2020, the U.S.A. National Oceanographic and Atmospheric Administration (NOAA) published a guideline that details specific metrics and criteria to consider when evaluating the success of coral outplanting as a strategy for restoring degraded coral reef ecosystems [13]. The guideline contains two categories, namely Universal Metrics and Goal-based Performance Metrics, which are designed to establish a comprehensive system of quantitative and qualitative measurements. These measurements encompass biological aspects, ranging from the molecular to the ecosystem level, as well as socio-economic factors such as coastal protection and increased opportunities for ecotourism. Nevertheless, many logistical and economic hurdles limit practitioners’ capacity to gather all the information specified in the guidelinewithin the often constrained timeframe of a single restoration project. As a result, practitioners must be selective in choosing metrics to determine project success. Selective use of metrics can hinder the progress of coral reef restoration science by restricting the information to what is considered a priority at a given time and location. Therefore, to comprehensively understand coral outplanting as a strategy for the ecological recovery of degraded coral reefs, collaborative efforts are indispensable.
One of the most common species used to restore coral reefs in the Caribbean is the threatened branching coral Acropora cervicornis [9]. This popularity can be attributed to various factors, including its rapid growth [14], ecological significance (i.e., habitat for fish, high calcium carbonate deposition, increased reef complexity [15,16]), and ease of propagation [14]. Numerous restoration programs utilizing A. cervicornis have been effectively implemented across the Greater Caribbean [9]. Most of these restoration programs define success based on outplants’ demographic performance (i.e., growth and survival [17,18]). Nevertheless, it is argued that the morphological complexity of this coral facilitates ecological processes (i.e., predation, herbivory) that promote the recruitment of reef-dwelling organisms (i.e., corals and fish), boost coral reef recovery, and strengthen resistance to biological and physical stressors [19]. Indeed, the recruitment of Scleractinian corals (as well as herbivore fish) into the restored reef is a major goal of the Ecological (Ecosystem) Restoration Goal-based Performance Metrics established in the NOAA’s guidelines, specifically the Community and Habitat Enhancement criteria [13]. Hence, the success of an A. cervicornis-based coral reef restoration program can also extend to the ecosystem level.
Despite the critical role of coral recruitment in coral reef dynamics, it has often been overlooked in the assessment of coral restoration programs. This oversight has led to ongoing debates regarding whether coral outplanting effectively promotes natural coral recruitment [12,20,21], creating a gap in our understanding of this vital aspect of coral reef health. In this study, we tested the hypothesis that restoring a population of the threatened coral A. cervicornis would promote coral recruitment. To do this, the rates of coral recruitment in a restored area was compared to that of a reference location where no action was taken. We further explored the relationship between A. cervicornis and recruitment by comparing the number of recruits established in the presence and absence of a coral outplant. Examining coral recruitment rates in the context of coral restoration will help us understand the potential impact of A. cervicornis reintroduction on the overall health of degraded coral reefs. If restoration efforts are shown to significantly boost coral recruitment, it will highlight the importance of continued support for such initiatives in protecting and rebuilding coral reefs. This study is a contribution towards such an end.
2. Study Site
This study was performed at the Punta Melones (PMEL) reef in the Island Municipality of Culebra, Puerto Rico (Figure 1). PMEL is a shallow-water reef, 1 to 5 m deep, located within the Canal Luis Peña no-take Natural Reserve. The site benefits from calm waters due to its sheltered location, protected by Luis Peña Island, and boasts good water quality [22]. A. cervicornis was common in the benthic community of PMEL. However, populations were decimated by several disease outbreaks in the early 2000s [23], episodes of water degradation (e.g., increased sedimentation) by unplanned coastal development (e.g., dirt road development), and the impacts of hurricanes [24]. In response, the not-for-profit organization Sociedad Ambiente Marino (SAM) started a restoration and rehabilitation program to reestablish viable A. cervicornis populations by outplanting nursery-reared coral colonies. During the outplanting phase, coral colonies were attached to the substrate using nails and cable ties (Figure 2A). This technique was chosen for its cost-effectiveness and minimal environmental disruption (i.e., change in substrate rugosity).
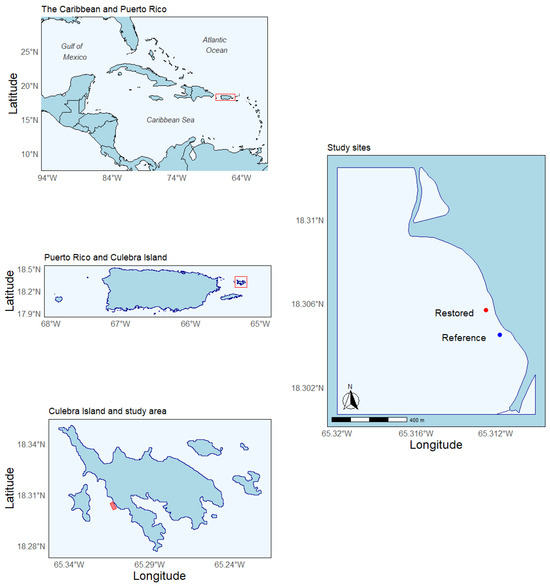
Figure 1.
The study was carried out at Punta Melones reef, located in the island municipality of Culebra, of the Caribbean island of Puerto Rico. Restored = site that received coral outplants; Reference = the control area where no coral were outplants.

Figure 2.
Example of an Acropora cervicornis outplant fixed to the reef substrate using a nail and a plastic cable tie (A), a quadrat receiving an A. cervicornis outplant after one year (B), and a control quadrat that does not receive a coral colony (C).
3. Data and Methods
3.1. Experimental Approach
To the hypothesis that restoring a population of A. cervicornis would lead to higher coral recruitment rates, we compared the number of corals recruits in the restored area to that of a reference location where no coral was outplanted (hereafter referred to as PMEL-Reference). PMEL-Reference was located approximately 200 m from the restored area (Figure 1). Despite the distance, there was no apparent variability in environmental conditions, such as water transparency and temperature. Indeed, a study by Hernández-Delgado and Ortiz-Flores [22] along the western coast of Culebra, where our study sites were located, reported consistent water quality conditions. Moreover, the study areas were similar in that both exhibited low coral cover (below 5% [25]), primarily dominated by Porites astreoides and P. porites.
In December 2020, we randomly placed a series of permanent quadrats, each measuring 0.25 m2, within a ~180 m2 reef section at each of the locations. We established 45 quadrats in the restored area and 30 quadrats at PMEL-Reference. The difference in sample size was primarily due to the limited available area at PMEL-Reference, which featured a higher presence of sandy patches. The smaller sample size at PMEL-Reference was deemed appropriate considering the site-specific constraints. During the establishment of the quadrats, we ensured that the selected area was devoid of any benthic organisms. If benthic organisms were found within the quadrats, they were disregarded, and the nearest denuded area was selected as the quadrat location. Each quadrat was marked with nails and assigned a unique numbered tag to facilitate re-localization during subsequent surveys.
To delve deeper into the correlation between A. cervicornis and coral recruitment, we conducted a field experiment. In this experiment, half of the quadrats in the restored area were seeded with a coral outplant, while the remaining half was left undisturbed (Figure 2B,C). Outplants (~15 cm long) were securely attached to the reef substrate using nails and cable ties.
In December 2021 we revisited the two locations to count and identify all coral colonies found within the quadrats [13,26]. Recruitment was defined as any new coral colony seen with the naked eye [26]. Since no coral was present in the fixed quadrats at the start of the study, any coral of any species observed at the end of the year was considered a recruit. In addition to the fixed quadrats, we also carried out a coral recruitment assessment in non-fixed quadrats. Following the methodology detailed by Hernández-Delgado et al. [27], non-fixed quadrats were haphazardly placed along three 30-meter-long transects that were established parallel to the coast both in the restored (15 quadrats per transect; n = 45) and non-restored sites (10 quadrats per transect; n = 30). To accurately identify coral recruits, we employed multiple criteria, including recruit orientation (horizontal vs. vertical), signs of fragmentation or basal detachment, and a thorough comparison of coral sizes [26,28]. This approach allowed us to distinguish recruits from established adult colonies within the quadrats. Unfortunately, we had to discontinue the study after December 2021 due to a series of events that significantly altered the reef structure. These events included a mass mortality of the sea urchin Diadema antillarum, multiple blooms of the alga Cottoniella filamentosa, and the impact of Hurricane Fiona. The dramatic changes in reef structure, including the mortality of many coral outplants, prompted the decision to halt the study. Continuing the study under such altered conditions could have introduced confounding factors that would have compromised the integrity of the results.
3.2. Statistical Analyses
We used the Mann–Whitney U-test to compare recruitment rates between the restored and non-restored areas. To assess if the presence of A. cervicornis significantly increased coral recruitment, we used the Kruskal–Wallis One-way ANOVA (KW), with the number of coral recruits per quadrat as the dependent variable. The independent variables included fixed quadrats that received a coral outplant (Acerv), fixed quadrats in the restored area with no corals (FRS), fixed quadrats in the non-restored area with no corals (FNRS), haphazard quadrats in the restored area with no corals (HRS), and haphazard quadrats in the non-restored area with no corals (HNRS). To assess whether quadrats with an A. cervicornis colony were more likely to be colonized by a coral recruit than those with no coral outplants, we conducted an odds ratio (OR) analysis. The odds ratio analysis, defined as OR = (N11 × N00)/(N10 × N01), measures the association between binary variables, which can be represented by a value of 0 and 1 [29], such as, for instance, fate (recruit = 1 or no recruit = 0) vs. condition (A. cervicornis present = 1 or A. cervicornis absent = 0). Thus, N00 represents the number of quadrats with no A. cervicornis where no recruits were found, N11 represents the number of quadrats with A. cervicornis and coral recruits, and so forth. In our case, OR values > 1 indicate that a coral recruit is more likely to colonize quadrats with an A. cervicornis colony.
4. Results
The number of coral recruits in the restored area was significantly higher than that at the reference location (W = 2281.5, p-value = 0.007, Figure 3A). At PMEL, the mean recruitment rate was 1.14 (±0.78 SD; Median = 1.00) per quadrat, while at PMEL-Reference, the mean rate was 0.53 (±1.39 SD; Median = 0.00). When comparing coral recruitment between quadrats receiving an A. cervicornis outplant and quadrats with no outplant, we found a statistically significant difference (Kruskal–Wallis’s chi-squared = 23.143, df = 4, p-value = 0.001; Figure 3B). The difference was primarily due to the positive association between A. cervicornis and coral recruits (Dunn test; p < 0.001) because no significant differences were found when comparing quadrats with no coral outplant (Dunn test; p > 0.05). Mean coral recruitment was at least 2.21 (up to 4.65)-times higher in the presence of A. cervicornis. The median number of recruits in quadrats with a coral outplant was 2, compared to a median value of 0 in the absence of the coral (Figure 3B). Indeed, the odds ratio analysis indicates that the likelihood of recruiting in proximity to A. cervicornis was greater than that of recruiting among non-coral quadrats (OR > 1; Table 1). Of the quadrats with a colony of A. cervicornis, 73% had at least one coral recruit, compared to only 45% in quadrats without the coral outplant (Figure 4). Six coral species were observed within the outplant quadrats compared to only three in non-coral quadrats (Figure 5). The most common coral recruiting into the study areas was Porites astreoides, which accounted for at least 57% of the recruits quantified (Figure 5 and Figure 6).
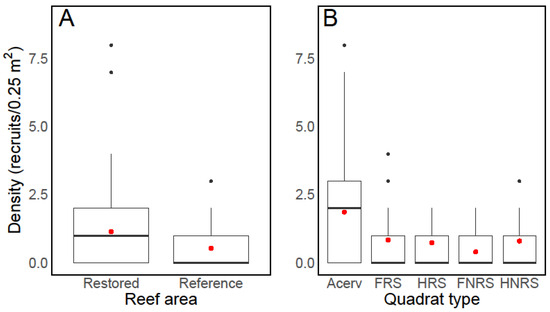
Figure 3.
Box plot comparing recruitment rates of Scleractinian corals between the restored area and the reference location (A) and among treatments (B). Acerv = quadrats that received a coral outplant; FRS = fixed quadrats in the restored area; HRS = haphazard quadrats in the restored area; NFRS = fixed quadrats in the non-restored area; HNRS = haphazard quadrats in the non-restored area. In the box plots, the bold horizontal line inside each box represents the median recruitment rate. The top and bottom lines of the box denote the 75th and 25th percentiles, respectively. The whiskers extend to the maximum value, while the dots within the box indicate mean values. Outlier values are represented by dots outside the whiskers.

Table 1.
Results of the odds ratio analysis performed to determine whether there is an association between Acropora cervicornis and coral recruits. A value larger than one indicates that there is a greater chance of a coral species recruiting in the proximity of Acropora cervicornis.
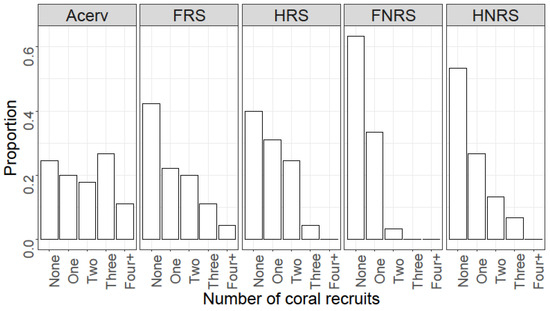
Figure 4.
Histograms displaying the distribution of quadrats based on the number of coral recruits per quadrat observed. Acerv = quadrats that received a coral outplant; FRS = fixed quadrats in the restored area; HRS = haphazard quadrats in the restored area; FNRS = fixed quadrats in the non-restored area; HNRS = haphazard quadrats in the non-restored area. Four+ refers to quadrats with four or more recruits.
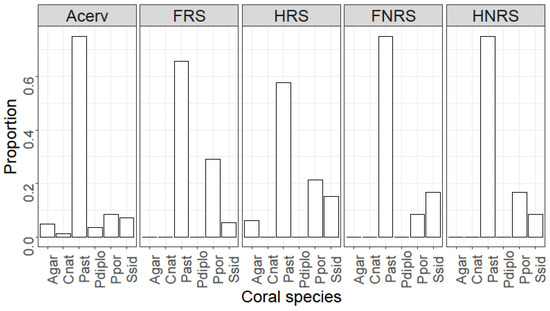
Figure 5.
Proportional contribution of coral species to the total observed number of recruits within the different quadrats evaluated. Coral species: Agar = Agaricia agaricites; Cnat = Colphophylia natans; Past = Porites astreoides; Ppor = Porites porites; Pstr = Pseudodiploria strigosa; Srad = Siderastrea siderea. Acerv = quadrats that received a coral outplant; FRS = fixed quadrats in the restored area; HRS = haphazard quadrats in the restored area; FNRS = fixed quadrats in the non-restored area; HNRS = haphazard quadrats in the non-restored area.

Figure 6.
An image showing two coral colonies (circles) of Porites astreoides recruiting in proximity to an Acropora cervicornis outplant. Quadrat is used for reference.
5. Discussion
The outplanting of nursery-reared coral colonies aims to mitigate the negative impacts of ecological degradation on coral reefs. Yet, the success of this coral restoration strategy is still a topic of discussion. For instance, a study by Ware et al. [30] found that the survivorship probabilities of A. cervicornis outplants over a seven-year period varied between 0% to 10%. Such low rates of survival raise questions about the overall effectiveness of coral outplanting in restoring degraded coral reef ecosystems. However, it is crucial to recognize that evaluating the success of a restoration program based solely on one metric, such as coral demographics, may lead to incomplete conclusions. Our results, together with recent research, including the work of Montoya-Maya et al. [12], have shed light on the broader ecosystem-level benefits of coral outplanting. Specifically, our study supports the contention that, compared to degraded reefs, coral recruitment rates are significantly higher in areas where coral outplants are present. Given that coral reef recovery heavily depends on coral recruitment [31,32], these findings prompt us to contemplate the multifaceted impact of A. cervicornis outplanting. While the long-term fate of individual outplanted colonies remains a topic of concern, it is becoming increasingly apparent that coral outplanting may contribute significantly to reef recovery in ways that extend beyond outplant survivorship rates.
A. cervicornis is a morphologically complex coral known to serve as a habitat for many reef species, i.e., fish [33,34]. Indeed, Calle-Triviño et al. [18] demonstrated that fish biodiversity in areas restored by outplanting A. cervicornis was much higher than in non-restored sites. During our study, we noted the evident abundance and diversity of herbivore fish at PMEL, such as parrotfish, surgeonfish, and damselfish. In contrast, at PMEL-Reference, fish species were limited to small labrid fishes, like Thalassoma spp., and juveniles of the territorial damselfish, Stegastes diencaeus. Enhanced herbivore fish populations play a crucial role in reducing macroalga cover and enhancing nutrient delivery [34,35], which, in turn, facilitates coral recruitment. The high number of coral recruits at PMEL (compared to PMEL-Reference) may support such a contention. However, it is important to note that recruitment rates did not statistically vary when A. cervicornis was absent from the quadrats, regardless of their restoration status (e.g., restored vs. non-restored). This finding suggests that coral recruitment dynamics at the studied sites cannot be explained solely by fish abundance or diversity.
Macroalgae can limit coral recruitment by reducing available settlement space for coral larvae. However, when comparing macroalga cover between coral- and non-coral quadrats, we found that the percent of alga cover, dominated by turf, did not vary statistically between the locations (PMEL = 46%; PMEL-Reference = 40%). It has been suggested that turf algae can inhibit coral recruitment, but mostly when combined with sediments [36,37]. It is possible, then, that at the two study locations, sedimentation rates are not at levels that, combined with turf alga, can inhibit coral recruitment.
Corals within the genus Acropora can produce chemical cues that attract various reef organisms, including other coral species [38]. If this phenomenon holds true for A. cervicornis, it raises the possibility that the observed positive association between A. cervicornis and coral recruits may be a result of coral larvae being attracted to the coral outplants. It is also conceivable that A. cervicornis promotes coral recruitment by modifying the local light conditions required for successful larval settlement, such as reducing light intensity [39]. For example, larvae of the coral Porites astreoides tend to settle in areas exposed to relatively lower light intensities and ultraviolet radiation [40,41]. Another plausible explanation is that the branching complexity of A. cervicornis protects the newly settled coral larvae against reef grazers (e.g., fish and sea urchins). These grazers could inadvertently consume and remove recruits when foraging on algal mats in open areas [42]. Further experimental studies are needed to determine whether the explanations proposed indeed play a role in shaping the recruitment pattern observed in this study.
Coral recruits exhibited both higher abundance and greater species diversity in the presence of A. cervicornis. This pattern is consistent with similar observations made at Punta Maguey reef in Culebra by Ruiz-Diaz and Mercado-Molina (unpublished data). Six coral species were identified to recruit near the coral outplants, whereas only three species were found within non-coral quadrats. The species associated with A. cervicornis were Agaricia agaricites, Colpophyllia natans, Porites astreoides, Porites porites, Pseudiploria strigosa, and Siderastrea siderea. Nevertheless, A. agaricites, C. natans, and P. strigosa contributed little to the number of recruits observed. Such recruitment patterns closely mirrored the broader benthic community structure at PMEL [30]. Specifically, the three most prevalent coral species, P. astreoides, P. porites, and S. siderea, accounted for a significant portion of the total adult coral species observed at both the restored (62%) and reference locations (100%).
P. astreoides showed the highest recruitment rate. This finding concurs with previous studies reporting that P. astreoides is one of the most common species recruiting on natural and artificial substrates [27,43,44,45]. The dominance of P. astreoides may be related to its reproductive strategy. As a brooder species, P. astreoides releases fully developed larvae that can rapidly settle into the reef, resulting in increased chances of recruiting within local populations [43]. Known for its stress tolerance and a weedy life history that facilitates rapid colonization of the reef substrate, P. astreoides can thrive even under seemingly unfavorable environmental conditions, such as growing over an algae-covered substrate [46]. Additionally, P. astreoides can be reproductively active throughout the year [47,48], which could increase the chances of the brooded larvae successfully settling into the reef substrate.
While there were very few recruits of the coral species P. strigosa, its presence within the restored area is encouraging. Populations of this coral were affected by the Stony Coral Tissue Loss Diseases (SCTLD), leading to over 70% colony mortality in one year (Rivera-Irizarry et al., unpublished data). Today, P. strigosa is rare in the study area. Healthier coral reef systems are known to attract coral larvae [25,49], suggesting that active outplanting efforts may, at least in part, counteract coral diseases like SCTLD.
Coral recruitment plays a vital role in coral restoration, as it replenishes populations and enhances the genetic diversity of reef ecosystems. However, the generalization of our findings may be limited because our study was confined to a single observation point and one coral reef. This constraint hinders our understanding of spatiotemporal recruitment dynamics, including settlement and survival rates in the context of coral reef restoration. Nevertheless, our findings support the argument that coral outplanting contributes positively to natural coral recruitment. Considering this evidence, the observed association between A. cervicornis outplants and coral recruits warrants further investigation. We advocate for the inclusion of recruitment monitoring as a valuable metric for evaluating restoration success at the ecosystem level. To gain a more comprehensive understanding of the benefits of coral outplanting, collaborative research from various Caribbean locations and time periods is crucial, and we hope this study serves as a contribution to such integrated research.
Author Contributions
Conceptualization, A.E.M.-M. and S.E.S.-R.; Methodology, A.E.M.-M. and S.E.S.-R.; Formal Analysis, A.E.M.-M.; Investigation, A.E.M.-M. and S.E.S.-R.; Resources, A.E.M.-M. and S.E.S.-R.; Data Curation, A.E.M.-M.; Writing—A.E.M.-M.; Writing—Review and Editing, A.E.M.-M. and S.E.S.-R.; Supervision, A.E.M.-M. and S.E.S.-R.; Project Administration, A.E.M.-M. and S.E.S.-R.; Funding Acquisition, A.E.M.-M. and S.E.S.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the USA National Oceanographic and Atmospheric Administration, Award # NA20NMF4630303.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are stored in Sociedad Ambiente Marino’s data repository as well as A.E.M.-M’s personal data files. Data will be freely available, in compliance with funding agencies’ requirements, after two years of embargo. Data will be available upon formal request.
Acknowledgments
We would like to thank members of Sociedad Ambiente Marino who provided logistical and field support. Special thanks to Claudia P. Ruiz-Diaz, Jeremy Velázquez, and Megan Considine. We would also like to thank the three anonymous reviewers for their comments and suggestions for this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eddy, T.D.; Lam, V.W.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Roff, G.; Mumby, P.J. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 2012, 27, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Delgado, E.A. The emerging threats of climate change on tropical coastal ecosystem services, public health, local economies and livelihood sustainability of small islands: Cumulative impacts and synergies. Mar. Pollut. Bull. 2015, 101, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Fezzi, C.; Ford, D.J.; Oleson, K.L. The economic value of coral reefs: Climate change impacts and spatial targeting of restoration measures. Ecol. Econ. 2023, 203, 107628. [Google Scholar] [CrossRef]
- Barton, J.A.; Willis, B.L.; Hutson, K.S. Coral propagation: A review of techniques for ornamental trade and reef restoration. Rev. Aquac. 2017, 9, 238–256. [Google Scholar] [CrossRef]
- Ishida-Castañeda, J.; Pizarro, V.; López-Victoria, M.; Zapata, F.A. Coral reef restoration in the Eastern Tropical Pacific: Feasibility of the coral nursery approach. Restor. Ecol. 2020, 28, 22–28. [Google Scholar] [CrossRef]
- Combillet, L.; Fabregat-Malé, S.; Mena, S.; Marín-Moraga, J.A.; Gutierrez, M.; Alvarado, J.J. Pocillopora spp. growth analysis on restoration structures in an Eastern Tropical Pacific upwelling area. PeerJ 2022, 10, e13248. [Google Scholar] [CrossRef] [PubMed]
- Calle-Triviño, J.; Rivera-Madrid, R.; León-Pech, M.G.; Cortés-Useche, C.; Sellares-Blasco, R.I.; Aguilar-Espinosa, M.; Arias-González, J.E. Assessing and genotyping threatened staghorn coral Acropora cervicornis nurseries during restoration in southeast Dominican Republic. PeerJ 2020, 8, e8863. [Google Scholar] [CrossRef]
- Bayraktarov, E.; Banaszak, A.T.; Montoya Maya, P.; Kleypas, J.; Arias-González, J.E.; Blanco, M.; Frías-Torres, S. Coral reef restoration efforts in Latin American countries and territories. PLoS ONE 2020, 15, e0228477. [Google Scholar] [CrossRef]
- Mercado-Molina, A.E.; Ruiz-Diaz, C.P.; Pérez, M.E.; Rodríguez-Barreras, R.; Sabat, A.M. Demography of the threatened coral Acropora cervicornis: Implications for its management and conservation. Coral Reefs 2015, 34, 1113–1124. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A.; Mercado-Molina, A.E.; Suleimán-Ramos, S.E.; Lucking, M.A. Multi-Disciplinary Lessons Learned from Low-Tech Coral Farming Reef Rehabilitation: II. Coral Demography and Social-Ecological Benefits. In Corals in a Changing World; IntechOpen: London, UK, 2018; pp. 245–268. [Google Scholar]
- Maya, P.H.M.; Smit, K.P.; Burt, A.J.; Frias-Torres, S. Large-scale coral reef restoration could assist natural recovery in Seychelles, Indian Ocean. Nat. Conserv. 2016, 16, 1–17. [Google Scholar] [CrossRef]
- Goergen, E.A.; Schopmeyer, S.; Moulding, A.L.; Moura, A.; Kramer, P.; Viehman, T.S. Coral Reef Restoration Monitoring Guide: Methods to Evaluate Restoration Success from Local to Ecosystem Scales; NOAA Technical Memorandum NOS NCCOS 279: Silver Spring, MD, USA, 2020; p. 145. [Google Scholar] [CrossRef]
- Tunnicliffe, V. Breakage and propagation of the stony coral Acropora cervicornis. Proc. Natl. Acad. Sci. USA 1981, 78, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.D.; Hall, B.R. Life history, growth habits, and constitutional roles of Acropora cervicornis in the patch reef environment. J. Sediment. Res. 1976, 46, 519–522. [Google Scholar]
- Agudo-Adriani, E.A.; Cappelletto, J.; Cavada-Blanco, F.; Croquer, A. Colony geometry and structural complexity of the endangered species Acropora cervicornis partly explains the structure of their associated fish assemblage. PeerJ 2016, 4, e1861. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Malé, S.; Mena, S.; Alvarado, J.J. Nursery-reared coral outplanting success in an upwelling-influenced area in Costa Rica. Rev. Biol. Trop. 2023, 71, e54879. [Google Scholar] [CrossRef]
- Calle-Triviño, J.; Muñiz-Castillo, A.I.; Cortés-Useche, C.; Morikawa, M.; Sellares-Blasco, R.; Arias-González, J.E. Approach to the Functional Importance of Acropora cervicornis in Outplanting Sites in the Dominican Republic. Front. Mar. Sci. 2021, 8, 668325. [Google Scholar] [CrossRef]
- Mumby, P.J.; Wolff, N.H.; Bozec, Y.M.; Chollett, I.; Halloran, P. Operationalizing the resilience of coral reefs in an era of climate change. Conserv. Lett. 2014, 7, 176–187. [Google Scholar] [CrossRef]
- Clark, S.; Edwards, A.J. Coral transplantation as an aid to reef rehabilitation: Evaluation of a case study in the Maldive Islands. Coral Reefs 1995, 14, 201–213. [Google Scholar] [CrossRef]
- Ferse, S.C.A.; Nugues, M.M.; Romatzki, S.B.C.; Kunzmann, A. Examining the use of mass transplantation of brooding and spawning corals to support natural coral recruitment in Sulawesi/Indonesia. Restor. Ecol. 2023, 21, 745–754. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A.; Ortiz-Flores, M.F. The Long and Winding Road of Coral Reef Recovery in the Anthropocene: A Case Study from Puerto Rico. Diversity 2022, 14, 804. [Google Scholar] [CrossRef]
- Mercado-Molina, A.E.; Sabat, A.M.; Hernández-Delgado, E.A. Population Dynamics of Diseased Corals: Effects of a Shut Down Reaction Outbreak in Puerto Rican Acropora cervicornis. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 61–82. [Google Scholar]
- Toledo-Hernández, C.; Ruiz-Diaz, C.P.; Hernández-Delgado, E.A.; Suleimán-Ramos, S.E. Devastation of 15-year old community-based coral farming and reef-restoration sites in Puerto Rico by major hurricanes Irma and María. Caribb. Nat. 2018, 53, 1–6. [Google Scholar] [CrossRef]
- Santiago-Padua, P.; Velázquez-Alvarado, J.; López-Pérez, A.D.M.; Nevárez-Mélendez, J.; Díaz-Druet, L.E.; Suleimán-Ramos, S.E.; Mercado-Molina, A.E. Demographic and population response of the threatened coral Acropora cervicornis (Scleractinia, Acroporidae) to fireworm corallivory. Rev. Biol. Trop. 2023, 71 (Suppl. S1), e54912. [Google Scholar] [CrossRef]
- Irizarry-Soto, E.; Weil, E. Spatial and temporal variability in juvenile coral densities, survivorship and recruitment in La Parguera, southwestern Puerto Rico. Caribb. J. Sci. 2009, 45, 269–281. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A.; González-Ramos, C.M.; Alejandro-Camis, P.J. Large-scale coral recruitment patterns on Mona Island, Puerto Rico: Evidence of a transitional community trajectory after massive coral bleaching and mortality. Rev. Biol. Trop. 2014, 62, 283–298. [Google Scholar]
- Wulff, J.L. Asexual fragmentation, genotype success, and population dynamics of erect branching sponges. J. Exp. Mar. Biol. Ecol. 1991, 149, 227–247. [Google Scholar] [CrossRef]
- Pearson, R.K. Exploring Data in Engineering, the Sciences, and Medicine; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Ware, M.; Garfield, E.N.; Nedimyer, K.; Levy, J.; Kaufman, L.; Precht, W.; Miller, S.L. Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting projects in the Florida Keys National Marine Sanctuary. PLoS ONE 2020, 15, e0231817. [Google Scholar] [CrossRef] [PubMed]
- Fisk, D.A.; Harriott, V.J. Spatial and temporal variation in coral recruitment on the Great Barrier Reef: Implications for dispersal hypotheses. Mar. Biol. 1990, 107, 485–490. [Google Scholar] [CrossRef]
- Lange, I.D.; Perry, C.T.; Stuhr, M. Recovery trends of reef carbonate budgets at remote coral atolls 6 years post-bleaching. Limnol. Oceanogr. 2023, 68, S8–S22. [Google Scholar] [CrossRef]
- Goldenberg, E.D. Outplanted Acropora Cervicornis Enhances the Fish Assemblages of Southeast Florida. Master’s Thesis, NOVA Southeastern University, Fort Lauderdale, FL, USA, May 2019. [Google Scholar]
- Holbrook, S.J.; Brooks, A.J.; Schmitt, R.J.; Stewart, H.L. Effects of sheltering fish on growth of their host corals. Mar. Biol. 2008, 155, 521–530. [Google Scholar] [CrossRef]
- Shantz, A.A.; Ladd, M.C.; Schrack, E.; Burkepile, D.E. Fish-derived nutrient hotspots shape coral reef benthic communities. Ecol. Appl. 2015, 25, 2142–2152. [Google Scholar] [CrossRef]
- Birrell, C.L.; McCook, L.J.; Willis, B.L. Effects of algal turfs and sediment on coral settlement. Mar. Pollut. Bull. 2005, 51, 408–414. [Google Scholar] [CrossRef]
- Speare, K.E.; Duran, A.; Miller, M.W.; Burkepile, D.E. Sediment associated with algal turfs inhibits the settlement of two endangered coral species. Mar. Pollut. Bull. 2019, 144, 189–195. [Google Scholar] [CrossRef]
- Dixson, D.L.; Hay, M.E. Corals chemically cue mutualistic fishes to remove competing seaweeds. Science 2012, 338, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Brakel, W.H. Small-scale spatial variation in light available to coral reef benthos: Quantum irradiance measurements from a Jamaican reef. Bull. Mar. Sci. 1979, 29, 406–413. [Google Scholar]
- Gleason, D.F.; Edmunds, P.J.; Gates, R.D. Ultraviolet radiation effects on the behavior and recruitment of larvae from the reef coral Porites astreoides. Mar. Biol. 2006, 148, 503–512. [Google Scholar] [CrossRef]
- Goodbody-Gringley, G.; Wong, K.H.; Becker, D.M.; Glennon, K.; De Putron, S.J. Reproductive ecology and early life history traits of the brooding coral, Porites astreoides, from shallow to mesophotic zones. Coral Reefs 2018, 37, 483–494. [Google Scholar] [CrossRef]
- Baria MV, B.; Guest, J.R.; Edwards, A.J.; Aliño, P.M.; Heyward, A.J.; Gómez, E.D. Caging enhances post-settlement survival of juveniles of the Scleractinia coral Acropora tenuis. J. Exp. Mar. Biol. Ecol. 2010, 394, 149. [Google Scholar] [CrossRef]
- Smith, S.R. Patterns of coral recruitment and post-settlement mortality on Bermuda’s reefs: Comparisons to Caribbean and Pacific reefs. Am. Zool. 1992, 32, 663–673. [Google Scholar] [CrossRef]
- Miller, M.W.; Barimo, J. Assessment of juvenile coral populations at two reef restoration sites in the Florida Keys National Marine Sanctuary: Indicators of success? Bull. Mar. Sci. 2001, 69, 395–405. [Google Scholar]
- Perera-Valderrama, S.; Hernández-Arana, H.; Ruiz-Zárate, M.; Alcolado, P.M.; Caballero-Aragón, H.; González-Cano, J.; Vega-Zepeda, A.; Cobián-Rojas, D. Condition assessment of coral reefs of two marine protected areas under different regimes of use in the north-western Caribbean. Ocean. Coast. Manag. 2016, 127, 16–25. [Google Scholar] [CrossRef]
- Olsen, K.; Sneed, J.M.; Paul, V.J. Differential larval settlement responses of Porites astreoides and Acropora palmata in the presence of the green alga Halimeda opuntia. Coral Reefs 2016, 35, 521–525. [Google Scholar] [CrossRef]
- Chornesky, E.A.; Peters, E.C. Sexual reproduction and colony growth in the scleractinian coral Porites astreoides. Biol. Bull. 1987, 172, 161–177. [Google Scholar] [CrossRef]
- McGuire, M.P. Timing of larval release by Porites astreoides in the northern Florida Keys. Coral Reefs 1998, 17, 369–375. [Google Scholar] [CrossRef]
- Gleason, D.F.; Danilowicz, B.S.; Nolan, C.J. Reef waters stimulate substratum exploration in planulae from brooding Caribbean corals. Coral Reefs 2009, 28, 549–554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).