Abstract
Rapid urbanization has substantially increased freshwater consumption and consequent wastewater generation. The produced wastewater is an abundant resource of phosphorus, nitrogen, and organics. Currently, well-established activated sludge processes are utilized in conventional wastewater treatment plants to remove organics. However, removing nitrogenous and phosphorus compounds continues to be challenging and energy-intensive for urban wastewater treatment plants. Therefore, the current study aims to understand how photosynthetic microalgae can recover phosphorus and nitrogen from urban wastewater and how wastewater-grown microalgae biomass may be used as a biofertilizer and biostimulant. Utilizing microalgae biomass treated with urban wastewater as a biofertilizer promotes plant growth in a manner similar to other organic manures and conventional fertilizers while minimizing nutrient loss to the soil. Furthermore, the microalgal recovery of nutrients from urban wastewater could have potential energy reductions of 47% and 240% for nitrogen and phosphorus, respectively. In addition to producing treated wastewater suitable for a variety of irrigation systems, microalgae biomass is a potential sustainable alternative resource that could reduce conventional inorganic fertilizer usage.
1. Introduction
Human population and food demand are predicted to grow by 60–100% by 2050, resulting in significant freshwater consumption. As a result of urbanization, industrialization, food security, and the need to support global economic growth, significant amounts of urban wastewater (UW) would be produced [1,2]. The generated UW comprises several organic, inorganic, and synthetic compounds, in addition to being an abundant resource of nitrogenous and phosphorus compounds [3,4,5,6]. Releasing the nitrogen and phosphorus-rich UW directly into the environment could result in eutrophication and the leaching of harmful pollutants into the soil and the air [7]. Therefore, wastewater treatment plants (WWTPs) are used to treat urban wastewater. Primary and secondary sludges are produced from the processing of urban wastewater. The combination of primary and secondary sludge is often further stabilized using an anaerobic digestion (AD) process. Methane is recovered as part of the proportion of organic carbon from the sludge [8]. Later, the anaerobically digested sludge is concentrated, and the liquid digestate is separated and returned to the WWTP’s treatment system, while the concentrated or thicker sludge, which is high in nitrogen and phosphorus, is dumped or landfilled in a designated disposal area [8]. WWTPs continue producing methane from wastewater sludge, even though aqueous phase reforming (APR) techniques are currently being studied to convert the oxygenated organic molecules in wastewater to hydrogen at milder operating conditions [9,10]. In addition to potentially contaminating groundwater and the soil when it is landfilled, the sludge could also be incinerated as a means of volume reduction [11]. Therefore, nitrogen and phosphorus from WWTPs continue to build up in the environment and act as a source of pollution for both air and water.
Furthermore, the concentrated sludge and supernatant that is recycled back into the wastewater treatment process contribute to the additional nitrogen and phosphorus that anaerobic digestion cannot remove. Therefore, photosynthetic microalgae could potentially be utilized to remove or lower the nitrogen and phosphorus from various streams that originate from WWTPs. In addition to being able to use atmospheric carbon dioxide (CO2), microalgae can generate biomass and high-value compounds (pigments and lipids) through the utilization of nitrogen (N) and phosphorus (P) in urban wastewater [12]. It has previously been shown that the microalgae could be successfully cultivated in open raceway ponds (ORPs), high-rate algal ponds (HRAPs), and different geometrically designed photobioreactors (PBRs) for achieving high biomass productivity and the simultaneous removal or reduction of N and P from several types of wastewater [13]. The microalgal biomass obtained through the phytoremediation of wastewater has undergone substantial research, mainly for producing gaseous and liquid biofuel and removing some recently emerging contaminants from urban wastewater [14,15]. However, the use of microalgae biomass cultivated in UWs as a potential feedstock for the production of biofertilizers and biostimulants needs more research. Additionally, phosphate and nitrogen fertilizers, which are often utilized in the form of urea and inorganic phosphorus forms, are needed by plants and crops. According to a report, the energy required to produce urea is around 30.1 GJ per ton [16]. The CO2 emissions for the production of N and P fertilizers comprise 2.1 tons of ammonia (NH3) and 0.343 tons of phosphorus pentoxide (P2O5), respectively [17]. The typical energy requirement for urban wastewater treatment is between 0.35 and 0.65 kWh/m3 or 1.26 and 2.34 MJ/m3 [18], whereas it takes 71,000 kWh/ton or 255.6 GJ/ton of energy to produce 1 ton of phosphorous at urban wastewater treatment plants combining sludge incineration and purification [19]. Thus, a potential strategy for recovering nitrogen and phosphorus from urban wastewater treatment facilities could involve the use of microalgae as a biofertilizer and biostimulant in soil and agricultural crops and plants. Therefore, one of the objectives of this review is to assess the potential of suitable microalgal strains to remove nitrogen and phosphate from urban wastewater. The review additionally studies the possibility of utilizing microalgae biomass subjected to urban wastewater treatment as a biofertilizer or biostimulant for various agricultural crops. The comparative energy economics of nitrogen and phosphorous are also studied, followed by a discussion of the potential and challenges associated with the use of microalgae-treated urban wastewater.
2. Potential Role of Microalgae in Conventional Urban Wastewater Treatment Process
Conventional WWTPs process wastewater by dividing it into a mainstream and a side stream [20]. The side streams could originate from the initial screening process or the primary clarifier just after the initial screening of the urban wastewater. The side streams are a rich source of N and P. However, the mainstream contains diluted N and P compounds and usually goes through an activated sludge process for the removal of water-soluble organics and a fraction of nitrogen and phosphorus. Later, the stream is passed on to the final clarifier to remove most of the biosolid before being discharged as a treated wastewater effluent (Figure 1). Thus, both the main and side streams finally result in effluents that could typically contain from 0.02 to 3 gL−1 N and from 0.002 to 0.4 gL−1 P [21,22]. As shown in Table 1, the urban wastewater originating from WWTPs in different countries contains 21 to 75 mgL−1 total nitrogen (TN), and 2.3 to 35.2 mgL−1 total phosphorus (TP).
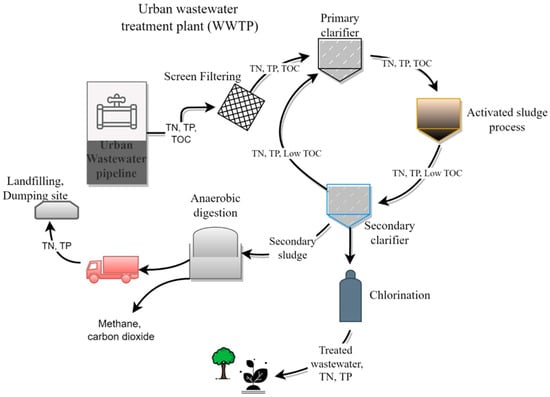
Figure 1.
The fate of total nitrogen (TN), total phosphorus (TP), and organic carbon as chemical oxygen demand (COD) in urban wastewater treatment plants.
UW contains varied amounts of total nitrogen and phosphorus (Table 1), even when it is collected and treated at different times inside the same WWTP. Urban wastewater has the potential to facilitate the growth of different species of photosynthetic microalgae. However, some forms of organic carbon and total nitrogen present in UW could also be taken up by microalgae in a mixotrophic mode, resulting in a higher microalgal biomass production that could eventually be utilized to produce not only biofuels but also biofertilizers or biostimulants [23]. The mechanisms by which various microalgae species take up nutrients and organic compounds like TN, TP, and COD from urban wastewater are briefly discussed in the section that follows.

Table 1.
Urban wastewater characteristics form different wastewater treatment plants (WWTPs).
Table 1.
Urban wastewater characteristics form different wastewater treatment plants (WWTPs).
| Country—Source | COD (ppm) | TN (ppm) | TP (ppm) | Reference |
|---|---|---|---|---|
| MWWTP, Zhoushan, China | 317.26 | 53.92 | 4.90 | [24] |
| WWTP, Koramangla region, South of Bangalore, India | 676 | 64.6 | 28 | [25] |
| MWWTP Wonju, South Korea | 591 | 56 | 15.8 | [26] |
| Greek Wastewater Treatment Plants (WWTPs), Greece | 528 | 57 | 11 | [27] |
| WWTP, Verona, Italy | 390.5 | 52.3 | 5.24 | [28] |
| WWTP, El Ejido, Spain | 3744 | 52 | 11 | [29] |
| WWTP, Beijing, China | 301.4 | 81.9 | 8.03 | [30] |
| WWTP, Mikkeli, Finland | 730.2 | 61.24 | 11.07 | [31] |
| WWTP, Rishikesh, Uttarakhand, India | 496 | 24.45 | 9.6 | [32] |
| WWTP, Santa Rosa Jauregui, Queretaro, Mexico | 517 | 86 | 43 | [33] |
| WWTP, Nestle Ghana Ltd., Ghana | 2388 | 65.6 | 35.2 | [34] |
| WWTP, Saint Paul, Minnesota, USA | 484.8 | 44.9 | 7.9 | [23] |
| WWTP, Turkey | 375 | 46 | 4.9 | [35] |
Nutrient Removal Mechanism from Urban Wastewater by Microalgae
The microalgal removal efficiency of nitrogenous and phosphate-containing components from urban wastewater is reliant on their concentrations and ratio and biotic and abiotic factors affecting microalgal growth [12,36]. Previous studies have shown that for high nitrogen and phosphate removal, as well as for maximal microalgal biomass productivity, the N/P ratio should fall between 5 and 30 [37]. As indicated in Table S1 (see Supplementary Materials), the biomass densities were found to be higher for N/P ratios between 15 and 20. The low biomass density for Scenedesmus sp. (Meyen) could be due to the low nitrogen concentration in the UW and the low light intensity for the flask culture [38]. Nevertheless, urban wastewater is usually a rich source of nutrients for microalgal cultivation.
The typical nitrogen and phosphorus contents in microalgae biomass with a biomass density of 0.5 gL−1 are 0.005–0.05 mg/L and 0.0005–0.003 mg/L, respectively [39,40]. As the microalgae grow in the wastewater, the cells consume these elements to produce their biomass; therefore, the higher the microalgal biomass density, the higher the recovery rates for nitrogen and phosphorus would be. However, the growth of any microalga and its biomass concentration in the wastewater would also depend on the concentration and types of nutrients (TN, TP, etc.), among other abiotic factors. Table 2 shows the potential of different microalgal strains in removing or recovering nitrogen and phosphorus from different UWs. The nitrogen and phosphorus in UW are consumed by microalgae for producing proteins, carbohydrates, and lipids containing biomass. Arthrospira platensis and Scenedesmus sp. cultivated in urban wastewater had protein contents of 42.6 and 32.6%, respectively, and carbohydrate contents of 32.4 and 22.8% [41]. In the absence of any external stress conditions, cyanobacterial species like Arthrospira platensis have been reported to produce 60% proteins; under stress or limiting conditions, proteins can be reduced, but lipid production is still lower than it is for microalgal strains like Scenedesmus sp., which tend to produce more lipids and carbohydrates [42]. Microalgal biomass acts as a source of carbon, nitrogen, phosphorus, and trace metals like iron, magnesium, zinc, and calcium [43]. Mixotrophic microalgae, such as Scenedesmus sp. and Tetraselmis sp. (H.J. Carter), assimilate the organic carbon and inorganic carbonate in the wastewater to yield biomass at concentrations ranging from 0.5 to 0.7 g/L [23,44].

Table 2.
(a) Nitrogen recovery by various microalgae from urban wastewaters. (b) Phosphorus recovery by various microalgae from urban wastewaters.
By utilizing the total nitrogen, total phosphorus, and organic carbon present in urban wastewaters, microalgae have shown the potential for generating biomass rich in macro- and microelements that later could be used as biofertilizers or biostimulants for various plants or crop production. Additionally, it was reported earlier that microalgae are suitable feedstock for biofertilizer applications and contain more nitrogen, phosphorus, and potassium (NPK) than conventional organic fertilizers. The following sections briefly explain how urban wastewater-cultivated biomass can be used as a possible raw material for biostimulant and biofertilizer applications.
3. Comparative Energy Assessment for Nitrogen and Phosphorus Recovery from Urban Wastewater Using Microalgae
In 2019, the global consumption levels of nitrogen and phosphorus fertilizers were 132 tons and 71 tons (as P2O5), respectively [17]. Moving forward, the need for these fertilizers is expected to increase to a compound annual growth rate (CAGR) of 0.6–1.5% to meet the growing demand for food and feed [17]. By the year 2030, the volume of global wastewater generation is expected to reach 470 billion m3, with average N and P concentrations of 43.7 and 7.8 mg/L, respectively [52]. Therefore, there is a huge potential to recycle N and P from UW, which could reduce the need for manufacturing fresh fertilizers and their associated environmental emissions.
The tertiary wastewater treatment process of conventional nitrification–denitrification and phosphorus removal from urban wastewater utilizes 8 to 16 kWh per capita per year in urban WWTPs [53,54]. Activated anaerobic sludge removal from the activated aerobic digestion sludge process was reported to require 2.3 kWh of energy per kilogram of N removed, and the recently discovered anammox process, which oxidizes ammonium under anaerobic conditions, requires 0.9 kWh of energy per kg of N [55,56]. The reported specific energy consumption for high-rate algal ponds (HRAPs) with biofertilizer production was reported to be 0.08 kWh/m3 to 0.1 kWh/m3 [57,58,59,60]. Depending on the nitrogen concentration (e.g., 65 mg/L) in the wastewater and the nitrogen content in microalgal strains (5%), the energy required for the efficient removal of nitrogen from urban wastewater using HRAPs is 1.23 kWh per kg N, which is 47% less energy than that previously reported, i.e., 2.3 kWh per kg N by the conventional process in the urban wastewater treatment plant.
The energy needed for conventional phosphorous removal in urban WWTPs to directly recover phosphorus and reuse it as fertilizer in agricultural fields was reported as 71 kWh per kg P [19]. Another study reported that by utilizing HRAPs, phosphorus could be removed from urban wastewater with an energy consumption of 0.23 kWh/m3 [61]. If the TP in urban wastewater is 7.8 ppm [45], then 1 m3 of wastewater would contain 7.8 g of TP; consequently, the reported energy usage as per the above-mentioned energy consumption of 0.23 kWh/m3 would be 29.4 kWh per kilogram P for phosphorus removal using HRAPs. Nonetheless, the energy required is 2.4 times less than the earlier reported 71 kWh/kgP for conventional extraction in WWTPs and reusing it as fertilizer in agriculture. Furthermore, HRAPs will simultaneously recover N and P as microalgal biomass while treating the urban wastewater. In addition, the energy needed to recover P from incinerated wastewater treatment sludge ash ranges from 45 to 70 kWh/kg P, which is substantially more than the energy used by HRAPs to recover 1 kg of phosphorus [19].
4. Valorization of Phycoremediated Microalgae Biomass from Urban Wastewater as Fertilizer
In addition to utilizing inorganic nitrogen and phosphate, microalgae can oxidize several organic substances, such as acetate, butyric acid, glucose (C6H12O6), and acetic acid (CH3COOH) present in UW. Several microalgae could mixotrophically and heterotrophically use these organics to produce their biomass [62,63]. On the contrary, most of the photosynthetic microalgae use inorganic carbon (e.g., carbonate) to produce their biomass. Microalgal biomass that are produced in different types of wastewater have undergone substantial research for the generation of biofuels, high-value chemicals, fish, and animal feeds [64,65]; compared to the above-mentioned products, urban wastewater cultivated microalgae biomass as a potential biofertilizer or biostimulant need to be studied more. The following section discusses the role of microalgae as a potential biofertilizer and biostimulant.
4.1. Microalgal Soil Fertilizers
As shown in Figure 2, the N- and P-rich microalgae biomass acts as a slow-release fertilizer in soil [66,67,68]. Additionally, it has been shown that the nitrogen content released by soil microalgae does not exceed the nutrient requirements of plants, minimizing the eutrophication of ground and surface waters brought on by the overuse and loss of conventional fertilizers [69,70]. Furthermore, it has been shown that phycoremediated NPK-rich microalgae biomass substantially improved corn and baby spinach crop yields, as it possesses NPK quantities greater than those found in vermicompost, de-oiled cakes, and waste slurries from biogas plants [71]. Microalgae biomass cultivated in urban wastewater rich in N and P could be directly used as soil fertilizers, as this could reduce the dependence on conventional fertilizers [72]. Various crops have recently been cultivated in urban and peri-urban areas to enhance food security, reduce poverty, and sustainable waste management and resource recovery [73]. Phycoremediated microalgae biomass could also be used as a biofertilizer in crops cultivated in urban or peri-urban areas [74]. In addition to having high NPK concentrations (Table 3), microalgae species have been found to contain substantial quantities of phytohormones [75,76].
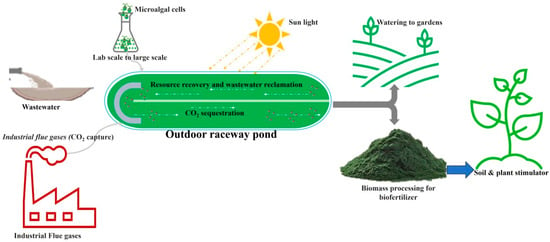
Figure 2.
Urban wastewater cultivated microalgae biomass as biofertilizer.

Table 3.
Potential of urban wastewater-cultivated microalgae biomass as soil fertilizer and organic fertilizer.
In a different study, the amounts of NPK (%) in farmyard manure combined with biochar for enhancing low-fertility tropical soil were 1.5, 0.0007, and 0.16, respectively. The values for the UW microalgae biomass were substantially higher in NPK content than those for the farmyard manure, as indicated in Table 3. [80]. Another study showed that palm oil mill effluent manure had N and P values (3.5 and 1.5) that were similar to certain microalgal biomass N and P values but relatively low compared to the N and P reported in some UW-cultivated microalgal biomass. [81]. Maize yield and soil fertility in Cameroon were shown to be significantly increased by the use of palm oil effluent manure in combination with other fertilizers. The UW-cultivated microalgae biomass may not only have low freshwater footprints but also be able to improve low-fertility soils and increase the yields of a variety of crops (Table 3), [81]. As depicted in Table 3, compared to potassium in organic fertilizers, the potassium level in wastewater-cultivated microalgae biomass is lower; this could be due to the microalgae requiring less potassium relative to nitrogen and phosphorus [70,82]. Although it has been shown that microalgae possess sufficient NPK to be utilized as biofertilizers, recent findings have revealed that microalgae may adsorb microplastics in UW, and this could negatively affect microalgal growth [83]. Additionally, the adsorbed microplastic may enter the soil during biofertilizer applications, but the effects of microplastic-adsorbed microalgal biofertilizers on crop productivity are still unclear and require extensive research [84].
4.2. Phytohormones Identification in Different Microalgae and Its Effect on Higher Plants
Urban wastewater-cultivated microalgae and cyanobacterial biomass have also been reported to contain a wide range of phytohormones, such as auxins, cytokinins, abscisic and gibberellic acids, ethylenes, brassinosteroids, jasmonates, and salicylic acid (Table 4) [85]. However, some microalgae species grown in urban wastewater have been reported to accumulate heavy metals [86]. As a result, the analysis of microalgal biomass before use in agriculture becomes a prerequisite, even though microalgae contain phytohormones that have been reported to have beneficial effects (Table 4) on plants [87].

Table 4.
Potential of various microalgae (Eukaryotes) and cyanobacteria (Prokaryotic microalgae) as phytohormone producers.
Extracting phytohormones from various microalgae and delivering them as foliar extracts (Figure 3) might be beneficial for the plants [119]. Tomato plant height and the number of flowers and branches can be effectively increased by applying the extracts of Tetradesmus dimorphus (Turpin) MJ Wyne as foliar sprays at 50% concentrations ranging from 0.35 to 3.75 gL−1 biomass [120].
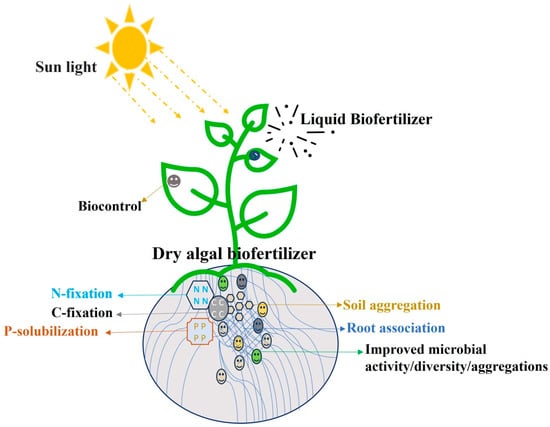
Figure 3.
Role of dry and liquid microalgal biofertilizer in soil and as foliar extracts on plants.
When foliar sprays of protein hydrolysates of Arthrospira sp. were sprayed on Gardenia petunia, the foliar extract stimulated enhanced root growth and increased the flowers per plant since it contains amino acids that are known to be biostimulants [76]. Another study found that spraying lettuce with Arthrospira sp. hydrolysate enhanced spermin content and promoted growth [121]. For producing microalgal biofertilizers and other biostimulants, using freshwater and commercial fertilizers would be counterproductive compared to their cultivation in nutrient-rich wastewater [119].
5. Applications of Microalgal-Treated Wastewater (MTW)
Urban wastewater from domestic households is considered important nowadays, with some countries, such as Malta and Cyprus, utilizing approximately 90 to 60% of treated effluent water due to the scarcity of freshwater [122]. In contrast, water-scarce countries, such as Israel and Jordan, utilize more than 90% of their treated urban wastewater for irrigation [123]. In existing urban WWTPs, the generated wastewater is subjected to tertiary wastewater treatment to remove microbiological contaminants, lower nitrogenous and phosphate compounds for reduced eutrophication potential, and have less adverse effects on the environment before being discharged for irrigation, fertigation, or disposal in areas other than eutrophication-sensitive zones. The conventional tertiary treatment is energy-intensive and generates greenhouse gases (CO2, ammonia, nitrous oxides, methane from nitrifying and denitrifying reactions, and sludge) in the removal of nitrogenous waste from UW, primarily ammonia and phosphate [124]. In this context, utilizing microalgae as a resource rather than a conventional tertiary treatment could lower the release of greenhouse gases and lead to the production of microalgae biomass, which can be used as biostimulants and fertilizers, as described in earlier sections.
Several PBRs and high-rate algal ponds (HRAPs) have been designed and implemented to lower energy costs, enhance biomass productivity, and lower eutrophying compounds [125,126].
Urban wastewater that microalgae have treated can be used as irrigation for containment areas, meadows, and terraces [126]. Escherichia coli Escherich contamination is the primary barrier to the reuse of wastewater treated by microalgae [127]. According to previous studies, there are certain approaches that can be used to minimize the presence of E. coli, such as the utilization of PBRs and ORPs, with high light penetration to attain maximum ultraviolet (UV) in the growth system to lower bacterial growth, as well as the cultivation of microalgae strains in high pH to help make wastewater unfavorable for bacterial growth [128]. The chemical and physical characteristics of wastewater treated by microalgae and prospective uses in a variety of sectors are shown in Table 5.
Although microalgae-treated wastewater has the potential to be utilized for irrigation purposes (Table 5), some requirements must be fulfilled before such treated wastewater can be used for irrigation. Some important water parameters required by the Food and Agriculture Organization (FAO) are soil bulletin water discharge criteria and FAO irrigation guidelines [129]. It was reported that increasing the microalgal biomass in urban wastewater might effectively lower the salinity because increased soil salinity and total dissolved solids (TDS) in UW could cause soil to become sodic [130]. As a result, UW that has undergone microalgal treatment is appropriate for fertigation or irrigation applications [131,132,133]. Even though the nutrient levels are reduced and the salinity is decreased, before being used for fertigation or irrigation after microalgal treatment, the presence of bacterial species, such as E. coli, intestinal nematodes, certain antibiotic-resistant bacteria, antibiotic-resistant genetic material, and several organic and toxic pharmaceutical compounds, may need to be maintained as per the prescribed regulation standards.

Table 5.
Microalgae-treated urban wastewater quality and potential applications.
Table 5.
Microalgae-treated urban wastewater quality and potential applications.
| Microalgae Strain | Cultivation System | Water Quality Parameters | Application Area | Reference |
|---|---|---|---|---|
| Microalgal natural consortium | Green dune PBR | TN-14 mgL−1, turbidity—29, E. coli—671, TP—1.8 mgL−1, NH4+—0.7 mgL−1 TSS—76.1 mgL−1 | Agriculture usage low/D * and usage low/E * Portugal classification areas | [126] |
| Chlorella vulgaris | 14 L transparent cylindrical PBR | pH—8.01, TN—12 mgL−1, TDS—3852 ppm, PO4—0.4 mgL−1, E. coli—n.a., EC—6.02 | Irrigation of low-quality soil for castor oil crop cultivation | [131] |
| Chlorella sp., Scenedesmus sp. | HRAP + wetlands | COD—73 ppm, TN—32 ppm, TP—2 ppm, TSS—24 ppm, turbidity—15 NTU | Irrigation of golf courts and municipal garden areas | [60] |
| Chlorella minutissima | PBR | pH—8.5, TDS—136 ppm, NH4+—2.9 mgL−1, DO—8.1 ppm, EC—0.25 dS/m | Suitable for crop irrigation | [132] |
| Chlorococcum sp., Oscillatoria sp., Scenedesmus sp., Phacus and Chlorella sps. | Conical open microalgal pond | pH—6.9, TDS—688, NH4+—7.6, TSS—4 | Amaranthus crop irrigation | [133] |
| Mixed microalgal consortia | HRAP combined with DAF and solar disinfection | Suspended solids 2.1–20 ppm, turbidity—9.6 NTU, Salmonella—absent, nematodes < 1 | Fit for agricultural use | [134] |
* Urban wastewater classification.
6. Life Cycle Impact Assessment (LCIA) of Microalgae Biofertilizer Production Using Urban Wastewater
Few recent LCIA studies on wastewater-cultivated microalgae biomass as biofertilizers have been published, despite this being a promising strategy for achieving sustainability through a circular bioeconomy. A recent life cycle assessment (LCA) assessed the environmental impacts of UW-cultivated biomass proposed for use as biofertilizers [59,135]. According to the LCA study, UW contains micropollutants, heavy metals, and pathogens that need to be regulated and would require social acceptance in order to function as UW-cultivated microalgal biofertilizers [59,135]. In contrast to conventional microalga cultivation, which has been shown to require more chemicals, and therefore, have higher impact values than using urban wastewater-cultivated microalgae biomass as biofertilizers, microalgae’s use of N and P from urban wastewater reduces negative environmental impacts and promotes a circular bioeconomy [59,135]. Additionally, another LCA study showed that utilizing microalgae cultivated in urban waste water in HRAPs as a biofertilizer directly was more cost-effective than using energy from biomethane cogeneration and residual digestate as a biofertilizer [59]. Additionally, the LCA study reported that, when compared to UW-cultivated microalgae biomass using HRAPs for biofertilizer applications, conventional activated sludge processes, from which the sludge is transported, incinerated, or landfilled, have significantly higher impact categories related to climate change, ozone layer depletion, fossil depletion, and photochemical oxidant formation [59].
7. Future Prospects and Challenges
- In order to lower the presence of heavy metals for biofertilizer applications, microalgal strains capable of complexing and precipitating heavy metals from UW must be developed.
- Microplastics that may contaminate soil or enter food crops may be present in certain microalgal biomass; hence, microalgal biofertilizers made from microalgae grown in UW must be thoroughly assessed for the presence of microplastics [83].
- Microalgal phytohormones need to be identified, and the phytohormone production in microalgal strains grown in UW needs to be improved to produce larger phytohormone yields, as opposed to using microalgal extracts directly on plants [5].
- Future studies should place greater emphasis on growing microalgae in HRAPs rather than PBRs. Additionally, when microalgae remove eutrophicating elements, CO2 might be used to compensate for the carbon removed during the secondary wastewater treatment step [136].
- In case the microalgae biomass needs to be separated, self-settling strains, such as Scenedesmus obliquus and Chroococcidiopsis sp., must be used for wastewater treatment, as this would eliminate the energy-consuming preliminary dewatering process [137,138,139]. Microalgal biomass harvested using inorganic metal-based and organic coagulants could interfere with their applications as biofertilizers [12]. The self-settled strain may then be centrifuged, sundried, and used as biofertilizer; alternatively, the phytohormones may be recovered from the centrifuged biomass and used as a biostimulant for plant growth.
- Additionally, microalgal co-cultivation systems need to be integrated into existing WWTPs. This requires support from governments and research organizations.
8. Conclusions
Nitrogenous, phosphorus, and organic substances that contribute to eutrophication can be found in different amounts in the process streams of traditional wastewater treatment facilities. The N/P ratios of different urban wastewaters are suitable for cultivating different microalgal species and converting eutrophying N and P compounds into protein, carbohydrate, and lipid-rich biomass. Compared to other organic manures, the microalgae biomass from treated UW has a higher NPK content, which, when used as soil fertilizers, improves crop productivity and reduces the potential for eutrophication caused by conventional inorganic fertilizers. In addition to possessing NPK, microalgae biomass from treated UW also contains numerous kinds of phytohormones or biostimulants that can stimulate the growth of specific crop parts. Additionally, microalgal-treated UW can be used for various irrigation or fertigation activities to increase crop yield after being disinfected to reduce the microbial load and meet certain FAO and European Union discharge criteria. Finally, it can be stated that using traditional procedures to remove nitrogenous and phosphate compounds from wastewater in WWTPs is far more expensive and energy-intensive than using microalgae to remove N and P from UW in HRAPs. This review concludes with future directions for a faster and more widespread acceptance of the utilization of microalgae as a sustainable resource for treating UW and producing biofertilizers and biostimulants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su152216019/s1. Table S1. Effect of N/P ratios on biomass densities of various microalgal strains cultivated in urban wastewaters.
Author Contributions
S.K.: Conceptualization, methodology, data curation, visualization, writing original draft. M.T.: data curation, writing original draft. M.A.: data curation, writing original draft M.F.: data curation. S.M.: data curation, writing original draft, visualization; M.A.A.A.-N.: funding acquisition, review, editing; H.A.-J.: review and editing, P.D.: conceptualization, methodology supervision, writing original draft, review and editing, project administration, funding acquisition All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Qatar University grant number IRCC-2022-537.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Avagyan, A.B. A contribution to global sustainable development: Inclusion of microalgae and their biomass in production and bio cycles. Clean Technol. Environ. Policy 2008, 10, 313–317. [Google Scholar] [CrossRef]
- Liu, X.; Beusen, A.H.W.; van Puijenbroek, P.J.T.M.; Zhang, X.; Wang, J.; van Hoek, W.J.; Bouwman, A.F. Exploring wastewater nitrogen and phosphorus flows in urban and rural areas in China for the period 1970 to 2015. Sci. Total Environ. 2024, 907, 168091. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Yang, Y.; Sonne, C.; Chen, X.; Yue, X.; Gu, H.; Lam, S.S.; Peng, W. Phytosphere purification of urban domestic wastewater. Environ. Pollut. 2023, 336, 122417. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-González, A.; Greque de Morais, E.; Planas-Carbonell, A.; Uggetti, E. Enhancing sustainability through microalgae cultivation in urban wastewater for biostimulant production and nutrient recovery. Sci. Total Environ. 2023, 904, 166878. [Google Scholar] [CrossRef] [PubMed]
- Teklehaimanot, G.Z.; Kamika, I.; Coetzee, M.A.A.; Momba, M.N.B. Population Growth and Its Impact on the Design Capacity and Performance of the Wastewater Treatment Plants in Sedibeng and Soshanguve, South Africa. Environ. Manag. 2015, 56, 984–997. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Abeyratne, W.M.L.K.; Bayat, H.; Delanka-Pedige, H.M.K.; Zhang, Y.; Brewer, C.E.; Nirmalakhandan, N. Multi-criteria evaluation of energy recovery from urban wastewater sludges by anaerobic digestion and hydrothermal liquefaction. J. Environ. Chem. Eng. 2023, 11, 109628. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Tito, E.; Zoppi, G.; Pipitone, G.; Miliotti, E.; Fraia, A.D.; Rizzo, A.M.; Pirone, R.; Chiaramonti, D.; Bensaid, S. Conceptual design and techno-economic assessment of coupled hydrothermal liquefaction and aqueous phase reforming of lignocellulosic residues. J. Environ. Chem. Eng. 2023, 11, 109076. [Google Scholar] [CrossRef]
- Achkir, A.; Aouragh, A.; El Mahi, M.; Lotfi, E.M.; Labjar, N.; EL Bouch, M.; Ouahidi, M.L.; Badza, T.; Farhane, H.; EL Moussaoui, T. Implication of sewage sludge increased application rates on soil fertility and heavy metals contamination risk. Emerg. Contam. 2023, 9, 100200. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; Abdulquadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Leong, Y.K.; Huang, C.-Y.; Chang, J.-S. Pollution prevention and waste phycoremediation by algal-based wastewater treatment technologies: The applications of high-rate algal ponds (HRAPs) and algal turf scrubber (ATS). J. Environ. Manag. 2021, 296, 113193. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Nanda, M.; Pruthi, V.; Chauhan, P.K. Small-scale phyco-mitigation of raw urban wastewater integrated with biodiesel production and its utilization for aquaculture. Bioresour. Technol. 2020, 297, 122489. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgal-based remediation of wastewater: A step towards environment protection and management. Environ. Qual. Manag. 2022, 32, 105–123. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Thaher, M.I.; AbdulQuadir, M.; Mahata, C.; Al Jabri, H. Utilization of nitrogen-rich agricultural waste streams by microalgae for the production of protein and value-added compounds. Curr. Opin. Green Sustain. Chem. 2023, 41, 100797. [Google Scholar] [CrossRef]
- Daramola, D.A.; Hatzell, M.C. Energy Demand of Nitrogen and Phosphorus Based Fertilizers and Approaches to Circularity. ACS Energy Lett. 2023, 8, 1493–1501. [Google Scholar] [CrossRef]
- Siatou, A.; Manali, A.; Gikas, P. Energy Consumption and Internal Distribution in Activated Sludge Wastewater Treatment Plants of Greece. Water 2020, 12, 1204. [Google Scholar] [CrossRef]
- Sylwan, I.; Zambrano, J.; Thorin, E. Energy demand for phosphorus recovery from municipal wastewater. Energy Procedia 2019, 158, 4338–4343. [Google Scholar] [CrossRef]
- Sheikh, M.; Harami, H.R.; Rezakazemi, M.; Cortina, J.L.; Aminabhavi, T.M.; Valderrama, C. Towards a sustainable transformation of municipal wastewater treatment plants into biofactories using advanced NH3-N recovery technologies: A review. Sci. Total Environ. 2023, 904, 166077. [Google Scholar] [CrossRef]
- Metcalf, W.; Eddy, C. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw Hill Education: New York, NY, USA, 2014; ISBN 978-0-07-340118-8. [Google Scholar]
- Sedlak, R. Phosphorus and Nitrogen Removal from Municipal Wastewater; Routledge: London, UK, 2018. [Google Scholar]
- Devi, N.D.; Sun, X.; Ding, L.; Goud, V.V.; Hu, B. Mixotrophic growth regime of novel strain Scenedesmus sp. DDVG I in municipal wastewater for concomitant bioremediation and valorization of biomass. J. Clean. Prod. 2022, 365, 132834. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-Y.; Zhao, Q.-L.; Chen, D.-Z.; Li, C.; Liu, M.; Yang, J.-S.; Liu, J.-Z.; Ge, Y.-M.; Chen, J.-M. Mixotrophic cultivation of microalgae coupled with anaerobic hydrolysis for sustainable treatment of municipal wastewater in a hybrid system of anaerobic membrane bioreactor and membrane photobioreactor. Bioresour. Technol. 2021, 337, 125457. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Bioremediation and lipid synthesis through mixotrophic algal consortia in municipal wastewater. Bioresour. Technol. 2014, 168, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Kabra, A.N.; Salama, E.-S.; Roh, H.-S.; Kim, J.R.; Lee, D.S.; Jeon, B.-H. Effect of mine wastewater on nutrient removal and lipid production by a green microalga Micratinium reisseri from concentrated municipal wastewater. Bioresour. Technol. 2014, 157, 84–90. [Google Scholar] [CrossRef]
- Koutsou, O.P.; Gatidou, G.; Stasinakis, A.S. Domestic wastewater management in Greece: Greenhouse gas emissions estimation at country scale. J. Clean. Prod. 2018, 188, 851–859. [Google Scholar] [CrossRef]
- Basset, N.; Katsou, E.; Frison, N.; Malamis, S.; Dosta, J.; Fatone, F. Integrating the selection of PHA storing biomass and nitrogen removal via nitrite in the main wastewater treatment line. Bioresour. Technol. 2016, 200, 820–829. [Google Scholar] [CrossRef]
- Posadas, E.; del Mar Morales, M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem. Eng. J. 2015, 265, 239–248. [Google Scholar] [CrossRef]
- Fan, Z.; Zeng, W.; Meng, Q.; Liu, H.; Ma, C.; Peng, Y. Achieving partial nitrification, enhanced biological phosphorus removal and in-situ fermentation (PNPRF) in continuous-flow system and mechanism analysis at transcriptional level. Chem. Eng. J. 2022, 428, 131098. [Google Scholar] [CrossRef]
- Kaur, P.; Park, Y.; Minami, I.; Imteaz, M.A.; Khan, M.A.; Al-Othman, A.A.S.; Alothman, Z.A.; Sillanpää, M.; Li, Y. Photoelectrocatalytic treatment of municipal wastewater with emerging concern pollutants using modified multi-layer catalytic anode. Chemosphere 2023, 339, 139575. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Kumar, A.; Bharti, R.K. An integrated approach for phycoremediation of municipal wastewater and production of sustainable transportation fuel using oleaginous Chlorella sp. J. Water Process Eng. 2021, 42, 102183. [Google Scholar] [CrossRef]
- Buitrón, G.; Coronado-Apodaca, K.G. Influence of the solids retention time on the formation of the microalgal-bacterial aggregates produced with municipal wastewater. J. Water Process Eng. 2022, 46, 102617. [Google Scholar] [CrossRef]
- Adonadaga, M.-G. Nutrient removal efficiency of activated sludge plants treating industrial and municipal wastewater in Ghana. J. Environ. Pollut. Hum. Health 2014, 2, 58–62. [Google Scholar]
- Isik, O.; Cengiz, A.I.; Abdelrahman, A.M.; Ozcelik, K.; Yuksekdag, A.; Koyuncu, I.; Ersahin, M.E.; Ozgun, H.; Demir, I. Impact of different inoculum sources on the performance of membrane bioreactors for municipal wastewater treatment: Dynamic membrane versus ultrafiltration membrane. J. Water Process Eng. 2022, 46, 102549. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Lage, S.; Toffolo, A.; Gentili, F.G. Microalgal growth, nitrogen uptake and storage, and dissolved oxygen production in a polyculture based-open pond fed with municipal wastewater in northern Sweden. Chemosphere 2021, 276, 130122. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hu, H.-Y.; Jia, Y. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.; AbdulQuadir, M.; Khan, S.; Chaudhary, A.; Al-Jabri, H. Long-term semi-continuous cultivation of a halo-tolerant Tetraselmis sp. using recycled growth media. Bioresour. Technol. 2019, 276, 35–41. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.I.; Hoekman, K.; Hawari, A.H. A comparison of bio-crude oil production from five marine microalgae–Using life cycle analysis. Energy 2022, 251, 123954. [Google Scholar] [CrossRef]
- Fan, H.; Wang, K.; Wang, C.; Yu, F.; He, X.; Ma, J.; Li, X. A comparative study on growth characters and nutrients removal from wastewater by two microalgae under optimized light regimes. Environ. Technol. Innov. 2020, 19, 100849. [Google Scholar] [CrossRef]
- Gao, L.; Ding, W.; Xi, J.; Gao, S.; Zhou, X.; Chen, Y.; Song, K.; Mao, X.; Tu, R.; Jiang, G. Effects of different nitrogen/phosphorus ratios on the growth and metabolism of microalgae Scenedesmus obliquus cultured in the mixed wastewater from primary settling tank and sludge thickener. Process Saf. Environ. Prot. 2023, 170, 824–833. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Wang, F. Mixotrophic Cultivation of Microalgae for Biodiesel Production: Status and Prospects. Appl. Biochem. Biotechnol. 2014, 172, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Slompo, N.D.M.; Quartaroli, L.; Fernandes, T.V.; da Silva, G.H.R.; Daniel, L.A. Nutrient and pathogen removal from anaerobically treated black water by microalgae. J. Environ. Manag. 2020, 268, 110693. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Quadir, M.A.; Thaher, M.I.; Alghasal, G.; Aljabri, H. Microalgal nutrients recycling from the primary effluent of municipal wastewater and use of the produced biomass as bio-fertilizer. Int. J. Environ. Sci. Technol. 2019, 16, 3355–3364. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.G.; Mehmood, M.A. A novel wastewater-derived cascading algal biorefinery route for complete valorization of the biomass to biodiesel and value-added bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Kiran, B.; Pathak, K.; Kumar, R.; Deshmukh, D. Cultivation of Chlorella sp. IM-01 in municipal wastewater for simultaneous nutrient removal and energy feedstock production. Ecol. Eng. 2014, 73, 326–330. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. In Natural Resources Forum; Blackwell Publishing Ltd.: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- Carrera, J.; Carbó, O.; Doñate, S.; Suárez-Ojeda, M.E.; Pérez, J. Increasing the energy production in an urban wastewater treatment plant using a high-rate activated sludge: Pilot plant demonstration and energy balance. J. Clean. Prod. 2022, 354, 131734. [Google Scholar] [CrossRef]
- Henze, M. Wastewater treatment: Biological and chemical processes. Choice Rev. Online 1996, 11, 383. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Qin, M.; Mao, Z.; Yuan, L.; Niu, Q.; Tan, X. Effects of temperature on anammox performance and community structure. Bioresour. Technol. 2018, 260, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Albuquerque, A.; Lisboa, I.; Murray, P.; Ermis, H. Economic Assessment of Energy Consumption in Wastewater Treatment Plants: Applicability of Alternative Nature-Based Technologies in Portugal. Water 2022, 14, 2042. [Google Scholar] [CrossRef]
- Garfí, M.; Flores, L.; Ferrer, I. Life Cycle Assessment of wastewater treatment systems for small communities: Activated sludge, constructed wetlands and high rate algal ponds. J. Clean. Prod. 2017, 161, 211–219. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Montero, N.; Ferrer, I.; Acién, F.G.; Gómez, C.; Garfí, M. Life cycle assessment of high rate algal ponds for wastewater treatment and resource recovery. Sci. Total Environ. 2018, 622, 1118–1130. [Google Scholar] [CrossRef]
- Saúco, C.; Cano, R.; Marín, D.; Lara, E.; Rogalla, F.; Arbib, Z. Hybrid wastewater treatment system based in a combination of high rate algae pond and vertical constructed wetland system at large scale. J. Water Process Eng. 2021, 43, 102311. [Google Scholar] [CrossRef]
- Robles, Á.; Capson-Tojo, G.; Gales, A.; Viruela, A.; Sialve, B.; Seco, A.; Steyer, J.-P.; Ferrer, J. Performance of a membrane-coupled high-rate algal pond for urban wastewater treatment at demonstration scale. Bioresour. Technol. 2020, 301, 122672. [Google Scholar] [CrossRef]
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Fendri, I.; et al. Chapter 12—Nonconventional treatments of agro-industrial wastes and wastewaters by heterotrophic/mixotrophic cultivations of microalgae and Cyanobacteria. In Valorization of Microalgal Biomass and Wastewater Treatment; Bandh, S.A., Malla, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–260. ISBN 978-0-323-91869-5. [Google Scholar]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Abdul Quadir, M.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation 2022, 8, 316. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Abdul Quadir, M.; Thaher, M.I.; Mahata, C.; Sayadi, S.; Al-Jabri, H. Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective. Fermentation 2023, 9, 281. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as Bio-fertilizers for Rice Growth and Seed Yield Productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Joshi, N.V.; Ramachandra, T.V.; Murthy, G.S. Algae-Based Biofertilizers: A Biorefinery Approach. In Microorganisms for Green Revolution: Volume 7: Microbes for Sustainable Agro-Ecosystem; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Das, P.; Khan, S.; Chaudhary, A.K.; AbdulQuadir, M.; Thaher, M.I.; Al-Jabri, H. Potential Applications of Algae-Based Bio-fertilizer. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.-S., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 41–65. ISBN 978-3-030-18933-4. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Ferrer, I.; Díez-Montero, R. Can microalgae grown in wastewater reduce the use of inorganic fertilizers? J. Environ. Manag. 2022, 323, 116224. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Bhattacharyya, R.; Sharma, A.; Gupta, D.K.; Kishore, P.; Gupta, N. Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. J. Environ. Manag. 2021, 287, 112295. [Google Scholar] [CrossRef]
- La Bella, E.; Baglieri, A.; Fragalà, F.; Puglisi, I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy 2022, 12, 234. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef]
- González, I.; Herrero, N.; Siles, J.Á.; Chica, A.F.; Ángeles Martín, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains1. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Sharma, G.K.; Malla, F.A.; Kumar, A.; Rashmi; Gupta, N. Microalgae based biofertilizers: A biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J. Clean. Prod. 2019, 211, 1412–1419. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different organic manure sources and NPK fertilizer on soil chemical properties, growth, yield and quality of okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef]
- Apori, S.O.; Byalebeka, J.; Murongo, M.; Ssekandi, J.; Noel, G.L. Effect of co-applied corncob biochar with farmyard manure and NPK fertilizer on tropical soil. Resour. Environ. Sustain. 2021, 5, 100034. [Google Scholar] [CrossRef]
- Ngone, M.A.; Ajoacha, D.M.-B.; Achiri, D.T.; Tchakounté, G.V.T.; Ruppel, S.; Tening, A.S.; Ngosong, C. Potential of bio-organic amendment of palm oil mill effluent manure and plant growth-promoting bacteria to enhance the yield and quality of maize grains in Cameroon. Soil Secur. 2023, 11, 100090. [Google Scholar] [CrossRef]
- Gupte, Y. Uptake of potassium by algae and potential use as biofertilizer. Indian J. Plant Physiol. 2015, 20, 285–288. [Google Scholar] [CrossRef]
- Cao, T.N.-D.; Mukhtar, H.; Le, L.-T.; Tran, D.P.-H.; Ngo, M.T.T.; Pham, M.-D.-T.; Nguyen, T.-B.; Vo, T.-K.-Q.; Bui, X.-T. Roles of microalgae-based biofertilizer in sustainability of green agriculture and food-water-energy security nexus. Sci. Total Environ. 2023, 870, 161927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef]
- Priya, A.K.; Jalil, A.A.; Vadivel, S.; Dutta, K.; Rajendran, S.; Fujii, M.; Soto-Moscoso, M. Heavy metal remediation from wastewater using microalgae: Recent advances and future trends. Chemosphere 2022, 305, 135375. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Maróti, G.; Ljung, K.; Turečková, V.; Strnad, M.; Ördög, V.; Van Staden, J. Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Mazur, H.; Konop, A.; Synak, R. Indole-3-acetic acid in the culture medium of two axenic green microalgae. J. Appl. Phycol. 2001, 13, 35–42. [Google Scholar] [CrossRef]
- Mazhar, S.; Cohen, J.D.; Hasnain, S. Auxin producing non-heterocystous Cyanobacteria and their impact on the growth and endogenous auxin homeostasis of wheat. J. Basic Microbiol. 2013, 53, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Krischke, M.; Roitsch, T.; Hasnain, S. Rapid determination of cytokinins and auxin in cyanobacteria. Curr. Microbiol. 2010, 61, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, E.; Liaimer, A.; Bergman, B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002, 215, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Čarná, M.; Repka, V.; Skůpa, P.; Šturdík, E. Auxins in defense strategies. Biologia 2014, 69, 1255–1263. [Google Scholar] [CrossRef]
- Lu, Y.; Tarkowská, D.; Turečková, V.; Luo, T.; Xin, Y.; Li, J.; Wang, Q.; Jiao, N.; Strnad, M.; Xu, J. Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga N annochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J. 2014, 80, 52–68. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Sakakibara, H. CYTOKININS: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Hartung, W.; Gimmler, H. Abscisic acid content of algae under stress. Bot. Acta 1989, 102, 326–334. [Google Scholar] [CrossRef]
- Maršálek, B.; Zahradníčková, H.; Hronková, M. Extracellular Abscisic Acid Produced by Cyanobacteria under Salt Stress. J. Plant Physiol. 1992, 139, 506–508. [Google Scholar] [CrossRef]
- Hartung, W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010, 37, 806–812. [Google Scholar] [CrossRef]
- Zahradníčková, H.; Maršálek, B.; Polišenská, M. High-performance thin-layer chromatographic and high-performance liquid chromatographic determination of abscisic acid produced by cyanobacteria. J. Chromatogr. A 1991, 555, 239–245. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Hanada, K.; Hase, T.; Toyoda, T.; Shinozaki, K.; Okamoto, M. Origin and evolution of genes related to ABA metabolism and its signaling pathways. J. Plant Res. 2011, 124, 455–465. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Mukai, M.; Hirata, K.; Miyamoto, K. Induction of tolerance to oxidative stress in the green alga, Chlamydomonas reinhardtii, by abscisic acid. Plant Cell Environ. 2003, 26, 451–457. [Google Scholar] [CrossRef]
- Kreslavsky, V.D.; Kobzar, E.F.; Muzafarov, E.N. Effect of red radiation, kinetin and linuron on growth and ethylene production in Chlorella. Biol. Plant. 1997, 39, 427–430. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Hormones and hormone-like substances of microorganisms: A review. Appl. Biochem. Microbiol. 2006, 42, 229–235. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plant. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; Van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.B.; Agarwal, P.R. Extraction, isolation, and bioassay of a gibberellin-like substance from Phormidium foveolarum. Ann. Bot. 1973, 37, 737–741. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Oklestkova, J.; Rárová, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and Metabolism of Brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef]
- Ueda, J.; Miyamoto, K.; Aoki, M.; Hirata, T.; Sato, T.; Momotani, Y. Identification of Jasmonic Acid in Chlorella and Spirulina. Bull. Univ. Osaka Prefect. Ser. B Agric. Biol. 1991, 43, 103–108. [Google Scholar]
- Ueda, J.; Miyamoto, K.; Sato, T.; Momotani, Y. Identification of jasmonic acid from euglena gracilis z as a plant growth regulator. Agric. Biol. Chem. 1991, 55, 275–276. [Google Scholar] [CrossRef]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci. 2010, 178, 307–311. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Gruz, J.; Strnad, M.; Ördög, V.; van Staden, J. Effect of gibberellins on growth and biochemical constituents in Chlorella minutissima (Trebouxiophyceae). S. Afr. J. Bot. 2019, 126, 92–98. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; González-López, C. Microalgae based wastewater treatment coupled to the production of high value agricultural products: Current needs and challenges. Chemosphere 2022, 291, 132968. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Unesco; UN-Water; World Water Assessment Programme. The United Nations World Water Development Report 2017: Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017; ISBN 9789231002014. [Google Scholar]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Tredici, M.R. Mass production of microalgae: Photobioreactors. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: Hoboken, NJ, USA, 2004. [Google Scholar]
- de Morais, E.G.; Amaro Marques, J.C.; Cerqueira, P.R.; Dimas, C.; Sousa, V.S.; Gomes, N.; Ribau Teixeira, M.; Nunes, L.M.; Varela, J.; Barreira, L. Tertiary urban wastewater treatment with microalgae natural consortia in novel pilot photobioreactors. J. Clean. Prod. 2022, 378, 134521. [Google Scholar] [CrossRef]
- Farhadkhani, M.; Nikaeen, M.; Yadegarfar, G.; Hatamzadeh, M.; Pourmohammadbagher, H.; Sahbaei, Z.; Rahmani, H.R. Effects of irrigation with secondary treated wastewater on physicochemical and microbial properties of soil and produce safety in a semi-arid area. Water Res. 2018, 144, 356–364. [Google Scholar] [CrossRef]
- Bacellar Mendes, L.B.; Vermelho, A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels 2013, 6, 152. [Google Scholar] [CrossRef]
- FAO. Climate-Smart Agriculture: Agriculture: Policies, Practices and Financing for Food Security, Adaptation and Mitigation; Food and Agriculture Organization: Rome, Italy, 2010. [Google Scholar]
- Jalali, M.; Merikhpour, H.; Kaledhonkar, M.J.; Van Der Zee, S.E.A.T.M. Effects of wastewater irrigation on soil sodicity and nutrient leaching in calcareous soils. Agric. Water Manag. 2008, 95, 143–153. [Google Scholar] [CrossRef]
- Reda, M.M.; El-Sayed, A.E.K.B.; Almutairi, A.W.; Hassoub, M.A. Fatty acid profiles and fuel properties of oils from castor oil plants irrigated by microalga-treated wastewater. Egypt. J. Bot. 2020, 60, 797–804. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Gupta, N.; Kumar, S.; Malav, L.C.; Nogiya, M.; Dubey, S.K. Bioremediatìon of sewage wastewater through microalgae (Chlorellaminutissima). Indian J. Agric. Sci. 2020, 90, 2024–2028. [Google Scholar] [CrossRef]
- Jothieswari, M.; Prabhakaran, N.; Krithika, A.; Swarnalatha, S. Reuse of Treated Domestic Sewage for Irrigation Purposes Using the Algal-based Treatment System. Water. Air. Soil Pollut. 2023, 234, 464. [Google Scholar] [CrossRef]
- Gutiérrez-Alfaro, S.; Rueda-Márquez, J.J.; Perales, J.A.; Manzano, M.A. Combining sun-based technologies (microalgae and solar disinfection) for urban wastewater regeneration. Sci. Total Environ. 2018, 619–620, 1049–1057. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Josa, I.; Ferrer, I.; Van Hulle, S.W.H.; Rousseau, D.P.L.; Garfí, M. Life cycle assessment of microalgae systems for wastewater treatment and bioproducts recovery: Natural pigments, biofertilizer and biogas. Sci. Total Environ. 2022, 847, 157615. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Thevarajah, B.; Nimarshana, P.H.V.; Prajapati, S.K.; Ariyadasa, T.U. Real-time integration of microalgae-based bioremediation in conventional wastewater treatment plants: Current status and prospects. J. Water Process Eng. 2023, 56, 104248. [Google Scholar] [CrossRef]
- Xu, X.; Lin, X.; Lin, J.; Wu, Y.; Zhao, Z.; He, Q.; Wu, Y.; Yang, J. Using the solution of self-settling microalgae Scenedesmus obliquus as processing medium for hydrothermal liquefaction of biomass. Biomass Bioenergy 2023, 173, 106784. [Google Scholar] [CrossRef]
- Das, P.; Quadir, M.A.; Chaudhary, A.K.; Thaher, M.I.; Khan, S.; Alghazal, G.; Al-Jabri, H. Outdoor continuous cultivation of self-settling marine cyanobacterium Chroococcidiopsis sp. Ind. Biotechnol. 2018, 14, 45–53. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Khan, S.; AbdulQuadir, M.; Chaudhary, A.K.; Alghasal, G.; Al-Jabri, H. Comparison of biocrude oil production from self-settling and non-settling microalgae biomass produced in the Qatari desert environment. Int. J. Environ. Sci. Technol. 2019, 16, 7443–7454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).