Recycling of Coal Combustion Waste through Production of Foamed Geopolymers with Improved Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
- -
- Sodium waterglass (an aqueous solution of sodium silicates) was used. The content of the main substance in the solution was 45 wt.%, silicate modulus = 2 (manufacturer: Palitra LLC, St. Petersburg, Leningrad region, Russian Federation);
- -
- In total, 12 M aqueous NaOH solution was prepared by dissolving granulated sodium hydroxide with the main substance content of 99 wt.% (producer: LLC SANTREID, Moscow, Russian Federation) in deionized water.
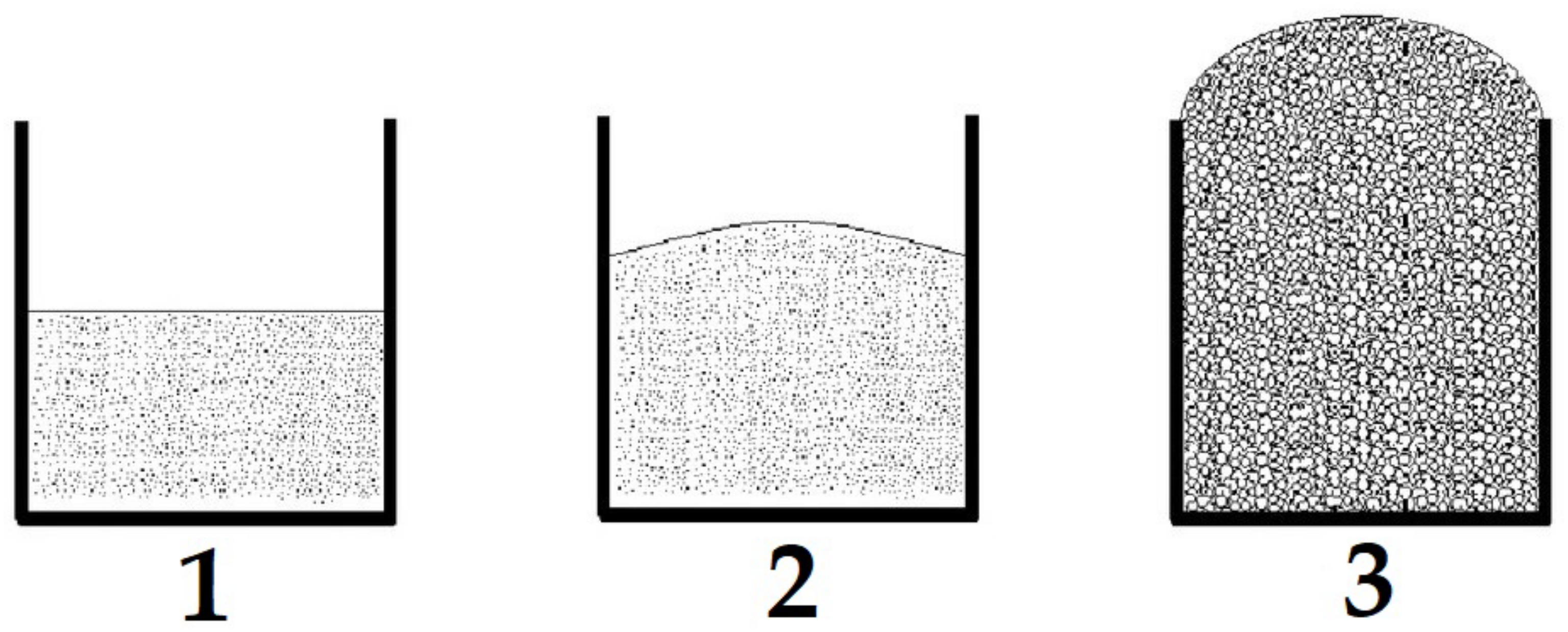
2.2. Synthesis of Foamed Geopolymer Materials with Strengthening Additives
2.3. Methods for Studying the Finished Materials
3. Results and Discussion
3.1. Composition and Structure of Coal Combustion Waste and Other Raw Materials
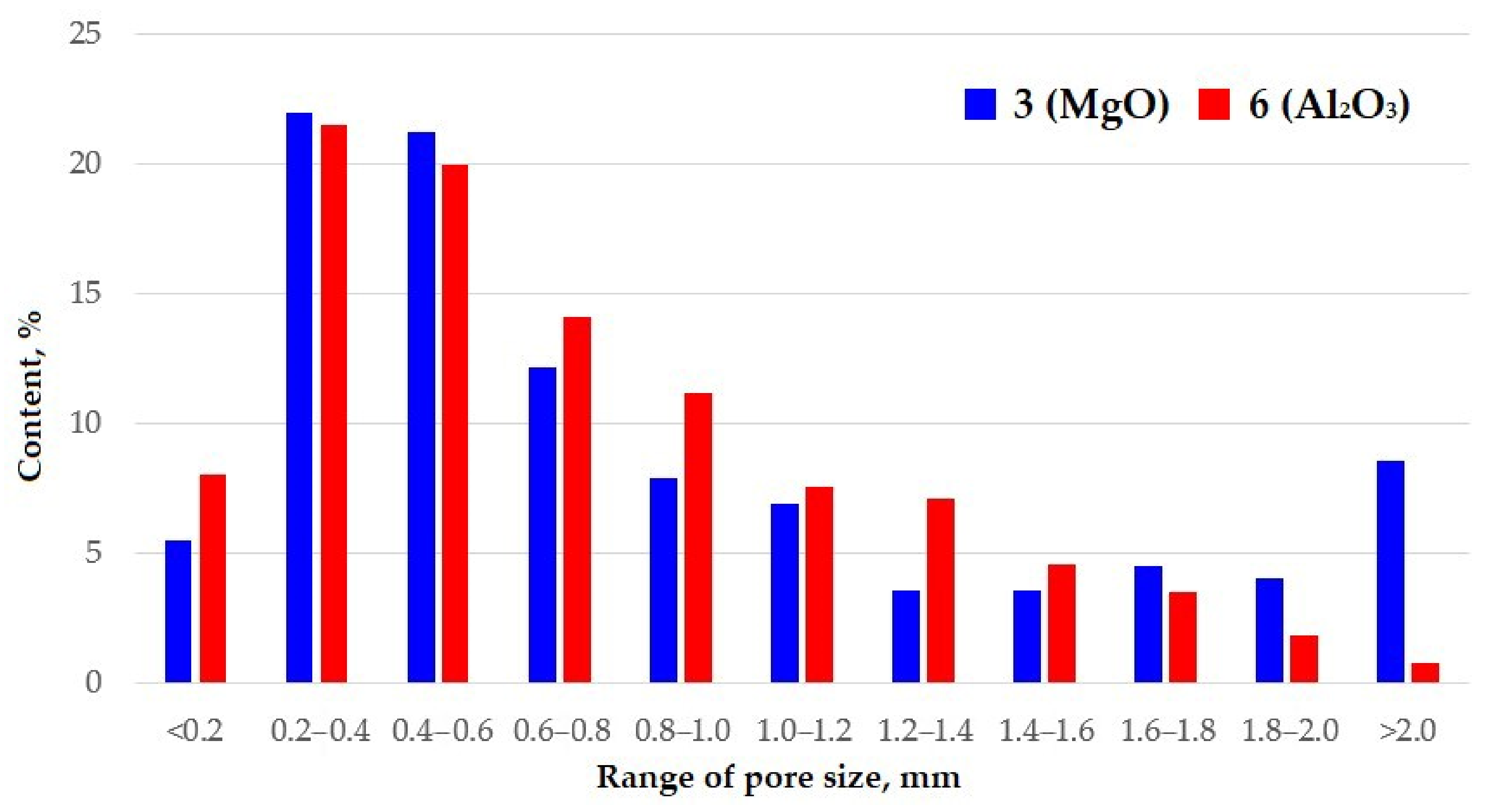
3.2. Investigation of the Properties of Synthesized Foamed Geopolymer Materials
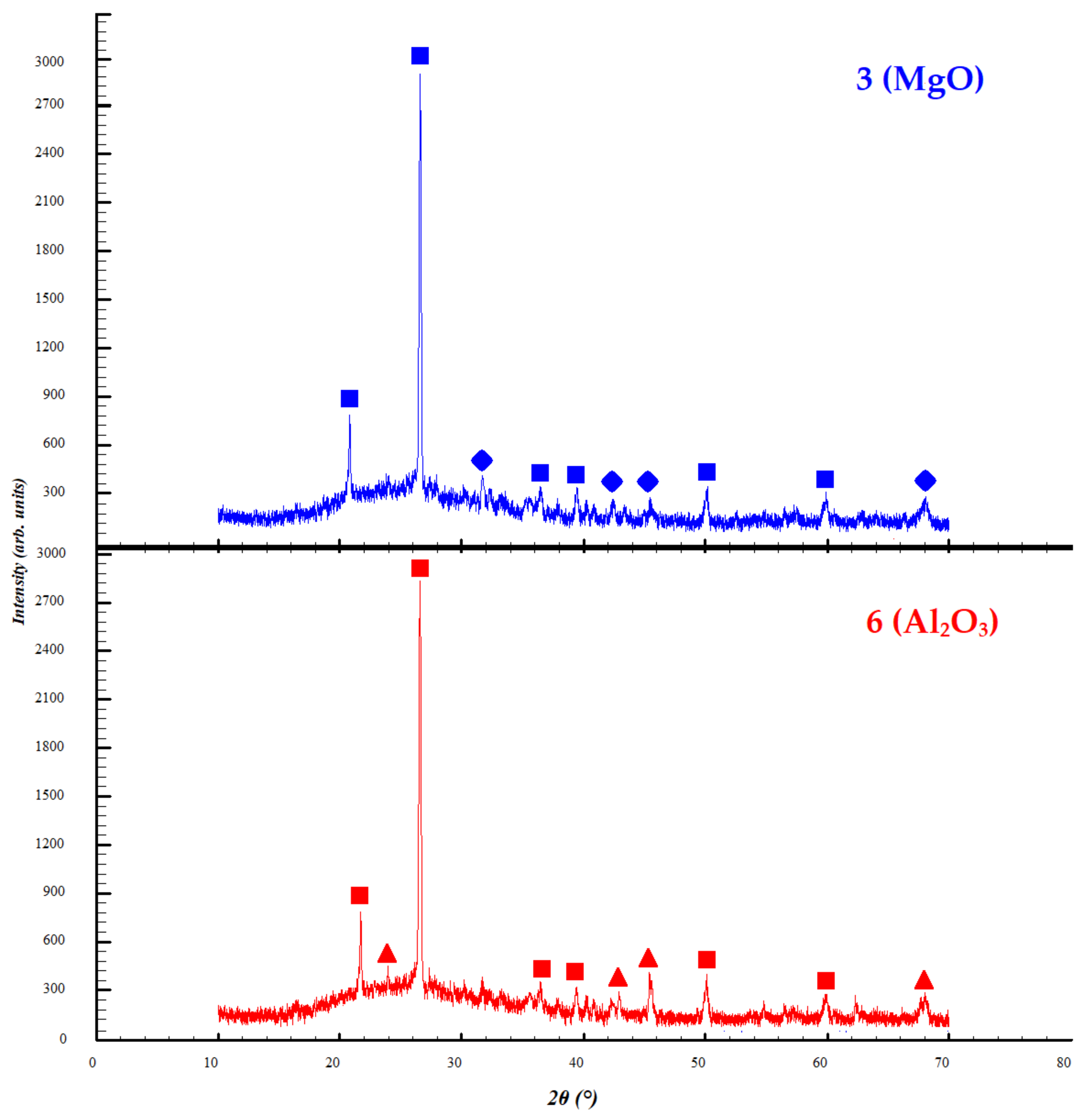
3.3. Study of X-ray Phase Analysis and Microstructure of Samples with Chosen Additives
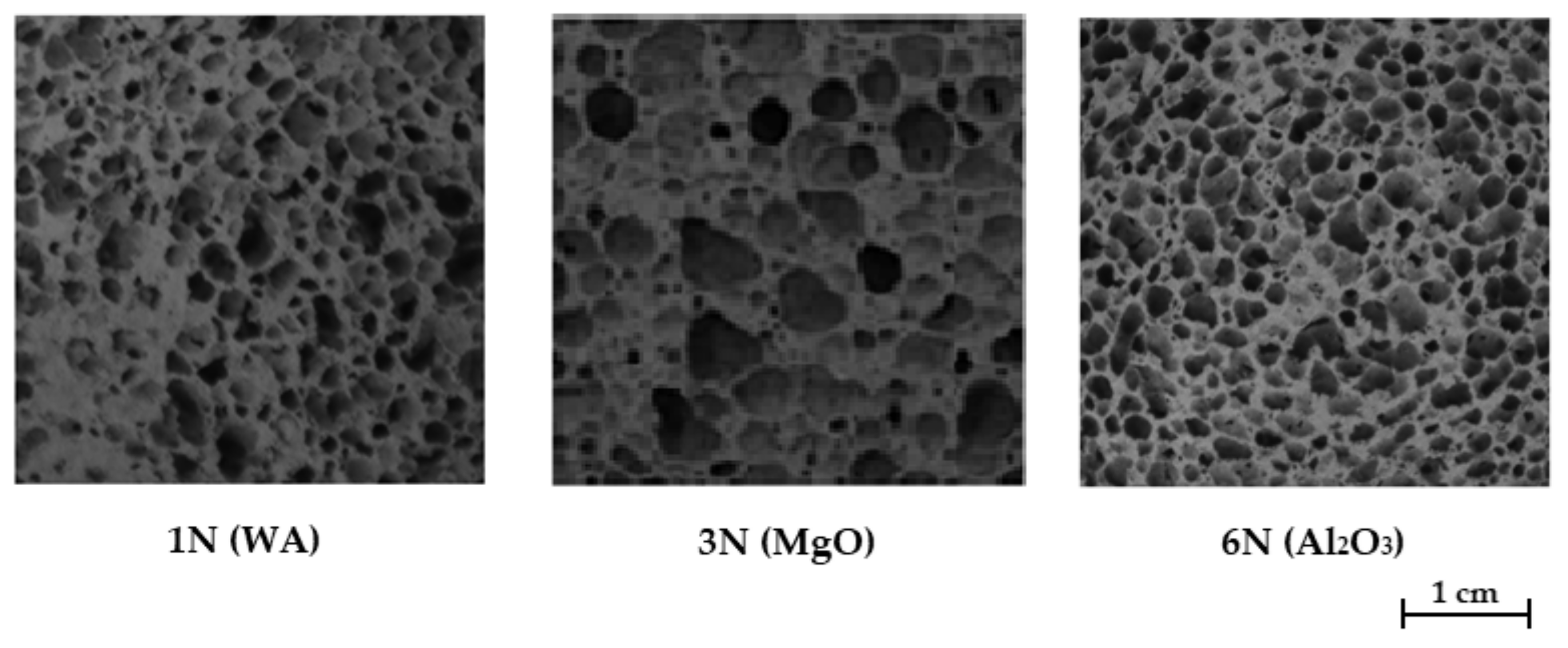
3.4. Study of the Influence of Additives on the Properties of Geopolymers Based on Other Raw Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Wu, Y.; Ma, S.; Zheng, S.; Zhang, Y.; Chu, P.K. Utilization of Coal Fly Ash in China: A Mini-Review on Challenges and Future Directions. Environ. Sci. Pollut. Res. 2021, 28, 18727–18740. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, E.A.; Goltsman, B.M.; Trofimov, S.V.; Kurdashov, V.M.; Novikov, Y.V.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition. Materials 2022, 15, 2587. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wan, Q.; Cang, D. Preparation of Anorthite-Based Porous Ceramics Using High-Alumina Fly Ash Microbeads and Steel Slag. Ceram. Int. 2019, 45, 22445–22451. [Google Scholar] [CrossRef]
- Cherian, C.; Siddiqua, S. Engineering and Environmental Evaluation for Utilization of Recycled Pulp Mill Fly Ash as Binder in Sustainable Road Construction. J. Clean. Prod. 2021, 298, 126758. [Google Scholar] [CrossRef]
- Kurtoglu, A.E.; Alzeebaree, R.; Aljumaili, O.; Nis, A.; Gulsan, M.E.; Humur, G.; Cevik, A. Mechanical and Durability Properties of Fly Ash and Slag Based Geopolymer Concrete. Adv. Concr. Constr. 2018, 6, 345. [Google Scholar]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of Fresh and Hardened Fly Ash/Slag Based Geopolymer Concrete: A Review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, J.R.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V. The Effect of Temperature on the Geopolymerization Process of a Metakaolin-Based Geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of Source Materials, Additives on the Mechanical Properties and Durability of Fly Ash and Fly Ash-Slag Geopolymer Mortar: A Review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S. Review on Characteristics of Metakaolin-Based Geopolymer and Fast Setting. J. Korean Ceram. Soc. 2020, 57, 368–377. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of Granulated Blast Furnace Slag on the Reaction, Structure and Properties of Fly Ash Based Geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. Mix Design Development of Fly Ash and Ground Granulated Blast Furnace Slag Based Geopolymer Concrete. J. Build. Eng. 2018, 20, 712–722. [Google Scholar] [CrossRef]
- Le, V.Q.; Do, M.Q.; Hoang, M.D.; Pham, V.T.H.Q.; Bui, T.H.; Nguyen, H.T. Effect of Alkaline Activators to Engineering Properties of Geopolymer-Based Materials Synthesized from Red Mud. Key Eng. Mater. 2018, 777, 508–512. [Google Scholar] [CrossRef]
- Longos, A., Jr.; Tigue, A.A.; Dollente, I.J.; Malenab, R.A.; Bernardo-Arugay, I.; Hinode, H.; Kurniawan, W.; Promentilla, M.A. Optimization of the Mix Formulation of Geopolymer Using Nickel-Laterite Mine Waste and Coal Fly Ash. Minerals 2020, 10, 1144. [Google Scholar] [CrossRef]
- Davidovits, J. Years of Successes and Failures in Geopolymer Applications. Market Trends and Potential Breakthroughs. In Proceedings of the Geopolymer 2002 Conference, Melbourne, Australia, 28–29 October 2002; Volume 28, p. 29. [Google Scholar]
- Malchik, A.G.; Litovkin, S.V.; Rodionov, P.V.; Kozik, V.V.; Gaydamak, M.A. Analyzing the Technology of Using Ash and Slag Waste from Thermal Power Plants in the Production of Building Ceramics. IOP Conf. Ser. Mater. Sci. Eng. 2016, 127, 12024. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.M.S.M.K. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.A.; Mohammed, A.S. The Role of Nanomaterials in Geopolymer Concrete Composites: A State-of-the-Art Review. J. Build. Eng. 2022, 49, 104062. [Google Scholar] [CrossRef]
- Imtiaz, L.; Kashif-ur-Rehman, S.; Alaloul, W.S.; Nazir, K.; Javed, M.F.; Aslam, F.; Musarat, M.A. Life Cycle Impact Assessment of Recycled Aggregate Concrete, Geopolymer Concrete, and Recycled Aggregate-Based Geopolymer Concrete. Sustainability 2021, 13, 13515. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental Impact Assessment of Fly Ash and Silica Fume Based Geopolymer Concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Das, S.; Saha, P.; Jena, S.P.; Panda, P. Geopolymer Concrete: Sustainable Green Concrete for Reduced Greenhouse Gas Emission-A Review. Mater. Today Proc. 2022, 60, 62–71. [Google Scholar] [CrossRef]
- SteinerOva, M. Mechanical Properties of Geopolymer Mortars in Relation to Their Porous Structure. Ceram.-Silikáty 2011, 55, 362–372. [Google Scholar]
- Škvára, F.; Jílek, T.; Kopecký, L. Geopolymer Materials Based on Fly Ash. Ceram.-Silik 2005, 49, 195–204. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Yatsenko, E.A.; Goltsman, B.M.; Trofimov, S.V.; Novikov, Y.V.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Influence of Various Coal Energy Wastes and Foaming Agents on Foamed Geopolymer Materials’ Synthesis. Materials 2022, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Y.; Li, Y.; Liu, W.; Wu, J.; Liu, C.; Yang, B.; Pang, Z. The Enhanced Surface Properties of Geopolymer Inorganic Coatings by Adding with MgO. J. Coat. Technol. Res. 2022, 19, 947–957. [Google Scholar] [CrossRef]
- Xavier, C.S.B.; Rahim, A. Nano Aluminium Oxide Geopolymer Concrete: An Experimental Study. Mater. Today Proc. 2022, 56, 1643–1647. [Google Scholar] [CrossRef]
- Baskara Sundararaj, J.; Kannan Rajkumar, P.R.; Sivasakthi, M.; Jegan, M. Effect of Mineral Admixtures on Mechanical and Thermal Properties of Geopolymer Mortar at Elevated Temperature. Innov. Infrastruct. Solut. 2022, 7, 354. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J.; Smith, J.D. Mechanism of Polysialation in the Incorporation of Zirconia into Fly Ash-Based Geopolymers. Ind. Eng. Chem. Res. 2000, 39, 2925–2934. [Google Scholar] [CrossRef]
- Zailan, S.N.; Bouaissi, A.; Mahmed, N.; Abdullah, M.M.A.B. Influence of ZnO Nanoparticles on Mechanical Properties and Photocatalytic Activity of Self-Cleaning ZnO-Based Geopolymer Paste. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2007–2016. [Google Scholar] [CrossRef]
- Sherin, K.; Saurabh, J.K. Review of Autoclaved Aerated Concrete:-Advantages and Disadvantages. In Proceedings of the National Conference: Advanced Structures, Materials and Methodology in Civil Engineering (ASMMCE-2018), Jalandhar, India, 3–4 November 2018; pp. 35–39. [Google Scholar]
- Lu, D.; Zheng, Y.; Liu, Y.; Xu, Z. Effect of Light-Burned Magnesium Oxide on Deformation Behavior of Geopolymer and Its Mechanism. J. Chin. Ceram. Soc. 2012, 40, 1625–1630. [Google Scholar]
- Jang, J.G.; Park, S.M.; Kim, G.M.; Lee, H.-K. Stability of MgO-Modified Geopolymeric Gel Structure Exposed to a CO2-Rich Environment. Constr. Build. Mater. 2017, 151, 178–185. [Google Scholar] [CrossRef]
- Zidi, Z.; Ltifi, M.; Ayadi, Z.B.; Mir, L.E.; Nóvoa, X.R. Effect of Nano-ZnO on Mechanical and Thermal Properties of Geopolymer. J. Asian Ceram. Soc. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Peng, R.; Wang, H. Geopolymer-TiO2 Nanocomposites for Photocsatalysis: Synthesis by One-Step Adding Treatment versus Two-Step Acidification Calcination. Minerals 2019, 9, 658. [Google Scholar] [CrossRef]
- Du, F.-P.; Xie, S.-S.; Zhang, F.; Tang, C.-Y.; Chen, L.; Law, W.-C.; Tsui, C.-P. Microstructure and Compressive Properties of Silicon Carbide Reinforced Geopolymer. Compos. Part B Eng. 2016, 105, 93–100. [Google Scholar] [CrossRef]
- Mohd Mortar, N.A.; Abdullah, M.M.A.B.; Abdul Razak, R.; Abd Rahim, S.Z.; Aziz, I.H.; Nabiałek, M.; Jaya, R.P.; Semenescu, A.; Mohamed, R.; Ghazali, M.F. Geopolymer Ceramic Application: A Review on Mix Design, Properties and Reinforcement Enhancement. Materials 2022, 15, 7567. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, H.; Li, L. A Review: The Comparison between Alkali-Activated Slag (Si+Ca) and Metakaolin (Si+Al) Cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Kouamo, H.T.; Elimbi, A.; Mbey, J.A.; Sabouang, C.J.N.; Njopwouo, D. The Effect of Adding Alumina-Oxide to Metakaolin and Volcanic Ash on Geopolymer Products: A Comparative Study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Sastry, K.V.S.G.K.; Sahitya, P.; Ravitheja, A. Influence of Nano TiO2 on Strength and Durability Properties of Geopolymer Concrete. Mater. Today Proc. 2021, 45, 1017–1025. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Izvarin, A.I.; Kurdashov, V.M.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L. V Recycling Ash and Slag Waste from Thermal Power Plants to Produce Foamed Geopolymers. Energies 2023, 16, 7535. [Google Scholar] [CrossRef]




| Sample | CCW | NaOH, 12 M Solution | Waterglass | Aluminum Powder * | CaO * | MgO * | ZnO * | TiO2 * | Al2O3 * | SiC * | ZrO2 * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (WA) | 70 | 5 | 25 | 2 | - | - | - | - | - | - | - |
| 2 (CaO) | 70 | 5 | 25 | 2 | 3 | - | - | - | - | - | - |
| 3 (MgO) | 70 | 5 | 25 | 2 | - | 3 | - | - | - | - | - |
| 4 (ZnO) | 70 | 5 | 25 | 2 | - | - | 3 | - | - | - | - |
| 5 (TiO2) | 70 | 5 | 25 | 2 | - | - | - | 3 | - | - | - |
| 6 (Al2O3) | 70 | 5 | 25 | 2 | - | - | - | - | 3 | - | - |
| 7 (SiC) | 70 | 5 | 25 | 2 | - | - | - | - | - | 3 | - |
| 8 (ZrO2) | 70 | 5 | 25 | 2 | - | - | - | - | - | - | 3 |
| Component | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CaO | TiO2 | MnO | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCW from (Severodvinsk CHPP-1) | 61.6 | 17.9 | 6.0 | 2.8 | 3.6 | 2.3 | 2.1 | 0.8 | 0.1 | 0.2 | 0.3 | 2.3 |
| CCW from Novocherkassk SDPP | 51.2 | 18.8 | 10.3 | 2.1 | 0.9 | 3.0 | 3.1 | 0.8 | 0.1 | 0.1 | 0.3 | 9.2 |
| Geopolymer | 57.8 | 18.4 | 5.1 | 2.3 | 9.5 | 2.0 | 1.8 | 0.7 | 0.1 | 0.2 | 0.2 | 1.9 |
| Sample | Density, kg/m3 | Compressive Strength, MPa | Porosity,% | Relative Compressive Strength, m2/s2·1000 | Average Pore Size (D50), mm |
|---|---|---|---|---|---|
| 1 (WA) | 304 ± 9 | 1.52 ± 0.04 | 85.0 ± 2.5 | 5.00 | 0.26 |
| 2 (CaO) | 658 ± 9 | 1.63 ± 0.05 | 68.1 ± 2.1 | 2.48 | 0.04 |
| 3 (MgO) | 285 ± 8 | 1.35 ± 0.04 | 86.0 ± 2.6 | 4.74 | 0.41 |
| 4 (ZnO) | 318 ± 9 | 1.42 ± 0.04 | 84.4 ± 2.5 | 4.47 | 0.33 |
| 5 (TiO2) | 314 ± 9 | 1.92 ± 0.06 | 84.6 ± 2.5 | 6.11 | 0.39 |
| 6 (Al2O3) | 320 ± 9 | 1.94 ± 0.06 | 84.3 ± 2.5 | 6.06 | 0.42 |
| 7 (SiC) | 371 ± 11 | 1.38 ± 0.04 | 81.8 ± 2.4 | 3.72 | 0.38 |
| 8 (ZrO2) | 326 ± 10 | 2.03 ± 0.06 | 84.0 ± 2.5 | 6.23 | 0.44 |
| Sample | Density, kg/m3 | Compressive Strength, Mpa | Porosity,% | Relative Compressive Strength, m2/s2·1000 |
|---|---|---|---|---|
| 1N (WA) | 320 ± 9 | 1.36 ± 0.02 | 85.1 ± 2.5 | 4.25 |
| 3N (MgO) | 296 ± 10 | 1.07 ± 0.02 | 87.7 ± 2.6 | 3.61 |
| 6N (Al2O3) | 332 ± 10 | 1.67 ± 0.03 | 86.3 ± 2.5 | 5.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsenko, E.A.; Goltsman, B.M.; Novikov, Y.V.; Trofimov, S.V.; Ryabova, A.V.; Smoliy, V.A.; Klimova, L.V. Recycling of Coal Combustion Waste through Production of Foamed Geopolymers with Improved Strength. Sustainability 2023, 15, 16296. https://doi.org/10.3390/su152316296
Yatsenko EA, Goltsman BM, Novikov YV, Trofimov SV, Ryabova AV, Smoliy VA, Klimova LV. Recycling of Coal Combustion Waste through Production of Foamed Geopolymers with Improved Strength. Sustainability. 2023; 15(23):16296. https://doi.org/10.3390/su152316296
Chicago/Turabian StyleYatsenko, Elena Alfredovna, Boris Mikhailovich Goltsman, Yuri Vladimirovich Novikov, Sergey Vyacheslavovich Trofimov, Anna Vladimirovna Ryabova, Victoria Alexandrovna Smoliy, and Lyudmila Vasilievna Klimova. 2023. "Recycling of Coal Combustion Waste through Production of Foamed Geopolymers with Improved Strength" Sustainability 15, no. 23: 16296. https://doi.org/10.3390/su152316296
APA StyleYatsenko, E. A., Goltsman, B. M., Novikov, Y. V., Trofimov, S. V., Ryabova, A. V., Smoliy, V. A., & Klimova, L. V. (2023). Recycling of Coal Combustion Waste through Production of Foamed Geopolymers with Improved Strength. Sustainability, 15(23), 16296. https://doi.org/10.3390/su152316296









