Analysis of Microbial Community in Circulating Cooling Water System of Coal Power Plant during Reagent Conversion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Agents and Carriers
2.2. Sample Source
2.3. System Transformation and Operation
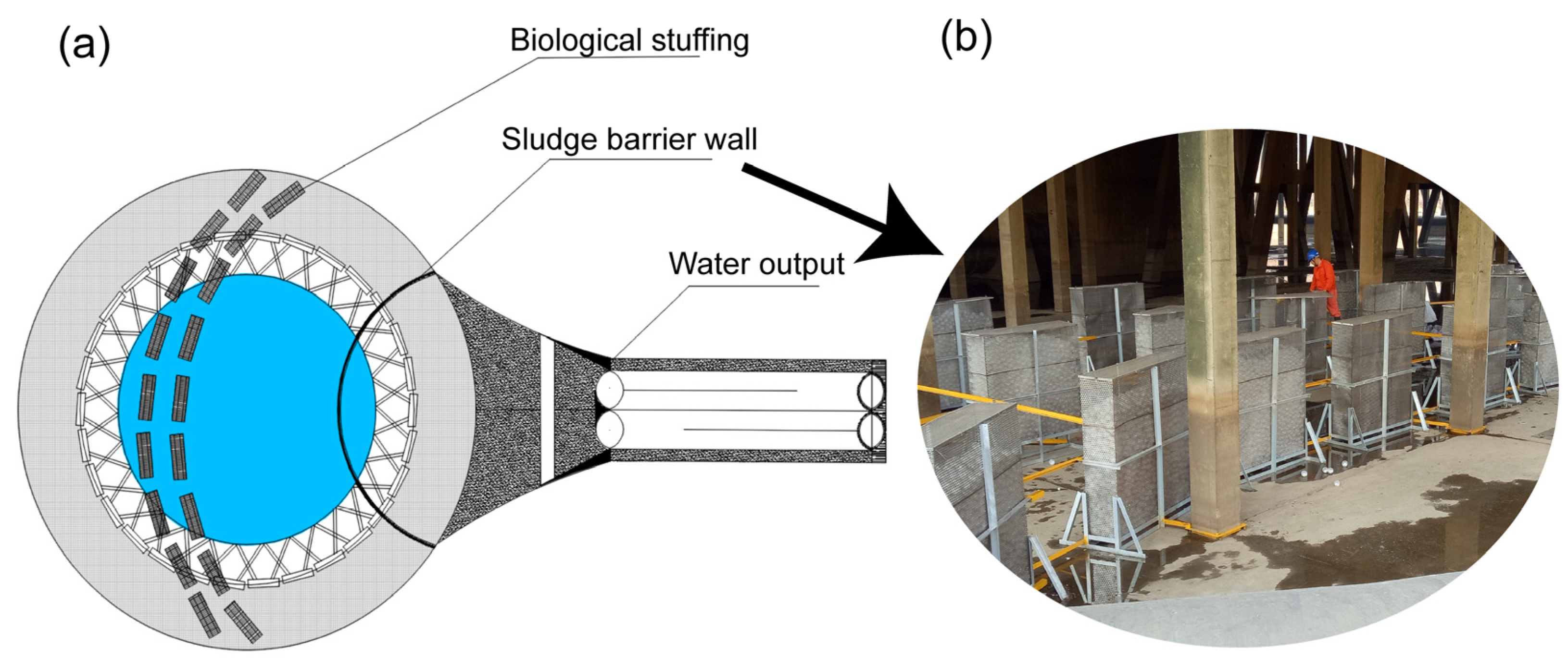
2.3.1. System Transformation
2.3.2. System Operation
2.4. Analysis Methods of Circulating Cooling Water
2.5. PCR Amplification of Samples and Illumina MiSeq Platform Sequencing
2.6. 16S Sequence Analysis of Samples
3. Results and Discussion
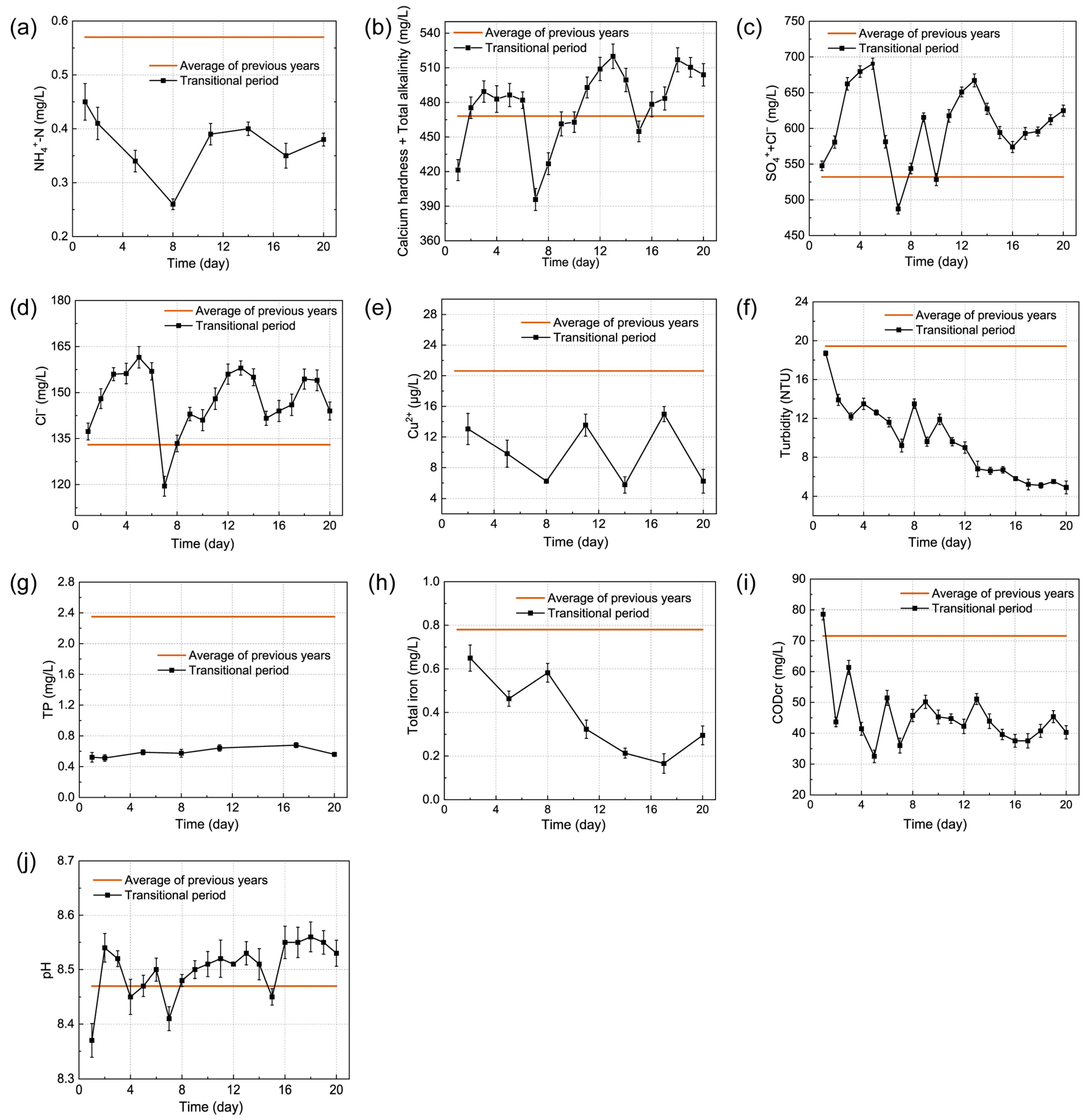
3.1. Comparison of Water Quality Stabilization Effects of Biological Agents and Chemical Agents
3.2. Analysis of Alpha Diversity and Beta Diversity of Microbial Community
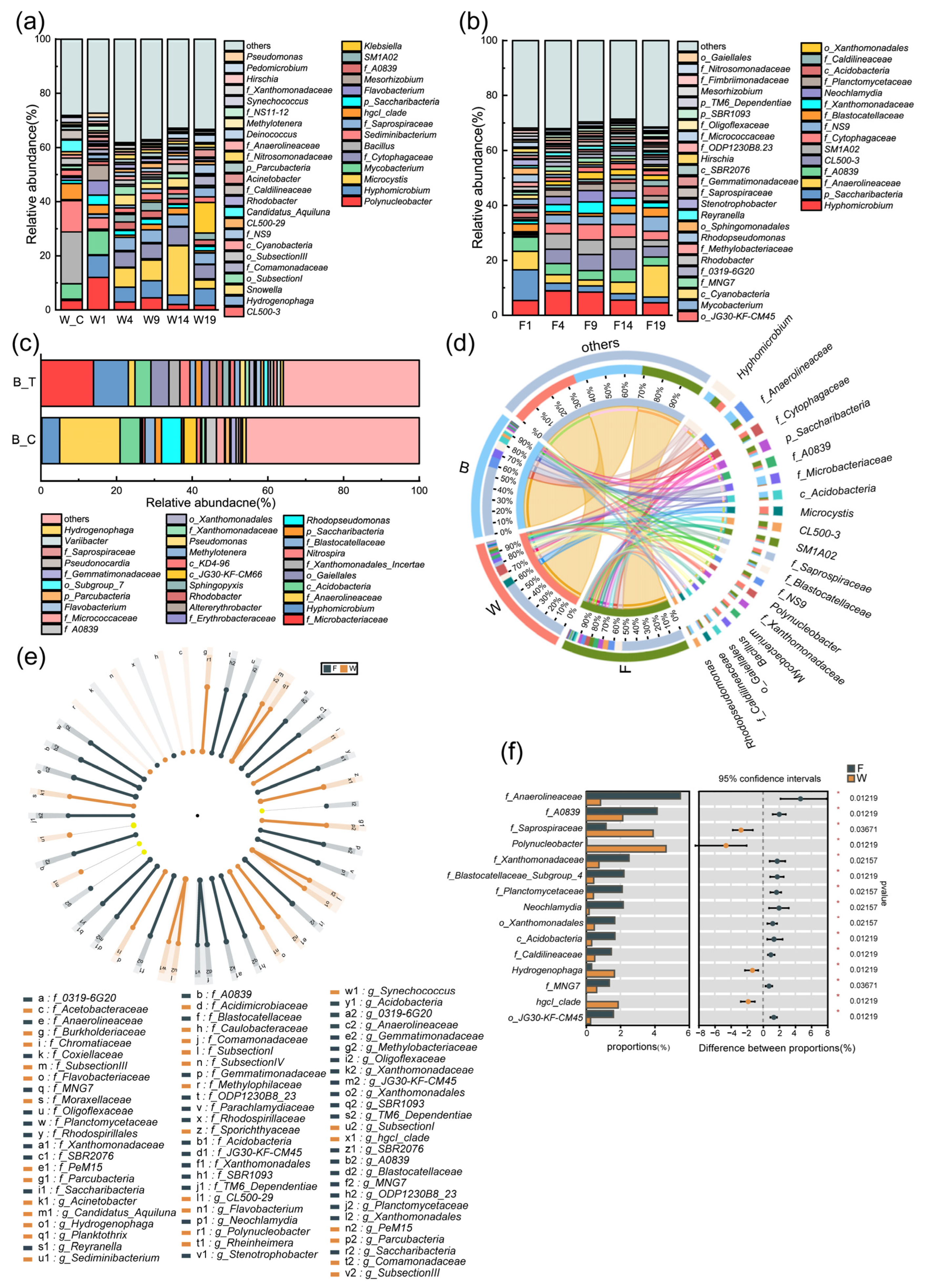
3.3. Similarities and Differences Analysis of Microbial Community Structure
3.4. Influence Analysis of Microbial Community Composition and Environmental Factors in Circulating Water System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, S.-Y.; Snyder, S.W.; Packman, A.I.; Lin, Y.J.; Chiang, P.-C. Cooling water use in thermoelectric power generation and its associated challenges for addressing water-energy nexus. Water-Energy Nexus 2018, 1, 26–41. [Google Scholar] [CrossRef]
- Liu, F.; Lu, X.H.; Yang, W.; Lu, J.J.; Zhong, H.Y.; Chang, X.; Zhao, C.C. Optimizations of inhibitors compounding and applied conditions in simulated circulating cooling water system. Desalination 2013, 313, 18–27. [Google Scholar] [CrossRef]
- Meesters, K.P.; Van Groenestijn, J.W.; Gerritse, J. Biofouling reduction in recirculating cooling systems through biofiltration of process water. Water Res. 2003, 37, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Doğruöz, N.; Göksay, D.; Ilhan-Sungur, E.; Cotuk, A. Pioneer colonizer microorganisms in biofilm formation on galvanized steel in a simulated recirculating cooling-water system. J. Basic Microbiol. 2009, 49 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.S.; Kora, A.J.; Chandramohan, P.; Panigrahi, B.S.; Narasimhan, S.V. Biofouling and microbial corrosion problem in the thermo-fluid heat exchanger and cooling water system of a nuclear test reactor. Biofouling 2009, 25, 581–591. [Google Scholar] [CrossRef]
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—Current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Yang, C.; Ueki, T.; Pittman, C.C.; Xu, D.; Woodard, T.L.; Holmes, D.E.; Gu, T.; Wang, F.; Lovley, D.R. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 2021, 15, 3084–3093. [Google Scholar] [CrossRef]
- Doğruöz Güngör, N.; Minnos, B.; Ilhan Sungur, E.; Cotuk, A. Biofilm Formation on Copper and Galvanized Steel Surfaces in a Cooling-Water System. IUFS J. Biol. Res. Artic. J Biol. 2009, 105, 105–111. [Google Scholar]
- Li, Y.; Gong, S.; Song, C.; Tian, H.; Yan, Z.; Wang, S. Roles of Microbial Extracellular Polymeric Substances in Corrosion and Scale Inhibition of Circulating Cooling Water. ACS EST Water 2023, 3, 743–755. [Google Scholar] [CrossRef]
- Choudhary, S.G. Emerging microbial control issues in cooling water systems. Hydrocarb. Process. 1998, 77, 91–102. [Google Scholar]
- Kokilaramani, S.; Al-Ansari, M.M.; Rajasekar, A.; Al-Khattaf, F.S.; Hussain, A.; Govarthanan, M. Microbial influenced corrosion of processing industry by re-circulating waste water and its control measures—A review. Chemosphere 2021, 265, 129075. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Khalek, D.E.; Abd-El-Nabey, B.A. Evaluation of sodium hexametaphosphate as scale and corrosion inhibitor in cooling water using electrochemical techniques. Desalination 2013, 311, 227–233. [Google Scholar] [CrossRef]
- Su, W.; Tian, Y.; Peng, S. The influence of sodium hypochlorite biocide on the corrosion of carbon steel in reclaimed water used as circulating cooling water. Appl. Surf. Sci. 2014, 315, 95–103. [Google Scholar] [CrossRef]
- Choi, D.-J.; You, S.-J.; Kim, J.-G. Development of an environmentally safe corrosion, scale, and microorganism inhibitor for open recirculating cooling systems. Mater. Sci. Eng. A 2002, 335, 228–235. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Hou, D. Hydroxyl carboxylate based non-phosphorus corrosion inhibition process for reclaimed water pipeline and downstream recirculating cooling water system. J. Environ. Sci. 2016, 39, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef]
- Li, S.L.; Qu, Q.; Li, L.; Xia, K.; Li, Y.; Zhu, T.T. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater. Water Res. 2019, 166, 11. [Google Scholar] [CrossRef]
- Qu, Q.; Yue, H.; Lei, W.; Xu, H.; Lei, L.; Chen, Y.; Ding, Z. Corrosion behavior of cold rolled steel in artificial seawater in the presence of Bacillus subtilis C2. Corros. Sci. 2015, 91, 321–329. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Z.; Liu, J.; Song, C.; Zhu, F.; Wang, S. The evaluation of Bacillus-secreted polyglutamic acid as anti-scaling treatment for circulating cooling water. Environ. Sci. Pollut. Res. 2022, 29, 82762–82771. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, C.C.; Xia, L.; Yang, F.; Chang, X.; Wang, Y.Q. Biofouling characteristics and identification of preponderant bacteria at different nutrient levels in batch tests of a recirculating cooling water system. Environ. Technol. 2011, 32, 901–910. [Google Scholar] [CrossRef]
- Lai, R.; Li, Q.; Cheng, C.; Shen, H.; Liu, S.; Luo, Y.; Zhang, Z.; Sun, S. Bio-competitive exclusion of sulfate-reducing bacteria and its anticorrosion property. J. Pet. Sci. Eng. 2020, 194, 107480. [Google Scholar] [CrossRef]
- Murshid, S.; Antonysamy, A.; Dhakshinamoorthy, G.; Jayaseelan, A.; Pugazhendhi, A. A review on biofilm-based reactors for wastewater treatment: Recent advancements in biofilm carriers, kinetics, reactors, economics, and future perspectives. Sci. Total Environ. 2023, 892, 164796. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, D.; Yang, J.; Wang, Z.; Dou, J. A Biological Agent for Maintaining the Stability of Circulating Cooling Water System and Its Preparation Method and Application. CN Patent CN106754583B, 21 March 2017. (In Chinese). [Google Scholar]
- Fang, D.; Zhao, G.; Xu, X.; Zhang, Q.; Shen, Q.; Fang, Z.; Huang, L.; Ji, F. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions. Bioresour. Technol. 2018, 249, 684–693. [Google Scholar] [CrossRef] [PubMed]
- GB/T 50050-2017; Code for Design of Industrial Recirculating Cooling Water Treatment. China Planning Press: Beijing, China, 2017.
- Yin, Y.; Wang, J. Predictive functional profiling of microbial communities in fermentative hydrogen production system using PICRUSt. Int. J. Hydrogen Energy 2021, 46, 3716–3725. [Google Scholar] [CrossRef]
- Magic-Knezev, A.; Wullings, B.; Van der Kooij, D. Polaromonas and Hydrogenophaga species are the predominant bacteria cultured from granular activated carbon filters in water treatment. J. Appl. Microbiol. 2009, 107, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.; Richert, I.; Szewzyk, U. Detection of iron-depositing Pedomicrobium species in native biofilms from the Odertal National Park by a new, specific FISH probe. J. Microbiol. Methods 2009, 79, 37–43. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Yu, J.; Cao, N.; Liu, R.; Yang, M. Characterization of bacterial community structure in a drinking water distribution system during an occurrence of red water. Appl. Environ. Microbiol 2010, 76, 7171–7180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, R.; Ma, Q. Study on the growth difference of Microcystis in different medium and its removal effect on N and P. Sci. Technol. Innov. Her. 2020, 17, 4. [Google Scholar]
- Baytshtok, V.; Kim, S.; Yu, R.; Park, H.; Chandran, K. Molecular and biokinetic characterization of methylotrophic denitrification using nitrate and nitrite as terminal electron acceptors. Water Sci. Technol. 2008, 58, 359–365. [Google Scholar] [CrossRef]
- Tsitko, I.; Lusa, M.; Lehto, J.; Parviainen, L.; Ikonen, A.T.K.; Lahdenperä, A.-M.; Bomberg, M. The Variation of Microbial Communities in a Depth Profile of an Acidic, Nutrient-Poor Boreal Bog in Southwestern Finland. Open J. Ecol. 2014, 04, 832–859. [Google Scholar] [CrossRef]
- Falkinham, J.O.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef]
- Liu, J.X.; Li, C.; Jing, J.H.; Jia, T.; Liu, X.G.; Wang, X.Y.; Chai, B.F. Composition and environmental adaptation of microbial community in shibahe copper tailing in zhongtiao mountain in Shanxi. Environ. Sci. 2017, 38, 318–326. (In Chinese) [Google Scholar]
- Vayenas, D. Attached Growth Biological Systems in the Treatment of Potable Water and Wastewater. Compr. Biotechnol. 2011, 6, 371–383. [Google Scholar] [CrossRef]
- Wang, J.; Gong, B.; Huang, W.; Wang, Y.; Zhou, J. Bacterial community structure in simultaneous nitrification, denitrification and organic matter removal process treating saline mustard tuber wastewater as revealed by 16S rRNA sequencing. Bioresour. Technol. 2017, 228, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kuramae, E.E.; de Assis Costa, O.Y. Acidobacteria. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.; Li, Y.; Tu, X.; Chen, Z.; Elrys, A.S.; Cheng, Y.; Ma, L. A shift from nitrification to denitrification-dominated N2O emission in an acidic soil following organic amendment. Biol. Fertil. Soils 2023, 59, 117–122. [Google Scholar] [CrossRef]
- James, S.N.; Vijayanandan, A. Recent advances in simultaneous nitrification and denitrification for nitrogen and micropollutant removal: A review. Biodegradation 2023, 34, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hou, L.; Zheng, Y.; Liu, M.; Yin, G.; Li, X.; Lin, X.; Yu, C.; Wang, R.; Jiang, X.; et al. nirS-Encoding denitrifier community composition, distribution, and abundance along the coastal wetlands of China. Appl. Microbiol. Biotechnol. 2016, 100, 8573–8582. [Google Scholar] [CrossRef]
- Huang, W.; Ertekin, E.; Wang, T.; Cruz, L.; Dailey, M.; Diruggiero, J.; Kisailus, D. Mechanism of water extraction from gypsum rock by desert colonizing microorganisms. Proc. Natl. Acad. Sci. USA 2020, 117, 10681–10687. [Google Scholar] [CrossRef]
- Bor, B.; Bedree, J.K.; Shi, W.; McLean, J.S.; He, X. Saccharibacteria (TM7) in the Human Oral Microbiome. J. Dent. Res. 2019, 98, 500–509. [Google Scholar] [CrossRef]


| Bacterial Genus | Percentage |
|---|---|
| Klebsiella | 38.76% |
| Prevotella | 24.16% |
| Streptococcus | 7.89% |
| Acinetobacter | 5.59% |
| Bacteroides | 3.42% |
| LactoBacillus | 3.07% |
| Enterococcus | 3.43% |
| PeptoStreptococcus | 2.82% |
| Kurthia | 1.30% |
| Others | 10.56% |
| Items | Fiber Carrier |
|---|---|
| Basic chemical composition | Polyethylene glycol terephthalate |
| Specification | 25–30 mm in sphere diameter |
| COD release capacity | Below the detection limit |
| Density | 1.38 kg/m3 |
| Specific surface area | 3000 m2/g |
| Filling ratio | 0.3 |
| Water Quality Parameters | Experimental Methods and Standards |
|---|---|
| Turbidity (NTU) | Portable turbidity plan method |
| pH | pH indicator electrode |
| NH4+-N (mg/L) | Nessler’s reagent spectrophotometry |
| CODcr (mg/L) | Dichromate method |
| TP (mg/L) | Ammonium molybdate spectrophotometry |
| Cl− (mg/L) | Nitrate radical titration method |
| SO42− (mg/L) | Gravimetric analysis |
| Total iron (mg/L) | Spectrophotometric o-phenanthroline |
| Calcium hardness (as mg/L CaCO3) | EDTA titration |
| Total alkalinity (as mg/L CaCO3) | Standard titration method |
| Index | Interim Period | The Same Time in Previous Years | Variable Quantity |
|---|---|---|---|
| Average Cl− in circulating water (mg/L) | 147.37 | 133.67 | 13.7 |
| Average Cl− in make-up water (mg/L) | 38.35 | 43.6 | 5.25 |
| Concentration multiple | 3.97 | 3.11 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ye, J.; Xu, M.; Li, Y.; Dou, J. Analysis of Microbial Community in Circulating Cooling Water System of Coal Power Plant during Reagent Conversion. Sustainability 2023, 15, 16359. https://doi.org/10.3390/su152316359
Wang Y, Ye J, Xu M, Li Y, Dou J. Analysis of Microbial Community in Circulating Cooling Water System of Coal Power Plant during Reagent Conversion. Sustainability. 2023; 15(23):16359. https://doi.org/10.3390/su152316359
Chicago/Turabian StyleWang, Yichao, Jiangyu Ye, Mingzhi Xu, Yunyi Li, and Jianjun Dou. 2023. "Analysis of Microbial Community in Circulating Cooling Water System of Coal Power Plant during Reagent Conversion" Sustainability 15, no. 23: 16359. https://doi.org/10.3390/su152316359
APA StyleWang, Y., Ye, J., Xu, M., Li, Y., & Dou, J. (2023). Analysis of Microbial Community in Circulating Cooling Water System of Coal Power Plant during Reagent Conversion. Sustainability, 15(23), 16359. https://doi.org/10.3390/su152316359








