Enhancement of Liquid Hot Water Pretreatment on Corn Stover with Ball Milling to Improve Total Sugar Yields
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Ball Milling Method
2.3. Liquid Hot Water Pretreatment
2.4. Particle Size Distribution
2.5. Enzymatic Hydrolysis
2.6. Sugar Yields
0.9]/(weight of glucose in raw corn stover) × 100%
(weight of xylose in raw corn stover) × 100%
(weight of xylose in acid and enzymatic hydrolysate) × 0.88]/(weight of glucose and
xylose in raw corn stover) × 100%
2.7. Statistical Analysis
3. Results
3.1. Chemical Compositions of All Corn Stover Samples
3.2. Particle Size Distribution
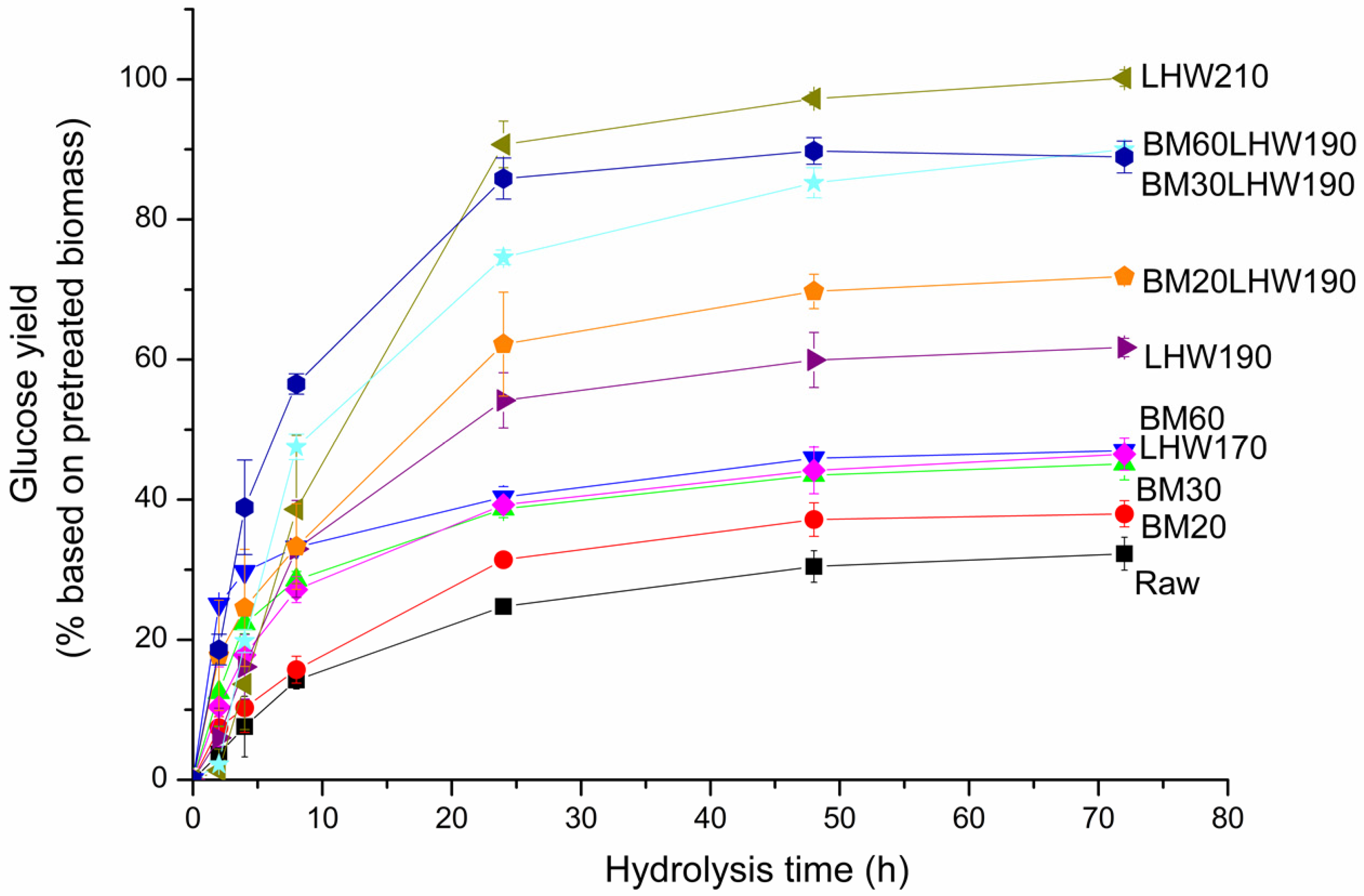
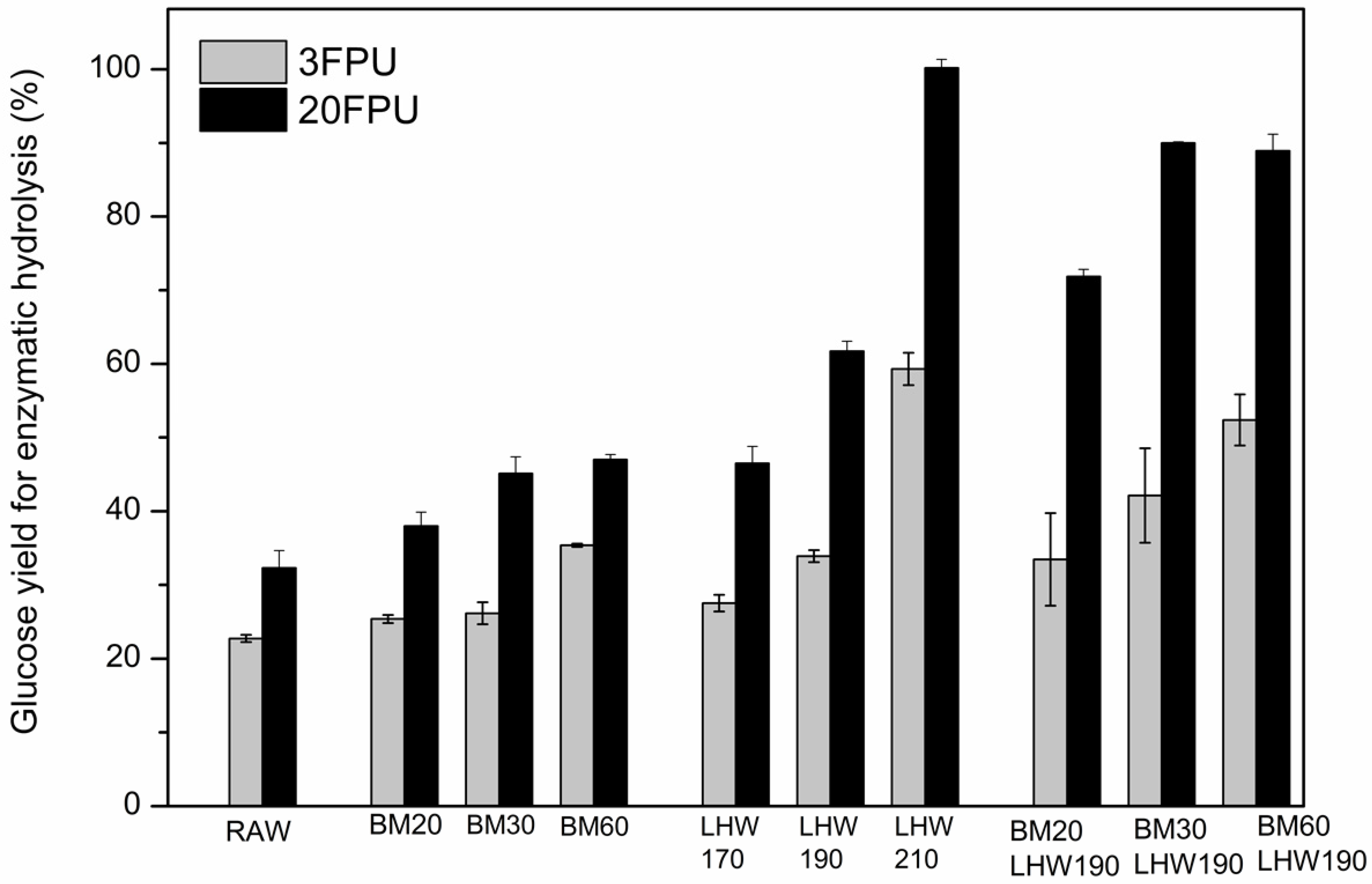
3.3. Enzymatic Hydrolysis
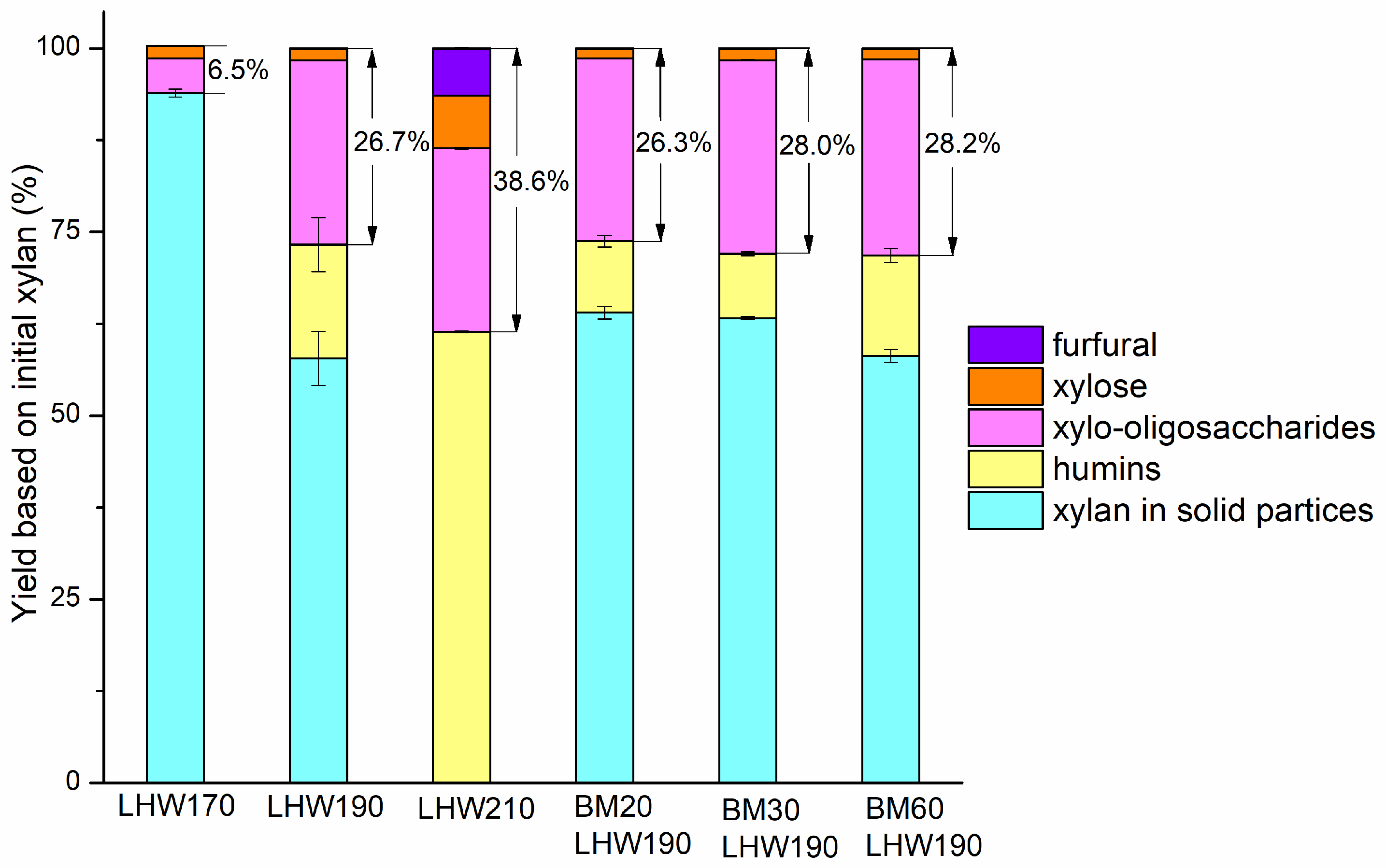
3.4. Total Sugar Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Alexandri, M.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Valorization of Grape Pomace for Trametes versicolor Mycelial Mass and Polysaccharides Production. Sustainability 2023, 15, 15080. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, X.; Bao, J. High solids loading pretreatment: The core of lignocellulose biorefinery as an industrial technology—An overview. Bioresour. Technol. 2023, 369, 128334. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Ximenes, E.; Ladisch, M.R.; Mosier, N.S.; Vermerris, W.; Huang, C.P.; Sherman, D.M. Tissue-specific biomass recalcitrance in corn stover pretreated with liquid hot-water: Enzymatic hydrolysis (part 1). Biotechnol. Bioeng. 2012, 109, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, M.; Su, J.; Hu, H.; Yang, M.; Huang, Z.; Chen, D.; Wu, J.; Feng, Z. Overcoming biomass recalcitrance by synergistic pretreatment of mechanical activation and metal salt for enhancing enzymatic conversion of lignocellulose. Biotechnol. Biofuels 2019, 12, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, N.; Wang, S.; Li, X.; Li, R.; Wu, Y. Study on Co-Pyrolysis of Coal and Biomass and Process Simulation Optimization. Sustainability 2023, 15, 15412. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Rajesh Banu, J.; Yoon, J.J.; Kant Bhatia, S.; Yang, Y.H.; Varjani, S.; Kim, S.H. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour. Technol. 2022, 344 Pt B, 126292. [Google Scholar] [CrossRef]
- Chu, Q.; Song, K.; Bu, Q.; Hu, J.; Li, F.; Wang, J.; Chen, X.; Shi, A. Two-stage pretreatment with alkaline sulphonation and steam treatment of Eucalyptus woody biomass to enhance its enzymatic digestibility for bioethanol production. Energy Convers. Manag. 2018, 175, 236–245. [Google Scholar] [CrossRef]
- Hajiali, F.; Jin, T.; Yang, G.; Santos, M.; Lam, E.; Moores, A. Mechanochemical Transformations of Biomass into Functional Materials. ChemSusChem 2022, 15, e202102535. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, e2108327. [Google Scholar] [CrossRef]
- Yun, Y.; Hongwei, W. Effect of ball milling on the hydrolysis of microcrystalline cellulose in hot-compressed water. AIChE J. 2011, 57, 793–800. [Google Scholar] [CrossRef]
- Barakat, A.; Rouau, X. New dry technology of environmentally friendly biomass refinery: Glucose yield and energy efficiency. Biotechnol. Biofuels 2014, 7, 138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.; Ge, S.; Xia, C.; Mei, C.; Kim, K.; Cai, L.; Smith, L.; Lee, J.; Shi, S.Q. Application of intermittent ball milling to enzy-matic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew. Sustain. Energy Rev. 2021, 136, 110442. [Google Scholar] [CrossRef]
- Mosier, N.; Hendrickson, R.; Ho, N.; Sedlak, M.; Ladisch, M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef]
- Youngmi, K.; Thomas, K.; Nathan, S.M.; Ladisch, M.R. Severity Factor Coefficients for Subcritical Liquid Hot Water Pretreatment of Hardwood Chips. Biotechnol. Bioeng. 2014, 111, 254–263. [Google Scholar]
- Hu, F.; Jung, S.; Ragauskas, A. Impact of Pseudolignin versus Dilute Acid-Pretreated Lignin on Enzymatic Hydrolysis of Cellulose. ACS Sustain. Chem. Eng. 2012, 1, 62–65. [Google Scholar] [CrossRef]
- Nazar, M.; Xu, L.; Ullah, M.W.; Moradian, J.M.; Wang, Y.; Sethupathy, S.; Iqbal, B.; Nawaz, M.Z.; Zhu, D. Biological delignification of rice straw using laccase from Bacillus ligniniphilus L1 for bioethanol production: A clean approach for agro-biomass utilization. J. Clean. Prod. 2022, 360, 132171. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fraction of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; Laboratory Analytical Procedure, NREL/TP-510-42628; National Renewable Energy Laboratory: Golden, CO, USA, 1996.
- Ji, G.; Han, L.; Gao, C.; Xiao, W.; Zhang, Y.; Cao, Y. Quantitative approaches for illustrating correlations among the mechanical fragmentation scales, crystallinity and enzymatic hydrolysis glucose yield of rice straw. Bioresour. Technol. 2017, 241, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Liu, J.; Zhang, Q.; Wang, C.; Zhan, H.; Ma, L. A review on the utilization of industrial biowaste via hydrothermal carbonization. Renew. Sustain. Energy Rev. 2022, 154, 111877. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Ma, Q.; An, S.; Li, M.; Jameel, H.; Chang, H.M. Pretreatment of corn stover for sugar production using a two-stage dilute acid followed by wet-milling pretreatment process. Bioresour. Technol. 2016, 211, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, R.; Cho, J.; Conner, J.W.C.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, W.; Ji, G.; Zhang, Y.; Cao, Y.; Han, L. Regularity and mechanism of wheat straw properties change in ball milling process at cellular scale. Bioresour. Technol. 2017, 241, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.; Venkatesh, B.; Dale, B.E. Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol. Bioeng. 2007, 96, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.G.D.; Rouau, S.G.X. Successive centrifugal grinding and sieving of wheat straw. Powder Technol. 2011, 208, 266–270. [Google Scholar] [CrossRef]
- Fadda, S.; Cincotti, A.; Concas, A.; Pisu, M.; Cao, G. Modelling breakage and reagglomeration during fine dry grinding in ball milling devices. Powder Technol. 2009, 194, 207–216. [Google Scholar] [CrossRef]
- Loustau-Cazalet, C.; Sambusiti, C.; Buche, P.; Solhy, A.; Bilal, E.; Larzek, M.; Barakat, A. Innovative Deconstruction of Biomass Induced by Dry Chemo-Mechanical Activation: Impact on Enzymatic Hydrolysis and Energy Efficiency. ACS Sustain. Chem. Eng. 2016, 4, 2689–2697. [Google Scholar] [CrossRef]
- Inoue, H.; Yano, S.; Endo, T.; Sakaki, T.; Sawayama, S. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels 2008, 1, 2. [Google Scholar] [CrossRef]
- Boissou, F.; Sayoud, N.; De Oliveira Vigier, K.; Barakat, A.; Marinkovic, S.; Estrine, B.; Jerome, F. Acid-Assisted Ball Milling of Cellulose as an Efficient Pretreatment Process for the Production of Butyl Glycosides. ChemSusChem 2015, 8, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- FYuan, Z.; Long, J.; Wang, T.; Shu, R.; Zhang, Q.; Ma, L. Process intensification effect of ball milling on the hydrothermal pretreatment for corn straw enzymolysis. Energy Convers. Manag. 2015, 101, 481–488. [Google Scholar]
- Wang, W.; Yuan, T.; Wang, K.; Cui, B.; Dai, Y. Combination of biological pretreatment with liquid hot water pretreatment to enhance enzymatic hydrolysis of Populus tomentosa. Bioresour. Technol. 2012, 107, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Pingali, S.V.; Urban, V.S.; Heller, W.T.; McGaughey, J.; O’Neill, H.; Foston, M.B.; Li, H.; Wyman, C.E.; Myles, D.A.; Langan, P.; et al. Understanding Multiscale Structural Changes During Dilute Acid Pretreatment of Switchgrass and Poplar. ACS Sustain. Chem. Eng. 2016, 5, 426–435. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Petridis, L.; Qi, X.; Schulz, R.; Lindner, B.; Smith, J.C. Mechanism of lignin inhibition of enzymatic biomass deconstruction. Biotechnol. Biofuels 2015, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Weiqi, W.; Shubin, W.; Liguo, L. Combination of liquid hot water pretreatment and wet disk milling to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Bioresour. Technol. 2013, 128, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Xiao, W.; Gao, C.; Cao, Y.; Zhang, Y.; Han, L. Mechanical fragmentation of wheat and rice straw at different scales: Energy requirement in relation to microstructure properties and enzymatic hydrolysis. Energy Convers. Manag. 2018, 171, 38–47. [Google Scholar] [CrossRef]


| Sample | Ball Milling | LHWP Temperature | ||
|---|---|---|---|---|
| Time (min) | Temperature (°C) | Time (min) | Severity Factor | |
| BM20 | 20 | N/A | N/A | N/A |
| BM30 | 30 | N/A | N/A | N/A |
| BM60 | 60 | N/A | N/A | N/A |
| LHW170 | N/A | 170 | 15 | 3.24 |
| LHW190 | N/A | 190 | 15 | 3.83 |
| LHW210 | N/A | 210 | 15 | 4.41 |
| BM20LHW190 | 20 | 190 | 15 | 3.83 |
| BM30LHW190 | 30 | 190 | 15 | 3.83 |
| BM60LHW190 | 60 | 190 | 15 | 3.83 |
| Solid Fraction (% of Initial) | Glucan (%) | Xylan (%) | Lignin (%) | |
|---|---|---|---|---|
| Raw | N/A | 33.2 ± 0.4 | 19.9 ± 0.3 | 11.8 ± 0.1 |
| LHW170 | 84.5 ± 0.3 | 39.2 ± 0.4 | 23.6 ± 0.2 | 17.5 ± 0.6 |
| LHW190 | 74.9 ± 3.8 | 46.9 ± 2.3 | 15.9 ± 1.9 | 19.3 ± 0.1 |
| LHW210 | 60.3 ± 0.4 | 48.0 ± 0.7 | 0 | 25.7 ± 0.1 |
| BM20LHW190 | 73.9 ± 0.2 | 44.7 ± 0.3 | 17.7 ± 0.6 | 18.7 ± 0.1 |
| BM30LHW190 | 76.2 ± 3.2 | 39.1 ± 0.6 | 17.4 ± 0.1 | 18.7 ± 0.1 |
| BM60LHW190 | 73.5 ± 1.0 | 37.3 ± 0.8 | 15.3 ± 0.8 | 19.7 ± 0.9 |
| Sample | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|
| Raw | 103.5 ± 3.5 | 568.0 ± 11.3 | 1360.0 ± 155.6 |
| BM20 | 27.8 ± 0.4 | 318.5 ± 6.4 | 1080.0 ± 56.6 |
| BM30 | 12.8 ± 0.7 | 108.0 ± 7.1 | 708.0 ± 32.5 |
| BM60 | 6.7 ± 0.1 | 37.3 ± 0.8 | 436.0 ± 83.4 |
| LHW170 | 155.0 ± 1.4 | 523.5 ± 17.7 | 1160.0 ± 70.7 |
| LHW190 | 155.0 ± 2.8 | 516.0 ± 7.1 | 1180.0 ± 28.3 |
| LHW210 | 95.1 ± 16.8 | 383.0 ± 38.2 | 919.5 ± 81.3 |
| BM20LHW190 | 97.0 ± 8.5 | 410.0 ± 18.4 | 1080.0 ± 14.1 |
| BM30LHW190 | 59.1 ± 13.8 | 353.0 ± 32.5 | 968.5 ± 23.3 |
| BM60LHW190 | 19.4 ± 1.2 | 95.7 ± 10.4 | 565.0 ± 96.2 |
| 20 FPU | 3 FPU | |||||
|---|---|---|---|---|---|---|
| Glucose (%) | Xylose (%) | Total Yields (%) | Glucose (%) | Xylose (%) | Total Yields (%) | |
| Raw | 32.3 | 22.1 | 28.6 | 22.7 | 11.9 | 18.8 |
| BM20 | 38.0 | 27.0 | 34.0 | 25.4 | 14.4 | 21.4 |
| BM30 | 45.1 | 31.7 | 40.2 | 26.2 | 15.3 | 22.2 |
| BM60 | 47.0 | 35.2 | 42.7 | 35.4 | 21.4 | 30.3 |
| LHW170 | 49.4 | 41.0 | 46.4 | 30.5 | 20.8 | 27.0 |
| LHW190 | 65.8 | 69.0 | 67.0 | 37.9 | 53.6 | 43.6 |
| LHW210 | 93.3 | 35.6 | 72.2 | 57.8 | 35.6 | 49.7 |
| BM20LHW190 | 75.7 | 73.3 | 74.9 | 37.5 | 52.4 | 42.9 |
| BM30LHW190 | 83.0 | 72.0 | 79.0 | 40.7 | 55.0 | 45.9 |
| BM60LHW190 | 79.8 | 76.9 | 78.7 | 49.3 | 59.4 | 53.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, G.; Zhang, B.; Niu, Q.; Liu, Y.; Yang, Q. Enhancement of Liquid Hot Water Pretreatment on Corn Stover with Ball Milling to Improve Total Sugar Yields. Sustainability 2023, 15, 16426. https://doi.org/10.3390/su152316426
Ji G, Zhang B, Niu Q, Liu Y, Yang Q. Enhancement of Liquid Hot Water Pretreatment on Corn Stover with Ball Milling to Improve Total Sugar Yields. Sustainability. 2023; 15(23):16426. https://doi.org/10.3390/su152316426
Chicago/Turabian StyleJi, Guanya, Bo Zhang, Qijian Niu, Yuxin Liu, and Qizhi Yang. 2023. "Enhancement of Liquid Hot Water Pretreatment on Corn Stover with Ball Milling to Improve Total Sugar Yields" Sustainability 15, no. 23: 16426. https://doi.org/10.3390/su152316426
APA StyleJi, G., Zhang, B., Niu, Q., Liu, Y., & Yang, Q. (2023). Enhancement of Liquid Hot Water Pretreatment on Corn Stover with Ball Milling to Improve Total Sugar Yields. Sustainability, 15(23), 16426. https://doi.org/10.3390/su152316426







