The Influence of Atmospheric Pressure and Organic Loading on the Sustainability of Simultaneous Nitrification and Denitrification
Abstract
:1. Introduction
2. Materials and Methods
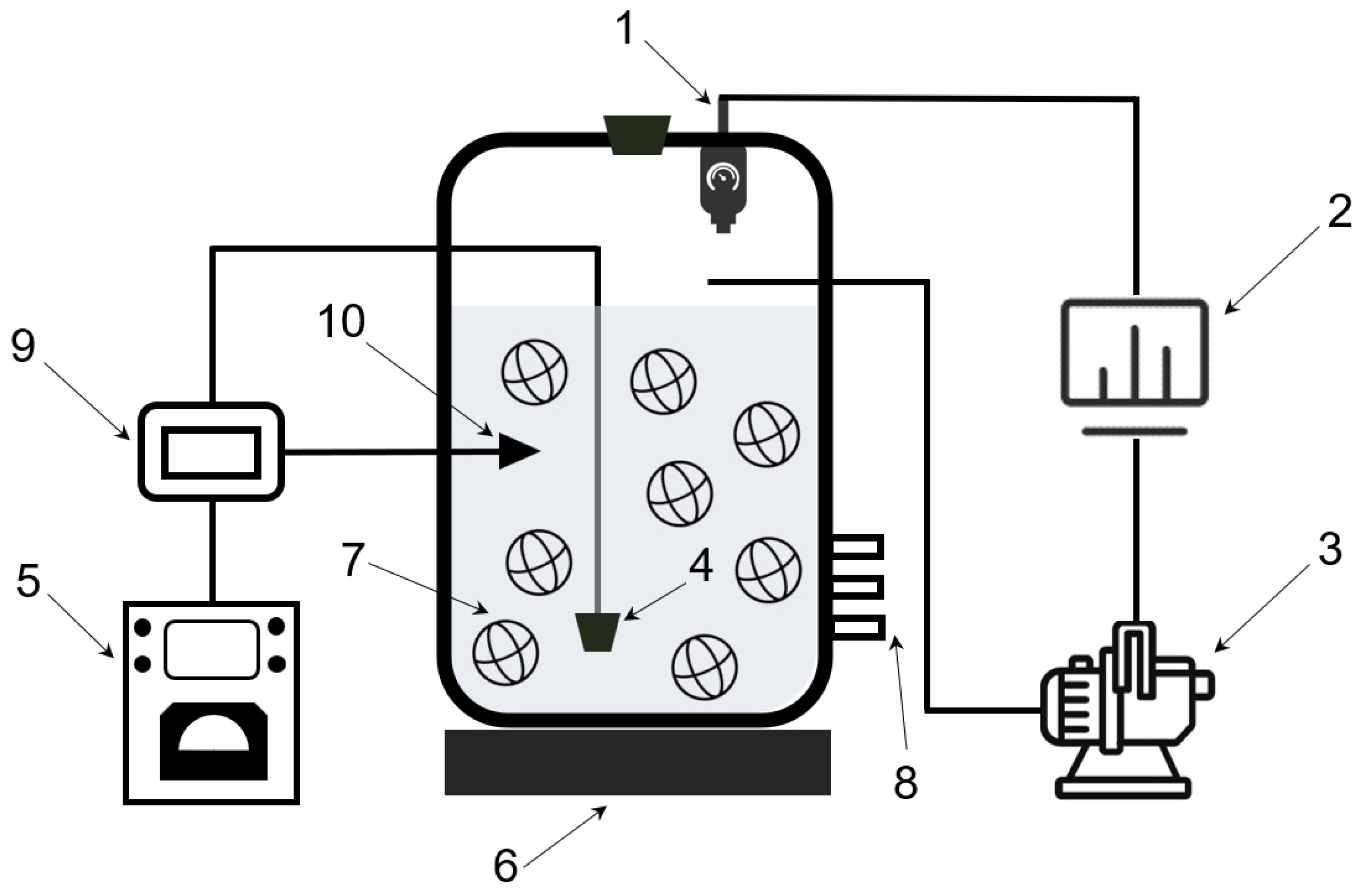
2.1. SND Reactor Setup and Operation
2.2. Analytical Methods
2.3. Microbial Community Analysis
2.4. Statistical Analysis
3. Results and Discussion
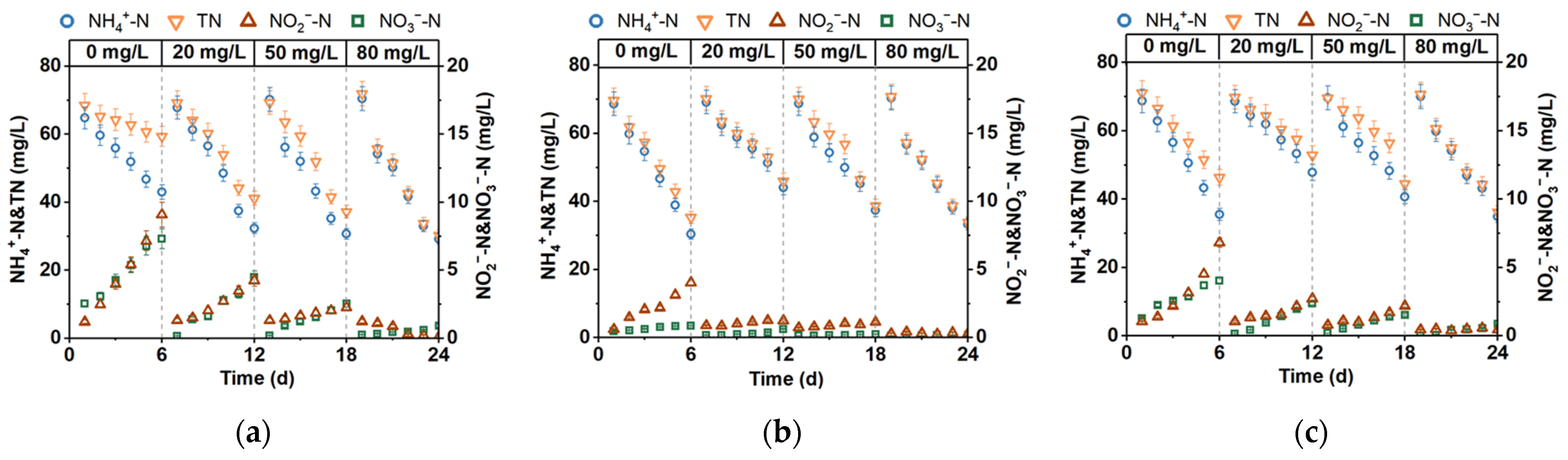
3.1. SND Reactor Performance
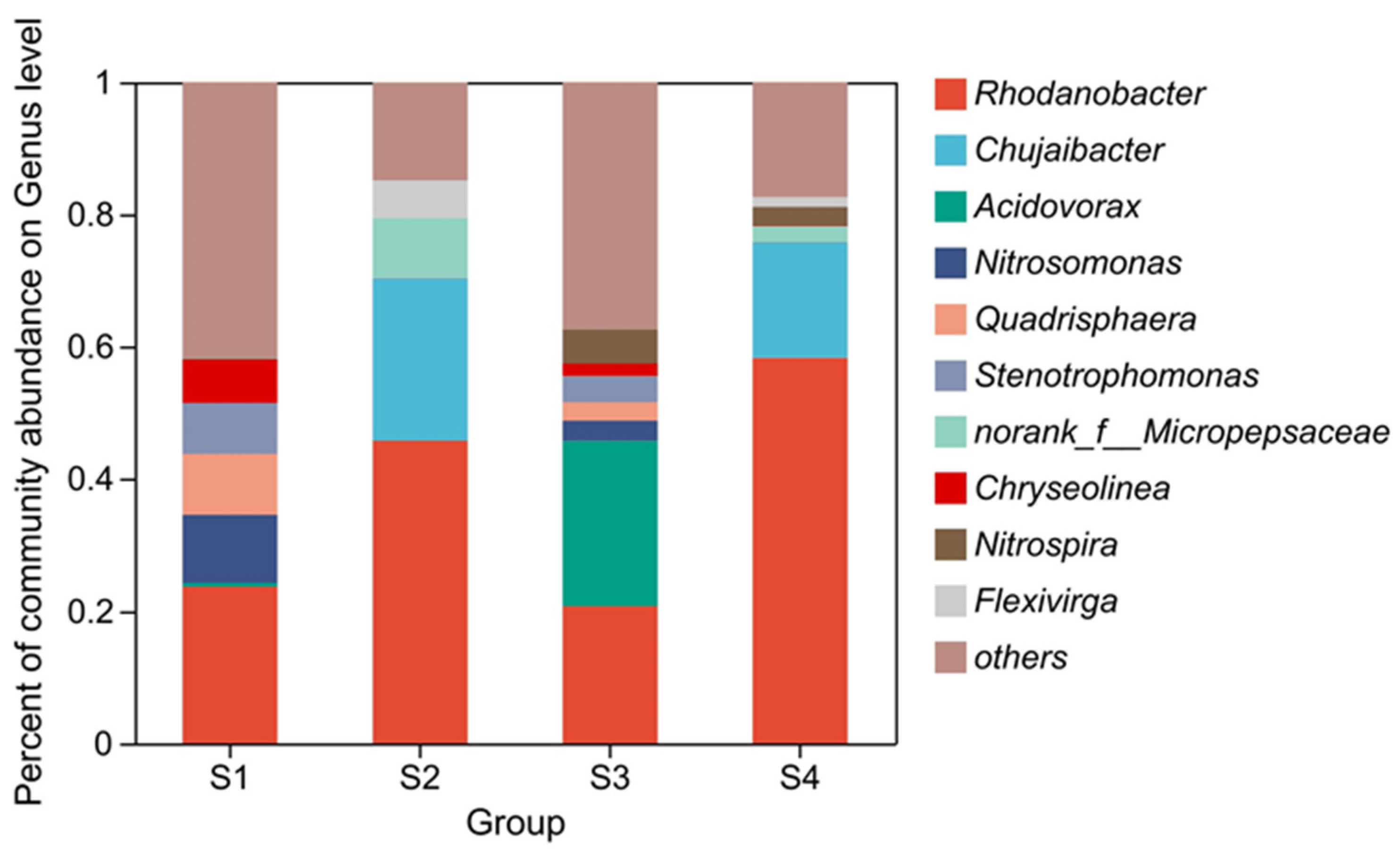
3.2. Variation of Microbial Diversity
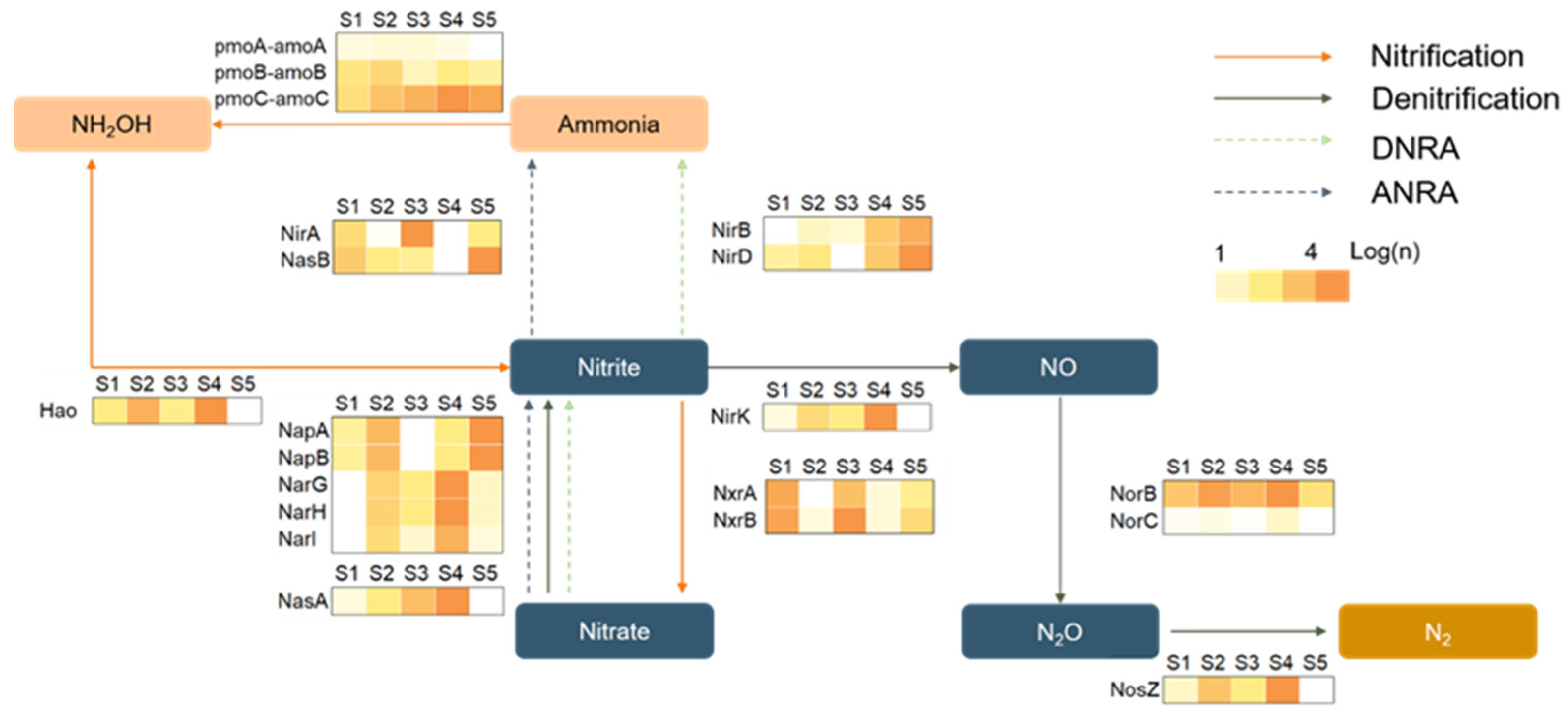
3.3. Correlation Analysis between Microbial Communities and Environmental Factors
3.4. SND as a Sustainable Wastewater Treatment Process in High-Altitude Environments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, D.; Ji, W.; Lin, B.; Chen, Y.; Huang, C.; Xiong, X.; Fu, M.; Mipam, T.D.; Ai, Y.; Zeng, B.; et al. Correlations between Gut Microbiota Community Structures of Tibetans and Geography. Sci. Rep. 2017, 7, 16982. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xin, Z.; Huang, Y.; Yu, J. Climate Suitability Assessment on the Qinghai-Tibet Plateau. Sci. Total Environ. 2022, 816, 151653. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Chang, H.; Yang, H.; Zhang, W.; Zhang, Y.; Sun, H. Deciphering the microbial community structures and functions of wastewater treatment at high-altitude area. Front. Bioeng. Biotechnol. 2023, 11, 1107633. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron Robustly Stimulates Simultaneous Nitrification and Denitrification Under Aerobic Conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef]
- He, T.; Li, Z.; Xie, D.; Sun, Q.; Xu, Y.; Ye, Q.; Ni, J. Simultaneous Nitrification and Denitrification with Different Mixed Nitrogen Loads by a Hypothermia Aerobic Bacterium. Biodegradation 2018, 29, 159–170. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous Nitrification, Denitrification and Phosphorus Removal in a Sequencing Batch Reactor (SBR) under Low Temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Lyu, Y.; Wang, Y. Nitrogen Removal by a Metal-Resistant Bacterium, Pseudomonas Putida ZN1, Capable of Heterotrophic Nitrification-Aerobic Denitrification. J. Chem. Technol. Biotechnol. 2019, 94, 1165–1175. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Xie, H.; Qi, P.; Ren, Y.; Hu, Z. Methane Emissions from a Full-Scale A/A/O Wastewater Treatment Plant. Bioresour. Technol. 2011, 102, 5479–5485. [Google Scholar] [CrossRef]
- Wang, L.; Niu, L.; Li, Y.; Zhang, P.; Zhang, W.; Zhang, H. Current State and Solution Proposal for Plateau Wastewater Treatment Plants: A Review. Desalination Water Treat. 2019, 155, 120–133. [Google Scholar] [CrossRef]
- Baird, R.; Eaton, A.D.; Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Lo, I.W.; Lo, K.V.; Mavinic, D.S.; Shiskowski, D.; Ramey, W. Contributions of Biofilm and Suspended Sludge to Nitrogen Transformation and Nitrous Oxide Emission in Hybrid Sequencing Batch System. J. Environ. Sci. 2010, 22, 953–960. [Google Scholar] [CrossRef]
- Gupta, P.; Sreekrishnan, T.R.; Ahammad, S.Z. Role of Sludge Volume Index in Anaerobic Sludge Granulation in a Hybrid Anaerobic Reactor. Chem. Eng. J. 2016, 283, 338–350. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Zhou, J.T.; Su, J.; Zhang, Z.Y. Integration of Nitrification and Denitrification in Airlift Bioreactor. Biochem. Eng. J. 2005, 23, 57–62. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Sobeck, D.C.; Higgins, M.J. Examination of Three Theories for Mechanisms of Cation-Induced Bioflocculation. Water Res. 2002, 36, 527–538. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Yu, H.-Q. Formation of Extracellular Polymeric Substances from Acidogenic Sludge in H-2-Producing Process. Appl. Microbiol. Biotechnol. 2007, 74, 208–214. [Google Scholar] [CrossRef]
- Zhang, B.; Lens, P.N.L.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. Enhancement of Aerobic Granulation and Nutrient Removal by an Algal-Bacterial Consortium in a Lab-Scale Photobioreactor. Chem. Eng. J. 2018, 334, 2373–2382. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing Soil Microbial Diversity Is Associated with Decreasing Microbial Biomass under Nitrogen Addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Fang, D.; Zhao, G.; Xu, X.; Zhang, Q.; Shen, Q.; Fang, Z.; Huang, L.; Ji, F. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions. Bioresour. Technol. 2018, 249, 684–693. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Wang, P.; Zhang, W.; Wang, C.; Wang, Q.; Voordouw, G. Understanding the Linkage between Elevation and the Activated-Sludge Bacterial Community along a 3,600-Meter Elevation Gradient in China. Appl. Environ. Microbiol. 2015, 81, 6567–6576. [Google Scholar] [CrossRef] [PubMed]
- Ondik, M.M.; Ooi, M.K.J.; Muñoz-Rojas, M. Soil Microbial Community Composition and Functions Are Disrupted by Fire and Land Use in a Mediterranean Woodland. Sci. Total Environ. 2023, 895, 165088. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wei, Z.; Xiao, X.; Tang, M.; Li, B.; Zhang, X. Nitrification/Denitrification Shaped the Mercury-Oxidizing Microbial Community for Simultaneous Hg0 and NO Removal. Bioresour. Technol. 2019, 274, 18–24. [Google Scholar] [CrossRef]
- Kostka, J.E.; Green, S.J.; Rishishwar, L.; Prakash, O.; Katz, L.S.; Mariño-Ramírez, L.; Jordan, I.K.; Munk, C.; Ivanova, N.; Mikhailova, N.; et al. Genome Sequences for Six Rhodanobacter Strains, Isolated from Soils and the Terrestrial Subsurface, with Variable Denitrification Capabilities. J. Bacteriol. 2012, 194, 4461–4462. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Liu, S.; Qin, J.; Ye, Z.; Liu, W.; Fang, S.; Yang, J. Performances of Simultaneous Enhanced Removal of Nitrogen and Phosphorus via Biological Aerated Filter with Biochar as Fillers under Low Dissolved Oxygen for Digested Swine Wastewater Treatment. Bioprocess Biosyst. Eng. 2021, 44, 1741–1753. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, D.-C. Nitrite oxidizing bacteria (NOB) dominating in nitrifying community in full-scale biological nutrient removal wastewater treatment plants. AMB Express 2017, 7, 25. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qu, Y.; Shen, W.; Zhang, Z.; Wang, J.; Liu, Z.; Li, D.; Li, H.; Zhou, J. Bacterial Community Compositions of Coking Wastewater Treatment Plants in Steel Industry Revealed by Illumina High-Throughput Sequencing. Bioresour. Technol. 2015, 179, 436–443. [Google Scholar] [CrossRef]
- Zhu, N.; Gao, J.; Liang, D.; Zhu, Y.; Li, B.; Jin, H. Thermal Pretreatment Enhances the Degradation and Humification of Lignocellulose by Stimulating Thermophilic Bacteria during Dairy Manure Composting. Bioresour. Technol. 2021, 319, 124149. [Google Scholar] [CrossRef]
- Hooper, A.B.; Vannelli, T.; Bergmann, D.J.; Arciero, D.M. Enzymology of the Oxidation of Ammonia to Nitrite by Bacteria. Antonie Van Leeuwenhoek 1997, 71, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ding, W.; Liu, D.; He, T.; Yoo, G.; Yuan, J.; Chen, Z.; Fan, J. Wheat Straw-Derived Biochar Amendment Stimulated N2O Emissions from Rice Paddy Soils by Regulating the amoA Genes of Ammonia-Oxidizing Bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mazumder, D. Simultaneous nitrification and denitrification in moving bed bioreactor and other biological systems. Bioprocess Biosyst. Eng. 2021, 44, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; Lee, M.D.; Detweiler, A.M.; Bebout, B.M. Millimeter-scale vertical partitioning of nitrogen cycling in hypersaline mats reveals prominence of genes encoding multi-heme and prismane proteins. ISME J. 2022, 16, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- James, S.N.; Vijayanandan, A. Recent advances in simultaneous nitrification and denitrification for nitrogen and micropollutant removal: A review. Biodegradation 2023, 34, 103–123. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Chen, W.; Ma, F.; Liu, H.; Tian, Y. The regulation and control strategies of a sequencing batch reactor for simultaneous nitrification and denitrification at different temperatures. Bioresour. Technol. 2013, 133, 59–67. [Google Scholar] [CrossRef]
- Ito, T.; Aoi, T.; Miyazato, N.; Hatamoto, M.; Fuchigami, S.; Yamaguchi, T.; Watanabe, Y. Diversity and abundance of denitrifying bacteria in a simultaneously nitrifying and denitrifying rotating biological contactor treating real wastewater at low temperatures. H2Open J. 2019, 2, 58–70. [Google Scholar] [CrossRef]
- Dawas-Massalha, A.; Gur-Reznik, S.; Lerman, S.; Sabbah, I.; Dosoretz, C.G. Co-metabolic oxidation of pharmaceutical compounds by a nitrifying bacterial enrichment. Bioresour. Technol. 2014, 167, 336–342. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Yang, Z.; Li, B.; Tang, C.; Chai, L.; Su, R. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]


| COD (mg/L) | SV (mL/L) | MLSS (g/L) | SVI (mL/g) |
|---|---|---|---|
| 0 | 216.9 ± 0.035 | 1.14 ± 0.008 | 190.26 |
| 20 | 63.0 ± 0.237 | 0.98 ± 0.011 | 64.29 |
| 50 | 41.0 ± 0.013 | 1.01 ± 0.006 | 40.59 |
| 80 | 12.2 ± 0.030 | 0.99 ± 0.010 | 12.32 |
| Sample | Ace | Chao1 | Coverage | Shannon | Simpson |
|---|---|---|---|---|---|
| S1 | 345.9 | 351.3 | 0.9985 | 3.297 | 0.0704 |
| S2 | 176.7 | 172.5 | 0.9991 | 2.087 | 0.2334 |
| S3 | 417.7 | 422.6 | 0.9985 | 3.209 | 0.1044 |
| S4 | 310.5 | 318.2 | 0.9982 | 2.095 | 0.3102 |
| S5 | 879.9 | 884.5 | 0.9985 | 5.141 | 0.0222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.-J.; Sun, J.; Zhang, W.-J.; Chen, Y.; Yang, J.-L.; Li, S.-P.; Zhu, G.-C.; Lu, Y.-Z. The Influence of Atmospheric Pressure and Organic Loading on the Sustainability of Simultaneous Nitrification and Denitrification. Sustainability 2023, 15, 15689. https://doi.org/10.3390/su152215689
Yu W-J, Sun J, Zhang W-J, Chen Y, Yang J-L, Li S-P, Zhu G-C, Lu Y-Z. The Influence of Atmospheric Pressure and Organic Loading on the Sustainability of Simultaneous Nitrification and Denitrification. Sustainability. 2023; 15(22):15689. https://doi.org/10.3390/su152215689
Chicago/Turabian StyleYu, Wei-Jia, Ji Sun, Wei-Jia Zhang, Yue Chen, Jun-Ling Yang, Shu-Ping Li, Guang-Can Zhu, and Yong-Ze Lu. 2023. "The Influence of Atmospheric Pressure and Organic Loading on the Sustainability of Simultaneous Nitrification and Denitrification" Sustainability 15, no. 22: 15689. https://doi.org/10.3390/su152215689
APA StyleYu, W.-J., Sun, J., Zhang, W.-J., Chen, Y., Yang, J.-L., Li, S.-P., Zhu, G.-C., & Lu, Y.-Z. (2023). The Influence of Atmospheric Pressure and Organic Loading on the Sustainability of Simultaneous Nitrification and Denitrification. Sustainability, 15(22), 15689. https://doi.org/10.3390/su152215689







