Abstract
Chrysanthemums (Chrysanthemum × morifolium) are highly valued for their ornamental and economic benefits. However, the commonly used growing medium for chrysanthemums, peat, is not renewable, and peatlands are facing depletion. Therefore, it is important to find sustainable alternatives to peat. This study aims to evaluate the potential of rice husks and leaf mold mixed with peat and perlite in different ratios (10–20–30–40–80% v/v/v/v) as substitute materials for peat in chrysanthemum production. The study examines the physical and chemical properties of the different growing media ratios, as well as their effects on plant growth, development, and physiological indicators. The results of the experiment demonstrate that the different ratios of the cultivation substrate significantly influence the physical and chemical properties of the growing medium, as well as the growth and physiological indicators of chrysanthemums. A 20–30% proportion of rice husks and leaf mold promotes the growth and photosynthetic activity of chrysanthemum cuttings, resulting in increased plant height, leaf area, total chlorophyll content, and net photosynthetic rate. The mixed substrates (10–40%) maintain suitable pH levels, electrical conductivity (EC), and nutrient content (nitrogen, phosphorus, and potassium). However, an 80% ratio of rice husks negatively affects plant survival and growth due to elevated EC and potassium levels. In conclusion, a peat medium containing 20% rice husks and leaf mold provides a more favorable cultivation substrate for producing high-quality chrysanthemums while promoting sustainable horticultural practices.
1. Introduction
Peat serves as an essential growing substrate for horticultural and ornamental plants cultivated in pots and on planting tables due to its excellent physicochemical properties [1,2]. However, it is not a renewable resource in the short term, and its exploitation has led to a rapid decline in availability. Consequently, some countries have had to resort to imports, resulting in a significant increase in the price of peat [3,4,5,6,7]. Moreover, the extensive use of peat has caused environmental issues such as imbalances in wetland ecosystems and an amplified greenhouse effect. As a result, the use of peat in horticulture has sparked intense controversy, leading certain countries to ban its usage. For instance, Poland aims to reduce peat usage by 50% by 2030 and phase it out completely thereafter [8]. Therefore, there is a pressing need to find an easily accessible and environmentally friendly substitute for peat that allows for large-scale production.
Agricultural and forestry waste represents the most abundant and underutilized natural resource for soil cultivation, holding great potential as a renewable resource [9]. However, due to limited technology, a significant amount of agroforestry waste is irresponsibly burned, resulting in wasted resources and environmental pollution [10]. The effective utilization of agroforestry waste is vital for resource development, environmental protection, ecological nutrient cycling, and sustainable resource development. Studies have demonstrated that agroforestry waste like corn stover, coconut husk, and sawdust can be used successfully instead of peat in the cultivation of geranium, calendula, garlic, and primula [10,11,12]. Some studies have shown that rotting leaf mold and rice husks can replace peat as a plant growth medium, where they not only help with adequate nutrients but also have better water retention and air permeability and offer lightweight substrates that reduce transportation costs and yield greater economic advantages [13,14,15,16,17,18].
Chrysanthemums (Chrysanthemum × morifolium), known for their vibrant colors and appealing shapes, are frequently utilized in garden landscaping, home horticulture, festive flower shows, and themed exhibitions due to their high economic value [19,20]. Given the extensive use of peat in chrysanthemum production, it is crucial to explore suitable substitutes to reduce peat usage and achieve sustainable horticultural practices while ensuring productivity and ornamental value. Some studies have shown that vermicompost and pecan wood chips can replace peat as a cultivation substrate for chrysanthemum growth [21,22]. However, the benefits of using rice husks and leaf mold as alternatives to peat in chrysanthemum cultivation remain unknown. In this study, we aimed to investigate the physicochemical properties and evaluate the effects of different growing media ratios on chrysanthemum morphology, photosynthesis, physiology, and biochemistry. The goal was to identify growing media that can promote plant growth and enhance ornamental value while reducing dependence on peat.
2. Materials and Methods
2.1. Experimental Materials
The experiment was conducted with three chrysanthemum varieties, C. morifolium “Huihuang” (A), “Boerduohong” (B), and “Huangfurong” (C), obtained via a pre-screening of the research group as the plant material and peat, perlite, leaf mold (oak leaves, pH = 6.45), and rice husk (fermented rice husk, pH = 7.06) as the substrate material; it was conducted from June to November 2022 in the garden nursery of Northeast Forestry University. For this experiment, well-grown, disease-free stem segments from the top of chrysanthemum plants were selected for propagation via cuttings, and the cuttings were grown in perlite substrate and then cultivated in a greenhouse. When the root length of the cuttings reached 1–2 cm, a single plant with uniform growth was selected for the experiment.
2.2. Experimental Design
The experiment included seven treatments of the composite substrate (Table 1) made from peat, perlite, and leaf mold with rice husk at different volume ratios, with conventional substrate peat/perlite = 8:2 (volume ratio) as the control. The peat was gradually replaced with agricultural and forestry wastes of decayed leaves and rice husk in the same proportion to formulate several formulations, in which perlite always accounted for two parts of the total amount, and the proportion of decayed leaves and rice husk was gradually increased until all the peat was replaced. The experiment first determined the physicochemical properties of the mixed substrates; then, the mixed substrates were loaded into pots, and C. morifolium cuttings with consistent and good growth were selected to be planted in each substrate. Each treatment was treated with 3 replicates and 4 experimental cuttings per replicate.

Table 1.
Different substrate ratios of cultivated C. morifolium substrates.
2.3. The Determination of the Physical and Chemical Properties of the Substrates
The substrate bulk density, total porosity, aeration porosity, water-holding porosity, and gas/water ratio were determined using the cutting ring method [23]. This means that a cutting ring of weight W0 was first filled with 100 mL of the air-dried substrate, the total weight of the cutting ring and the air-dried weight was recorded as W1, and the weight of the air-dried substrate was recorded as W2 after wiping off external water stains following the immersion of the substrate in water for 24 h. After natural draining, the weight W3 was recorded, and lastly, it was put into an oven and baked at 65 °C until a constant weight was achieved; the weight W4 was then recorded. We calculated the relevant indexes via the following formula: Bulk density (g/cm3) = (W4 − W0)/100; Total porosity (%) = (W2 − W4)/100 × 100%; Aeration porosity (%) = (W2 − W3)/100 × 100%, Water-holding porosity (%) = Total porosity-Aeration porosity; Gas/water ratio = Aeration porosity/Water-holding porosity.
The pH and EC values of the substrate were determined via Ahmet and Zhang’s method using a pH meter and conductivity meter (DDS-11A) [24,25]. The available nitrogen, available phosphorus, and available calcium contents of the substrate were determined via the alkaline diffusion method, 0.5 mol/L sodium hydrogen carbonate solution–Mo-Sb flame spectrophotometric method, and ammonium acetate extraction–flame photometric method, respectively, with reference to the methods of Cheng and Ksenija [26,27].
2.4. Measurement of the Growth Indexes of the Potted Chrysanthemums
The survival rate of potted chrysanthemum cuttings was determined after 10 days of greenhouse planting, and the plant height, stem diameter, and crown width were determined every 10 d. Survival rate (%) = number of surviving plants/total number of plants × 100%. Plant height was measured via the straightedge method, stem diameter via the vernier caliper method, and crown width via straightedge using the cross method. After the chrysanthemums entered the reproductive growth stage, measurements of blooming period, inflorescence diameter, and inflorescence number were added to the experiment. The maximum fully open inflorescences were measured after the full blooming period with a straightedge using the cross method and the number of inflorescences was recorded via counting. The leaf width and leaf length of the plants were determined using a tape measure, and the leaf area was determined using a leaf area meter LI-3000C.
Samples were taken at full bloom, and chrysanthemum biomass-related indicators were determined. The same weighing method as before was used to determine the fresh mass and dry mass. Firstly, the shoot and root were separated to determine the fresh mass, and then, the dry mass was weighed after being fixed at 105 °C for 15 min and then dried in the oven at 70 °C until a constant weight was achieved.
The following formulas were used to calculate the root/shoot ratio, health index, and G value. Root/shoot ratio = Dry mass of root/Dry mass of shoot; Health index = (Stem diameter/Plant height + Dry mass of root/Dry mass of shoot) × Dry mass of plant; and G value = Dry mass of plant/Number of cutting days.
2.5. Measurement of the Physiological Indexes of the Potted Chrysanthemums
The samples were taken at the peak growth period of chrysanthemums, and the 3rd–5th functional leaves counted from the top of the plant were selected and stored in a refrigerator at −80 °C for measurement. The chlorophyll content was determined via acetone extraction; soluble protein content was determined with Coomassie brilliant blue G-250; and soluble sugar content was determined via the colorimetric method of anthrone [28,29,30].
2.6. Measurement of Photosynthetic Indexes
The net photosynthetic rate (Pn) was measured during the peak growth period with an Li-6400 (LiCor, Lincoln, NE, USA), using a red and blue light source leaf chamber set at a light intensity of 800 μmol/(m2·s), a temperature of 25 °C, and a flow rate of 500 mL/min.
2.7. Data Processing and Analysis
The data were organized and calculated using Excel 2013 and subjected to analysis of variance (ANOVA) using SPSS 26.0; one-way ANOVA was performed on the processed results and multiple comparisons were performed using Duncan’s method, a significance test was performed for each parameter at the 0.05 level, and cluster analysis graphs were plotted using Origin 2022.
The principal component analysis method was used to construct the minimum dataset index; the growth status of the potted chrysanthemums under each treatment substrate was evaluated by combining the membership function method; and then, the advantages and disadvantages of the cultivation substrate used in the potted chrysanthemums were compared. Then, we calculated the comprehensive evaluation index (CEI) of each species and each treatment according to Formulas (1)−(4), and the larger the CEI, the better the cutting–raising effect.
Zi = (X − Xmin)/(Xmax − Xmin),
Zi = 1 − (X − Xmin)/(Xmax − Xmin),
In the formula, n is the number of indicators; Wi and Zi are the weights and the value of the affiliation function of the ith indicator, respectively. X is the measured value of an indicator under a certain substrate condition, and Xmax and Xmin are the maximum and minimum values of the indicator measurement, respectively. Indicators positively correlated with growth performance were used to calculate affiliation values using Formula (3) and vice versa using Formula (4).
3. Results
3.1. Analysis of the Physical Properties of Different Formula Substrates
As shown in Table 2, replacing peat with leaf mold and rice husks significantly reduced the bulk density of the substrates (p < 0.05). The bulk densities of the seven substrates ranged from 0.13 to 0.40 g/cm3, all within the ideal range (0.1–0.8 g/cm3), with Q1–Q6 differing significantly from Q0 (p < 0.05). The Q5 and Q6 treatments had the lowest bulk density of 0.13 g/cm3, which was 67.5% lower than that of Q0. The total porosity of the seven substrates ranged from 71.93% to 74.98%, which was within the ideal range (54%–96%), and the Q2–Q4 treatments were all significantly (p < 0.05) different from Q0. The aeration porosity of each treatment group was significantly higher than Q0, and the water-holding porosity was significantly lower than Q0 (p < 0.05), with the largest difference in the Q6 treatment. The gas/water ratio of the seven substrates was higher than Q0 and differed significantly from Q0 (p < 0.05), with the highest one in the Q6 treatment, which was 9.9 times that of Q0, followed by the second highest one in Q5, which was 7.3 times that of Q0.

Table 2.
Physical properties of different formula substrates.
3.2. Analysis of the Chemical Properties of Different Formulated Substrates
As shown in Table 3, the pH values of the seven substrates were in the range of 5.06–7.12, except for the Q6 treatment, which was acidic, and all the treatment groups differed significantly from Q0 (p < 0.05). The EC values of the seven substrates ranged from 0.28 to 2.60 mS/cm, and all of them differed significantly from Q0, except for the Q1 and Q5 treatments (p < 0.05). Among them, the Q6 treatment had the highest, which was 9.3 times higher than that of Q0. Nitrogen, phosphorus, and potassium are very important nutrients for plant growth. In this experiment, the treatment group’s substrate had much higher amounts of alkaline nitrogen, available phosphorus, and available kalium potassium than the Q0 group. This meant that they could give plants more nutrients. The Q6 treatment had the highest nutrient content, with the contents of the three nutrients being 1.3, 12.2, and 32.6 times that of Q0, respectively.

Table 3.
Chemical properties of different formula substrates.
3.3. The Effects of Different Formulated Substrates on the Survival Rate of Potted Chrysanthemums
As shown in Table S1, the survival rate of three species of potted chrysanthemums in Q0, Q1, Q2, Q3, and Q5 treatments was 100%. The survival rate of all three potted chrysanthemums, C. morifolium “Huihuang”, “Boerduohong”, and “Huangfurong”, was the lowest in the Q6 treatment, which was 33.33%, 41.67%, and 27.27%, in that order.
3.4. The Effects of Different Formulated Substrates on the Plant Height of Potted Chrysanthemums
As shown in Figure 1, the Q1, Q2, and Q3 treatment groups promoted plant growth, and the Q5 treatment group significantly inhibited plant growth. When cultivated for 120 days, all varieties were the highest in the Q2 treatment, and in the Q1–Q5 treatments, the lowest plant height of the three potted chrysanthemums was the Q5 treatment.
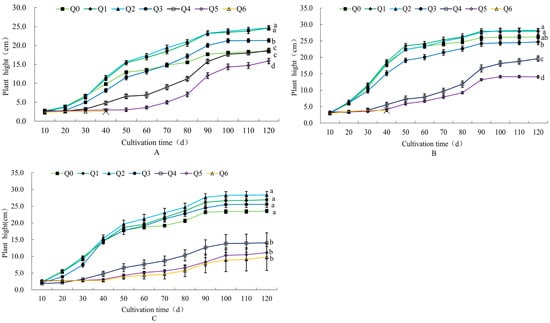
Figure 1.
Effects of different formula substrates on plant height of potted chrysanthemums. (A) Effect on “Huihuang” plant height; (B) effect on “Boerduohong” plant height; (C) effect on “Huangfurong” plant height. Different lowercase letters for the same species at the same time indicate significant differences between treatments (p < 0.05); “╳” indicates that plants died after this node.
3.5. The Effects of Different Formulated Substrates on the Stem Thickness of Potted Chrysanthemums
As shown in Figure 2, the order of the stem diameters of “Huihuang” and “Boerduohong” at 120 d of cultivation, from thick to thin, was Q2, Q3, Q1, Q0, Q4, and Q5. The order of the stem diameters of “Huangfurong”, from thick to thin, was Q2, Q1, Q3, Q0, Q4, Q5, and Q6. It can be seen that the stem diameters of the potted chrysanthemums in the three formulations of Q1–Q3 were better than those of Q0. The stem diameter of the three potted chrysanthemums was elevated by 22.3%, 37.8%, and 24.9%, respectively, in the Q2 treatment, while it was reduced by 26.1%, 44.6%, and 46.9%, respectively, in the Q5 treatment compared with Q0.
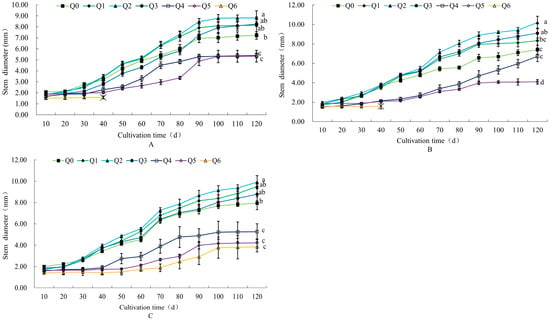
Figure 2.
Effects of different formula substrates on stem diameter of potted chrysanthemums. (A) Effect on “Huihuang” stem diameter; (B) effect on “Boerduohong” stem diameter; (C) effect on “Huangfurong” stem diameter. Different lowercase letters for the same species at the same time indicate significant differences between treatments (p < 0.05); “╳” indicates that plants died after this node.
3.6. The Effects of Different Formulated Substrates on the Crown Width of Potted Chrysanthemums
As can be seen from Figure 3, at 120 d of cultivation, the crown widths of the three potted chrysanthemums were all higher than Q0 in the Q1–Q4 treatments. Both “Huihuang” and “Boerduohong” had significantly higher crown widths in Q1–Q3 treatments than in Q0 (p < 0.05), and the highest ones were in the Q2 treatments, which were 39.3% and 38.4% higher than Q0, respectively. In addition, the crown width of “Huangfurong” in the Q2 treatment was significantly higher (by 16.8%) than that of Q0. In the same period, the crown widths of the three species of potted chrysanthemums in the Q5 treatment were significantly different from Q0: 17.0%, 30.7%, and 30.3% lower.
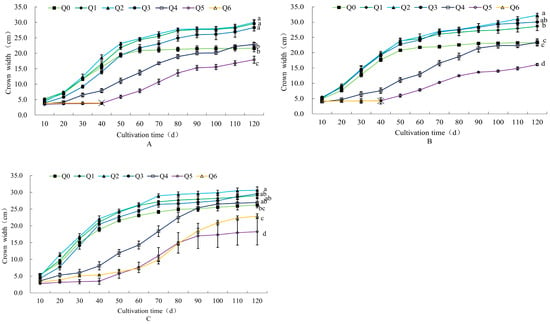
Figure 3.
Effects of different formula substrates on crown width of potted chrysanthemums. (A) Effect on “Huihuang” crown width; (B) effect on “Boerduohong” crown width; (C) effect on “Huangfurong” crown width. Different lowercase letters for the same species at the same time indicate significant differences between treatments (p < 0.05); “╳” indicates that plants died after this node.
3.7. The Effects of Different Formulated Substrates on the Leaf Morphology of Potted Chrysanthemums
As shown in A, B, and C in Figure 4, both “Huihuang” and “Boerduohong” had a higher leaf length, leaf width, and leaf area in Q1–Q4 treatments than in Q0, and the three indexes were lower in the Q5 treatment than in Q0. “Huihuang” had a higher leaf length, leaf width, and leaf area in all treatment groups than in Q0, especially the Q6 treatment, which had the greatest difference from Q0: 87.8%, 85.2%, and 240.1% higher, respectively. All the groups, except the Q5 treatment, had significant differences from Q0 (p < 0.05).
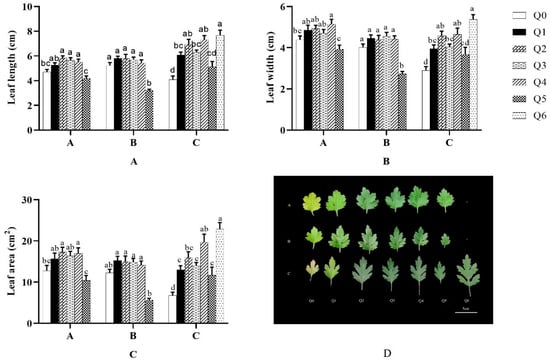
Figure 4.
Effects of different formula substrates on leaf morphology of potted chrysanthemums. (A) Effect on chrysanthemum leaf length; (B) effect on chrysanthemum leaf width; (C) effect on chrysanthemum leaf area; (D) effect on chrysanthemum leaf morphology. Different lowercase letters for the same species at the same time indicate significant differences between treatments (p < 0.05).
As shown in D in Figure 4, the leaves of the three potted chrysanthemums were clearly yellowish in Q0, yellow-green in the Q1 treatment, and dark green in the Q2–Q6 treatments. The leaves of the three potted chrysanthemums were small in the Q0 and Q5 treatments, and there were plants of “Huangfurong” surviving in the Q6 treatment with clearly enlarged leaves.
3.8. The Effects of Different Formulated Substrates on the Flowering of Potted Chrysanthemums
As shown in Figure 5, under different formulated substrate treatments, “Boerduohong” had the earliest blooming period, “Huihuang” was the second earliest, and “Huangfurong” was the latest. For each species, the blooming period was earlier in the Q0–Q3 treatments, later in the Q4 and Q5 treatments than in the Q0 treatment, and the latest in the Q5 treatment. Of the three potted chrysanthemums, only “Huangfurong” demonstrated plant survival in the Q6 treatment, but it failed to flower.
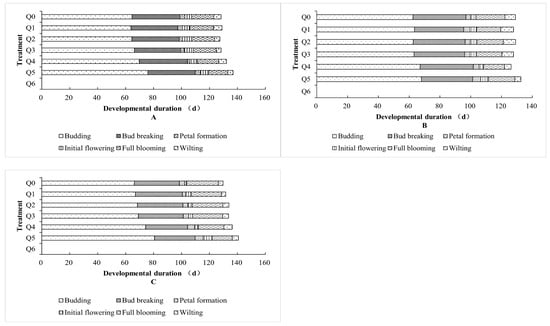
Figure 5.
Comparison of potted chrysanthemum development duration under different formula substrates. (A) Effect on “Huihuang” flowering; (B) effect on “Boerduohong” flowering; (C) effect on “Huangfurong” flowering.
As shown in A and B in Figure 6, the different formulated substrate treatments had significant effects on the flowering quality of the three potted chrysanthemums. The flower diameter of “Huihuang” in the Q1–Q5 treatments was higher than that in Q0 (9.98%, 12.06%, 16.42%, 6.65%, and 11.02%) and was the highest in the Q3 treatment; the amount of flower setting in the Q1–Q3 treatments was higher than that in Q0 (80.23%, 91.86%, and 75%) and was the highest and lowest in the Q5 treatment. The flower size of “Boerduohong” was significantly higher than that in Q0 only in the Q5 treatment (p < 0.05), while the amount of flower set was higher than that in Q0 in the Q1–Q3 treatments (127.01%, 200.43%, and 206.22%). In “Huangfurong”, the flower diameters in the Q1–Q5 treatments were all significantly greater than in Q0 (p < 0.05), but the differences between them were not significant (p > 0.05), and the amount of flowering was significantly greater than in Q0 in the Q2 treatment only (p < 0.05), which was 2.10 times that of Q0.
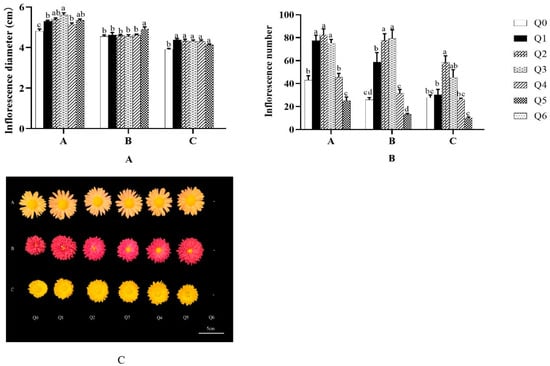
Figure 6.
Effects of different formula substrates on flowering of potted chrysanthemums. (A) Effect on chrysanthemum flower stems; (B) effect on chrysanthemum flower attachment; (C) effect on chrysanthemum flower morphology. Different lowercase letters for the same species at the same time indicate significant differences between treatments (p < 0.05).
As shown in C of Figure 6, there were some differences in the flowering of the three potted chrysanthemums under different substrates, with slightly lighter flowers in Q0 and slightly brighter flowers in the other treatments, as well as small, poorly formed flowers in Q0.
3.9. The Effects of Different Formulated Substrates on the Biomass, Cutting Strength Index, and G Value of Potted Chrysanthemums
In treatments Q1–Q3, the plant’s dry mass and the G value of “Huihuang” and “Boerduohong” were significantly higher than those in Q0 with different formulated substrates (p < 0.05), as shown in Table 4 and Table 5. “Huangfurong” was significantly higher in the Q1 treatment than in Q0, except for the root/shoot ratio (p < 0.05), and the dry mass of the plant, health index, and G value were significantly higher in the Q2 and Q3 treatments than in Q0 (p < 0.05). “Huihuang” and “Boerduohong” had a significantly lower dry mass of plant, health index, and G value in the Q5 treatment than in Q0 (p < 0.05), while “Huangfurong” did not differ significantly from Q0 (p > 0.05).

Table 4.
Effects of different formula substrates on the biomass of potted chrysanthemums.

Table 5.
Effects of different formula substrates on root/shoot ratio, health index, and G value of potted chrysanthemums.
3.10. The Effects of Different Formulated Substrates on the Physiological Indexes of Potted Chrysanthemums
As shown in Figure 7, “Huihuang” had the highest Pn rate in the Q1 treatment and the lowest in the Q6 treatment. The total chlorophyll content in the Q1–Q3 treatments was higher than in Q0 (28.42%, 15.78%, and 15.79%), with the Q1 treatment having the highest, while the carotenoid content in treatments Q1 and Q2 was higher than that in Q0 (23.07%). The soluble protein content in the Q2 treatment and the soluble sugar content in the Q3 treatment were the highest. In the Q6 treatment, the total chlorophyll, carotenoid, soluble protein, and soluble sugar contents were significantly lower than in Q0 (p < 0.05).
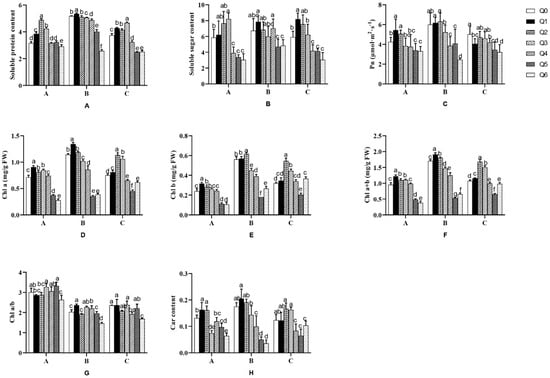
Figure 7.
Effects of different formula substrates on different physiological indicators of potted chrysanthemums. (A) Effect on chrysanthemum soluble protein; (B) effect on chrysanthemum soluble sugar; (C) effect on chrysanthemum net photosynthesis; (D) effect on chlorophyll a of chrysanthemums; (E) effect on chrysanthemum chlorophyll b; (F) effect on total chlorophyll of chrysanthemums; (G) effect on chrysanthemum chlorophyll a/b; (H) effect on chrysanthemum carotenoids. Different lowercase letters for the same species at the same time indicate significant differences between treatments (p < 0.05).
In the Q1 and Q2 treatments, the Pn rate of “Boerduohong” was higher than that in Q0, but the difference was not significant (p > 0.05). In treatments Q4–Q6, the Pn rate was significantly lower than that in Q0 (p < 0.05). Moreover, the total chlorophyll content was higher in treatments Q1 and Q2 than in Q0 (7.5% and 57%), the soluble protein content was higher in treatments Q1–Q3 than in Q0 (14.1%, 10.7%, and 24.53%), and all indexes except soluble sugar content were significantly lower in treatment Q6 than in Q0 (p < 0.05).
It was found that “Huangfurong” had significantly more total chlorophyll and soluble protein in treatments Q1–Q3 than in treatment Q0 (p < 0.05). Among them, the total chlorophyll content was highest in the Q2 treatment, which was 57.0% higher than in Q0. The soluble protein content was highest in the Q3 treatment, which was 24.5% higher than in Q0. However, all the indexes in the Q5 treatment were lower than in Q0.
3.11. Principal Component Analysis of the Growth Morphology and Physiological Indexes of Potted Chrysanthemums under Different Formulated Substrates
The results of the principal component analysis of 24 plant growth morphological and physiological indexes of each variety and each treatment, respectively, are shown in Table S2. The contribution rates of principal component 1 and principal component 2 in “Huihuang” were 75.077% and 11.835%, respectively, with a cumulative contribution rate of 86.912%. This indicates that these two principal components contain 86.912% of the information in the 24 indicators analyzed. In “Boerduohong”, the contribution rates of principal components 1 and 2 were 78.291% and 10.986%, respectively, and the cumulative contribution rate was 89.277%.
The contributions of principal component 1 and principal component 2 in “Huangfurong” were 67.383% and 18.813%, respectively, with a cumulative contribution of 86.197%. This indicates that these two principal components contain 86.197% of the information in the 25 indicators analyzed.
All three potted chrysanthemums had two principal components with eigenvalues greater than 1, which could reflect the response of potted chrysanthemum growth to different formulated substrates. From the principal component analysis, it can be seen that in “Huihuang”, the dry mass of the plant loaded the most in principal component 1, and the carotenoid content loaded the most in principal component 2. Therefore, the two indicators of plant dry mass and carotenoid content were included in the minimum dataset, which constituted the indicators for the comprehensive evaluation of the growth of potted chrysanthemums. In “Boerduohong”, the health index had the largest loading in principal component 1, and the full blooming period had the largest loading in principal component 2, which constituted the indexes for the comprehensive evaluation of potted chrysanthemum growth. In “Huangfurong”, the fresh mass of shoots and leaf width were screened to constitute the indexes for the comprehensive evaluation of potted chrysanthemum growth.
3.12. The Effects of Different Light-Renewable Substrates on the Growth of Potted Chrysanthemums
Figure S1 shows the growth effects of potted chrysanthemums under different light-renewable substrates. In Q0, the leaves of the potted chrysanthemums were yellow and the inflorescence was loose; in the Q1–Q3 treatments, the potted chrysanthemums’ growth was vigorous and the inflorescence was dense; and in the Q4–Q6 treatments, the growth was poor and the plants were short. The method of affiliation function (Table S3) evaluated the effect of potted chrysanthemums on each cultivation substrate for growth. The results showed that “Huihuang” had a composite evaluation index of 0.973, 0.991, and 0.792 in the Q1, Q2, and Q3 treatments, respectively, which were higher than that in Q0 (0.627). However, the Q4, Q5, and Q6 treatments had a composite evaluation index of 0.560, 0.276, and 0.000, respectively, which were lower than that of Q0. In the Q1–Q3 treatments, “Boerduohong” was also higher than in Q0, while for the Q4–Q5 treatments, it was lower than in Q0. For “Huangfurong”, all treatment groups were higher than Q0 with 0.324, except for the Q5 and Q6 treatments. The three potted chrysanthemum treatments, Q1, Q2, and Q3, all ranked above Q0 and can be used as ideal cultivation substrates. Among them all, the Q2 treatment had the highest comprehensive evaluation index and the best performance.
4. Discussion
As far as plant growth is concerned, the nature of the cultivation substrate is considered to be an important medium that directly influences the state of plant growth. Therefore, any medium used as a cultivation substrate should have specific physical properties with good permeability and water retention [31]. For example, forestry and agricultural waste reduces the substrate capacity compared to peat, and agroforestry waste decomposes easily. After decomposition, not only can the nutrients needed by plants be obtained, but also the permeability and water retention of the soil can be improved [32]. In this experiment, leaf mold and rice husk were added to optimize the aeration and water retention of the cultivation substrate while reducing the bulk density. Furthermore, when rice hulls were used as a cultivation substrate for growing peach trees, it was discovered that their high water retention not only increased the biomass and canopy ratio of the trees but also contributed to water conservation [33].
The external morphology and growth indexes of plants are usually two of the most intuitive indicators of plant growth and development. This study found that adding rice husk and dead leaves to the growing medium changed the dry weight, leaf area, and other characteristics of chrysanthemums. The addition of 20% rice husk and dead leaves had the most significant effects on the plant’s height, stem thickness, and leaf area. The biomass of the plant’s above- and below-ground parts also increased the most. These results may be due to the enhanced metabolism and increased photosynthetic capacity of the plant. In a study on Pinus halepensis M., it was found that peat/rice husk (7:3) promoted the morphological characteristics of seedlings such as shoots, plant height, and roots; in a study on stevia, it was also found that the addition of rice hulls and rotting leaves to the cultivation substrate increased the dry weight of the plant and promoted plant growth [17,18]. This suggests that suitable rice husk and leaf litter as a substitute for peat can promote plant growth.
Different plants exhibit different preferences for soil, making soil pH a crucial factor that directly influences plant growth [34]. Soil pH is not only an indicator of soil acidity and alkalinity but is also closely related to the effectiveness of nutrients needed for plant growth [35]. C. morifolium prefers slightly acidic-to-neutral conditions. In this study, all substrate formulations were in weakly acidic or neutral conditions. Meanwhile, it was found that with the reduction in peat, the pH and EC values of the cultivation substrates increased, which was the same as the results of a previous study [36]. It was also found that the appropriate pH value can effectively promote the effective utilization of nutrients such as N and P by plants, thus achieving sustainable plant production [37].
Seventeen elements have been identified as essential for plant growth, and they are categorized into major and minor elements based on the specific elemental requirements of each plant [38]. Among them, C, H, and O generally come from the air and water, and the rest of the elements originate from soil or fertilizers [39]. Moreover, plants’ demand for N, P, and K is almost constant throughout their life cycle [40]. Nevertheless, it is challenging to meet plants’ nutrient demands solely through the soil. Therefore, growers arefocused on increasing the content of N, P, and K in the cultivation substrate. In this study, the addition of rice husk and leaf mold to the cultivation substrate increased the content of alkaline nitrogen, available phosphorus, and rapidly available kalium, thereby reducing the use of chemical fertilizers and other additives. In addition, rice husk and leaf mold, as low-cost soil conditioners rich in mineral elements such as N, P, and K, can be added to the cultivation substrate to promote the ecological cycle. However, the plant’s own demand for nutrients is also a factor. When the ratio of elements is not appropriate, the plant shows symptoms such as dwarfing, dark green or yellow leaves, delayed maturity, flower and fruit drop, poor resistance, and even death [41,42]. In this study, it was found that the appropriate ratio in the substrate could prolong the flowering period of C. morifolium and make the plant enter the blooming period earlier. At the same time, C. morifolium in the Q6 treatment had a low survival rate and failed to enter the full bloom stage. This could be attributed to the excessive increase in potassium content, resulting in elevated EC values and leading to stress in chrysanthemums. Moreover, the study by He also proved that too much kalium fertilizer affects plant growth [43].
Chlorophyll, soluble sugar, and soluble protein contents in plants are important physiological and biochemical indicators. In this study, it was found that there were differences in the indexes among different species and different substrate ratios, but the Q3 and Q2 groups showed higher indexes than the rest; the results of the affiliation function also indicated that Q2 and Q3 were better than the other groups, and Q2 had the best performance. This may be inseparably related to the increased content of N, P, and K. Because nitrogen is an important component of chlorophyll and protein in plants, phosphorus is the main component of protein, phospholipids, and other substances, and kalium can promote sugar conversion and transportation [44,45,46,47,48]. Therefore, this shows that a reasonable substrate ratio can effectively improve the physical properties of the substrate, thus making organic substances accumulate in chrysanthemums. This is essentially consistent with the results of related studies, such as the effect of olive branch residues on potted olive cuttings and the effect of rice husk charcoal on azaleas [49,50].
5. Conclusions
In summary, a reasonable ratio of leaf mold and rice husk mixed with peat and perlite promotes the growth and development of Chrysanthemums. This demonstrates that 20% rice husk and leaf mold can replace peat as a substrate for the cultivation of chrysanthemums, providing a cost-effective cultivation option for countries facing peat scarcity. Simultaneously, adding leaf mold and rice husks significantly reduces the weight of the cultivation substrate, greatly facilitating the long-distance transportation of C. morifolium products and reducing transportation costs. However, the total replacement of peat with rice husk and rotting leaves remains a matter of inquiry. This will further reduce the weight of the substrate if it is completely replaced.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su152316137/s1, Table S1: Effects of different formula substrates on the survival rate of potted chrysanthemum; Table S2: Coefficient, eigenvalue and contribution rate of different indexes; Table S3: Comprehensive evaluation of growth effects of different formula substrates on potted chrysanthemum; Figure S1: Growth effect of potted chrysanthemum with different formula substrates. (A) Effect on ‘Huihuang’ growth; (B) Effect on ‘Boerduohong’ rowth; (C) Effect on ‘Huangfurong’ growth.
Author Contributions
L.Y. and Y.Z. designed the experiments; S.L., M.L., S.C., X.N. and K.Z. carried out the experiments and analyses; S.L. and L.Y. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2019YFD1001500).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to this manuscript can be obtained by contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fascella, G.; Mammano, M.M.; D’angiolillo, F.; Pannico, A.; Rouphael, Y. Coniferous wood biochar as substrate component of two containerized Lavender species: Effects on morpho-physiological traits and nutrients partitioning. Sci. Hortic. 2020, 267, 109356. [Google Scholar] [CrossRef]
- Farru, G.; Dang, C.H.; Schultze, M.; Kern, J.; Cappai, G.; Libra, J.A. Benefits and limitations of using hydrochars from organic residues as replacement for peat on growing media. Horticulturae 2022, 8, 325. [Google Scholar] [CrossRef]
- Rozas, A.; Aponte, H.; Maldonado, C.; Contreras-Soto, R.; Medina, J.; Rojas, C. Evaluation of Compost and Biochar as Partial Substitutes of Peat in Growing Media and Their Influence in Microbial Counts, Enzyme Activity and Lactuca sativa L. Seedling Growth. Horticulturae 2023, 9, 168. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Q.; Yang, R.; Lin, W.; Wang, H.; Zhou, W.; Qi, Z.; Ouyang, L. Slight carbonization as a new approach to obtain peat alternative. Ind. Crops Prod. 2023, 202, 117041. [Google Scholar] [CrossRef]
- Bao, K.; Liu, T.; Chen, M.; Lin, Z.; Zhong, J.; Neupane, B. Peat records of atmospheric environmental changes in China: A brief review and recommendations for future research perspectives. Catena 2023, 229, 107234. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Lempinen, H.; Vainio, A. Lost in transition: Peat workers’ experiences of Finland’s low carbon transition policies. Extr. Ind. Soc. 2023, 15, 101312. [Google Scholar] [CrossRef]
- Gupta, J.; Kumari, M.; Mishra, A.; Swati; Akram, M.; Thakur, I.S. Agro-forestry waste management—A review. Chemosphere 2022, 287, 132321. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop residue burning in India: Policy challenges and potential solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef]
- Yasin, M.; Jabran, K.; Afzal, I.; Iqbal, S.; Nawaz, M.A.; Mahmood, A.; Asif, M.; Nadeem, M.A.; Rahman, Z.U.; Adnan, M.; et al. Industrial sawdust waste: An alternative to soilless substrate for garlic (Allium sativum L.). J. Appl. Res. Med. Aromat. Plants 2020, 18, 100252. [Google Scholar] [CrossRef]
- Bagci, S.; Cayci, G.; Kütük, C. Growth of Primula plant in coir dust and peat-based growing media. J. Plant Nutr. 2011, 34, 909–919. [Google Scholar] [CrossRef]
- Gong, X.; Li, S.; Sun, X.; Wang, L.; Cai, L.; Zhang, J.; Wei, L. Green waste compost and vermicompost as peat substitutes in growing media for geranium (Pelargonium zonale L.) and calendula (Calendula officinalis L.). Sci. Hortic. 2018, 236, 186–191. [Google Scholar] [CrossRef]
- Bonaguro, J.E.; Coletto, L.; Zanin, G. Environmental and agronomic performance of fresh rice hulls used as growing medium component for Cyclamen persicum L. pot plants. J. Clean. Prod. 2017, 142, 2125–2132. [Google Scholar] [CrossRef]
- Kordi, M.; Farrokhi, N.; Pech-Canul, M.I.; Ahmadikhah, A. Rice Husk at a Glance: From Agro-Industrial to Modern Applications. Rice Sci. 2023. [Google Scholar] [CrossRef]
- Eastman, B.A.; Adams, M.B.; Peterjohn, W.T. The path less taken: Long-term N additions slow leaf litter decomposition and favor the physical transfer pathway of soil organic matter formation. Soil Biol. Biochem. 2022, 166, 108567. [Google Scholar] [CrossRef]
- González, I.; Sixto, H.; Rodríguez-Soalleiro, R.; Cañellas, I.; Fuertes, A.; Oliveira, N. How can leaf-litter from different species growing in short rotation coppice contribute to the soil nutrient pool? For. Ecol. Manag. 2022, 520, 120405. [Google Scholar] [CrossRef]
- Sardar, H.; Waqas, M.; Naz, S.; Ejaz, S.; Ali, S.; Ahmad, R. Evaluation of different growing media based on agro-industrial waste materials for the morphological, biochemical and physiological characteristics of stevia. Clean. Waste Syst. 2022, 3, 100038. [Google Scholar] [CrossRef]
- Berruti, A.; Scariot, V. Efficacy of flurprimidol and peat alternatives on growth control of potted camellias. N. Z. J. Crop Hortic. Sci. 2013, 41, 230–239. [Google Scholar] [CrossRef]
- Hadizadeh, H.; Samiei, L.; Shakeri, A. Chrysanthemum, an ornamental genus with considerable medicinal value: A comprehensive review. S. Afr. J. Bot. 2022, 144, 23–43. [Google Scholar] [CrossRef]
- Kaur, G.; Jhanji, S. Plant growth regulators preserved the longevity of cut stems of Chrysanthemum morifolium by orchestrating physio-biochemical and anatomical responses. Plant Physiol. Biochem. 2023, 196, 1098–1110. [Google Scholar] [CrossRef]
- Picchioni, G.A.; Martinez, S.A.; Mexal, J.G.; VanLeeuwen, D.M. Vegetative Growth and Leaf Nutrient Status of ‘Carpino’ Chrysanthemum on a Pecan Wood-amended Commercial Substrate. HortScience 2016, 51, 177–185. [Google Scholar] [CrossRef]
- Hidalgo, L.P.R. Vermicompost as a substrate amendment for poinsettia and chrysanthemum production. HortScience 2002, 37, 304–308. [Google Scholar] [CrossRef]
- Heng, T.; Hermansen, C.; de Jonge, L.W.; Chen, J.; Yang, L.; Zhao, L.; He, X. Differential responses of soil nutrients to edaphic properties and microbial attributes following reclamation of abandoned salinized farmland. Agric. Ecosyst. Environ. 2023, 347, 108373. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.; Luo, Y. Interactive effects of soil pH and substrate quality on microbial utilization. Eur. J. Soil Biol. 2020, 96, 103151. [Google Scholar] [CrossRef]
- Maltas, A.S.; Tavali, I.E.; Uz, I.; Kaplan, M. Monitoring the effects of pH and EC regulated drip fertigation on microbial dynamics of calcareous soil in tomato (Solanum lycopersicum L.) cultivation under greenhouse conditions in a Mediterranean climate. Sci. Hortic. 2022, 306, 111448. [Google Scholar] [CrossRef]
- Jakovljević, K.; Mišljenović, T.; Djordjević, V.; van der Ent, A.; Ćosić, M.; Andrejić, G.; Šinžar-Sekulić, J. Elemental and ecophysiological profiles of orchid Dactylorhiza sambucina show distinct responses to contrasting geological substrates. Flora 2023, 303, 152276. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, L.; Wang, X.; Ou, Y.; Liu, H.; Hou, X.; Yan, L.; Li, X. The various effect of cow manure compost on the degradation of imazethapyr in different soil types. Chemosphere 2023, 337, 139325. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; You, X.; Li, Y.; Long, S.; Wen, S.; Liu, Q.; Liu, T.; Guo, H.; Xu, Y. Hydrogen sulfide improves tall fescue photosynthesis response to low-light stress by regulating chlorophyll and carotenoid metabolisms. Plant Physiol. Biochem. 2022, 170, 133–145. [Google Scholar] [CrossRef]
- Kristensen, H.T.; Juul, L.; Amer, B.; Møller, A.H.; Danielsen, M.; Dalsgaard, T.K. Co-precipitation of red clover soluble protein with caseinate in the presence of antioxidant. LWT 2023, 182, 114895. [Google Scholar] [CrossRef]
- Van Dyck, I.; Vanhoudt, N.; i Batlle, J.V.; Horemans, N.; Van Gompel, A.; Nauts, R.; Vangronsveld, J. Effects of environmental parameters on starch and soluble sugars in Lemna minor. Plant Physiol. Biochem. 2023, 200, 107755. [Google Scholar] [CrossRef]
- Fan, H.-M.; Wang, X.-W.; Sun, X.; Li, Y.-Y.; Sun, X.-Z.; Zheng, C.-S. Effects of humic acid derived from sediments on growth, photosynthesis and chloroplast ultrastructure in chrysanthemum. Sci. Hortic. 2014, 177, 118–123. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Lordan, J.; Pascual, M.; Fonseca, F.; Villar, J.; Rufat, J. Use of rice husk to enhance peach tree performance in soils with limiting physical properties. Soil Tillage Res. 2013, 129, 19–22. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Z.; Li, X.; Shi, X.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z. Precipitation-mediated responses of plant biomass production and allocation to changing soil pH in semiarid grasslands. Agric. Ecosyst. Environ. 2022, 339, 108123. [Google Scholar] [CrossRef]
- Sultana, S.; Bell, R.W.; Vance, W.H. Genotypic variation among chickpea and wild Cicer spp. in nutrient uptake with increasing concentration of solution Al at low pH. Plant Physiol. Biochem. 2020, 157, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Delvasto, N.; Garcia-Ibañez, P.; Olmos-Ruiz, R.; Bárzana, G.; Carvajal, M. Substrate composition affects growth and physiological parameters of blueberry. Sci. Hortic. 2023, 308, 111528. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Yang, G.; Ding, C.; Lü, X. Increases in substrate availability and decreases in soil pH drive the positive effects of nitrogen addition on soil net nitrogen mineralization in a temperate meadow steppe. Pedobiologia 2021, 89, 150756. [Google Scholar] [CrossRef]
- Mahler, R.L. Nutrients plants require for growth. Iniversity of Idaho. Coll. Agric. Life Sci. CIS 2004, 1124, 1–4. [Google Scholar]
- FAO (Food and Agriculture Organization). Plant nutrition for food security: A guide for integrated nutrient management. In FAO Fertilizer and Plant Nutrient Bulletin 16; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Francis, B.; Aravindakumar, C.; Brewer, P.B.; Simon, S. Plant nutrient stress adaptation: A prospect for fertilizer limited agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar, M.; Cheema, K.L.; Rasool, I.; Zahid, M.A.; Hussain, A.; Qadeer, Z.; Khalid, M.J. Identification of operative dose of NPK on yield enhancement of desi and kabuli chickpea (Cicer arietinum L.) in diverse milieu. Saudi J. Biol. Sci. 2021, 28, 1063–1068. [Google Scholar] [CrossRef]
- Xu, X.; Liu, G.; Liu, J.; Lyu, M.; Wang, F.; Xing, Y.; Meng, H.; Li, M.; Jiang, Y.; Tian, G.; et al. Potassium Alleviated High Nitrogen-Induced Apple Growth Inhibition by Regulating Photosynthetic Nitrogen Allocation and Enhancing Nitrogen Utilization Capacity. Hortic. Plant J. 2023. [Google Scholar] [CrossRef]
- He, A.; Feng, J.; Yu, Q.; Jiang, J.; Ding, J.; Qian, K.; Tian, H. Enhanced phytotoxicity of 4-chloro-3-methyphenol and lindane under sodium and potassium salt stresses. Chemosphere 2023, 335, 139111. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, T.; Wen, X.; Liao, Y.; Liu, Y. Effect of potassium foliage application post-anthesis on grain filling of wheat under drought stress. Field Crops Res. 2017, 206, 95–105. [Google Scholar] [CrossRef]
- Niemiec, M.; Cupiał, M.; Szeląg-Sikora, A. Efficiency of Celeriac Fertilization with Phosphorus and Potassium Under Conditions of Integrated Plant Production. Agric. Agric. Sci. Procedia 2015, 7, 184–191. [Google Scholar] [CrossRef]
- Wu, K.; Hu, C.; Wang, J.; Guo, J.; Sun, X.; Tan, Q.; Zhao, X.; Wu, S. Comparative effects of different potassium sources on soluble sugars and organic acids in tomato. Sci. Hortic. 2023, 308, 111601. [Google Scholar] [CrossRef]
- Bingham, I.J.; Garzon, D.C. Relative contribution of soil N availability and grain sink demand to the control of post-anthesis N uptake by field-grown spring barley. Field Crops Res. 2023, 292, 108829. [Google Scholar]
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Endeshaw, S.T.; Lodolini, E.M.; Neri, D. Effects of olive shoot residues on shoot and root growth of potted olive plantlets. Sci. Hortic. 2015, 182, 31–40. [Google Scholar] [CrossRef]
- Bu, X.; Ji, H.; Ma, W.; Mu, C.; Xian, T.; Zhou, Z.; Wang, F.; Xue, J. Effects of biochar as a peat-based substrate component on morphological, photosynthetic and biochemical characteristics of Rhododendron delavayi Franch. Sci. Hortic. 2022, 302, 111148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).