Acute Ecotoxicity Potential of Untreated Tannery Wastewater Release in Arequipa, Southern Peru
Abstract
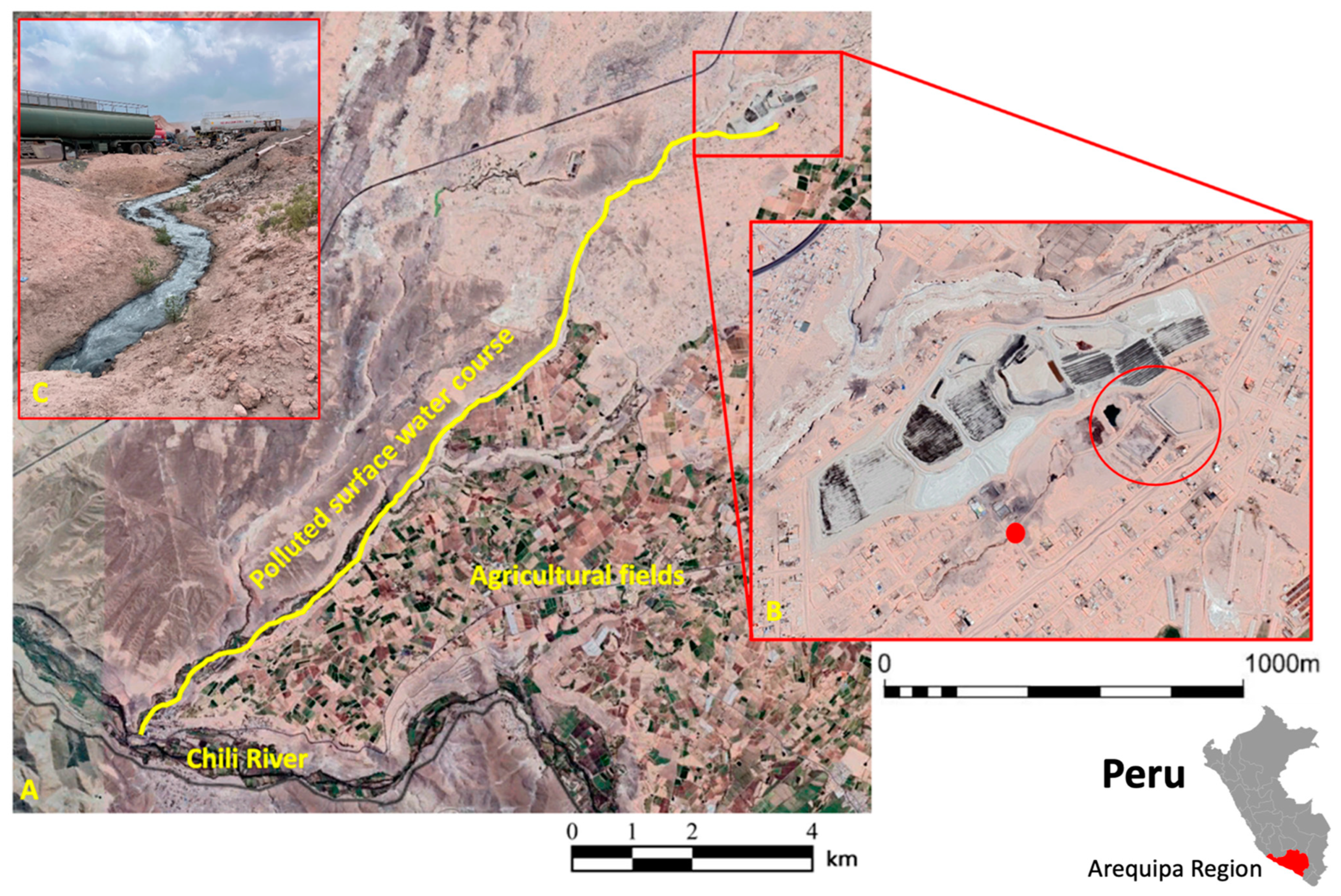
:1. Introduction
2. Materials and Methods
2.1. Determination of Physicochemical Parameters for Tannery Wastewater
2.2. Ecotoxicity Bioassays and Trophic Organisms
2.3. Quantification of Acute Toxicity
2.4. Statistical Analyses
3. Results
3.1. Physicochemical Parameters of the Harvested RSIP Effluent Used in Toxicology Experiments
3.2. Acute Mortality and Ecotoxic Indices for Model Organisms Exposed to Dilluted RSIP Tannery Wastewater
3.2.1. Lemna Minor
3.2.2. Daphnia Magna
3.2.3. Physa Venustula
3.2.4. Xenopus Laevis (Embryos and Larvae)
3.3. Determination of Acute Toxicity Units (ATU) and Toxicological Classification of Tannery Wastewater
4. Discussion
4.1. Physicochemical Parameters
4.2. Determination of Accumulated Mortality and Ecotoxic Indices in Model Organisms Due to the Effect of Exposure to Dilluted RSIP Tannery Wastewater
4.2.1. Lemna Minor
4.2.2. Daphnia Magna
4.2.3. Physa Venustula
4.2.4. Xenopus Laevis
4.3. Determination of Acute Toxicity Units (ATU) and Toxicological Classification of Tannery Wastewater
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilardi, G.; Di Palma, L.; Verdone, N. On the Critical Use of Zero Valent Iron Nanoparticles and Fenton Processes for the Treatment of Tannery Wastewater. J. Water Process Eng. 2018, 22, 109–122. [Google Scholar] [CrossRef]
- Younas, F.; Niazi, N.K.; Bibi, I.; Afzal, M.; Hussain, K.; Shahid, M.; Aslam, Z.; Bashir, S.; Hussain, M.M.; Bundschuh, J. Constructed Wetlands as a Sustainable Technology for Wastewater Treatment with Emphasis on Chromium-Rich Tannery Wastewater. J. Hazard. Mater. 2022, 422, 126926. [Google Scholar] [CrossRef] [PubMed]
- García-Valero, A.; Martínez-Martínez, S.; Faz, Á.; Terrero, M.A.; Muñoz, M.Á.; Gómez-López, M.D.; Acosta, J.A. Treatment of Wastewater from the Tannery Industry in a Constructed Wetland Planted with Phragmites australis. Agronomy 2020, 10, 176. [Google Scholar] [CrossRef]
- Joseph, K.; Nithya, N. Material Flows in the Life Cycle of Leather. J. Clean. Prod. 2009, 17, 676–682. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Picos-Benítez, A.; Pacheco-Alvarez, M.; Pérez, T.; Bandala, E.R.; Peralta-Hernández, J.M. Tannery Wastewater Treatment Using Combined Electrocoagulation and Electro-Fenton Processes. J. Environ. Chem. Eng. 2022, 10, 107290. [Google Scholar] [CrossRef]
- Mustapha, S.; Oladejo, T.J.; Muhammed, N.M.; Saka, A.A.; Oluwabunmi, A.A.; Abdulkabir, M.; Joel, O.O. Fabrication of Porous Ceramic Pot Filters for Adsorptive Removal of Pollutants in Tannery Wastewater. Sci. Afr. 2021, 11, e00705. [Google Scholar] [CrossRef]
- Pérez-Vidal, A.; Rivera-Sanchez, S.P.; Florez-Elvira, L.J.; Silva-Leal, J.A.; Diaz-Gomez, J.; Herrera-Cuero, L.F.; Lopez Botero, L.P. Removal of E. coli and Salmonella in Pot Ceramic Filters Operating at Different Filtration Rates. Water Res. 2019, 159, 358–364. [Google Scholar] [CrossRef]
- Saxena, S.; Saharan, V.K.; George, S. Enhanced Synergistic Degradation Efficiency Using Hybrid Hydrodynamic Cavitation for Treatment of Tannery Waste Effluent. J. Clean. Prod. 2018, 198, 1406–1421. [Google Scholar] [CrossRef]
- Tarcan, G.; Akıncı, G.; Danışman, M.A. Assessment of the Pollution from Tannery Effluents upon Waters and Soils in and around Kula Vicinity, Turkey. Water Air Soil. Pollut. 2010, 213, 199–210. [Google Scholar] [CrossRef]
- Singh, K.; Kumari, M.; Prasad, K.S. Tannery Effluents: Current Practices, Environmental Consequences, Human Health Risks, and Treatment Options. CLEAN–Soil Air Water 2023, 51, 2200303. [Google Scholar] [CrossRef]
- Mengistie, E.; Ambelu, A.; Van Gerven, T.; Smets, I. Impact of Tannery Effluent on the Self-Purification Capacity and Biodiversity Level of a River. Bull. Environ. Contam. Toxicol. 2016, 96, 369–375. [Google Scholar] [CrossRef]
- Gupta, P.; Rani, R.; Chandra, A.; Kumar, V. Potential Applications of Pseudomonas sp. (Strain CPSB21) to Ameliorate Cr6+ Stress and Phytoremediation of Tannery Effluent Contaminated Agricultural Soils. Sci. Rep. 2018, 8, 4860. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, A.S.; Nogueira, M.A.; Martines, A.M.; Dos Santos, C.A.; Baldesin, L.F.; Marschner, P.; Cardoso, E.J.B.N. Effects of Tannery Sludge Application on Physiological and Fatty Acid Profiles of the Soil Microbial Community. Appl. Soil Ecol. 2012, 61, 92–99. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Machuca-Martínez, F.; Barajas-Solano, A.F. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules 2021, 26, 3222. [Google Scholar] [CrossRef] [PubMed]
- OEFA. Informe Complementario de Evaluación Ambiental En El Ámbito; Del Parque Industrial de Río Seco, Provincia y Departamento de Arequipa: Lima, Peru, 2017. [Google Scholar]
- Lazo, E.A. Evaluación de La Contaminación Ambiental Generada Por Efluentes Industriales En El Proceso Productivo de Una Curtiembre de Mediana Capacidad Del Parque Industrial de Rio Seco, Arequipa. Bachelor’s Thesis, Universidad Nacional de San Agustín, Arequipa, Peru, 2017. [Google Scholar]
- Sackey, L.N.A.; Kočí, V.; van Gestel, C.A.M. Ecotoxicological Effects on Lemna minor and Daphnia magna of Leachates from Differently Aged Landfills of Ghana. Sci. Total Environ. 2020, 698, 134295. [Google Scholar] [CrossRef]
- Hu, Y.; Lei, D.; Wu, D.; Xia, J.; Zhou, W.; Cui, C. Residual β-Lactam Antibiotics and Ecotoxicity to Vibrio fischeri, Daphnia magna of Pharmaceutical Wastewater in the Treatment Process. J. Hazard. Mater. 2022, 425, 127840. [Google Scholar] [CrossRef] [PubMed]
- Tigini, V.; Giansanti, P.; Mangiavillano, A.; Pannocchia, A.; Varese, G.C. Evaluation of Toxicity, Genotoxicity and Environmental Risk of Simulated Textile and Tannery Wastewaters with a Battery of Biotests. Ecotoxicol. Environ. Saf. 2011, 74, 866–873. [Google Scholar] [CrossRef]
- Foudhaili, T.; Jaidi, R.; Neculita, C.M.; Rosa, E.; Triffault-Bouchet, G.; Veilleux, É.; Coudert, L.; Lefebvre, O. Effect of the Electrocoagulation Process on the Toxicity of Gold Mine Effluents: A Comparative Assessment of Daphnia magna and Daphnia pulex. Sci. Total Environ. 2020, 708, 134739. [Google Scholar] [CrossRef]
- Park, S.; Jo, A.; Choi, J.; Kim, J.; Zoh, K.-D.; Choi, K. Rapid Screening for Ecotoxicity of Plating and Semiconductor Wastewater Employing the Heartbeat of Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 186, 109721. [Google Scholar] [CrossRef]
- Diaz-Sosa, V.R.; Tapia-Salazar, M.; Wanner, J.; Cardenas-Chavez, D.L. Monitoring and Ecotoxicity Assessment of Emerging Contaminants in Wastewater Discharge in the City of Prague (Czech Republic). Water 2020, 12, 1079. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Guo, X.; Yang, R.; Si, X.; Zhou, J. Humic Acid Alleviates the Ecotoxicity of Graphene-Family Materials on the Freshwater Microalgae Scenedesmus obliquus. Chemosphere 2018, 197, 749–758. [Google Scholar] [CrossRef]
- Elersek, T.; Ženko, M.; Filipič, M. Ecotoxicity of Disinfectant Benzalkonium Chloride and Its Mixture with Antineoplastic Drug 5-Fluorouracil towards Alga Pseudokirchneriella subcapitata. PeerJ 2018, 6, e4986. [Google Scholar] [CrossRef]
- Adochite, C.; Andronic, L. Aquatic Toxicity of Photocatalyst Nanoparticles to Green Microalgae Chlorella vulgaris. Water 2020, 13, 77. [Google Scholar] [CrossRef]
- Arenazas, R.A.J. Evaluación Ecotoxicológica de Contaminantes Minero-Industriales En El Desarrollo Embrionario de Especies Bioindicadoras Carassius auratus “Goldfish” y El Caracol Physa venustula (Gould 1847). Ph.D. Thesis, Universidad Nacional de San Agustín, Arequipa, Peru, 2018. [Google Scholar]
- Caja-Molina, A.V.; Iannacone, J. Evaluación Del Riesgo Ambiental Por Petróleo Crudo En Las Especies Acuáticas Lemna minor, Daphnia magna y Danio rerio. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2021, 45, 777–794. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Adamcová, D.; Radziemska, M.; Zloch, J.; Brtnický, M.; Šindelář, O.; Maxiánová, A.; Mazur, Z. Ecotoxicity of In-Situ Produced Compost Intended for Landfill Restoration. Environments 2018, 5, 111. [Google Scholar] [CrossRef]
- Li, X.-J.; Jiang, L.; Chen, L.; Chen, H.-S.; Li, X. Neurotoxicity of Dibutyl Phthalate in Brain Development Following Perinatal Exposure: A Study in Rats. Environ. Toxicol. Pharmacol. 2013, 36, 392–402. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Monteiro, J.A.; da Cunha, L.A.; da Costa, M.H.P.; Dos Reis, H.S.; da Silva Aguiar, A.C.; de Oliveira-Bahia, V.R.L.; Burbano, R.M.R.; da Rocha, C.A.M. Mutagenic and Histopathological Effects of Hexavalent Chromium in Tadpoles of Lithobates catesbeianus (Shaw, 1802) (Anura, Ranidae). Ecotoxicol. Environ. Saf. 2018, 163, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Swarnakar, S.; Mukhopadhyay, A.; Ghosh, S. Exposure of Composite Tannery Effluent on Snail, Pila globosa: A Comparative Assessment of Toxic Impacts of the Untreated and Membrane Treated Effluents. Ecotoxicol. Environ. Saf. 2016, 126, 45–55. [Google Scholar] [CrossRef]
- Tišler, T.; Zagorc-Končan, J.; Cotman, M.; Drolc, A. Toxicity Potential of Disinfection Agent in Tannery Wastewater. Water Res. 2004, 38, 3503–3510. [Google Scholar] [CrossRef]
- Cooman, K.; Gajardo, M.; Nieto, J.; Bornhardt, C.; Vidal, G. Tannery Wastewater Characterization and Toxicity Effects on Daphnia Spp. Environ. Toxicol. Int. J. 2003, 18, 45–51. [Google Scholar] [CrossRef]
- Apha, A.W.; Greenberg, W.E.F.I.A.E.; Clesceri, L.S.; Eaton, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- OECD. Test No. 221: Lemna Sp. Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2006; ISBN 9789264016194. [Google Scholar]
- OECD. Daphnia magna Reproduction Test (OECD TG 211); Book of Organization for Economic Cooperation and Development: Paris, France, 2018; pp. 253–263. [Google Scholar]
- OECD. Lymnaea stagnalis Reproduction Test (OECD TG 243); Book of Organization for Economic Cooperation and Development: Paris, France, 2018; pp. 231–239. [Google Scholar]
- Nieuwkoop, P.D.; Faber, J.; Gerhart, J.; Kirschner, M. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis; Garland Science: New York, NY, USA, 1994; ISBN 1003064566. [Google Scholar]
- Saldaña, P.; Lerdo, T.A.; Gómez, M.A.; López, R. La Importancia de Incluir Análisis de Toxicidad En Descargas Industriales y Municipales Que Afectan a Los Cuerpos Receptore; Instituto Mexicano de Tecnología del Agua: Jutepec, Mexico, 2002; pp. 1–11. [Google Scholar]
- Roig, N.; Sierra, J.; Nadal, M.; Moreno-Garrido, I.; Nieto, E.; Hampel, M.; Gallego, E.P.; Schuhmacher, M.; Blasco, J. Assessment of Sediment Ecotoxicological Status as a Complementary Tool for the Evaluation of Surface Water Quality: The Ebro River Basin Case Study. Sci. Total Environ. 2015, 503, 269–278. [Google Scholar] [CrossRef]
- Darko, A.P.; Antwi, C.O.; Asamoah, K.O.; Opoku-Mensah, E.; Ren, J. A Probabilistic Reliable Linguistic PROBID Method for Selecting Electronic Mental Health Platforms Considering Users’ Bounded Rationality. Eng. Appl. Artif. Intell. 2023, 125, 106716. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Chapter NR 217: Effluent Standards and Limitations for Phosphorus. In Effluent Standards and Limitations; Environmental Protection Agency: Washington, DC, USA, 2013; 8p. [Google Scholar]
- Chowdhury, M.; Mostafa, M.G.; Biswas, T.K.; Saha, A.K. Treatment of leather industrial effluents by filtration and coagulation processes. Water Res. Ind. 2013, 3, 11–22. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, K.; Xia, Q.; Yu, S.; Zhang, M.; An, Y.; Zhao, X.; Zhou, Z. Up-Concentration of Nitrogen from Domestic Wastewater: A Sustainable Strategy from Removal to Recovery. Chem. Eng. J. 2023, 451, 138789. [Google Scholar] [CrossRef]
- Vo, T.-K.-Q.; Dang, B.-T.; Ngo, H.H.; Nguyen, T.-T.; Nguyen, V.-T.; Vo, T.-D.-H.; Ngo, T.-T.-M.; Nguyen, T.-B.; Lin, C.; Lin, K.-Y.A.; et al. Low Flux Sponge Membrane Bioreactor Treating Tannery Wastewater. Environ. Technol. Innov. 2021, 24, 101989. [Google Scholar] [CrossRef]
- Vo, T.D.H.; Bui, X.T.; Dang, B.T.; Nguyen, T.T.; Nguyen, V.T.; Tran, D.P.H.; Nguyen, P.T.; Boller, M.; Lin, K.Y.A.; Varjani, S.; et al. Influence of Organic Loading Rates on Treatment Performance of Membrane Bioreactor Treating Tannery Wastewater. Environ. Technol. Innov. 2021, 24, 101810. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Effluent Guidelines Program Plan; U.S. Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Wilhelm, F.M. Pollution of Aquatic Ecosystems I; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Lefebvre, O.; Vasudevan, N.; Torrijos, M.; Thanasekaran, K.; Moletta, R. Halophilic Biological Treatment of Tannery Soak Liquor in a Sequencing Batch Reactor. Water Res. 2005, 39, 1471–1480. [Google Scholar] [CrossRef]
- Rezgui, S.; Ghazouani, M.; Bousselmi, L.; Akrout, H. Efficient Treatment for Tannery Wastewater through Sequential Electro-Fenton and Electrocoagulation Processes. J. Environ. Chem. Eng. 2022, 10, 107424. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Borba, F.H.; Manenti, D.R. Performance Evaluation of an Integrated Photo-Fenton–Electrocoagulation Process Applied to Pollutant Removal from Tannery Effluent in Batch System. Chem. Eng. J. 2012, 197, 1–9. [Google Scholar] [CrossRef]
- Saxena, S.; Rajoriya, S.; Saharan, V.; George, S. An Advanced Pretreatment Strategy Involving Hydrodynamic and Acoustic Cavitation along with Alum Coagulation for the Mineralization and Biodegradability Enhancement of Tannery Waste Effluent. Ultrason. Sonochem. 2018, 44, 299–309. [Google Scholar] [CrossRef]
- Moradi, M.; Moussavi, G. Enhanced Treatment of Tannery Wastewater Using the Electrocoagulation Process Combined with UVC/VUV Photoreactor: Parametric and Mechanistic Evaluation. Chem. Eng. J. 2019, 358, 1038–1046. [Google Scholar] [CrossRef]
- Munz, G.; De Angelis, D.; Gori, R.; Mori, G.; Casarci, M.; Lubello, C. The Role of Tannins in Conventional and Membrane Treatment of Tannery Wastewater. J. Hazard. Mater. 2009, 164, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Lara, A.D.; Álvarez, F.; Gamiño-Arroyo, Z.; Navarro, R.; Peralta-Hernández, J.M.; Fuentes, R.; Pérez, T. Electrocoagulation Treatment of Industrial Tannery Wastewater Employing a Modified Rotating Cylinder Electrode Reactor. Chemosphere 2021, 264, 128491. [Google Scholar] [CrossRef]
- Haruna, A.; Uzairu, A.; Harrison, G. Monitoring of Sewage Quality for Small–Scale Irrigation: Case Studies in Some Fadama Lands in Zaria City, Nigeria. Cont. J. Appl. Sci. 2009, 4, 8–17. [Google Scholar]
- Kannan, P.R.; Deepa, S.; Yasothai, A.; Kanth, S.V.; Rao, J.R.; Chandrasekaran, B. Phytoremediation of Tannery Wastewater Treated Lands. Part II: Using Harvested Salicornia brachiata Plants for the Preservation of Sheepskins. J. Soc. Leather. Technol. Chem. 2009, 93, 240–244. [Google Scholar]
- Hughes, I.R. Temporary Preservation of Hides Using Boric Acid. J. Soc. Leather Technol. Chem. 1974, 58, 100–103. [Google Scholar]
- Mandal, T.; Dasgupta, D.; Mandal, S.; Datta, S. Treatment of Leather Industry Wastewater by Aerobic Biological and Fenton Oxidation Process. J. Hazard. Mater. 2010, 180, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Karahan, Ö.; Dogruel, S.; Dulekgurgen, E.; Orhon, D. COD Fractionation of Tannery Wastewaters—Particle Size Distribution, Biodegradability and Modeling. Water Res. 2008, 42, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Doumbi, R.T.; Noumi, G.B.; Ngobtchok, B. Tannery Wastewater Treatment by Electro-Fenton and Electro-Persulfate Processes Using Graphite from Used Batteries as Free-Cost Electrode Materials. Case Stud. Chem. Environ. Eng. 2022, 5, 100190. [Google Scholar] [CrossRef]
- Lofrano, G.; Belgiorno, V.; Gallo, M.; Raimo, A.; Meric, S. Toxicity Reduction in Leather Tanning Wastewater by Improved Coagulation Flocculation Process. Glob. NEST J. 2006, 8, 151–158. [Google Scholar]
- Kurt, U.; Apaydin, O.; Gonullu, M.T. Reduction of COD in Wastewater from an Organized Tannery Industrial Region by Electro-Fenton Process. J. Hazard. Mater. 2007, 143, 33–40. [Google Scholar] [CrossRef]
- Ganesh, R.; Balaji, G.; Ramanujam, R.A. Biodegradation of Tannery Wastewater Using Sequencing Batch Reactor—Respirometric Assessment. Bioresour. Technol. 2006, 97, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, Ö.; Kurt, U.; Gonullu, M.T. An Investigation on the Treatment of Tannery Wastewater by Electrocoagulation. Glob. NEST J. 2009, 11, 546–555. [Google Scholar]
- Alonso, A.; Camargo, J.A. Long-Term Effects of Ammonia on the Behavioral Activity of the Aquatic Snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Arch. Environ. Contam. Toxicol. 2009, 56, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Melgar, D. Tecnología En El Cuero: Tomo I Procesos de Curtición Control de Calidad y Maquinarias; Ministry of Foreign Trade and Tourism (MINCETUR): Huancayo, Peru, 2000. [Google Scholar]
- Suthanthararajan, R.; Ravindranath, E.; Chitra, K.; Umamaheswari, B.; Ramesh, T.; Rajamani, S. Membrane Application for Recovery and Reuse of Water from Treated Tannery Wastewater. Desalination 2004, 164, 151–156. [Google Scholar] [CrossRef]
- Niculescu, M.; Ionitţǎ, A.D.; Filipescu, L. Removal Chromium Pollution from Leather Industries Wastes. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2010, 72, 99–114. [Google Scholar]
- Obaidullah, S.; Mahmood, A.L. Chemical Treatment Options for Tannery Wastewater. Master’s Thesis, Bangladesh University of Engineering and Technology, Dhaka, Bangladesh, 2008. [Google Scholar]
- Minas, F.; Chandravanshi, B.S.; Leta, S. Chemical Precipitation Method for Chromium Removal and Its Recovery from Tannery Wastewater in Ethiopia Chemical Composition of Food and Environmental Samples View Project Developing High Strength Wastewater Treatment Technology in Ethiopia View Project. Chem. Int. 2017, 3, 291–305. [Google Scholar]
- Sharma, S.K. Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014; ISBN 1782620176. [Google Scholar]
- Chaves Quintero, C.M. Caracterización y Modelación Del Transporte de Cromo Total En La Cuenca Alta Del Río Bogotá Tramo-Stock 440-Puente Hacienda. Bachelor’s Thesis, Departamento de Ingeniería Civil y Ambiental, Universidad de Los Andes, Bogotá, Colombia, 2016. [Google Scholar]
- Li, T.; Guan, Y.; Guo, C.; Yang, T.; Yu, Z.; Xu, G. Pilot Scale Experiment of an Innovative Magnetic Bar Magnetic Separator for Chromium Removal from Tannery Wastewater. Process Saf. Environ. Prot. 2021, 149, 575–580. [Google Scholar] [CrossRef]
- Moradi, G.; Zinadini, S.; Rajabi, L. Development of the Tetrathioterephthalate Filler Incorporated PES Nanofiltration Membrane with Efficient Heavy Metal Ions Rejection and Superior Antifouling Properties. J. Environ. Chem. Eng. 2020, 8, 104431. [Google Scholar] [CrossRef]
- Zapana, J.S.P.; Arán, D.S.; Bocardo, E.F.; Harguinteguy, C.A. Treatment of Tannery Wastewater in a Pilot Scale Hybrid Constructed Wetland System in Arequipa, Peru. Int. J. Environ. Sci. Technol. 2020, 17, 4419–4430. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Simultaneous Removal of Organic and Inorganic Pollutants in Tannery Wastewater Using Electrocoagulation Technique. Korean J. Chem. Eng. 2008, 25, 703–709. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, W.; Huang, Z.; Feng, X.; Ma, L.; Qi, X.; Li, Z. Enhanced Removal of Toxic Cr (VI) in Tannery Wastewater by Photoelectrocatalysis with Synthetic TiO2 Hollow Spheres. Appl. Surf. Sci. 2017, 405, 102–110. [Google Scholar] [CrossRef]
- Huber, W.; Sankhla, N. Effect of Sodium Chloride on Photosynthesis of Lemna minor L. Zeitschrift für Pflanzenphysiologie 1979, 91, 147–156. [Google Scholar] [CrossRef]
- Bošnir, J.; Puntarić, D.; Cvetković, Z.; Pollak, L.; Barušić, L.; Klarić, I.; Miškulin, M.; Puntarić, I.; Puntarić, E.; Milošević, M. Effects of Magnesium, Chromium, Iron and Zinc from Food Supplements on Selected Aquatic Organisms. Coll. Antropol. 2013, 37, 965–971. [Google Scholar] [PubMed]
- Tkalec, M.; Vidaković-Cifrek, Ž.; Regula, I. The Effect of Oil Industry “High Density Brines” on Duckweed Lemna minor L. Chemosphere 1998, 37, 2703–2715. [Google Scholar] [CrossRef]
- Reale, L.; Ferranti, F.; Mantilacci, S.; Corboli, M.; Aversa, S.; Landucci, F.; Baldisserotto, C.; Ferroni, L.; Pancaldi, S.; Venanzoni, R. Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere 2016, 145, 98–105. [Google Scholar] [CrossRef]
- Augustynowicz, J.; Kołton, A.M.; Baran, A.M.; Kostecka-Gugała, A.M.; Lasek, W.W. Strategy of Cr Detoxification by Callitriche cophocarpa. Cent. Eur. J. Chem. 2013, 11, 295–303. [Google Scholar] [CrossRef]
- Arambašić, M.B.; Bjelić, S.; Subakov, G. Acute Toxicity of Heavy Metals (Copper, Lead, Zinc), Phenol and Sodium on Allium cepa L., Lepidium sativum L. and Daphnia magna St.: Comparative Investigations and the Practical Applications. Water Res. 1995, 29, 497–503. [Google Scholar] [CrossRef]
- Biesinger, K.E.; Christensen, G.M. Effects of Various Metals on Survival, Growth, Reproduction, and Metabolism of Daphnia magna. J. Fish. Res. Board Can. 1972, 29, 1691–1700. [Google Scholar] [CrossRef]
- Jo, H.-J.; Son, J.; Cho, K.; Jung, J. Combined Effects of Water Quality Parameters on Mixture Toxicity of Copper and Chromium toward Daphnia magna. Chemosphere 2010, 81, 1301–1307. [Google Scholar] [CrossRef]
- Sawasdee, B.; Köhler, H.R. Embryo Toxicity of Pesticides and Heavy Metals to the Ramshorn Snail, Marisa cornuarietis (Prosobranchia). Chemosphere 2009, 75, 1539–1547. [Google Scholar] [CrossRef]
- de Freitas Tallarico, L.; Borrely, S.I.; Hamada, N.; Grazeffe, V.S.; Ohlweiler, F.P.; Okazaki, K.; Granatelli, A.T.; Pereira, I.W.; de Bragança Pereira, C.A.; Nakano, E. Developmental Toxicity, Acute Toxicity and Mutagenicity Testing in Freshwater Snails Biomphalaria glabrata (Mollusca: Gastropoda) Exposed to Chromium and Water Samples. Ecotoxicol. Environ. Saf. 2014, 110, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Khangarot, B.S.; Das, S. Effects of Copper on the Egg Development and Hatching of a Freshwater Pulmonate Snail Lymnaea luteola L. J. Hazard. Mater. 2010, 179, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Factor, C.J.B.; de Chavez, E.R.C. Toxicity of Arsenic, Aluminum, Chromium and Nickel to the Embryos of the Freshwater Snail, Radix quadrasi von Möellendorf 1898. Phillipine J. Sci. 2012, 141, 207–216. [Google Scholar]
- Soucek, D.J.; Dickinson, A. Full-Life Chronic Toxicity of Sodium Salts to the Mayfly Neocloeon Triangulifer in Tests with Laboratory Cultured Food. Environ. Toxicol. Chem. 2015, 34, 2126–2137. [Google Scholar] [CrossRef]
- Hillyard, S.D.; Willumsen, N.J. Chemosensory Function of Amphibian Skin: Integrating Epithelial Transport, Capillary Blood Flow and Behaviour. Acta Physiol. 2011, 202, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Haywood, L.K.; Alexander, G.J.; Byrne, M.J.; Cukrowska, E. Xenopus laevis Embryos and Tadpoles as Models for Testing for Pollution by Zinc, Copper, Lead and Cadmium. Afr. Zool. 2004, 39, 163–174. [Google Scholar] [CrossRef]
- Garber, E.A.E.; Erb, J.L.; Magner, J.; Larsen, G. Low Levels of Sodium and Potassium in the Water from Wetlands in Minnesota That Contained Malformed Frogs Affect the Rate of Xenopus Development. Environ. Monit. Assess. 2004, 90, 45–64. [Google Scholar] [CrossRef]
- Meindl, G.A.; Schleissmann, N.; Sander, B.; Lam, M.; Parker, W.; Fitzgerald, C.; Oltmer, R.; Hua, J. Exposure to Metals (Ca, K, Mn) and Road Salt (NaCl) Differentially Affect Development and Survival in Two Model Amphibians. Chem. Ecol. 2020, 36, 194–204. [Google Scholar] [CrossRef]
- Tornabene, B.J.; Breuner, C.W.; Hossack, B.R. Relative Toxicity and Sublethal Effects of NaCl and Energy-Related Saline Wastewaters on Prairie Amphibians. Aquat. Toxicol. 2020, 228, 105626. [Google Scholar] [CrossRef]
- Hossack, B.R.; Puglis, H.J.; Battaglin, W.A.; Anderson, C.W.; Honeycutt, R.K.; Smalling, K.L. Widespread Legacy Brine Contamination from Oil Production Reduces Survival of Chorus Frog Larvae. Environ. Pollut. 2017, 231, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Prati, M.; Gornati, R.; Boracchi, P.; Biganzoli, E.; Fortaner, S.; Pietra, R.; Sabbioni, E.; Bernardini, G. A Comparative Study of the Toxicity of Mercury Dichloride and Methylmercury, Assayed by the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). Altern. Lab. Anim. 2002, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Tarazona, J.V.; Pablos, M.V. Are Fish and Standardized FETAX Assays Protective Enough for Amphibians? A Case Study on Xenopus laevis Larvae Assay with Biologically Active Substances Present in Livestock Wastes. Sci. World J. 2012, 2012, 605804. [Google Scholar] [CrossRef]
- Aich, A.; Chattopadhyay, B.; Datta, S.; Mukhopadhyay, S.K. Impact of Composite Tannery Effluent on the Amino-Transferase Activities in a Fish Biosystem, Using Guppy Fish (Poecilia reticulata) as an Experimental Model. Toxicol. Environ. Chem. 2011, 93, 85–91. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Bharagava, R.N. Environmental Hazards and Toxicity Profile of Organic and Inorganic Pollutants of Tannery Wastewater and Bioremediation Approaches. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Springer: Singapore, 2020; pp. 381–398. [Google Scholar]
- Bharagava, R.N.; Saxena, G.; Mulla, S.I.; Patel, D.K. Characterization and Identification of Recalcitrant Organic Pollutants (ROPs) in Tannery Wastewater and Its Phytotoxicity Evaluation for Environmental Safety. Arch. Environ. Contam. Toxicol. 2018, 75, 259–272. [Google Scholar] [CrossRef]






| Organism | Effect | Effluent Concentrations (%) |
|---|---|---|
| Lemna minor | Number of fronds, dry and wet weight | 0, 1.5, 3.0, 4.5 |
| Daphnia magna | Mortality | 0, 1.5, 3.0, 4.5 |
| Physa venustula | Egg hatching, development delay | 0, 1.5, 3.0, 4.5 |
| Xenopus laevis embryos and larvae | Mortality and morphological effects | 0, 1.5, 3.0, 4.5 |
| Parameter | Unit | Result ± SD |
|---|---|---|
| pH | - | 8.6 ± 0.4 |
| Total dissolved solids (TDS) | mg/L | 67,700 ± 15,600 |
| Total suspended solids (TSS) | mg/L | 650 ± 190 |
| Total nitrogen | mg/L | 490 ± 10 |
| Biochemical oxygen demand | mg/L | 1530 ± 290 |
| Chemical oxygen demand | mg/L | 3030 ± 50 |
| Sulfate | mg/L | 4850 ± 430 |
| Turbidity | NTU | 180 ± 10 |
| Chlorides | mg/L | 12,300 ± 400 |
| Metal | Concentration Values ± SD (mg/L) | Metal | Concentration Values ± SD (mg/L) |
|---|---|---|---|
| Aluminum | 0.68 ± 0.05 | Lithium | 2.1 ± 0.4 |
| Antimony | 0.003 ± 0.001 | Magnesium | 230 ± 50 |
| Arsenic | 0.42 ± 0.10 | Manganese | 0.24 ± 0.05 |
| Barium | 0.12 ± 0.03 | Molybdenum | 0.021 ± 0.004 |
| Bismuth | 0.0004 ± 0.0001 | Nickel | 0.016 ± 0.003 |
| Boron | 7.2 ± 1.5 | Lead | 0.032 ± 0.007 |
| Cadmium | 0.0012 ± 0.0003 | Potassium | 183 ± 6 |
| Calcium | 470 ± 120 | Rubidium | 1.2 ± 0.3 |
| Cerium | 0.0009 ± 0.0001 | Silica | 99 ± 26 |
| Cesium | 0.017 ± 0.003 | Silicon | 46 ± 12 |
| Cobalt | 0.014 ± 0.002 | Sodium | 26,000 ± 5500 |
| Copper | 0.062 ± 0.013 | Thallium | 0.001 ± 0.0001 |
| Chromium | 10.4 ± 0.4 | Titanium | 0.33 ± 0.00 |
| Strontium | 5.0 ± 1.1 | Uranium | 0.0016 ± 0.0003 |
| Phosphorous | 6.8 ± 0.3 | Vanadium | 0.020 ± 0.003 |
| Gallium | 0.16 ± 0.03 | Tungsten | 0.0007 ± 0.0001 |
| Germanium | 0.013 ± 0.002 | Zinc | 0.49 ± 0.10 |
| Iron | 1.7 ± 0.4 | Zirconium | 0.0007 ± 0.0002 |
| Organism | ET (h) | LC50 | %S | ATU | CS | CR |
|---|---|---|---|---|---|---|
| Lemna minor | 168 | - | - | - | - | - |
| Daphnia magna hatchlings | 48 | 1.1 | 2.5 | 89 | Very toxic | Highly toxic |
| Physa venustula embryos | 216 | - | 0 | - | - | Highly toxic |
| Xenopus laevis embryos | 96 | 1.6 | 10 | 63 | Very toxic | Highly toxic |
| Xenopus laevis larvae | 96 | 1.8 | 5 | 60 | Very toxic | Highly toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Meza, K.; Arenazas-Rodríguez, A.; Garcia-Chevesich, P.A.; Flores-Farfan, C.; Morales-Paredes, L.; Romero-Mariscal, G.; Ticona-Quea, J.; Vanzin, G.; Sharp, J.O. Acute Ecotoxicity Potential of Untreated Tannery Wastewater Release in Arequipa, Southern Peru. Sustainability 2023, 15, 15240. https://doi.org/10.3390/su152115240
Tejada-Meza K, Arenazas-Rodríguez A, Garcia-Chevesich PA, Flores-Farfan C, Morales-Paredes L, Romero-Mariscal G, Ticona-Quea J, Vanzin G, Sharp JO. Acute Ecotoxicity Potential of Untreated Tannery Wastewater Release in Arequipa, Southern Peru. Sustainability. 2023; 15(21):15240. https://doi.org/10.3390/su152115240
Chicago/Turabian StyleTejada-Meza, Kevin, Armando Arenazas-Rodríguez, Pablo A. Garcia-Chevesich, Carmen Flores-Farfan, Lino Morales-Paredes, Giuliana Romero-Mariscal, Juana Ticona-Quea, Gary Vanzin, and Jonathan O. Sharp. 2023. "Acute Ecotoxicity Potential of Untreated Tannery Wastewater Release in Arequipa, Southern Peru" Sustainability 15, no. 21: 15240. https://doi.org/10.3390/su152115240
APA StyleTejada-Meza, K., Arenazas-Rodríguez, A., Garcia-Chevesich, P. A., Flores-Farfan, C., Morales-Paredes, L., Romero-Mariscal, G., Ticona-Quea, J., Vanzin, G., & Sharp, J. O. (2023). Acute Ecotoxicity Potential of Untreated Tannery Wastewater Release in Arequipa, Southern Peru. Sustainability, 15(21), 15240. https://doi.org/10.3390/su152115240








