Impact of Excess Magnesium Salt Supply on Rice Yield, Physiological Response, and Grain Mineral Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial 1: Biomass and Yield Analysis
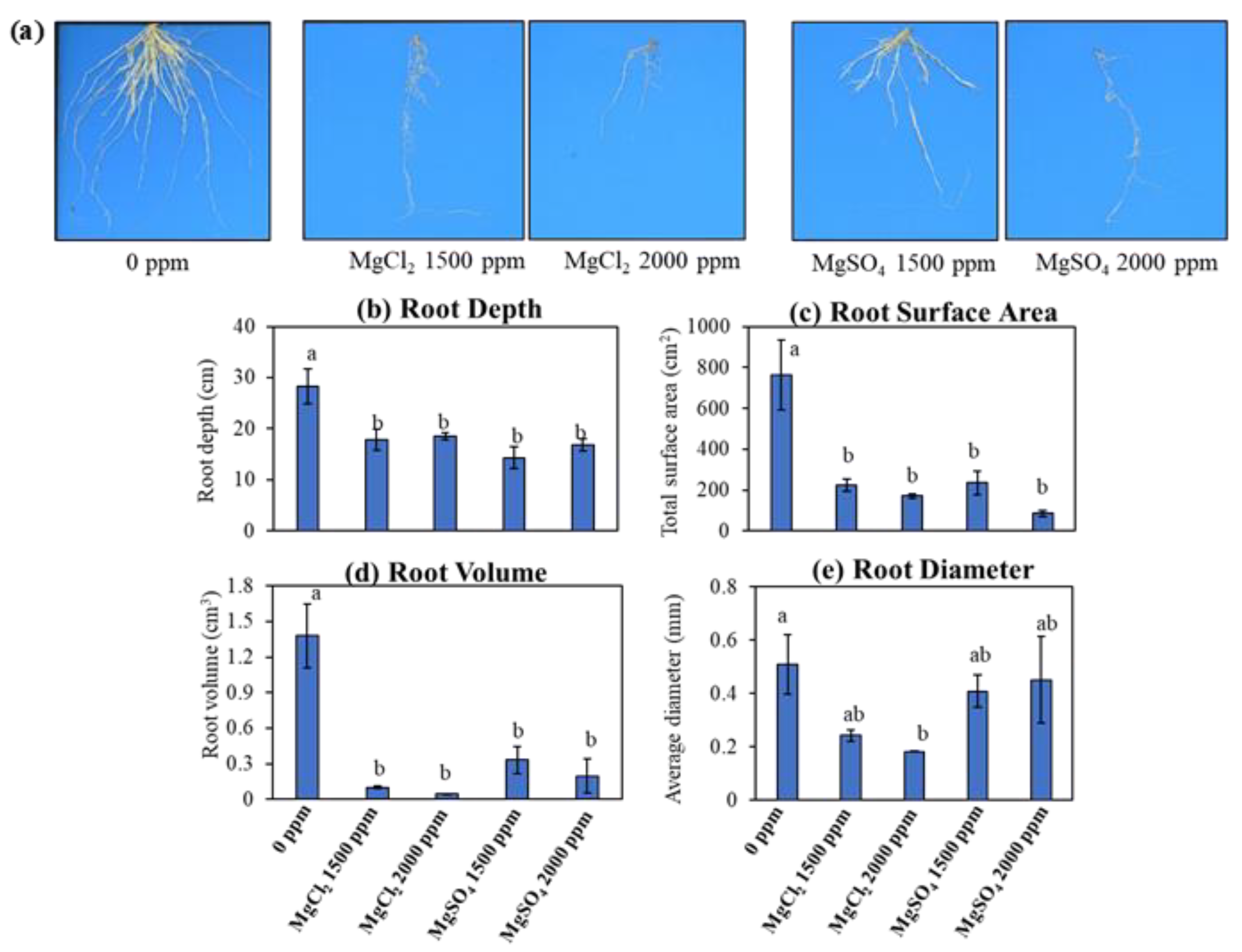
2.2. Trial 2: Root Morphology Anlaysis
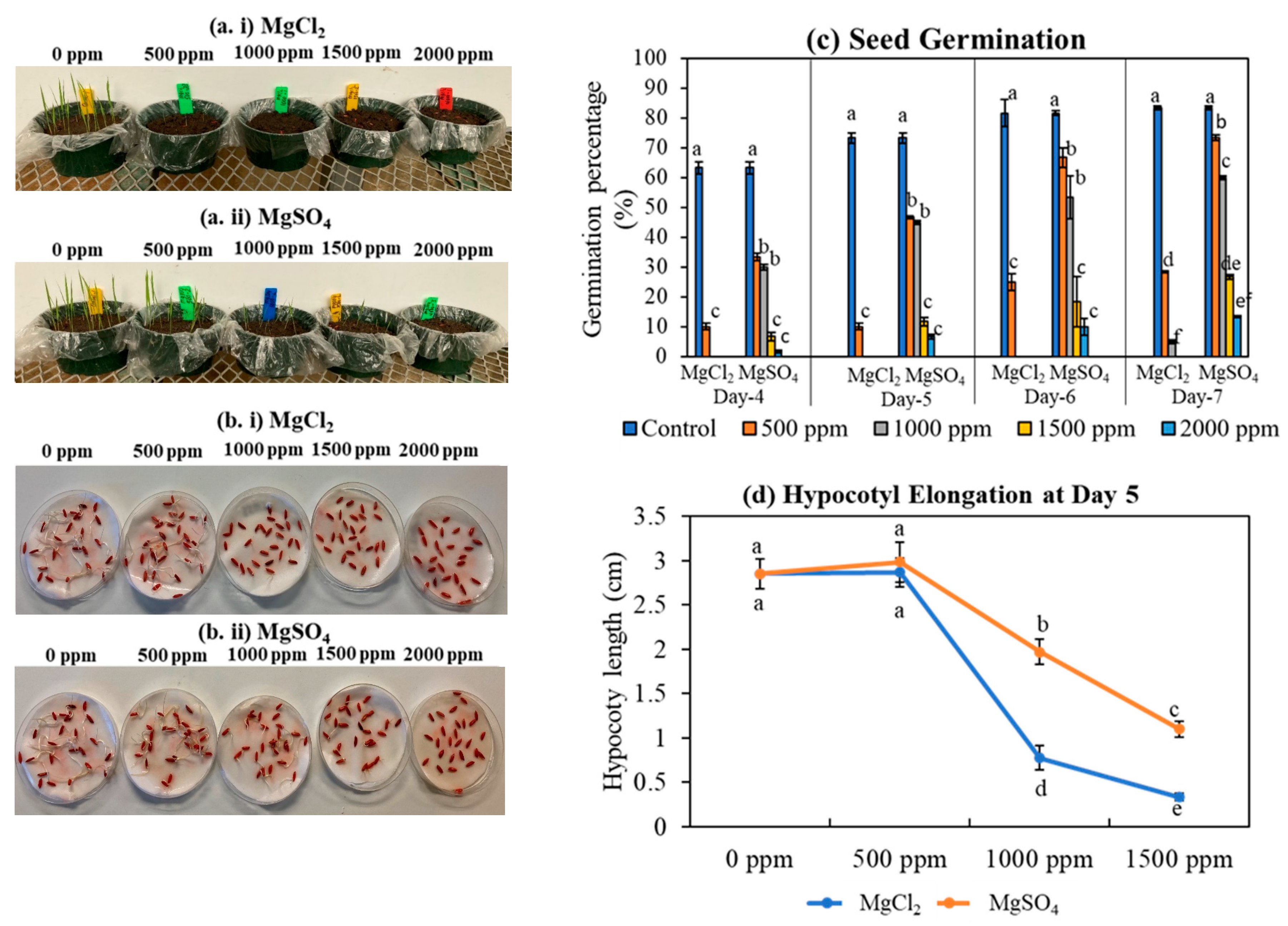
2.3. Trial 3: Germination and Hypocotyl Elongation
2.4. Trial 4: Stress Evaluation Experiemnt
2.5. Statistical Analysis
3. Results
3.1. Germination and Hypocotyl Elongation
3.2. Early Vegetative Growth and Biomass
3.3. Root Morphology
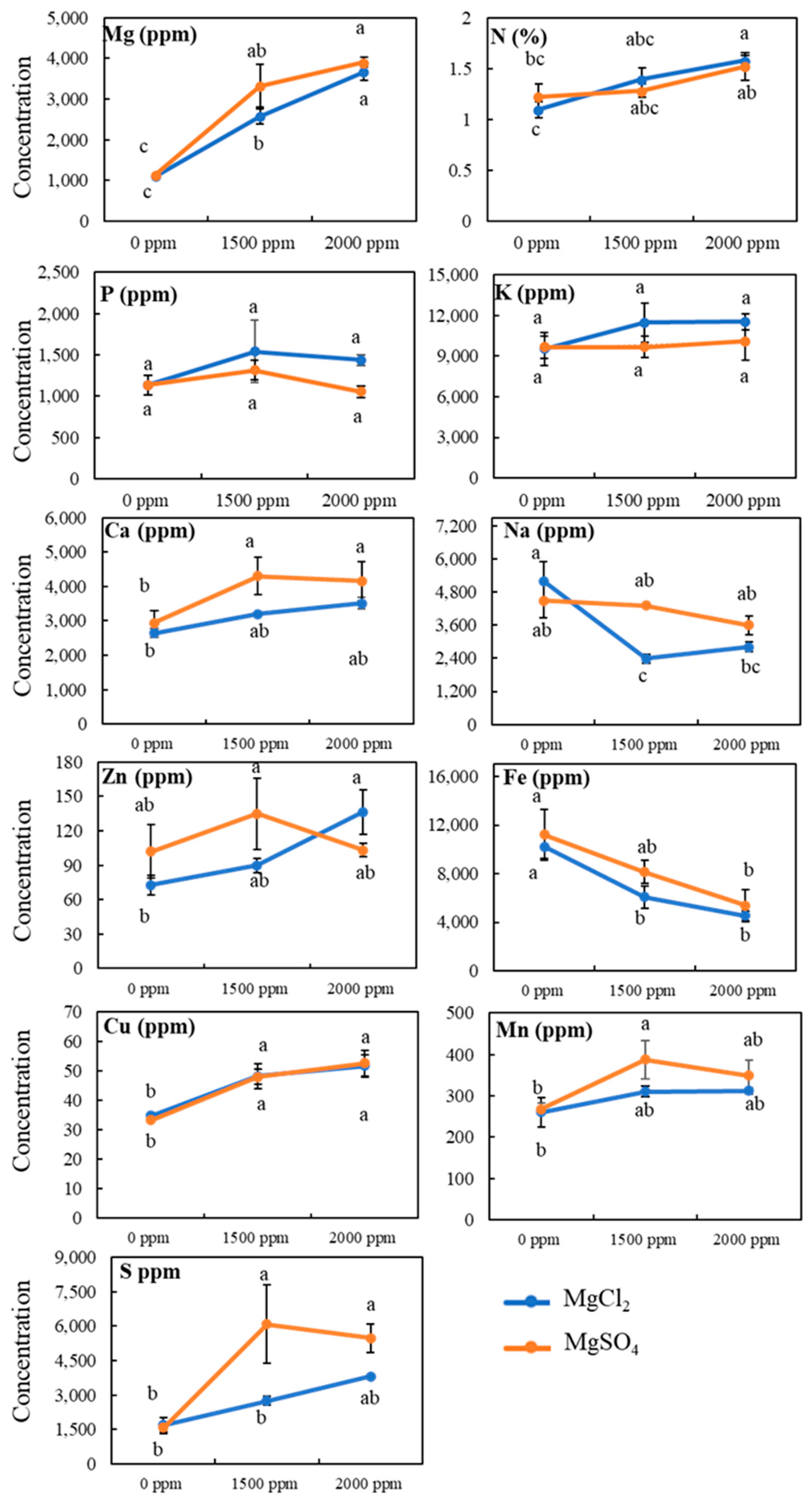
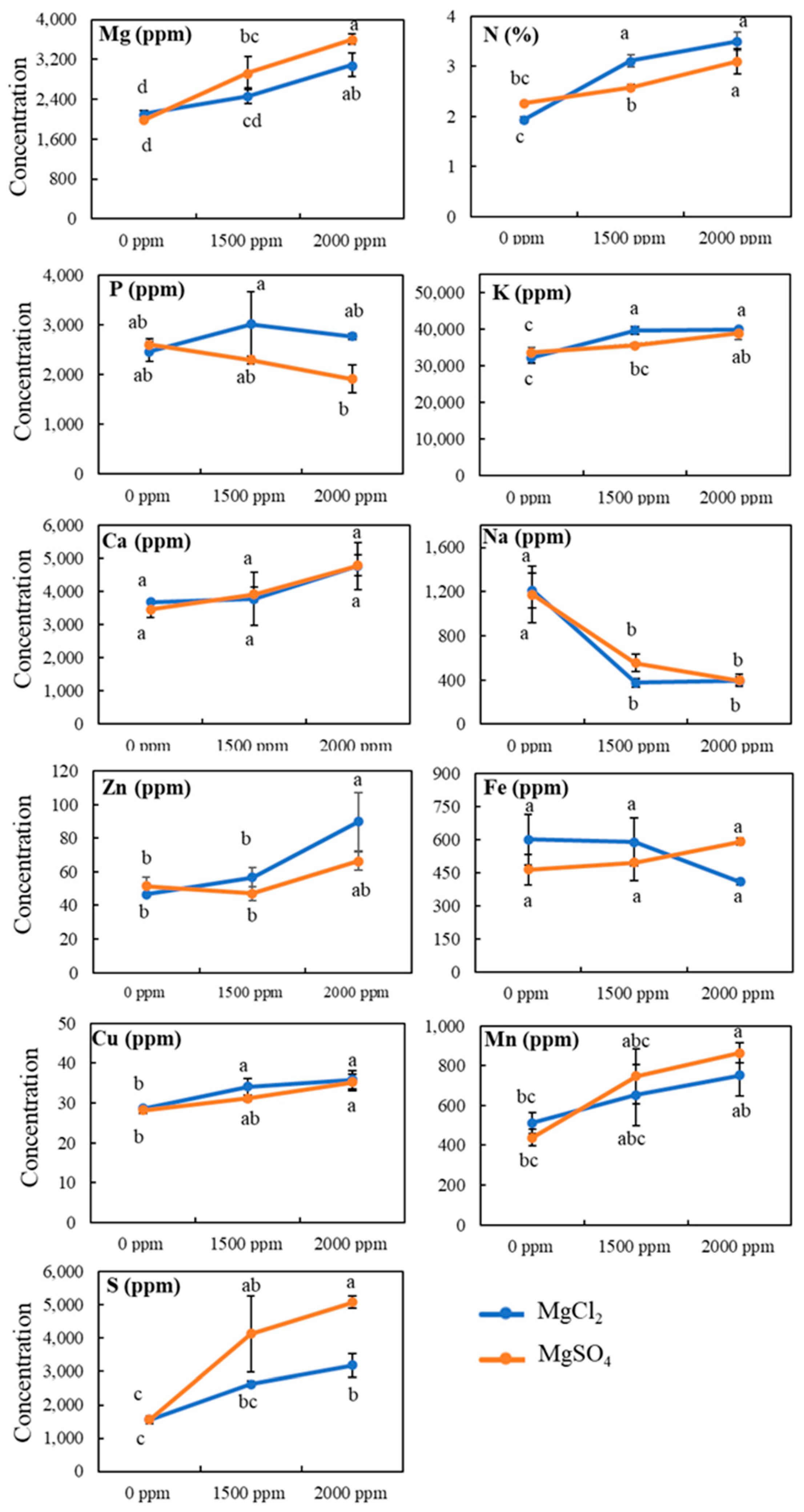
3.4. Tissue Mineral Contents
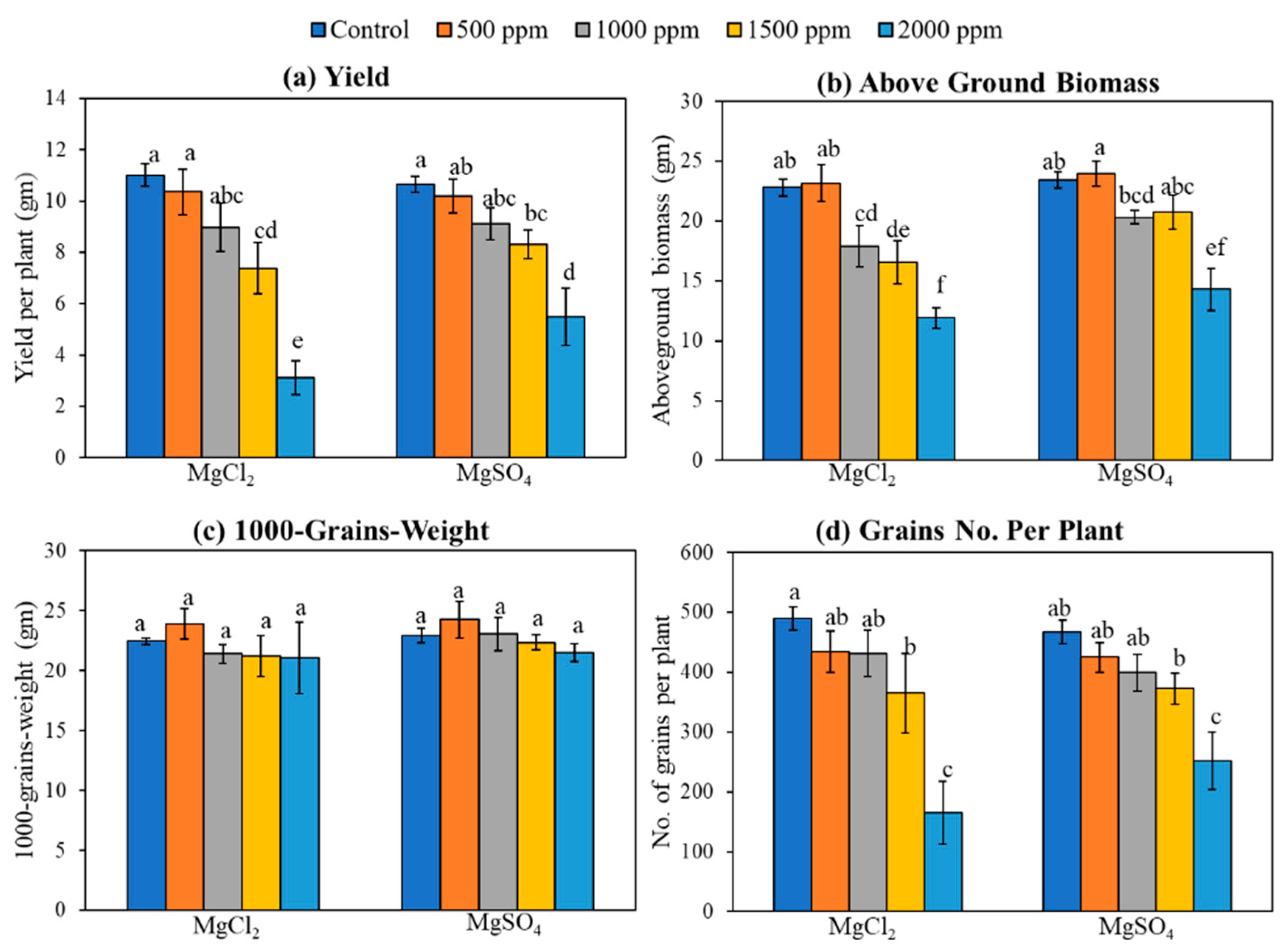
3.5. Yield and Biomass
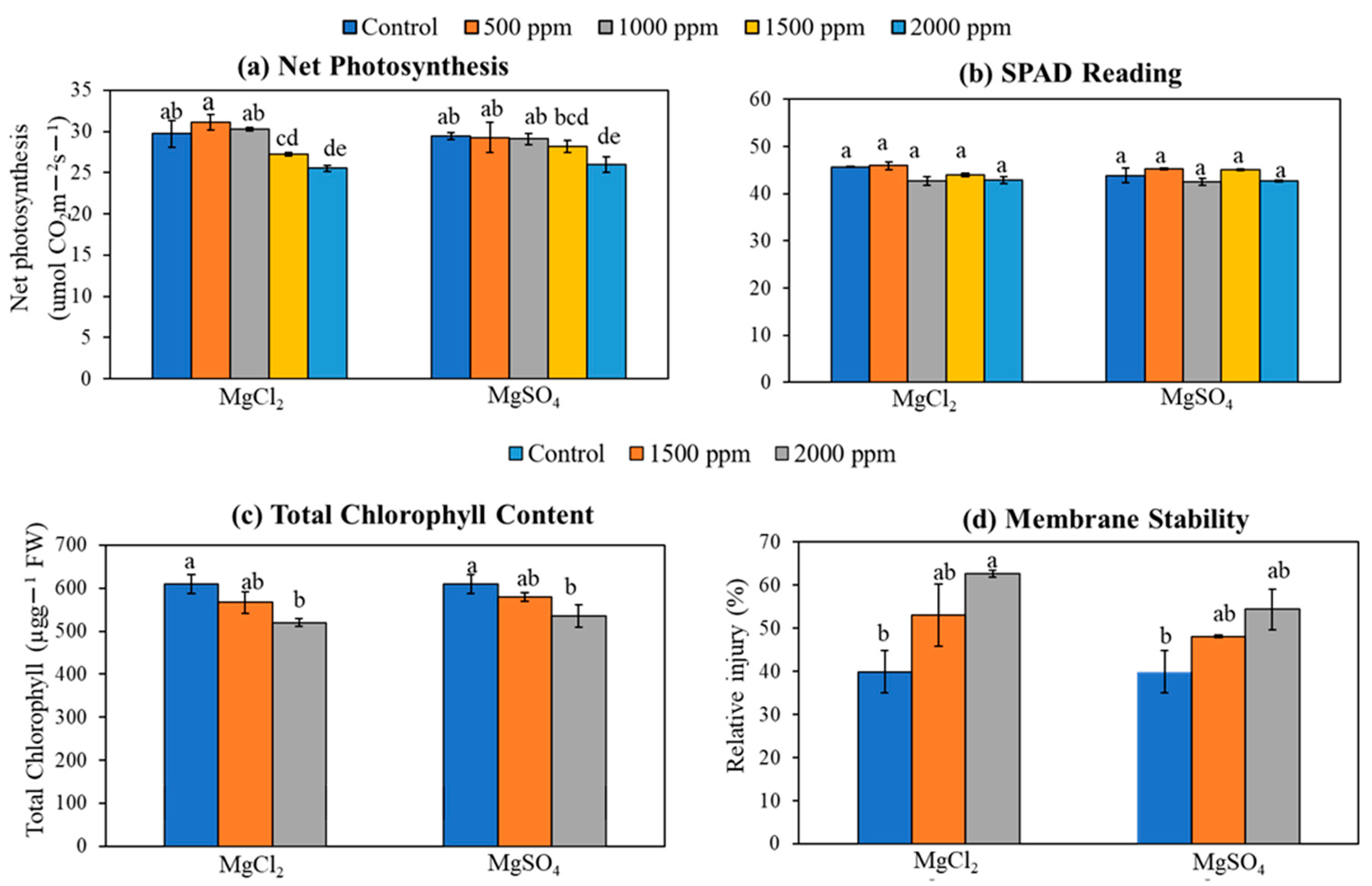
3.6. Photosynthesis, SPAD, Total Chlorophyll Content, and Membrane Damage
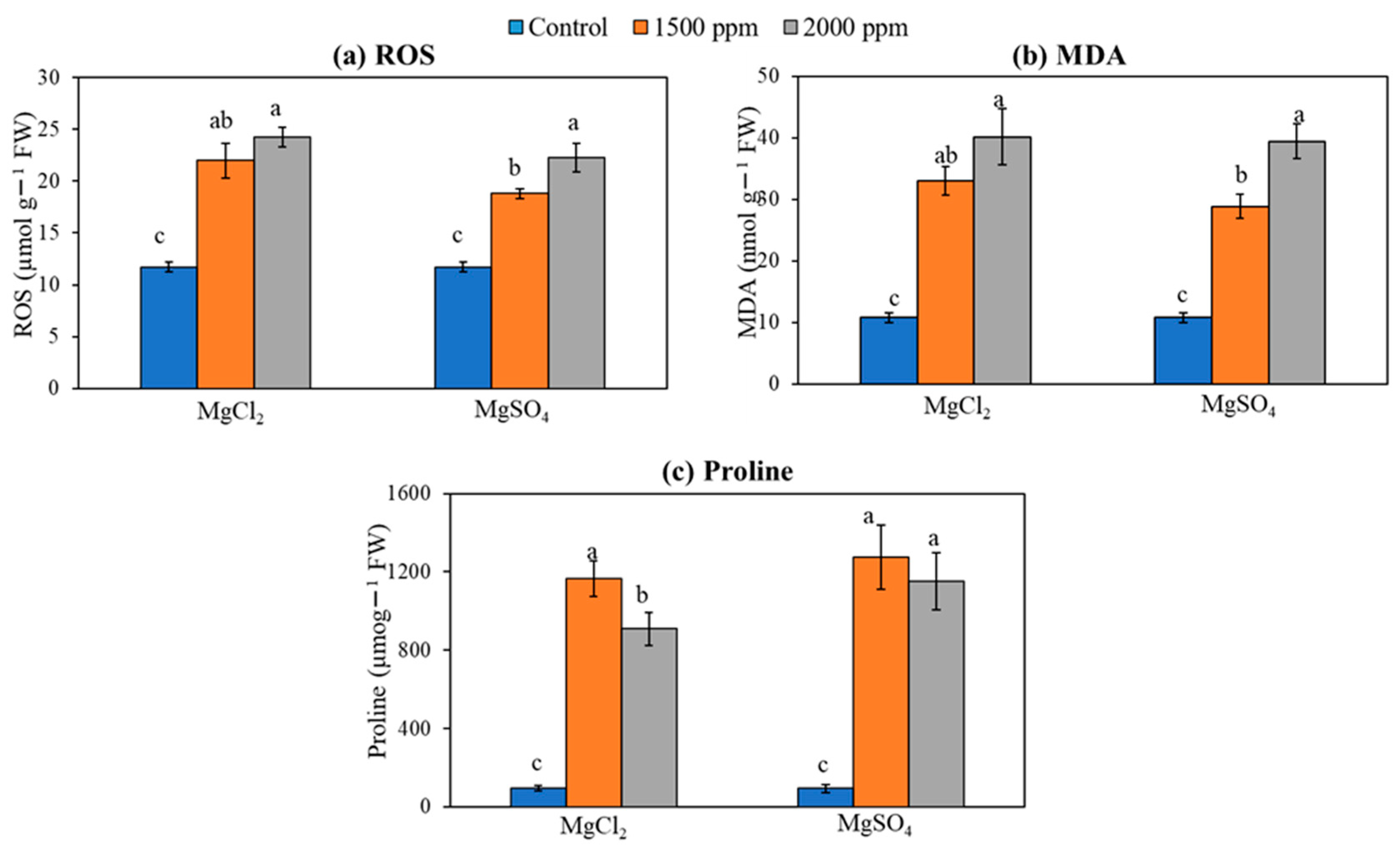
3.7. ROS, Free Proline, and Membrane Lipid Peroxidation (MDA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Cell Develop. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, e992287. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Vyshpolsky, F.; Qadir, M.; Karimov, A.; Mukhamedjanov, K.; Bekbaev, U.; Paroda, R.; Karajeh, F. Enhancing the productivity of high-magnesium soil and water resources in Central Asia through the application of phosphogypsum. Land Degrad. Develop. 2008, 19, 45–56. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, W.; Xu, G. Characterization of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar] [CrossRef]
- Tobe, K.; Li, X.; Omasa, K. Effects of sodium, magnesium and calcium salts on seed germination and radicle survival of a halophyte, Kalidium caspicum (Chenopodiaceae). Aust. J. Bot. 2002, 50, 163–169. [Google Scholar] [CrossRef]
- Kant, C.; Aydin, A.; Turan, M. Ameliorative effect of hydro gel substrate on growth, inorganic ions, proline, and nitrate contents of bean under salinity stress. J. Plant Nutr. 2008, 31, 1420–1439. [Google Scholar] [CrossRef]
- Nukaya, A.; Masui, M.; Ishida, A. Salt tolerance of muskmelons as affected by various salinities in nutrient solution culture. J. Jpn. Soc. Hort. Sci. 1983, 52, 167–173. [Google Scholar] [CrossRef][Green Version]
- Kwon, M.C.; Kim, Y.X.; Lee, S.; Jung, E.S.; Singh, D.; Sung, J.; Lee, C.H. Comparative metabolomics unravel the effect of magnesium oversupply on tomato fruit quality and associated plant metabolism. Metabolites 2019, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.X.; Kwon, M.C.; Lee, S.; Jung, E.S.; Lee, C.H.; Sung, J. Effects of nutrient and water supply during fruit development on metabolite composition in tomato fruits (Solanum lycopersicum L.) grown in magnesium excess soils. Front. Plant Sci. 2020, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Masaoka, Y.; Sato, S. Effects of excess magnesium on the growth and mineral content of rice and Echinochloa. Plant Prod. Sci. 2005, 8, 38–43. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. ROS and oxidative stress: Origin and implication. In Reactive Oxygen Species in Plant Biology; Springer: New Delhi, India, 2019; pp. 1–31. [Google Scholar]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.L.; Soltanpour, P.N. A nitric acid and plant digest method for use with inductively coupled plasma spectrometry. Commun. Soil Sci. Plant. 1989, 14, 969–980. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae Tutorial; Version 1.3-5; Universidad Nacional Agraria: La Molina, Peru, 2021. [Google Scholar]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Alpuerto, J.B.; Han, A.; Fukao, T. The central negative regulator of flooding tolerance, the PROTEOLYSIS 6 branch of the N-degron pathway, adversely modulates salinity tolerance in Arabidopsis. Plants 2020, 9, 1415. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Peng, W.T.; Qi, W.L.; Nie, M.M.; Xiao, Y.B.; Liao, H.; Chen, Z.C. Magnesium supports nitrogen uptake through regulating NRT2.1/2.2 in soybean. Plant Soil 2020, 457, 97–111. [Google Scholar] [CrossRef]
- Choudhury, T.M.A.; Khanif, Y.M. Evaluation of effects of nitrogen and magnesium fertilization on rice yield and fertilizer nitrogen efficiency using 15N tracer technique. J. Plant Nutr. 2001, 24, 855–871. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Liu, J.; Islam, M.T.; Sherif, S.M. Changes in reactive oxygen species, antioxidants and carbohydrate metabolism in relation to dormancy transition and bud break in apple (Malus × domestica Borkh) cultivars. Antioxidants 2021, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Das Bhowmik, S.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L.T.M. Rapid accumulation of proline enhances salinity tolerance in Australian wild rice Oryza australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhao, L.Y.; Wu, C.W.; Shen, B.; Zhu, A.Y. Exogenous proline reduces NaCl-induced damage by mediating ionic and osmotic adjustment and enhancing antioxidant defense in Eurya emarginata. Acta Physiol. Plant. 2015, 37, 181. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, S.; Tarpley, L.; Dou, F. Impact of Excess Magnesium Salt Supply on Rice Yield, Physiological Response, and Grain Mineral Content. Sustainability 2023, 15, 15741. https://doi.org/10.3390/su152215741
Lamichhane S, Tarpley L, Dou F. Impact of Excess Magnesium Salt Supply on Rice Yield, Physiological Response, and Grain Mineral Content. Sustainability. 2023; 15(22):15741. https://doi.org/10.3390/su152215741
Chicago/Turabian StyleLamichhane, Suman, Lee Tarpley, and Fugen Dou. 2023. "Impact of Excess Magnesium Salt Supply on Rice Yield, Physiological Response, and Grain Mineral Content" Sustainability 15, no. 22: 15741. https://doi.org/10.3390/su152215741
APA StyleLamichhane, S., Tarpley, L., & Dou, F. (2023). Impact of Excess Magnesium Salt Supply on Rice Yield, Physiological Response, and Grain Mineral Content. Sustainability, 15(22), 15741. https://doi.org/10.3390/su152215741





