Abstract
In Argentina, the excessive use of fertilizers is common in intensively cultivated zones around highly populated areas. Bioinoculants based on plant growth promoting rhizobacteria (PGPR) could be effective for crop production improvement without negative effects on the environment. The objective of this work was to evaluate an alternative inoculation method, namely the application of the biofilm produced by Bacillus subtilis as a growth promoter on seeds of three varieties of Lactuca sativa, and to compare it with the common planktonic approach. Biofilm was obtained under static culture conditions, while planktonic inoculum was produced at 150 rpm. The major biofilm effects were observed with Bacillus subtilis subsp. spizizenii, that showed antifungal activity against phytopathogens, synthesized plant growth regulators (IAA, cytokinin and ABA) and solubilized phosphates. The Grand Rapid variety inoculated with biofilm showed the best results, with 30% and 37% higher aerial and root biomass, respectively, compared to the planktonic form. Moreover, the biofilm positive effects were observed through successive plant development stages until harvest, when the bacterium was recovered from the interior of the roots. The biofilm of B. subtilis subsp. spizizenii behave as a superior growth-promoting inoculant compared to the traditional planktonic inoculation technique.
1. Introduction
Agriculture is considered one of the essential human activities. This is not only because of its main role in food supply, but also because of its economic, social, and environmental importance. Although Argentina is a vast country, the asymmetry in the population distribution leads to the presence of intensively cultivated areas around the largest cities, which are considered as horticultural strips or green belts [1]. Various horticultural species are produced in these urban and peri-urban zones. Urban and peri-urban horticulture requires soils with high and sustained fertility, a low incidence of pathogens and very low concentrations of pollutants to guarantee high production rates and optimal final crop quality. However, urban soils often do not constitute the ideal substrate for horticultural activities; this is because they may have poor structure, may be formed by layers of different contrasting origins and may have high concentrations of heavy metals [2]. Moreover, intensive crops frequently are associated with the use of excessive inorganic fertilizers, which can be harmful for the environment and contribute to climate change. For example, the overapplication of nitrogen mostly contribute to the greenhouse gas (GHG) emissions from the agricultural sector, which represent between 65 and 80% of the total emissions worldwide [3]. Therefore, the implementation of agronomic techniques that avoid or reduce pollution and gas emissions, have been shown to improve soil physical and chemical conditions, as well as crop nutrition, yield and safety, so that they are essential to achieve sustained development. A common crop in the Argentinean horticultural belts is Lactuca sativa. Worldwide, the largest producing countries of this vegetable are China, the USA, Spain, Italy and India [4]
The incorporation of microorganisms into the soil, using bioinoculants, is becoming increasingly relevant in sustainable agriculture as a promising ecological and friendly alternative to promote plant growth and health, and to enhance soil quality [5,6]. Among these microorganisms, a group of plant-beneficial bacteria, referred to as Plant Growth Promoting Rhizobacteria (PGPR), have been found to survive in the presence of native soil microbiota and to play a role as biofertilizers, phytostimulators, biocontrolers, rhizoremediators and stress controllers [7,8]. PGPR action as biofertilizers boosts nutrient acquisition and improves the structure of degraded soils through different mechanisms, such as biological nitrogen fixation, phosphates solubilization], potassium mobilization or iron sequestration [9,10,11,12]. These kinds of bacteria also act as biocontrolers by releasing antibiotics, lytic enzymes and other metabolites capable of controlling pathogenic microorganisms’ proliferation in the soil, thereby enhancing plant development [13,14,15,16]. Therefore, PGPR not only have emerged as an important and promising tool for sustainable agriculture [17,18,19] but also could be a solution to meet challenges of global food security and environmental stability [20,21].
Bacillus is a genus of interest as PGPR [10], since its wide physiological diversity allows it to live in different habitats. Also, these bacteria are recognized for their action as biofertilizers, phytostimulants, and biological control agents since they produce various antibiotics [22,23]. There are several species of Bacillus, including B. subtilis subs. spizizenii and B. subtilis var. natto. Another important feature of this genus is its ability to produce biofilm. Thus, in the laboratory, B. subtilis can develop biofilms at the air–liquid interface or grow as a free-living planktonic form, depending on the culture conditions [24]. Bacterial biofilms consist primarily of a three-dimensional exopolysaccharide matrix, with minor amounts of protein, DNA and lysate products [25,26,27]. Also, lipopeptides are induced and accumulate, some of them with antibacterial and antifungal properties [27]. In nature, biofilm represents a sheltered mode of growth that protects cells from environmental fluctuations in humidity, temperature, pH and nutrients concentrations, allowing for cell-waste removal [28,29].
Again, the genus Bacillus presents versatility for its application, which makes it an excellent candidate for the development of bioinoculants [10]. On the market, bioinoculants exist mainly as liquid- and solid-supported formulations. In Argentina, most of the formulations with bacteria are in liquid form, that is, the bacteria are in planktonic form. The main problems with this type of formulation are the low viability of the bacteria and the need to store them at low temperatures. L sativa is one of the most important leafy vegetables, and the third most productive crop after potatoes and tomatoes [5]. There are several varieties of L. sativa, and its characteristics and widespread use worldwide make it a good vegetable for testing the capacities of an inoculant. Based on the above rationale, the objective of this work was to evaluate the use of B. subtilis biofilm as a bioinoculant, i.e., a plant-growth promoter, in different varieties of L. sativa by comparing its effects with those of the traditional liquid inoculation of the bacterium, also referred to as planktonic cell method.
2. Materials and Methods
2.1. Microorganisms and Culture Media for Strain Activation
B. subtilis subsp. spizizenii and B. subtilis var. natto were obtained from the AGRAL collection of Faculty of Agronomy, Buenos Aires University. Phytopathogens Fusarium solani and Pythium ultimum were provided by San Pedro INTA Experimental Station (Plant Pathology Laboratory). The bacterial strains were activated in nutritive agar media at 30 °C for 24 h and, fungi were seeded in Potato Dextrose Agar (PDA) and incubated at 25 °C for 10 days.
2.2. Antifungal Activity of Bacillus
The bacteria B. subtilis subsp. spizizenii and B. subtilis var. natto, previously activated, were grown on potato dextrose liquid medium at 30 °C and 150 rpm. Aliquots were taken at 24, 48, 72 and 96 h and were centrifuged at 3500 g, separating the cell package and supernatant.
The spore suspension of F. solani and P. ultimum were spread uniformly on Petri dishes with PDA. Then, 0.5 cm sterile filter paper discs were placed on the seeded surface and instilled twice with 10 µL of the bacterial supernatants. The plates were incubated at 25 °C for 7 days. The inhibition of fungal growth was evaluated by measuring the diameter of the inhibition halo. The procedure was performed in triplicate.
2.3. Growth Curves of Bacillus Strains in Different Carbon Sources
B. subtilis subsp. spizizenii and B. subtilis var. natto were cultivated in Minimal Salts Medium (MSM), containing 1 g/L K2HPO4; 0.3 g/L KH2PO4; 0.5 g/L NH4Cl; 0.1 g/L NH4NO3; 0.1 g/L Na2SO4; 0.01 g/L MgSO4 7H2O; 1 mg/L MnSO4 4H2O; 1mg/L FeSO4 7H2O; 0.5 g/L CaCl2; and 0.01 g/L EDTA in deionized water pH = 7 ± 0.4 [30], with 35 mM L-glutamic acid and 1% glucose or 1% glycerol as a carbon source. The bacteria were incubated in a rotary shaker (New Brunswick Scientific, Edison, NJ, USA) at 150 rpm and 30 °C. Aliquots of 5 mL were taken at 0, 24, 48, 72 and 96 h to measure the optical density at 610 nm. The assays were performed in triplicate.
2.4. Cultures of Bacillus in Presence of Phytopathogen Fungi
The bacterium B. subtilis subsp. spizizenii was cultivated for 24 h in MSM with 1% glycerol and 35 mM L-glutamic acid at 30 °C and 150 rpm. Then, 5 buttons of 1 cm diameter of PDA containing fungi, i.e., Fusarium solani or Phytium ultimum, were added to each Erlenmeyer flask. These cultures were incubated at 30 °C and 150 pm for 96 h. 5 mL aliquots were extracted every 24 h to measure pH, optical density at 610 nm and antifungal activity.
2.5. Quantification of Plant Growth Regulators Produced by B. subtilis subsp. spizizenii
The bacterium was grown in MSM with 1% glycerol and 35 mM L-glutamic acid at 30 °C and 150 rpm for 96 h, reaching a concentration of 108 CFU/mL. Vials containing 5 mL of this culture were lyophilized and used to determine plant hormones by liquid chromatography. For the extraction process, the following modified Bieleski solvent was used: MeOH-HCO2H-H2O 15:1:4 (v/v/v). The lyophilized material was resuspended with cold extraction solvent and homogenized for one hour in the cold. It was centrifugated at 13,000× g for 20 min at 4 °C. In order to remove pigments and lipids, the extracts were filtrated using Sep-Pak Plus C18 columns, Merck, Darmstadt, Germany. Subsequently, it was evaporated under vacuum at 40 °C near dryness. For the purification process, the method of Dobrev [31] was used. Extracts were diluted with 5 mL of 1 M formic acid and passed through OASIS MCX columns, Thermo Fisher Scientific, Waltham, MA, USA. The column was washed with 5 mL of 1 M formic acid. Abscisic acid and indole acetic acid were eluted with 5 mL of methanol. The phosphate riboside cytokinins were then eluted with 5 mL of 0.35 M ammonia in water. Subsequently, the basic cytokinins, ribosides and glycosides were eluted with 5 mL of 0.35 M ammonium in 60% v/v of methanol. Finally, the solvents were evaporated using a rotavap at 40 °C. The samples were dissolved in 100 µL of acetonitrile/water (50:50) (v/v). Then, 5 µL were injected into an Agilent 1100 Series HPLC (High Performance Liquid Chromatography) through an Eclipse XDB-C18 column, Agilent Biotek, Santa Clara, CA, USA at a flow rate of 0.5 mL/min using a linear gradient of acetonitrile (B) in 0.0005% v/v acidified water with acetic acid (A): 10% B for 5 min, 17% B for 10 min, then 50% B for 11 min, finally increased to 90% B and maintained for 5 min. The areas were read at a wavelength of 270 nm. Standards purchased from Sigma were used for calibration, and the retention times were ABA (16.8 min), IAA (16.4 min), trans zeatin (tZ) (5.5 min) and trans zeatin riboside (tZR) (13.9 min).
2.6. Phosphate Solubilization in Liquid Medium
A culture of B. subtilis subsp. spizizenii in broth NBRIP (National Botanical Research Institute’s phosphate growth medium) [32] was used. Samples of 1.5 mL were taken at 24, 48, 72 and 96 h of culture. They were centrifuged in a microcentrifuge at 12,000 rpm, and the supernatants were used for phosphate determination through the vanadate-molybdate method [33] by spectrophotometry at 460 nm.
2.7. Preparation of Planktonic and Biofilm Inoculate for Growth-Promotion Assays
B. subtilis subsp. spizizenii was cultivated in liquid MSM with 1% glycerol and 35 mM L-glutamic acid. For the production of bacteria in a planktonic state, they were cultivated at 30 °C with agitation at 150 rpm for 96 h in a rotatory agitation incubator, obtaining a count of 108 CFU/mL. To produce biofilm, the culture was maintained under static conditions at 30 °C for 96 h, obtaining a count of 109 CFU/g. In both cases, the bacterial counts were carried out by the serial dilution method and colony count on nutritive agar plate [34].
2.8. Seed Germination Assays
Seeds of L. sativa (lettuce) of three varieties were used: Waldman’s Green, Crimor and Grand Rapid. The seeds were disinfected by washing with 70% alcohol and then three times with sterile distilled water. A layer of sterilized cotton covered with sterile filter paper with the pore size equivalent to Whatman Grade 3 was placed in sterile Petri dishes and moistened with 5 mL of sterile distilled water. Ten seeds were placed in each Petri dish. Each seed was inoculated with 0.1 mL of planktonic culture and maintained under dark conditions at 22 °C. The seeds that received the treatment with bacterial biofilm were mixed with it due to its great adherence on the seeds. Simultaneously, a control with seeds that received 0.1 mL of distilled water was performed (Figure S1).
A completely randomized design with three replicates per treatment was used. Observations were made 4 and 7 days after inoculation without uncovering the boxes, and a visible radicle length of at least 2 mm was the criterion for germination [35]. The germination percentage (G%) was determined according to Araya [36].
At 15 days, the length of hypocotyl and the radicle were measured in each seedling.
2.9. Greenhouse Assays
Seeds of L. sativa of Crimor and Grand Rapid varieties were soaked for 15 min with planktonic inoculum or biofilm. Subsequently, they were sown in seedling trays with cells of 5 cm in diameter and 10 cm in depth with commercial substrate and compost (3:1). Twenty-five seeds were placed at each treatment. The assay was carried out at an average temperature of 20 °C. After 25 days, the seedlings were harvested and separated into aerial biomass and roots, cutting each seedling at the height of the neck. Each part of seedling was weighed (using an analytical balance with 0.0001 g precision) as fresh biomass due to its small size (Figure S2).
On the other hand, seeds of L. sativa of the Grand Rapid variety were inoculated with planktonic inoculum or biofilm and sown in seedling trays as describe above. After 20 days, the seedlings were transplanted into 2 L pots, placing one seedling per pot and filling with the same substrate (commercial substrate and compost, 3:1). The assay was carried out in greenhouse at the Faculty of Agronomy (University of Buenos Aires) (34°45′ S, 60°31′ W) at an average temperature of 24 °C. Twenty-five plants were used per treatment. After 60 days, the plants were harvested and dried in an oven at 70 °C until constant weight. Then, the plants were separated in aerial biomass and root, and each part was weighed.
2.10. Statistical Analysis
The simple ANOVA test was used. Means were compared using Tukey’s test at a significance level of 0.05.
3. Results
3.1. Bacillus as a Biocontrol Agent
3.1.1. Antifungal Activity of Liquid Culture Supernatants of B. subtilis subsp. spizizenii and B. subtilis var. natto
For both Bacillus strains, the synthesis of water-soluble metabolites with the capacity to inhibit the growth of the fungi F. solani and P. ultimum (measured by the inhibition halo) was observed after 48 h of bacterial culture (Table 1). The maximum antifungal inhibition halo of B. subtilis subsp. spizizenii was at 96 h of bacterial culture and was similar for both fungi (1.9 ± 2.1 mm for F. solani and 16.2 ± 1.4 mm for P. ultimum). In the case of B. subtilis var. natto, there was not a significant increase in the antifungal activity after 48 h of bacterial culture. The antifungal activity of B. subtilis subsp. spizizenii was always higher than that of B. subtilis var. natto, at 96 h of culture B. subtilis subsp. spizizenii was 274% higher for P. solani and more than 352% for P. ultimum, with respect to B. subtilis var. natto in both cases.

Table 1.
Antifungal activities of cell-free supernatants of two B. subtilis strains grown in liquid potato dextrose medium.
3.1.2. Growth and Antifungal Activity of B. subtilis subsp. spizizenii and B. subtilis var. natto in Simple Liquid Media
Both bacteria were also capable of synthesizing antifungal metabolites in simple culture media by using glucose or glycerol as a carbon source (Table 2). In those media, the antifungal activity was detected after 72 h of culture, which corresponds to late stationary phase (Figure 1). B. subtilis subsp. spizizenii showed its maximum antifungal activity in this system at 96 h of culture, while it remained constant for B. natto. Like in the complex medium, B. subtilis subsp. Spizizenii antifungal activities were always superior to those of B. subtilis var. natto. For both bacteria, the fungal growth inhibition with glucose or glycerol was similar, with a halo of approximately 15 mm, although the growth in glucose was double that in glycerol (Figure 1). Glycerol is a promising carbon source for a biofertilizer production, and given that glycerol is a byproduct of the biodiesel industry, its use may reduce biofertilizer manufacturing costs. For these reasons, the following assays were made with B. subtilis subsp. spizizenii in simple media with glycerol as a carbon source.

Table 2.
Antifungal activities of cell-free supernatants of two B. subtilis strains grown in liquid glucose or glycerol media.
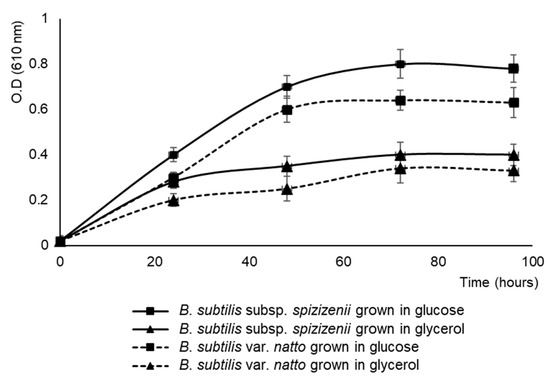
Figure 1.
Growth curves of B. subtilis strains grown in MSM, 35 mM L-glutamic acid and 1% glycerol or 1% glucose. Data are the means of the three experiments.
3.1.3. Effect of Culture of B. subtilis subsp. spizizenii in Presence Phytopathogenic Fungi
The presence of phytopathogens fungal buttons (P. ultimum or F. solani) in the culture medium of B. subtilis subsp. spizizenii did not affect the bacterial growth (Figure 2A) but caused an increase in the pH of the media (Figure 2B). After 60 h of culture, the pH of the bacterium alone was 6.5 ± 0.3 mm, while with P. ultimum, the button was 8.8 ± 0.3 mm, and 8.6 ± 0.2 mm with F. solani button.
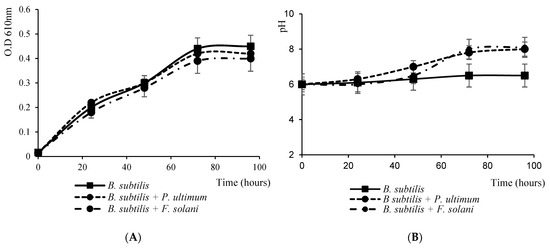
Figure 2.
(A) Growth curve and (B) pH of the media of B. subtilis subsp. spizizenii cultured in the presence of buttons of P. ultimum or F. solani in 1% MSM, 35 mM L-glutamic acid and 1% glycerol. Data are the means of the three replicated experiments.
Antifungal activities of the cell free supernatants of B. subtilis subsp. spizizenii cultures were also not affected by the presence of the fungi in the culture. This is due to the inhibitory effect of bacteria cell-free supernatants cultured alone being similar to that of the cultures in presence of F. solani or P. ultimum. The antifungal activities were detected at 72 h of culture, with the maximum at 96 h (Table 3).

Table 3.
Antifungal activity of cell-free supernatants of B. subtilis subsp. spizizenii cultured alone or with P. ultimum or F. solani.
3.2. Biofertilization Mechanisms
3.2.1. Quantitative Evaluation of Inorganic Phosphorus Solubilization by B. subtilis subsp. spizizenii
The bacterium was able to solubilize phosphorus, as an increase in phosphorus concentration in the incubation medium was observed with a maximum of 20.0 ± 2.1 mg/L at 48 h and an abrupt decrease after that time (Figure 3), i.e., when the bacterium enters in its stationary phase (Figure 1).
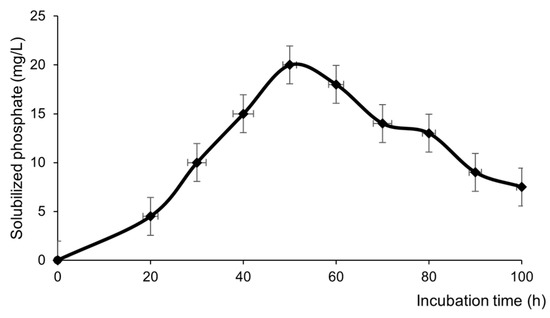
Figure 3.
Solubilization of phosphate by B. subtilis subsp. spizizenii grown in NBRIP broth. Data are the means of three experiments.
3.2.2. Synthesis of Plant Growth Regulators by B. subtilis subsp. spizizenii
Indole acetic acir (IAA), two cytokinins, i.e., trans zeatin T (tZ) and trans zeatin riboside (tZR), and abscisic acid (ABA) production in a culture of B. subtilis subsp. spizizenii in its stationary phase was quantified. The mean IAA concentration was 0.38 µg/mL. Among the cytokinins, only the synthesis of tZ was detected (0.14 µg/mL), and the average concentration of ABA was 0.29 µg/mL (Table 4).

Table 4.
Quantification of growth hormones in a culture of B. subtilis subsp. spizizenii.
3.3. Effects of B. subtilis subsp. spizizenii Inoculation as Planktonic Form or Biofilm on Three Varieties of L. sativa
3.3.1. Effects on Seed Germination
The effect of the bacterium on three varieties of L. sativa was tested. For all varieties, the non-inoculated seeds reached their maximum germination after four days (Table 5). The Crimor variety presented the lowest germination capacity. For this variety, the planktonic inoculum caused a 12% increase in germination. For Waldman’s Green and Grand Rapid varieties, the planktonic inoculum had no effect. The inoculation with biofilm caused a delay in the germination of all lettuce varieties, and after 4 days, none of the seeds had germinated; however, after 7 days, Waldman’s Green and Grand Rapid reached the control values, while for the Crimor variety, its germination was similar to that of the planktonic inoculum.

Table 5.
Germination percentages of L. sativa seeds inoculated with planktonic form or biofilm of B. subtilis subsp. spizizenii.
3.3.2. Effects on Seedlings
L. sativa seed inoculation with B. subtilis subsp. spizizenii had a positive effect on seedlings for Crimor and Grand Rapid varieties, the positive effect was depended on the mode of application. The greatest effect was on the seeds of the Grand Rapid variety. Compared with the control, the inoculation of plankton or biofilm produced an increase in the radicle length of 30% and 53%, respectively (p < 0.05). Hypocotyl development was also higher with both types of inoculation (p < 0.05), showing increases of 19% with respect to the control for planktonic inoculation and 31% with the biofilm. The biofilm application was significantly more effective than the planktonic one, showing an increase of 17% in the radicle and 10% in the hypocotyl (Figure 4).
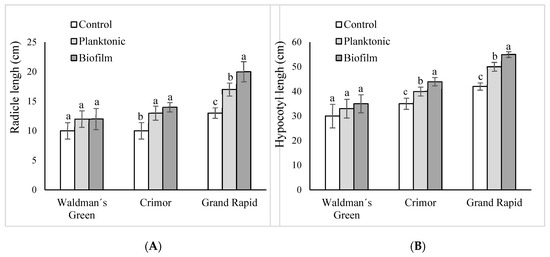
Figure 4.
Morphological parameters of seedlings of different varieties of L. sativa grown from seeds inoculated with B. subtilis subsp. spizizenii in its planktonic or biofilm form. (A) Radicle length. (B) Hypocotyl length. Different letters correspond to significant differences between treatments for each L. sativa variety (p < 0.05).
In the Crimor variety, as in Grand Rapid varieties, the effect was also dependent on the form of inoculation. Compared to the control, inoculation with plankton or biofilm produced a 30% and 40% increase in radicle length, respectively (p < 0.05), while hypocotyl length increases were 14% and 26%, respectively (p < 0.05). As in Grand Rapid, the application of the biofilm turned out to be more effective than the planktonic inoculation, with increases of 8% in the growth of the radicle and 10% in the development of the hypocotyl (Figure 4).
In the case Waldman’s Green, although the differences with the control were not significant, it showed an increase of 20% in the radicle for both inoculation methods, and increases in the hypocotyl of 10% for planktonic inoculum and 17% for biofilm inoculum respect to control (Figure 4).
3.3.3. Effect on the Growth of 25-Day-Old Plants of the Crimor and Grand Rapid Varieties
Due to the higher PGPR activity observed with the Crimor and Grand Rapid, the trials continued with these varieties. For both, root development was favored with seed inoculation (Figure 5A). For the Crimor variety, there was an increase of 42% with planktonic inoculation and 57% with biofilm application; there were no significant differences between the inoculation methods. In the case of the Grand Rapid variety, the mode of inoculum application induced differences in root development, with greater growth for biofilm (82%) than for planktonic inoculum (39%); being the former 33% more effective than planktonic inoculum.
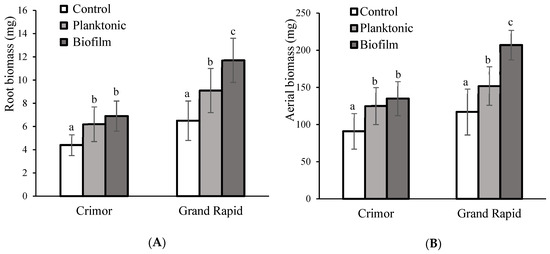
Figure 5.
Biomass of different varieties of L. sativa grown from seeds inoculated with Bacillus subtilis subsp. spizizenii in its planktonic or biofilm form. (A) Root biomass. (B) Aerial biomass. Different letters correspond to significant differences between treatments for each L. sativa variety (p < 0.05).
The inoculation of seeds with B. subtilis subsp. spizizenii also exerted a positive effect on the aerial biomass of L. sativa (Figure 5B). In the Crimor variety, an increase of 37% was observed with the planktonic inoculum and 47% with the biofilm, there was no significant difference between the inoculation methods. In the Grand Rapid variety, increases of 30% were found with the planktonic inoculum and 77% with the application of the biofilm. As in the case of the roots, the effect of the biofilm was 36% higher than with the planktonic inoculum.
3.3.4. Effect on the Growth of Plants of the Grand Rapid Variety at Harvest Time
To determine if the positive effect of the inoculation was prolonged over time, the growth achieved by the Grand Rapid variety at the time of harvest (60 days) was studied. At that time, the positive effects of the inoculation on roots and aerial parts continued (Figure 6). In root development, the application of the bacteria in a planktonic state or as a biofilm showed increases of 58% and 95%, respectively. In the case of the aerial part, the application of the bacteria in a planktonic state or as a biofilm compared to control showed increases of 56% and 86%, respectively. Significant differences between inoculation treatments also persist, with the biofilm being the most effective method, showing an increase of 24% in root development and 20% in the aerial part compared to planktonic inoculation.
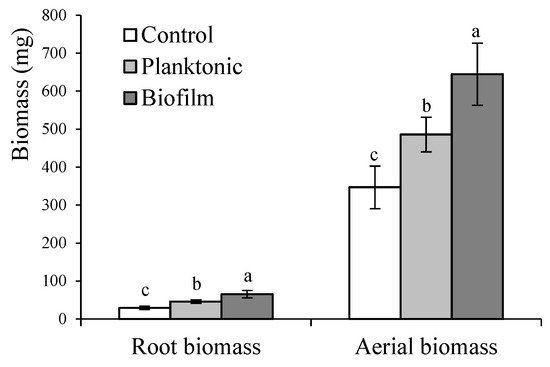
Figure 6.
Biomass of Grand Rapid L. sativa variety at harvest time (60 days) grown from seeds inoculated with B. subtilis subsp. spizizenii in its planktonic or biofilm form. Different letters correspond to significant differences between treatments (p < 0.05).
3.4. Bacterial Endophytism
The presence of B. subtilis subsp. spizizenii in the roots of L. sativa var. Grand Rapid was evaluated at 60 days of seed inoculation. In total, 4.104 CFU/g of fresh root weight in roots from seeds inoculated with biofilm was detected, while no bacteria were recovered in control roots.
4. Discussion
PGPR bacteria are known to have a positive interaction with plant roots, either directly by influencing plant growth, or indirectly by modifying the rhizosphere environment [37,38]. The latter effect could be mediated by the release of substances that can act as biocontrols, for example, antibiotics, lithic enzymes, and other metabolites, which are able to control the proliferation of soil pathogenic microorganisms, resulting in the improvement of plant development and growth [13,16]. In this study, both, B. subtilis subsp. spizizenii and B. subtilis var. natto showed antifungal activity (measured by inhibition halos) against P. ultimum and F. solani in complex and simple media. Moreover, the produced metabolites were present in cell-free supernatants, implying that they were not attached to the bacterial cell. Walker [39] obtained similar results with aqueous supernatants of B. subtilis, strains J7, B3 and C1, which are active against Botrytis cinerea. Also, the antifungal activity was similar in complex medium and in simple media using glucose or glycerol as carbon sources [40,41]. The similar bacterial behavior in different media suggests that this could also happen in natural environments. The antifungal metabolites would be synthesized when the bacterial density is high enough to act against pathogens [42]. Moreover, Hultberg [43] showed that the application of these kind of metabolites does not affect the indigenous flora at the rhizosphere level. These facts place the studied strains as an alternative to synthetic fungicides [44].
Both B. subtilis subsp. spizizenii and B. subtilis var. natto showed antifungal activity in the late phase of bacterial growth, with the maximum amount in the late stationary phase, and also in all the assayed media. Another bacterium of the genus Bacillus, Bacillus sp. B209, also synthesizes antifungal metabolites in the stationary phase [45]. In all cases, B. subtilis subsp. spizizenii always showed higher fungal activity than B. subtilis var. natto, which makes it superior as a bioinoculant. The growth of B. subtilis subsp. spizizenii was not affected by the presence of P. ultimun or F. Solani, although the pH of the media was higher when the fungus was present. The fact that the antifungal activity of B. subtilis subsp. spizizenii was not induced by the culture in the presence of fungi suggests that the synthesis of the antifungal metabolites would be innate.
Other types of PGPR effects are associated with the capacity of the microorganisms to enhance nutrient availability for plant growth, for example, phosphates solubilization. We showed that B. subtilis subsp. spizizenii was able to solubilize inorganic phosphate, which is fundamental for plant nutrition. The main phosphate-dissolving activity was in the exponential growth stage, and decreased during the stationary phase, suggesting that this activity could be regulated by the bacterium. Growth processes in plants are controlled by internal signals that depend on the adequate supply of minerals through the roots; therefore, it is expected that increasing levels of different minerals by PGPR actions contribute to plant growth [9].
Another way for microorganisms to act as a PGPR is through the production of phytohormones, such as indole acetic acid (AIA), gibberellins and cytokines, which modify the plant morphogenesis and cellular proliferation [15]. In this work, metabolites with plant-growth activity (IAA and cytokinin) were detected in the stationary phase of growth of B. subtilis subsp. spizizenii in a minimum medium, with glycerol and L-glutamic acid as carbon and nitrogen sources, respectively. These plant-growth factors could easily be assimilated by the plant, so phytostimulation could be one of the possible mechanisms of this bacterium to promote plant development. Also, other authors have attributed the PGPR effect exerted by certain bacteria on plants to the production of IAA, as indicated by Deshwal [46] for Oryza sativa L. plants inoculated with Pseudomonas sp. Similarly, Ahmed [47] working with IAA-producing Bacillus strains, found an increase of 40% in Solanum tuberosum seedlings inoculated with the bacteria. Moreover, auxin- and cytokinin-type phytohormones promoted root development [48].
The various PGPR actions (the synthesis of antifungal metabolites and plant-growth hormones, and its ability to dissolve phosphates) suggest that B subtilis subsp. spizizenii is a good candidate to produce bioinoculants. Currently, most bioinoculants are produced in liquid form (i.e., in the planktonic form). The main challenge of this type of formulation is that a number of viable cells are needed, and they must be preserved over time to hold its effectiveness throughout the entire marketing chain. Furthermore, when the bioinoculant is used in seeds there, must be a close PGPR microorganism–seed interaction, which is generally achieved by adding several substances, making it more expensive [49].
Considering these two aspects, namely the cell viability and the close interaction between microorganism and seed, the bacterial biofilm would be an innovative and promising proposal. In this sense, B. subtilis subsp. spizizenii, can produce a biofilm [50,51], a characteristic that differentiates it from other microorganisms used as bioinoculants. The biofilm cells are held together by an extracellular matrix composed of exopolysaccharides, proteins, and nucleic acids. This exopolysaccharide network may provide an anchoring site that would protect the bacterial cells. In addition, the bacteria would be in permanent contact with the seed, so the physicochemical characteristics of the biofilm would allow it to function as a mucilage, enabling greater adherence to the seed, with a longer seed–microorganism contact time. Gonzalez [52] found that the presence of a mucilage in seeds can favor the adhesion of microorganisms and the assimilation of the products synthesized by them, as is the case of gibberellins or other hormones that facilitate germination. Furthermore, this matrix can be degraded by the biofilm microorganisms using it as a nutrient source, which would increase their survival [53].
Another aspect to consider when a biofilm is used as a bioinoculant lies in that the growth-promoting effect exerted by bacteria depends on the relationship between the bacterial strain and the plant species; thus, a bacterium may show excellent effects on one plant species but not on another. This plant–microorganism interaction is so particular that different responses have been observed with the application of the same microorganism between two varieties of the same plant species [54]. Each plant species produces its own chemical molecules that attract certain microorganisms (chemotaxis) and generate the appropriate conditions for their establishment and multiplication [55]. Bacillus mycoides showed a marked PGPR effect on papaya, rice, cassava and sunflower seeds, with an increase in the germination in all cases; however, it did not have a beneficial effect on L. sativa seeds. Even more, other microorganisms, such as Trichoderma harziarum, Enterobacter aerogenes and Microbacterium sp., showed deleterious effects on L. sativa, although they were effective PGPR on other plant species [52]. These facts emphasize the great specificity that exists between the microbial strain and the plant species. In the case of L. sativa, the literature cites that bacterium belonging to the genera Hafnia and Beijerinckia as PGPRs. Díaz-Vargas [56] indicated that the strains HP-3; HP-27, corresponding to Hafnia alvei; and the strain S4BE of the genus Beijerinckia showed increases greater than 50% in germination tests.
The International Seed Testing Association (ISTA) has established that the germination process is a metabolically active state that is physiologically manifested by cell division and differentiation, with the emergence of the radicle being considered a sign of seed germination [57]. According to this, the effect of B. subtilis subsp. spizizenii inoculation, in its planktonic and biofilm form, on the radicle emergency of three varieties of L. sativa, Crimor, Waldman´s Green and Grand Rapid, was studied. The effect of both types of inoculation on germination was dependent on the L. sativa variety, with a positive effect only for Crimor, which is probably due to its low germination power, unlike the high power of the other varieties. The delay observed with biofilm inoculation could be due to the fact that the biofilm would have initially been like a barrier between the seed and the environmental signals (humidity, temperature and gaseous environment) that induce germination [58]. Once this barrier is overcome, the exopolysaccharide matrix of the biofilm would allow for closer contact with the bacteria. It should be noted that although the application of the biofilm delayed the germination of all seed varieties, it had no negative effect on any of them, reaching similar germination percentages in all cases after 7 days.
The effect of seed inoculation was also dependent on lettuce variety. The most prominent effect was observed with the Grand Rapid variety; it was less with Crimor and none with Waldman’s Green. In the case of Grand Rapid, the positive effect of seed inoculation was prolonged in time during the successive vegetable development stages; it was first observed at radicle and hypocotyl, them along the plant growth cycle until the harvest time. The biofilm always had a superior effect compared to the planktonic inoculum. Both roots and aerial-part development were positively affected. Root development was enhanced by bacteria inoculation, which increased its ability to absorb nutrients. This allowed for a greater growth of the aerial part. Our results are in accordance with those reported by Pereira [59] and Kloepper [38], which found that growth-promoting bacteria P. fluorescens increased root development, directly affecting crop yield. Díaz-Vargas [56] reported that a liquid inoculant with Pseudomonas aeruginosa SP5 and Azospirillum brasilense T2P010, which was applied to L. sativa, stimulated both germination and vegetative development. Furthermore, these plant-growth effects could also be related to the production of metabolites with plant-growth activity (IAA and cytokinin) and phosphate solubilization, as previously indicated. Plant development is controlled by internal signals that depend on the adequate supply of minerals through the roots; therefore, the action of PGPRs by increasing different minerals levels in plant tissues contributes to growth [46]. Therefore, considering that auxin- and cytokinin-type phytohormones promote root development [48] and allow the plant to absorb a greater amount of soil minerals, it could be assumed that these would be efficient mechanisms of action used by B. subtilis subsp. spizizenii to execute its growth-promoting activity on L. sativa. In this work, B. subtilis subsp. spizizenii was recovered from the interior of the roots of Grand Rapid inoculated with biofilm, which indicates that the bacterium was able to leave the biofilm, and penetrate and proliferate in the interior of L. sativa roots, persisting until the harvest time. Initial bacterial infection and colonization does not mean that it will continue over time. Wulff [60] observed in cabbage plants after 10 days of inoculation with B. subtilis, the presence of the bacterium in root tissues (abundantly) and in its aerial part. However, after 35 days they did not detect the bacterium in any plant tissue. Other authors also detected a decrease in the bacterial population of the inoculated microorganism. For example, Kloepper [61], when examining the ability of six strains with PGPR activity to internally colonize cucumber roots, found that bacterial populations fell after 21 days of inoculation. Lamb [62] found that Pseudomonas aureofaciens did not remain viable inside corn plants grown in hydroponics; however, when the plant grew in soil, the bacterium was able to persist inside the plant. The environment within host cells may be repressive enough to restrict the growth rate of endophytic bacteria. The presence of B. subtilis subsp. spizizenii in L. sativa roots could be another way by which the bacterium can exert a beneficial effect on this crop [63].
The procedure of biofilm application used in this work was different from that carried out by other authors. Ricci [64] applied biofilms of Bacillus sp., Pseudomonas sp. or both on the roots of cultivated tomato (Solanum lycopersicum L. cv Trust). Domínguez-González [65] inoculated Triticum aestivum with a biofilm of Streptomyces spp. induced on a perlite carrier, while Gorodylova [66] used a microbial consortium biofilm grown on Fe-modified zeolite grains for the treatment of contaminated soils. Unlike these works, the form of biofilm application presented here would allow the bacteria to implement its positive effect after the stimulation of seed germination by environmental signals.
5. Conclusions
Seed inoculation with biofilm of B. subtilis subsp. spizizenii obtained under static culture conditions resulted in a significantly higher biomass yield of L. sativa than inoculation with the traditional planktonic technique. The Grand Rapid variety showed the highest biomass increases for all the different stages of the plant life cycle, including the formation of radicle and the hypocotyl, the development stage as well as harvest time; both root and aerial parts of the plant showed biomass increases.
Biofilm inoculation showed other positive effects, including a large phosphorus concentration in the incubation medium, as well as increments in the production of plant-growth regulators, namely, indole acetic acid, cytokinin and abscisic acid.
Altogether, the biofilm of B. subtilis subsp. spizizenii not only behaved as a biofertilizer and phytostimulator of L. sativa, but also produced metabolites with proven antifungal activity against common soil phytopathogens specific of this vegetable. Therefore, the biofilm also performed as a biocontrol agent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su152115406/s1, Figure S1: Scheme of the seed germination assays; Figure S2: Scheme of the greenhouse assays.
Author Contributions
Conceptualization, G.C.S., M.E.G. and A.P.-G.; methodology, G.C.S., S.A., J.A.E.C.-M. and A.P.-G.; formal analysis, G.C.S., M.E.G., J.A.E.C.-M. and A.P.-G.; investigation, G.C.S., M.E.G. and J.A.E.C.-M.; resources, G.C.S. and A.P.-G.; writing—original draft preparation, G.C.S., M.E.G., S.A. and A.P.-G.; writing—review and editing, G.C.S., M.E.G., J.A.C. and A.P.-G.; supervision, G.C.S., M.E.G., J.A.C. and A.P.-G.; project administration, G.C.S.; funding acquisition, G.C.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad de Buenos Aires, Proyectos de Ciencia y Técnica, grant number (UBACyT) N° 20020170100080BA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paladino, I.; Sokolowski, A.; Prack Mc Cormick, B.; Wolski, E.; Rodríguez, H.; Navas, M. Soil Quality Problems Associated with Horticulture in the Southern Urban and Peri-Urban Area of Buenos Aires, Argentina. Chapter 8. In Urban Horticulture—Necessity of the Future; Solankey, S., Akhtar, S., Maldonado, A., Rodriguez-Fuentes, H., Vidales Contreras, J., Márquez, R., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Giuffré, L.; Ratto, S.; Marbán, L.; Schonwald, J.; Romaniuk, R. Heavy Metal Risks in Urban Agriculture. Cienc. Suelo Argent. 2005, 23, 101–106. (In Spanish) [Google Scholar]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen Fertilization. A Review of the Risks Associated with the Inefficiency of Its Use and Policy Responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Viteri, M.L.; Ghezán, G.; Iglesias, D. Tomato and lettuce production marketing and comsumption. Estud. Socioeconómico De Los Sist.: INTA Balcarce Argent. 2013, 14, 185–190. [Google Scholar]
- Sammauria, S.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020, 202, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Lokesh Kumar Jat, L.K. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture: Perspectives and Challenges. In PGPR Amelioration in Sustainable Agriculture. Food Security and Environmental Management; Singh, K.A., Kumar, A., Singh, P.K., Eds.; Elevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Pérez, J.; Carmona, S.; Zamudio, E.; Rivera, N.; Calva, G. Bioremediation of soils from oil spill impacted sites using biosurfactants producing, native, free-living nitrogen fixing bacteria. Rev. Int. Contam. Amb. 2017, 33, 105–114. [Google Scholar] [CrossRef]
- Gomez Ramirez, L.F.; Uribe Velez, D. Phosphorus solubilizing and mineralizing Bacillus spp. Contribute to rice growth promotion using soil amended with rice Straw. Curr. Microbiol. 2021, 78, 932–943. [Google Scholar] [CrossRef]
- Sarti, G.; Miyazaki, S. Bacillus subtilis crude extracts with antifungal activity against soybean (Glycine max) phytopathogens and Bradyrhizobium japonicum coinoculation effect. Agrociencia 2013, 47, 373–383. (In Spanish) [Google Scholar]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.H.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef] [PubMed]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The role of beneficial microorganisms in soil quality and plant health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 287. [Google Scholar] [CrossRef]
- Mekonnen, H.; Kibret, M. The roles of plant growth promoting rhizobacteria in sustainable vegetable production in Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 15. [Google Scholar] [CrossRef]
- Miao, S.; Liang, J.; Xu, Y.; Yu, G.; Shao, M. Bacillaene, Sharp objects consist in the arsenal of antibiotics produced by Bacillus. Cell. Physio. 2023. early view. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Massa, F.; Defez, R.; Bianco, C. Exploitation of Plant Growth Promoting Bacteria for Sustainable Agriculture: Hierarchical Approach to Link Laboratory and Field Experiments. Microorganisms 2022, 10, 865. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive secondary metabolites from Bacillus subtilis: A comprehensive review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef]
- Tran, C.; Cock, I.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and their mode of action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Sarti, G.; Miguez Cristóbal, J.; Curá, A. Optimization of culture conditions for development of a bacteria biofilm and its application as a biofertilizer in Solanum lycopersicum L.var. Río grande. Rev. Protección Veg. Cuba. 2019, 34, 2224–4697. [Google Scholar]
- Hobley, L.; Harkins, C.; MacPhee, C.; Stanley-Wall, N. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef]
- Penha, R.; Vandenberghe, L.; Faulds, C.; Soccol, V.; Soccol, C. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 70. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thumheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed]
- Daboor, S.; John, R.; Rohde, A.; Zhenyu, C. Disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate lyase enhances pathogen eradication by antibiotics. J. Cyst. Fibros. 2020, 30, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, P.; Murray, R.; Wood, W.; Krieg, N. Methods of General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Dobrev, P.; Kaminek, M. Fast and Efficient Separation of Cytokinins from Auxin and Abscisic Acid and Their Purification. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Kitson, R.; Mellon, M. Colorimetric Determination of Phosphorus as molybdivanadophosphoric acid. Industrial and Engineering. Chem. Anal. Edit. 1994, 16, 379–383. [Google Scholar] [CrossRef]
- Frioni, L. Soil Microbial Ecology; Universidad de la República: Montevideo, Uruguay, 1990; pp. 90–94. (In Spanish) [Google Scholar]
- Marti, L. Effect of Saninity and Temperature on Seed Germination of Linonium mansaltrarum. Ph.D. Thesis, Universidad Politécnica Superior de Valencia, Gandía, Spain, 2010. (In Spanish). [Google Scholar]
- Araya, E.; Gómez, L.; Hidalgo, N.; Valverde, R. Efecto de la luz y del ácido giberélico sobre la germinación in vitro de (Alunus Acuminata). Agron. Costarric. 2000, 24, 75–80. [Google Scholar]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Kloepper, J.; Zablotowicz, R.; Tipping, E.; Lifshitz, R. Plant Growth Promotion Mediated by Bacterial Rhizosphere Colonizer. In The Rhizosphere and Plant Growth; Keister, D.L., Cregan, P.B., Eds.; Kluwer: Dordrecht, The Netherlands, 1991; pp. 315–326. [Google Scholar]
- Walker, R.; Powell, A.; Seddon, B. Bacillus isolates from the spermosphere of peas and dwarf French beans with antifungal activity against Botrytis cinerea and Pythium species. J. Applied Microbiol. 1998, 84, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, M.; Del Gallo, M. Cell-free supernatants of plant growth-promoting bacteria: A review of their use as biostimulant and microbial biocontrol agents in sustainable agriculture. Sustainability 2020, 12, 9917. [Google Scholar] [CrossRef]
- Kiesewalter, H.; Lozano-Andrade, C.; Wibowo, M.; Strube, M.; Maróti, G.; Snyder, D.; Jørgensen, T.; Larsen, T.; Cooper, V.; Weber, T.; et al. Genomic and chemical diversity of Bacillus subtilis secondary metabolites against plant pathogenic fungi. mSystems 2021, 6, e00770–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential agents for controlling cellular communication, motility, and antagonism. Front. Mol. Biosci. 2021, 8, 727070. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, M.; Alsberg, T.; Khalil, S.; Alsanius, B. Suppression of disease in tomato infected by Pythium ultimum with a biosurfactant produced by Pseudomonas koreensis. BioControl 2010, 55, 435–444. [Google Scholar] [CrossRef]
- Ambrico, A.; Trupo, M. Efficacy of cell free supernatant from Bacillus subtilis ET-1, an Iturin a producer strain, on biocontrol of green and gray mold. Postharvest Biol. Technol. 2017, 134, 5–10. [Google Scholar] [CrossRef]
- Cornea, C.; Grebenisan, I.; Mateescu, R.; Campeanu, E. Isolation and characterization of new Bacillus spp. Strains useful as biocontrol agents of plants pathogens. Roumanian Biotechnol. Lett. 2003, 8, 1115–1122. [Google Scholar]
- Deshwal, V.; Thapliyal, N. Plant growth promoting Pseudomonas strains effectively enhance plant growth of Oryza sativa. JPDS 2019, 11, 471–474. [Google Scholar]
- Ahmed, A.; Hasnain, S. Auxin-producing Bacillus sp.: Auxin quantification and effect on the growth of Solanum tuberosum. Pure Appl. Chem. 2010, 82, 313–319. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sanches Santos, M.; Nogueira, M.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Expr. 2019, 9, 205. [Google Scholar] [CrossRef]
- Galelli, M.; Sarti, G.; Miyazaki, S. Lactuca sativa biofertilization using biofilm from Bacillus with PGPR activity. J. Appl. Hortic. 2015, 17, 186–191. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant growth-promoting bacteria (PGPB) with biofilm-forming ability: A multifaceted agent for sustainable agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- González, F.; Fuentes, N. Mechanism of action of five microorganisms promoting plant growth. Rev. Col. de Ciencias Agr. 2016, 34, 17–22. (In Spanish) [Google Scholar]
- Tsai, A.; McGee, R.; Dean, G.; Haughn, G.; Sawa, S. Seed mucilage: Biological functions and potential applications in biotechnology. Plant Cell Physiol. 2021, 62, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Arellano, C.; Zúñiga, D. Efecto de diferentes bacterias aisladas de rizósfera de Caesalpina spinosa en la germinación de diferentes especies vegetales culivados. Zonas Áridas 2008, 12, 137–153. [Google Scholar]
- Dakora, F.; Phillips, D. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Díaz Vargas, P.; Ferrera-Cerrato, R.; Almaraz-Suárez, J.; Alcántar, G. Inoculation of growth promoting bacteria in lettuce. Terra 2001, 19, 231–238. (In Spanish) [Google Scholar]
- Mis, F.; Ermis, S.; Powell, A.; Demir, I. Radicle emergence (RE) test identifies differences in normal germination percentages (NG) of watermelon, lettuce and carrot seed lots. Seed Sci. Technol. 2022, 50, 257–267. [Google Scholar] [CrossRef]
- Qasem, J. Weed Seed Dormancy: The Ecophysiology and Survival Strategies. Chapter 2. In Seed Dormancy and Germination; Jimenez-Lopez, J., Ed.; Intechopen Limited: London, UK, 2020. [Google Scholar]
- Pereira, J.; Cavalcante, V.; Baldani, J.; Dobereiner, J. Sorghum and rice inoculation with Azospirillum sp. and Herbaspirillum seropedicae in field. Plant Soil 1988, 110, 269–274. [Google Scholar] [CrossRef]
- Wulff, E.; van Vuurde, L.; Hockenhull, J. The ability of the biological control agent Bacillus subtilis, strain BB, to colonise vegetable brassicas endophytically following seed inoculation. Plant Soil 2003, 255, 463–474. [Google Scholar] [CrossRef]
- Kloepper, W.; Wei, G.; Tuzun, S. Rhizosphere Population Dynamics and Internal Colonisation of Cucumber by Plant Growth-Promoting Rhizobacteria Which Induce Systemic Resistance to Colletotrichum orbiculare. In Biological Control of Plant Diseases; Tjamos, E.C., Papavizas, G.C., Cook, R.J., Eds.; Springer: Cham, Switzerland, 1992; pp. 185–191. [Google Scholar]
- Lamb, T.; Tonkyn, D.; Kluepfel, D. Movement of Pseudomonas aerofaciens from the rhizosphere to aerial plant tissue. Can. J. Microbiol. 1996, 42, 1112–1120. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwaria, Z.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Schwinghamer, T.; Fan, D.; Smith, D.; Gravel, V. Growth promotion of greenhouse tomatoes with Pseudomonas sp. and Bacillus sp. biofilms and planktonic cells. Appl. Soil Ecol. 2019, 138, 61–68. [Google Scholar] [CrossRef]
- Domínguez-González, K.; Robledo-Medrano, J.; Valdez-Alarcón, J.; Hernández Cristobal, O.; Martínez-Flores, H.; Cerna-Cortés, J.; Garnica-Romo, M.; Cortés-Martínez, R. Streptomyces spp. biofilmed solid inoculant improves microbial survival and plant-growth efficiency of Triticum aestivum. Appl. Sci. 2022, 12, 11425. [Google Scholar] [CrossRef]
- Gorodylova, N.; Seron, A.; Michel, K.; Joulian, C.; Delorme, F.; Soulier, C.; Bresch, S.; Garreau, C.; Giovannelli, F.; Michel, C. Zeolite-supported biofilms as inoculants for the treatment of MCPA-polluted soil and sand by bioaugmentation: A microcosm study. Appl. Soil Ecol. 2022, 180, 10461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).