Preparation of Ti4O7 Reactive Electrochemical Membrane for Electrochemical Oxidation of Coking Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Synthesis of Ti4O7 REM
2.4. Reactor Configuration
2.5. Analytical Techniques
3. Results
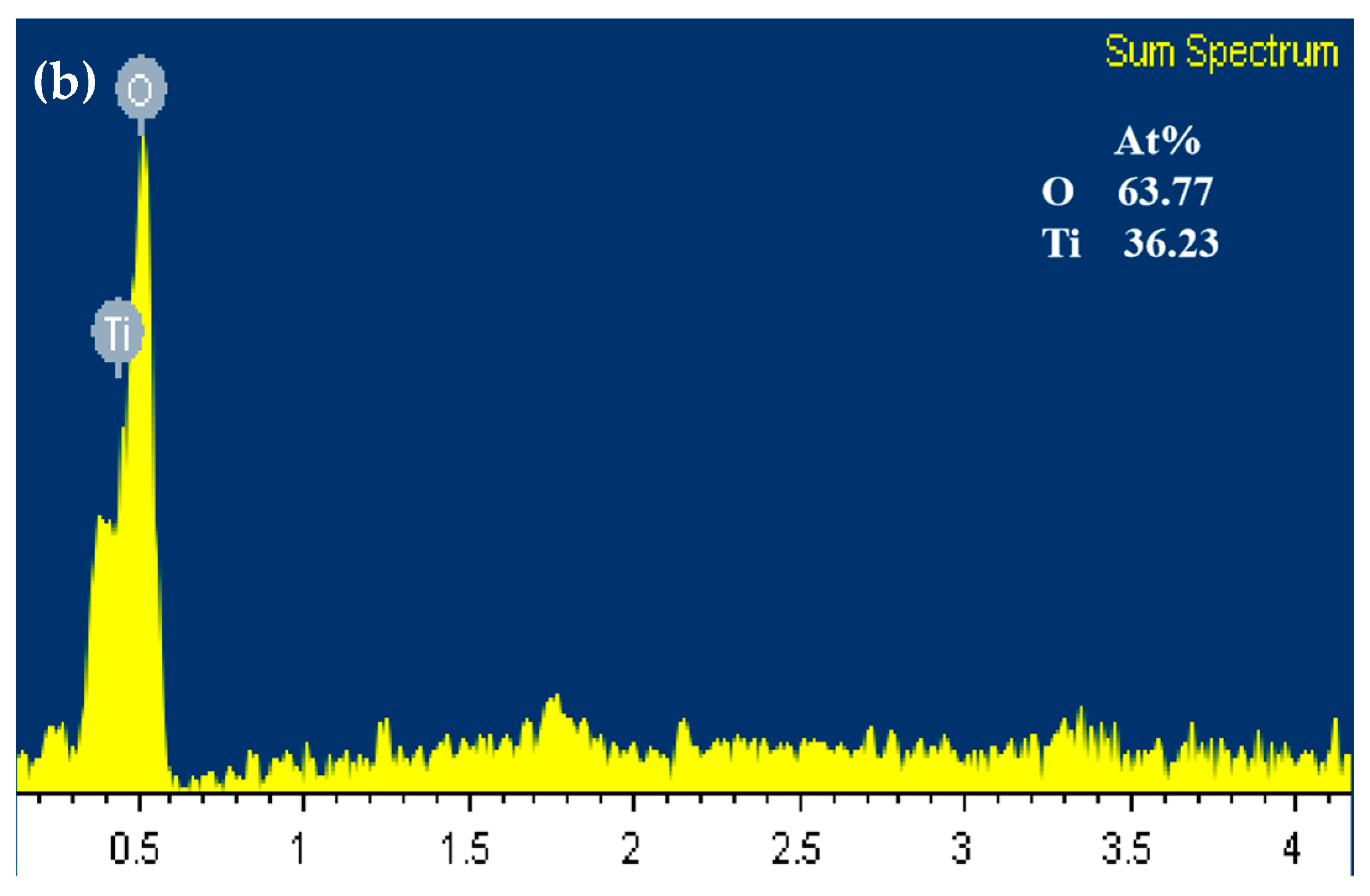
3.1. Characterization of the Ti4O7 REM
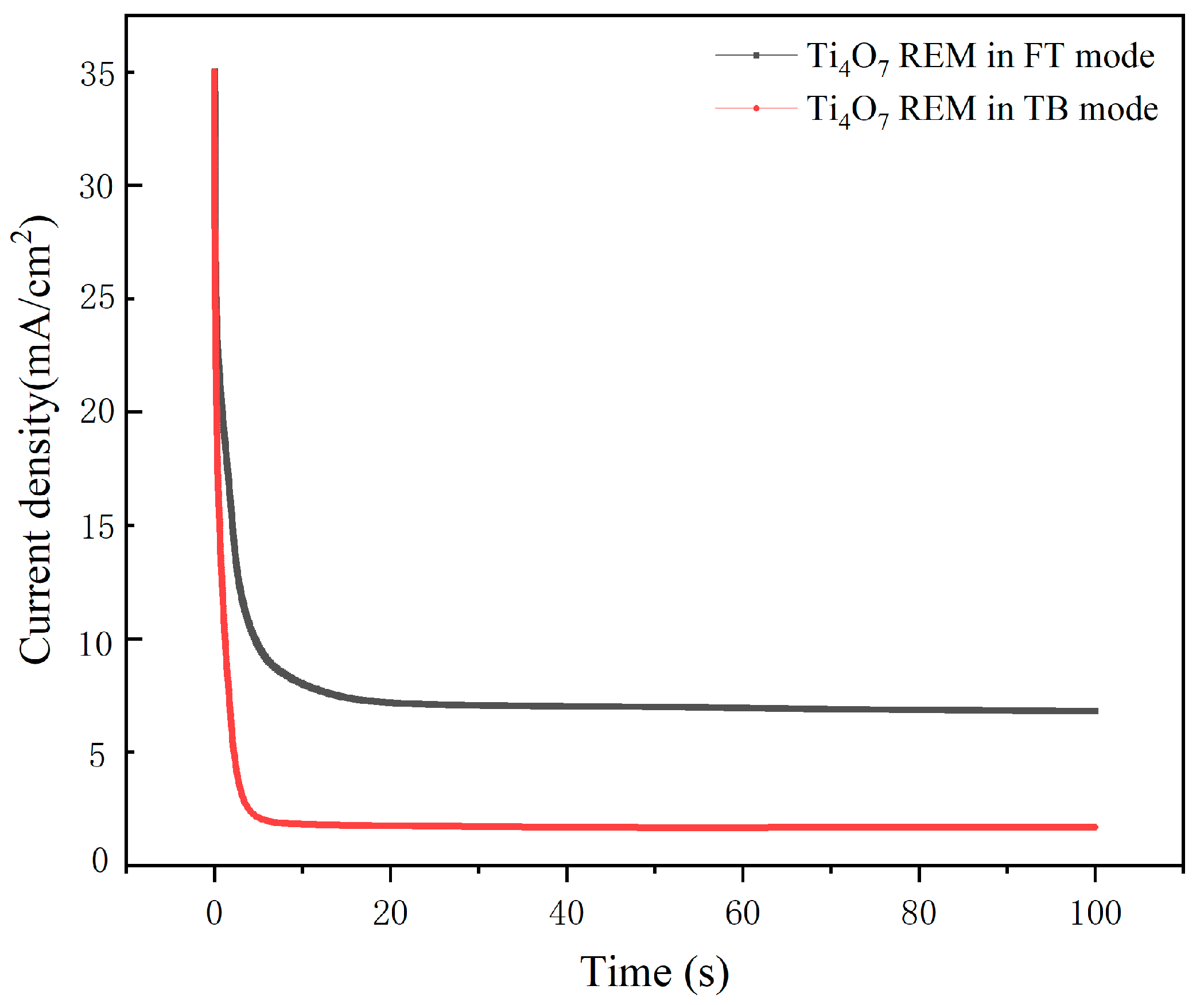
3.2. Mass Transfer Performance
3.3. Effect of Current Density
3.4. Effect of Anode–Cathode Distance
3.5. Energy Consumption
3.6. Electrode Stability and Cathodic Polarization
3.7. Treatment of Real Wastewater
4. Discussion
5. Conclusions
- The surface of the prepared Ti4O7 REM was proven to be highly pure and uniform by the characterization of XRD and SEM.
- CFD simulation was employed to compare the mass transfer performance of the Ti4O7 REM in TB mode and FT mode, indicating that the FT operation was more conducive to enhance mass transfer, which correlated well with the CA result.
- In the FT mode, the effect of current density and anode–cathode distance on the COD removal efficiency was investigated. The results showed that the COD removal efficiencies increased with the applied current density and decreased with the increase in the anode–cathode distance. The COD removal efficiency of the Ti4O7 REM could reach 76.2% with an energy consumption of 110.5 kWh kg−1 COD under the optimal condition.
- The Ti4O7 REM exhibited satisfactory stability during cycling experiments. However, the effect of cathodic polarization could result in better long-term electrode performance.
- According to the three-dimensional fluorescence analysis, the characteristic peak of humic acid in the coking wastewater disappeared, which suggested that the Ti4O7 REM had excellent catalytic properties for dissolved organic matters. Based on the UV–vis analysis, the aromatic compounds and heterocyclic organic compounds were significantly degraded.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Pitas, V.; Somogyi, V.; Karpati, A.; Thury, P.; Frater, T. Reduction of chemical oxygen demand in a conventional activated sludge system treating coke oven wastewater. J. Clean. Prod. 2020, 273, 122482. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Liu, W.; Ye, M.; Su, F. Effluent characteristics of advanced treatment for biotreated coking wastewater by electrochemical technology using BDD anodes. Environ. Sci. Pollut. Res. 2015, 22, 6827–6834. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Sun, H.; Guo, D. The removal of COD from coking wastewater using extraction replacement-biodegradation coupling. Desalination 2012, 289, 45–50. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Xu, Z.; Liu, Y.; Cheng, F. Simultaneous anti-fouling and flux-enhanced membrane distillation via incorporating graphene oxide on PTFE membrane for coking wastewater treatment. Appl. Surf. Sci. 2020, 531, 147349. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations. Sustainability 2021, 13, 6759. [Google Scholar] [CrossRef]
- Yang, K.; Lin, H.; Liang, S.; Xie, R.; Lv, S.; Niu, J.; Chen, J.; Hu, Y. A reactive electrochemical filter system with an excellent penetration flux porous Ti/SnO2-Sb filter for efficient contaminant removal from water. Rsc Adv. 2018, 8, 13933–13944. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef]
- Rada, E.C.; Istrate, I.A.; Ragazzi, M.; Andreottola, G.; Torretta, V. Analysis of Electro-Oxidation Suitability for Landfill Leachate Treatment through an Experimental Study. Sustainability 2013, 5, 3960–3975. [Google Scholar] [CrossRef]
- Xu, D.; Song, X.; Qi, W.; Wang, H.; Bian, Z. Degradation mechanism, kinetics, and toxicity investigation of 4-bromophenol by electrochemical reduction and oxidation with Pd-Fe/graphene catalytic cathodes. Chem. Eng. J. 2018, 333, 477–485. [Google Scholar] [CrossRef]
- Kumar, A.; Barbhuiya, N.H.; Singh, S.P. Magneli phase titanium sub-oxides synthesis, fabrication and its application for environmental remediation: Current status and prospect. Chemosphere 2022, 307, 135878. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Lee, J.-M.; Fu, G. Recent advances in rare-earth-based materials for electrocatalysis. Chem Catal. 2022, 2, 967–1008. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Hao, W.; Zhang, T.; Liu, Y.; Yu, L.; Li, W. Preparation of Ti-based Yb-doped SnO2-RuO2 electrode and electrochemical oxidation treatment of coking wastewater. J. Rare Earths 2022, 40, 763–771. [Google Scholar] [CrossRef]
- Hossain, M.K.; Rubel, M.H.K.; Akbar, M.A.; Ahmed, M.H.; Haque, N.; Rahman, M.F.; Hossain, J.; Hossain, K.M. A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram. Int. 2022, 48, 32588–32612. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Porous Substoichiometric TiO2 Anodes as Reactive Electrochemical Membranes for Water Treatment. Environ. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef]
- Guo, L.; Jing, Y.; Chaplin, B.P. Development and Characterization of Ultrafiltration TiO2 Magneli Phase Reactive Electrochemical Membranes. Environ. Sci. Technol. 2016, 50, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Gayen, P.; Chen, C.; Abiade, J.T.; Chaplin, B.P. Electrochemical Oxidation of Atrazine and Clothianidin on Bi-doped SnO2-TinO2n−1 Electrocatalytic Reactive Electrochemical Membranes. Environ. Sci. Technol. 2018, 52, 12675–12684. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef] [PubMed]
- Donaghue, A.; Chaplin, B.P. Effect of Select Organic Compounds on Perchlorate Formation at Boron-doped Diamond Film Anodes. Environ. Sci. Technol. 2013, 47, 12391–12399. [Google Scholar] [CrossRef]
- Napoles-Armenta, J.; Vidales-Contreras, J.A.; Leyva-Soto, L.A.; Meza-Escalante, E.R.; Diaz-Tenorio, L.M.; Garcia-Gomez, C.; Martinez-Orozco, E.; De La Mora-orozco, C.; Gortares-Moroyoqui, P.; Salcedo-Gastelum, L.A. The Influence of the Configuration of Two Electrochemical Reactors on the Process of Removing Atrazine from Water. Sustainability 2021, 13, 5267. [Google Scholar] [CrossRef]
- Schnoor, M.H.; Vecitis, C.D. Quantitative Examination of Aqueous Ferrocyanide Oxidation in a Carbon Nanotube Electrochemical Filter: Effects of Flow Rate, Ionic Strength, and Cathode Material. J. Phys. Chem. C 2013, 117, 2855–2867. [Google Scholar] [CrossRef]
- Nayak, S.; Chaplin, B.P. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes. Electrochim. Acta 2018, 263, 299–310. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Causserand, C.; Oturan, M.A. Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol: In different electrolyte media. Sep. Purif. Technol. 2019, 208, 142–152. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yang, Z.; Qiu, Y.; Yang, B.; Wang, A.; Yu, G.; Zhang, Z. A facile synthesis of a novel Ti4O7 anode rich in oxygen defects and its electrochemical oxidation of florfenicol: Performance and mechanism. Sep. Purif. Technol. 2023, 310, 123134. [Google Scholar] [CrossRef]
- Yang, Y.; Hoffmann, M.R. Synthesis and Stabilization of Blue-Black TiO2 Nanotube Arrays for Electrochemical Oxidant Generation and Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11888–11894. [Google Scholar] [CrossRef]
- Shukla, P.; Singh, K.K.; Tewari, P.K.; Gupta, P.K. Numerical simulation of flow electrolysers: Effect of obstacles. Electrochim. Acta 2012, 79, 57–66. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Zhou, M.; Li, X.; Yu, J. Characterization of hydrodynamics and mass transfer in two types of tubular electrochemical reactors. Electrochim. Acta 2015, 173, 698–704. [Google Scholar] [CrossRef]
- Silva Barni, M.F.; Doumic, L.I.; Procaccini, R.A.; Ayude, M.A.; Romeo, H.E. Layered platforms of Ti4O7 as flow-through anodes for intensifying the electro-oxidation of bentazon. J. Environ. Manag. 2020, 263, 110403. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, C.; Chen, X.; Jiang, L.; Ji, Y.; Li, H.; Liu, Y.; Wang, J. Preparation of Tin-Antimony anode modified with carbon nanotubes for electrochemical treatment of coking wastewater. Chemosphere 2022, 288, 132362. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Z.; Lv, L.; Song, J.; Yin, Z. Electro-Fenton with peroxi-coagulation as a feasible pre-treatment for high-strength refractory coke plant wastewater: Parameters optimization, removal behavior and kinetics analysis. Chemosphere 2020, 238, 124649. [Google Scholar] [CrossRef]
- Waraksa, C.C.; Chen, G.Y.; Macdonald, D.D.; Mallouk, T.E. EIS studies of porous oxygen electrodes with discrete particles—II. Transmission line modeling. J. Electrochem. Soc. 2003, 150, E429–E437. [Google Scholar] [CrossRef]
- Bejan, D.; Malcolm, J.D.; Morrison, L.; Bunce, N.J. Mechanistic investigation of the conductive ceramic Ebonex (R) as an anode material. Electrochim. Acta 2009, 54, 5548–5556. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Ding, G.; Wang, H. Electrochemical Degradation Characteristics of Refractory Organic Pollutants in Coking Wastewater on Multiwall Carbon Nanotube-Modified Electrode. J. Nanomater. 2012, 2012, 614032. [Google Scholar] [CrossRef]
- Wei, C.; Wu, H.; Kong, Q.; Wei, J.; Feng, C.; Qiu, G.; Wei, C.; Li, F. Residual chemical oxygen demand (COD) fractionation in bio-treated coking wastewater integrating solution property characterization. J. Environ. Manag. 2019, 246, 324–333. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Liang, S.; Wang, C.; Wang, Y.; Jin, F.; Luo, Q.; Chiang, S.-Y.D.; Huang, Q. Development of macroporous Magneli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances. Chem. Eng. J. 2019, 359, 827. [Google Scholar] [CrossRef]








| Condition | Cycle | ||||||
|---|---|---|---|---|---|---|---|
| COD Removal (%) | 1 | 2 | 3 | 4 | 5 | 6 | |
| Without cathodic polarization | 76.2 | 75.8 | 74.9 | 73.6 | 73.4 | 73.0 | |
| With cathodic polarization | 76.3 | 77.6 | 77.8 | 77.4 | 77.1 | 76.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Yu, H.; Wang, C.; Ma, J.; Wang, J. Preparation of Ti4O7 Reactive Electrochemical Membrane for Electrochemical Oxidation of Coking Wastewater. Sustainability 2023, 15, 15488. https://doi.org/10.3390/su152115488
Yu J, Yu H, Wang C, Ma J, Wang J. Preparation of Ti4O7 Reactive Electrochemical Membrane for Electrochemical Oxidation of Coking Wastewater. Sustainability. 2023; 15(21):15488. https://doi.org/10.3390/su152115488
Chicago/Turabian StyleYu, Jifang, Huijun Yu, Chunhui Wang, Jingyun Ma, and Jianbing Wang. 2023. "Preparation of Ti4O7 Reactive Electrochemical Membrane for Electrochemical Oxidation of Coking Wastewater" Sustainability 15, no. 21: 15488. https://doi.org/10.3390/su152115488
APA StyleYu, J., Yu, H., Wang, C., Ma, J., & Wang, J. (2023). Preparation of Ti4O7 Reactive Electrochemical Membrane for Electrochemical Oxidation of Coking Wastewater. Sustainability, 15(21), 15488. https://doi.org/10.3390/su152115488





