Insights into Opposite and Positive Effects of Biochar and Organic Fertilizer on Red Soil Properties and Growth of Pennisetum giganteum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Soil Sampling, and Amendments
2.2. Experimental Setup
2.3. Soil Physicochemical Properties
2.4. Soil Enzyme Activity
2.5. Microbial Diversity Detection
2.6. Data Statistical Analysis
3. Results
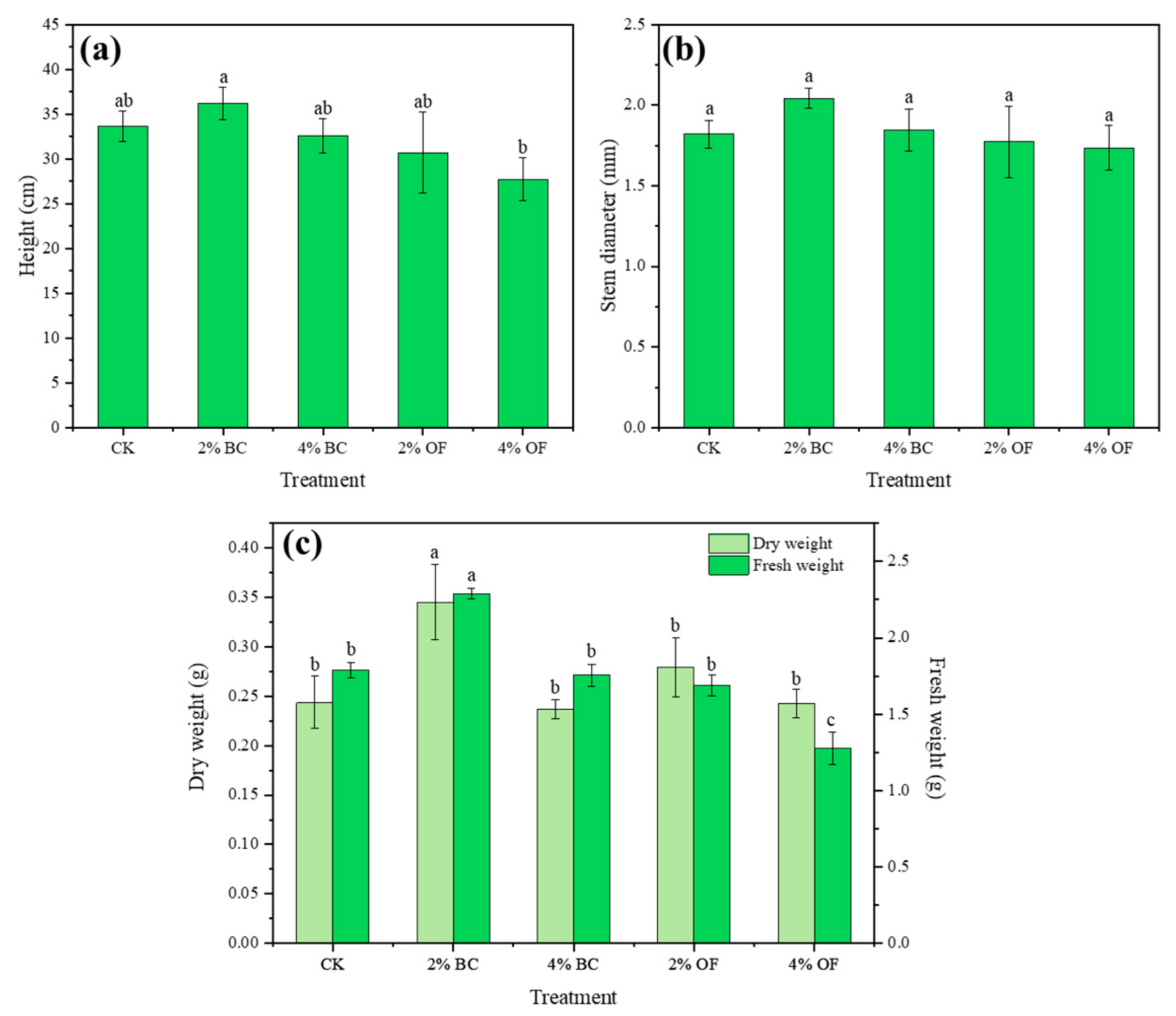
3.1. Analysis of the Growth of P. giganteum
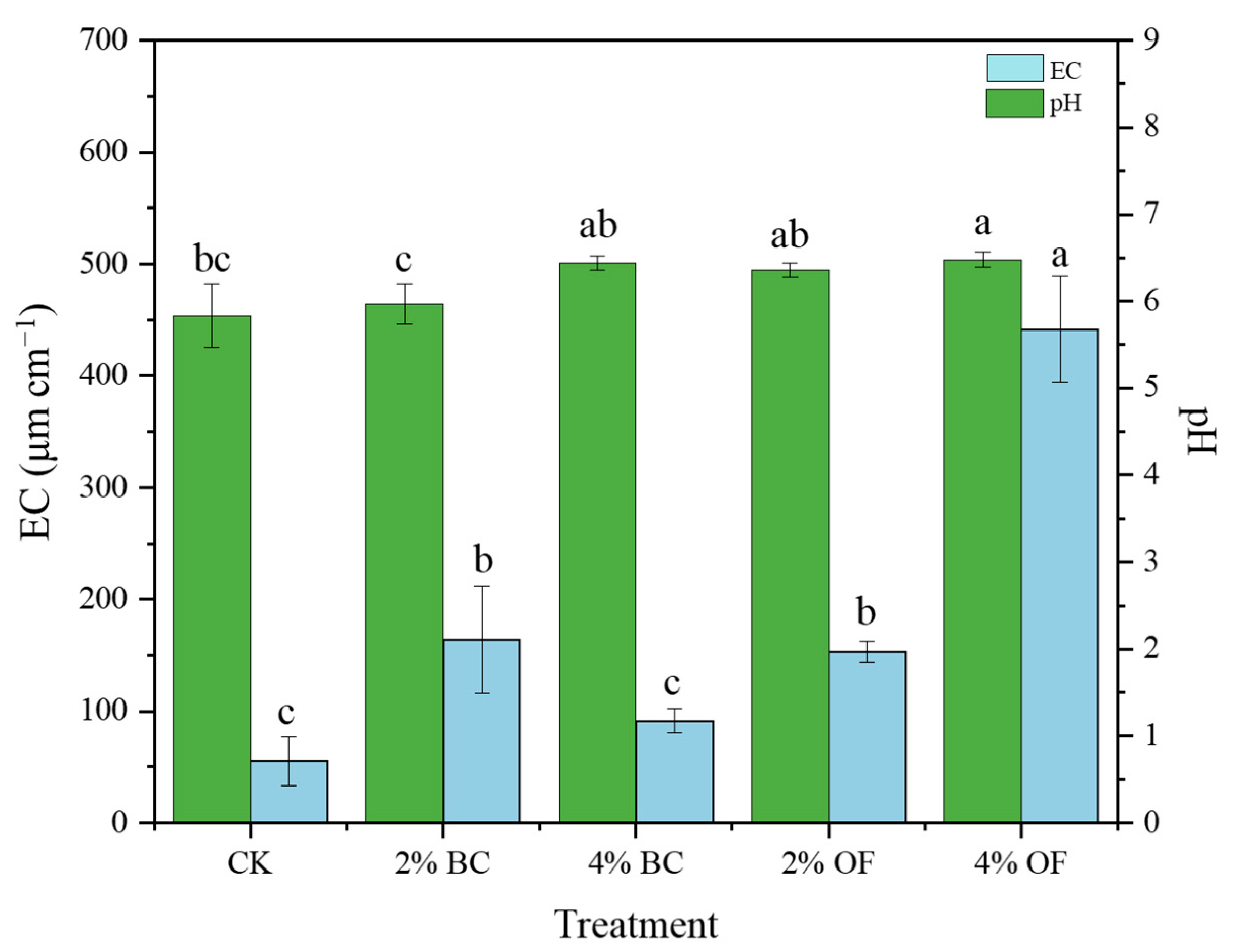
3.2. Soil Physicochemical Properties
3.3. Analysis of Enzymes Present in the Soil
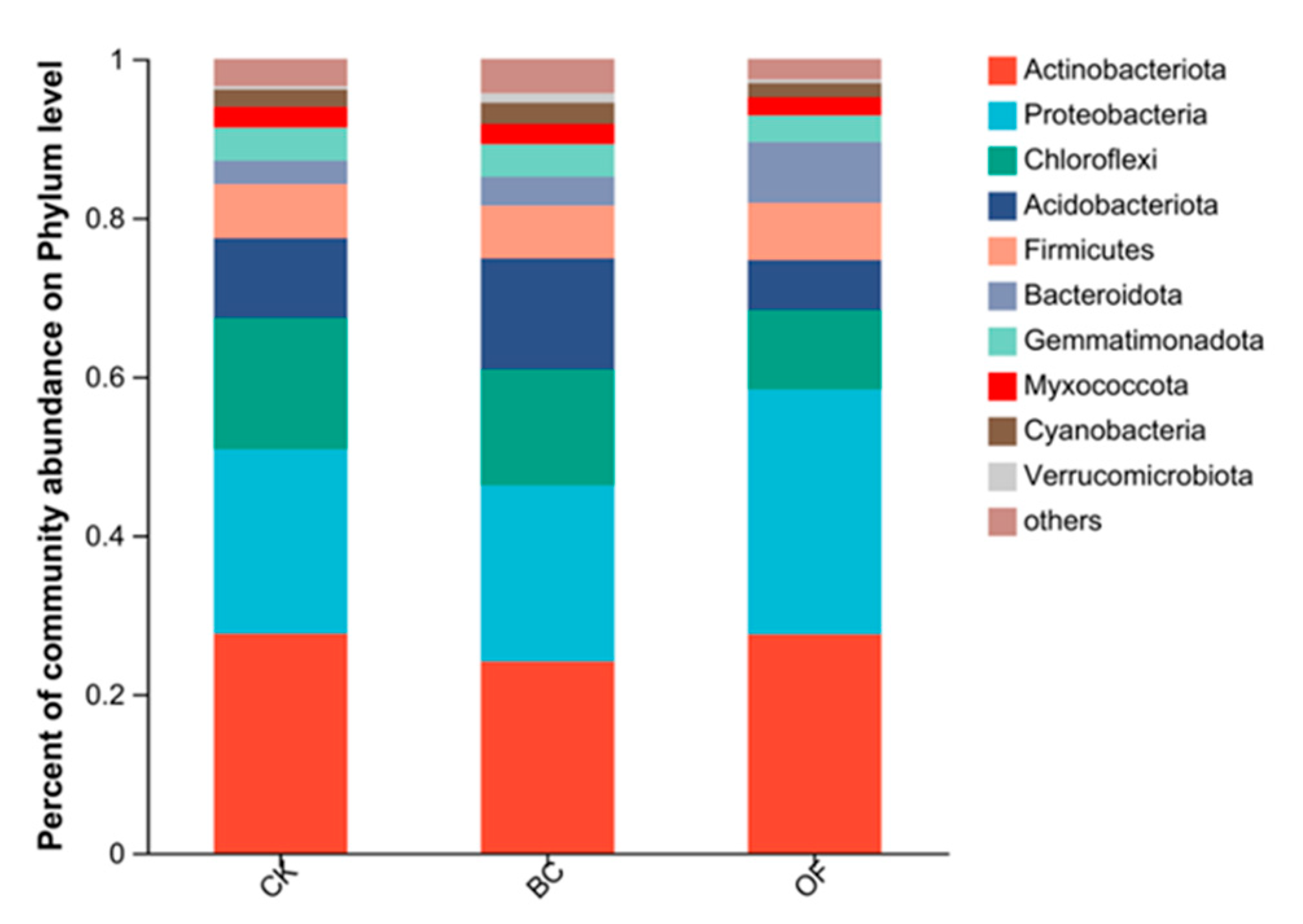
3.4. Analysis of Soil Bacterial Diversity
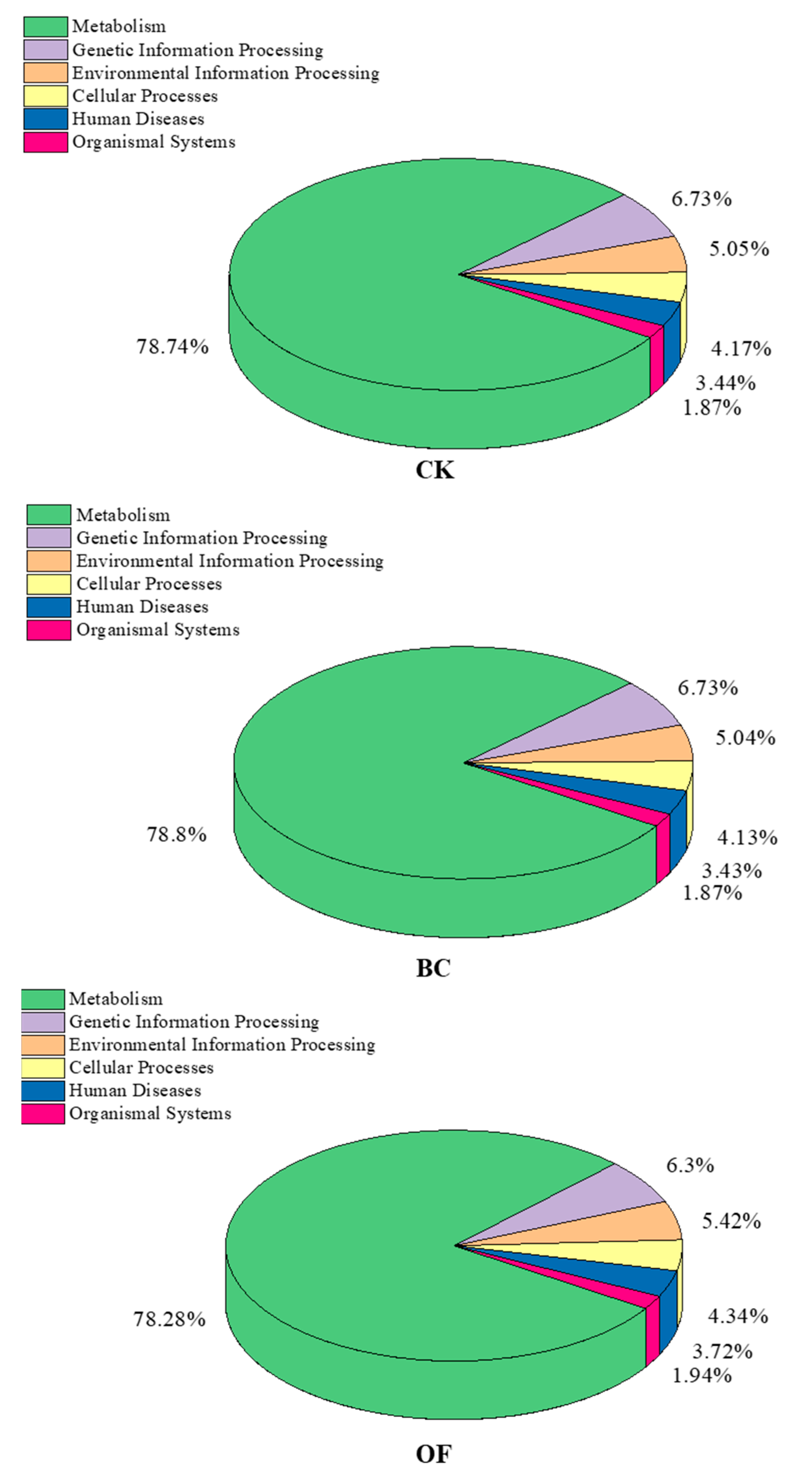
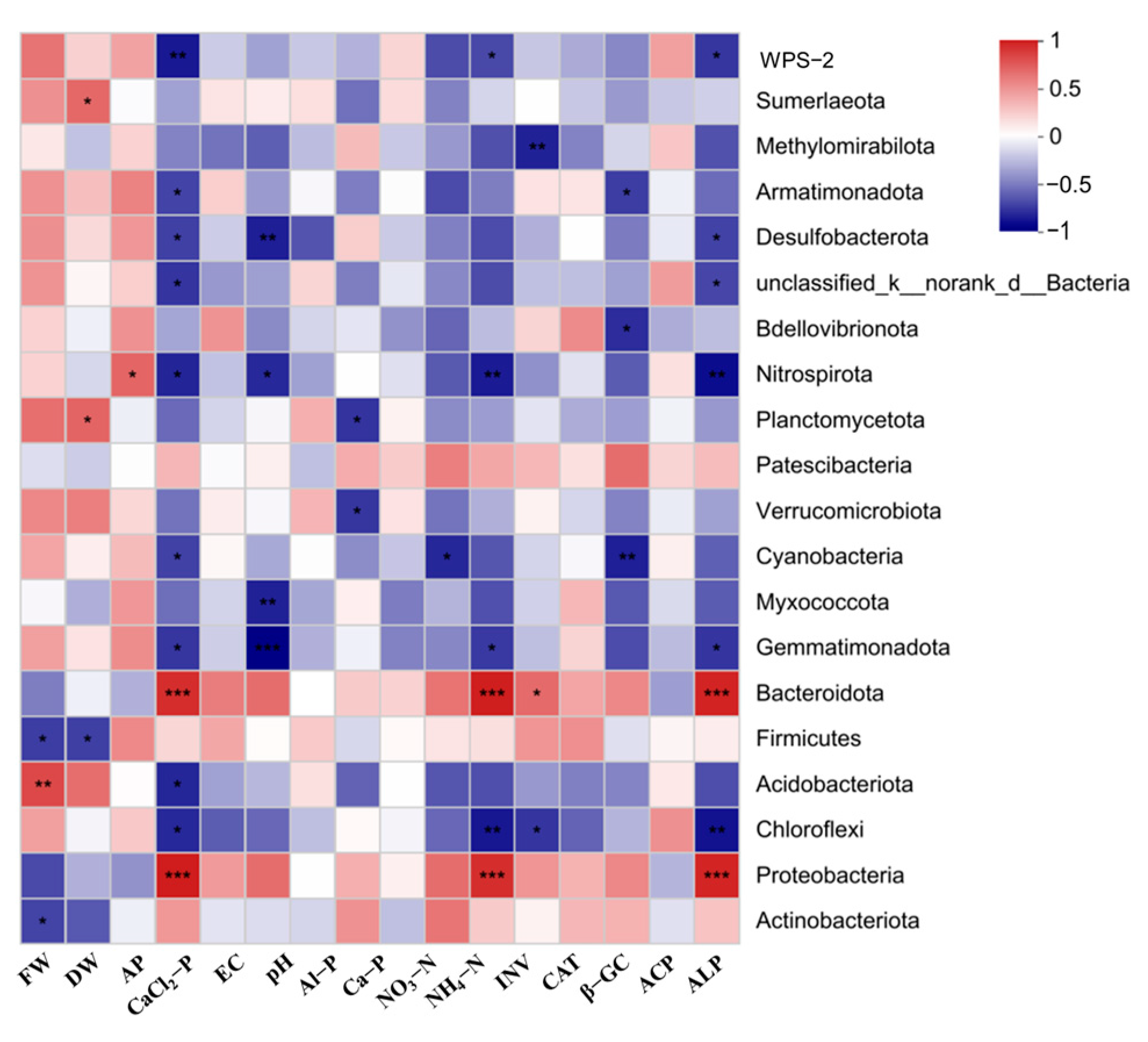
3.5. Functions of Bacterium
3.6. Relationships among Diverse Soil Physicochemical Properties
4. Discussion
4.1. Soil Nutrients and Properties
4.2. Soil Microbial Abundance and Enzyme Acclivity
4.3. Soil Microbe Community and Result Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, M.; Li, X.; Liu, Q.; Tang, F. Rice husk ash addition to acid red soil improves the soil property and cotton seedling growth. Sci. Rep. 2022, 12, 1704. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Le, L.B.; Pham, L.P.; Nguyen, H.T.; Tran, T.D.; Van Thai, N. The effects of biochar on the biomass yield of elephant grass (Pennisetum Purpureum Schumach) and properties of acidic soils. Ind. Crops Prod. 2021, 161, 113224. [Google Scholar] [CrossRef]
- Baligar, V.; Fageria, N.; Eswaran, H.; Wilson, M.; He, Z. Nature and properties of red soils of the world. In The Red Soils of China: Their Nature, Management and Utilization; Springer: Berlin/Heidelberg, Germany, 2004; pp. 7–27. [Google Scholar]
- Gong, X.; Liu, Y.; Li, Q.; Wei, X.; Guo, X.; Niu, D.; Zhang, W.; Zhang, J.; Zhang, L. Sub-tropic degraded red soil restoration: Is soil organic carbon build-up limited by nutrients supply. For. Ecol. Manag. 2013, 300, 77–87. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Bo-Ren, W.; Ming-Gang, X.; Ting-Lu, F. Crop yield and soil responses to long-term fertilization on a red soil in southern China. Pedosphere 2009, 19, 199–207. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Kang, G.; Zhu, B.; Li, H.; Hu, F.; Jiao, J. Soil quality response to organic amendments on dryland red soil in subtropical China. Geoderma 2020, 373, 114416. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Peng, Y.; Xiang, P.; Zhou, Y.; Yao, B.; Zhou, Y.; Sun, C. Co-application of biochar and organic fertilizer promotes the yield and quality of red pitaya (Hylocereus polyrhizus) by improving soil properties. Chemosphere 2022, 294, 133619. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Rong, X.; Han, Y.; Yang, Z.; Hou, K.; Zhao, H.; Hu, W. Responses of maize yield, nitrogen and phosphorus runoff losses and soil properties to biochar and organic fertilizer application in a light-loamy fluvo-aquic soil. Agric. Ecosyst. Environ. 2021, 314, 107433. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Nsubuga, D.; Kabenge, I.; Zziwa, A.; Yiga, V.A.; Mpendo, Y.; Harbert, M.; Kizza, R.; Banadda, N.; Wydra, K.D. Optimization of adsorbent dose and contact time for the production of jackfruit waste nutrient-enriched biochar. Waste Dispos. Sustain. Energy 2023, 5, 63–74. [Google Scholar] [CrossRef]
- Abukari, A.; Kaba, J.S.; Dawoe, E.; Abunyewa, A.A. A comprehensive review of the effects of biochar on soil physicochemical properties and crop productivity. Waste Dispos. Sustain. Energy 2022, 4, 343–359. [Google Scholar] [CrossRef]
- Are, K.S. Biochar and soil physical health. In An Imperative Amendment for Soil and the Environment; InTech Open: London, UK, 2019; pp. 21–33. [Google Scholar]
- Adhikari, S.; Timms, W.; Mahmud, M.P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment–A review. Sci. Total Environ. 2022, 851, 158043. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Fu, Q.; Li, T.; Liu, D.; Hou, R.; Li, Q.; Li, M.; Meng, F. Effects of biochar and straw application on the soil structure and water-holding and gas transport capacities in seasonally frozen soil areas. J. Environ. Manag. 2022, 301, 113943. [Google Scholar] [CrossRef] [PubMed]
- Lopes, É.M.G.; Reis, M.M.; Frazão, L.A.; da Mata Terra, L.E.; Lopes, E.F.; dos Santos, M.M.; Fernandes, L.A. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 2021, 21, 101270. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Noyce, G.L.; Jones, T.; Fulthorpe, R.; Basiliko, N. Phosphorus uptake and availability and short-term seedling growth in three Ontario soils amended with ash and biochar. Can. J. Soil Sci. 2017, 97, 678–691. [Google Scholar] [CrossRef]
- Du, T.; Hu, Q.; He, H.; Mao, W.; Yang, Z.; Chen, H.; Sun, L.; Zhai, M. Long-term organic fertilizer and biofertilizer application strengthens the associations between soil quality index, network complexity, and walnut yield. Eur. J. Soil Biol. 2023, 116, 103492. [Google Scholar] [CrossRef]
- Xiao, Q.; He, B.; Wang, S. Effect of the Different Fertilization Treatments Application on Paddy Soil Enzyme Activities and Bacterial Community Composition. Agronomy 2023, 13, 712. [Google Scholar] [CrossRef]
- Rubagumya, I.; Komakech, A.J.; Kabenge, I.; Kiggundu, N. Potential of organic waste to energy and bio-fertilizer production in Sub-Saharan Africa: A review. In Waste Disposal & Sustainable Energy; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Shah, M.N.; Wright, D.L.; Hussain, S.; Koutroubas, S.D.; Seepaul, R.; George, S.; Ali, S.; Naveed, M.; Khan, M.; Altaf, M.T. Organic fertilizer sources improve the yield and quality attributes of maize (Zea mays L.) hybrids by improving soil properties and nutrient uptake under drought stress. J. King Saud Univ. Sci. 2023, 35, 102570. [Google Scholar] [CrossRef]
- Bondarenko, A.; Kachanova, L.; Chelbin, S. Formation of an organisational and technological mechanism for the development of the fertiliser market in the Russian Federation. In E3S Web of Conferences; EDP Sciences: Ulys, France, 2023. [Google Scholar]
- Sharma, A.; Chetani, R. A review on the effect of organic and chemical fertilizers on plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 677–680. [Google Scholar] [CrossRef]
- Singh, T.B.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Role of organic fertilizers in improving soil fertility. In Contaminants in Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 61–77. [Google Scholar] [CrossRef]
- Vengadaramana, A.; Jashothan, P.T.J. Effect of organic fertilizers on the water holding capacity of soil in different terrains of Jaffna peninsula in Sri Lanka. Sch. Res. Libr. 2021, 2, 500–503. [Google Scholar]
- Cooperband, L.; Bollero, G.; Coale, F. Effect of poultry litter and composts on soil nitrogen and phosphorus availability and corn production. Nutr. Cycl. Agroecosyst. 2002, 62, 185–194. [Google Scholar] [CrossRef]
- Jia, Y.; Liao, Z.; Chew, H.; Wang, L.; Lin, B.; Chen, C.; Lu, G.; Lin, Z. Effect of Pennisetum giganteum zx lin mixed nitrogen-fixing bacterial fertilizer on the growth, quality, soil fertility and bacterial community of pakchoi (Brassica chinensis L.). PLoS ONE 2020, 15, e0228709. [Google Scholar] [CrossRef]
- Zhao, J.; Jing, Z.-D.; Yin, X.-J.; Li, J.-F.; Dong, Z.-H.; Wang, S.-R.; Shao, T. Sustainable utilization of residual grass: Effect of anaerobic storage days on chemical composition, fermentation performance, microbial community, and functional profiles of Pennisetum giganteum. In Environmental Science and Pollution Research; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Q.; Yang, R.; Zhang, B.; Yang, Z.; Chen, X.; Wang, X.; Du, M.; Tang, L. Typical JUNCAO Overwintering Performance and Optimized Cultivation Conditions of Pennisetum sp. in Guizhou, Southwest China. Sustainability 2022, 14, 4086. [Google Scholar] [CrossRef]
- Qiao-Hong, Z.; Xin-Hua, P.; Huang, T.-Q.; Zu-Bin, X.; Holden, N. Effect of biochar addition on maize growth and nitrogen use efficiency in acidic red soils. Pedosphere 2014, 24, 699–708. [Google Scholar]
- Raza, M.A.; Feng, L.Y.; van der Werf, W.; Iqbal, N.; Khalid, M.H.B.; Chen, Y.K.; Wasaya, A.; Ahmed, S.; Ud Din, A.M.; Khan, A.; et al. Maize leaf-removal: A new agronomic approach to increase dry matter, flower number and seed-yield of soybean in maize soybean relay intercropping system. Sci. Rep. 2019, 9, 13453. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Ling, N.; Zhu, C.; Chi, F.; Li, W.; Hao, X.; Zhang, W.; Bian, J.; Chen, L. Exploring soil factors determining composition and structure of the bacterial communities in saline-alkali soils of Songnen Plain. Front. Microbiol. 2020, 10, 2902. [Google Scholar] [CrossRef]
- Jiang, B.; Gu, Y. A suggested fractionation scheme of inorganic phosphorus in calcareous soils. Fertil. Res. 1989, 20, 159–165. [Google Scholar] [CrossRef]
- Zhang, H.; Kovar, J.L. Phosphorus fractionation. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, Waters; North Carolina State University: Manhattan, KS, USA, 2000; p. 50. [Google Scholar]
- Moir, J.; Tiessen, H. Characterization of Available P by Sequential Extraction; Lewis Publishers: Boca Rato, FL, USA, 2007; p. 14. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Sánchez-Alcalá, I.; Del Campillo, M.; Torrent, J. Extraction with 0.01 M CaCl2 underestimates the concentration of phosphorus in the soil solution. Soil Use Manag. 2014, 30, 297–302. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, B.; Liu, C.; Lin, Y.; Fu, Q.; Li, N.; Li, H. Soil fertility, enzyme activity, and microbial community structure diversity among different soil textures under different land use types in coastal saline soil. J. Soils Sediments 2021, 21, 2240–2252. [Google Scholar] [CrossRef]
- Hayano, K. A method for the determination of β-glucosidase activity in soil. Soil Sci. Plant Nutr. 1973, 19, 103–108. [Google Scholar] [CrossRef]
- Aguilar-Chávez, Á.; Díaz-Rojas, M.; del Rosario Cárdenas-Aquino, M.; Dendooven, L.; Luna-Guido, M. Greenhouse gas emissions from a wastewater sludge-amended soil cultivated with wheat (Triticum spp. L.) as affected by different application rates of charcoal. Soil Biol. Biochem. 2012, 52, 90–95. [Google Scholar] [CrossRef]
- Schulz, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bird, M.I.; Nelson, P.N.; Bass, A.M. The ameliorating effects of biochar and compost on soil quality and plant growth on a Ferralsol. Soil Res. 2015, 53, 1–12. [Google Scholar] [CrossRef]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The impact of biochar application on soil properties and plant growth of pot grown lettuce (Lactuca sativa) and cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef]
- Mohammadifar, A.; Gholami, H.; Golzari, S.; Collins, A.L. Spatial modelling of soil salinity: Deep or shallow learning models? Environ. Sci. Pollut. Res. 2021, 28, 39432–39450. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical fertilizer reduction with organic fertilizer effectively improve soil fertility and microbial community from newly cultivated land in the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Y.; Zhao, F.; He, X.; Liu, H.; Yu, K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 2020, 10, 9568. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202, 183–191. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Min, K.; Liu, T.; Li, C.; Xu, J.; Hu, F.; Li, H. The potential role of fertilizer-derived exogenous bacteria on soil bacterial community assemblage and network formation. Chemosphere 2022, 287, 132338. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Monitoring soil enzymes activity before and after animal manure application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Höglund, E. The Effects of Biochar on Soil Biota in Temperate and Boreal Agricultural Soils. Helsingin Yliopiston. 2022. Available online: https://helda.helsinki.fi/server/api/core/bitstreams/5dd23027-ae59-4add-9454-aa335b722c64/content (accessed on 11 June 2023).
- Kruczyńska, A.; Kuźniar, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Marzec-Grządziel, A.; Gałązka, A.; Wolińska, A. Bacteroidota structure in the face of varying agricultural practices as an important indicator of soil quality—A culture independent approach. Agric. Ecosyst. Environ. 2023, 342, 108252. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, J.; Ongena, M.; Liu, W.; Wei, D.; Zhao, B.; Guan, D.; Jiang, X.; Li, J. Effect of long-term fertilization strategies on bacterial community composition in a 35-year field experiment of Chinese Mollisols. Amb Express 2018, 8, 20. [Google Scholar]
- Rousk, J.; Brookes, P.C.; Bååth, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 516–520. [Google Scholar] [CrossRef]
- Preston, G.M.; Studholme, D.J.; Caldelari, I. Profiling the secretomes of plant pathogenic Proteobacteria. FEMS Microbiol. Rev. 2005, 29, 331–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, J.; Wu, H.; Zhong, Q.; Liu, W.; He, S.; Zhang, L. Diversity patterns and drivers of soil microbial communities in urban and suburban park soils of Shanghai, China. PeerJ 2021, 9, e11231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Romanyà, J.; Li, N.; Xiang, Y.; Yang, J.; Han, X. Biochar fertilization effects on soil bacterial community and soil phosphorus forms depends on the application rate. Sci. Total Environ. 2022, 843, 157022. [Google Scholar] [CrossRef]
- Shao, T.; Zhao, J.; Liu, A.; Long, X.; Rengel, Z. Effects of soil physicochemical properties on microbial communities in different ecological niches in coastal area. Appl. Soil Ecol. 2020, 150, 103486. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, T.; Van Zwieten, L.; Zhu, Q.; Yan, T.; Xue, J.; Wu, Y. Soil microbial community structure shifts induced by biochar and biochar-based fertilizer amendment to Karst calcareous soil. Soil Sci. Soc. Am. J. 2019, 83, 398–408. [Google Scholar] [CrossRef]
- Du, T.-Y.; He, H.-Y.; Zhang, Q.; Lu, L.; Mao, W.-J.; Zhai, M.-Z. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar]
- Gao, L.; Wang, R.; Shen, G.; Zhang, J.; Meng, G.; Zhang, J. Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils. J. Soil Sci. Plant Nutr. 2017, 17, 884–896. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, N.; Zhou, G.; Dang, P.; Yang, X.; Qiu, L.; Huang, M.; Gong, Y.; Zhao, S.; Chen, J. Response of soil microbial community to plant composition changes in broad-leaved forests of the karst area in Mid-Subtropical China. PeerJ 2022, 10, 12739. [Google Scholar] [CrossRef]
- Sato, T.; Ezawa, T.; Cheng, W.; Tawaraya, K. Release of acid phosphatase from extraradical hyphae of arbuscular mycorrhizal fungus Rhizophagus clarus. Soil Sci. Plant Nutr. 2015, 61, 269–274. [Google Scholar] [CrossRef]
- Gaume, A.; Mächler, F.; De León, C.; Narro, L.; Frossard, E. Low-P tolerance by maize (Zea mays L.) genotypes: Significance of root growth, and organic acids and acid phosphatase root exudation. Plant Soil 2001, 228, 253–264. [Google Scholar] [CrossRef]
- Solans, M.; Messuti, M.I.; Reiner, G.; Boenel, M.; Vobis, G.; Wall, L.G.; Scervino, J.M. Exploring the response of Actinobacteria to the presence of phosphorus salts sources: Metabolic and co-metabolic processes. J. Basic Microbiol. 2019, 59, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Dasila, H.; Sah, V.; Jaggi, V.; Kumar, A.; Tewari, L.; Taj, G.; Chaturvedi, S.; Perveen, K.; Bukhari, N.A.; Siang, T.C. Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status. Front. Microbiol. 2023, 14, 1135693. [Google Scholar] [CrossRef]
- Azizoglu, U. Bacillus thuringiensis as a biofertilizer and biostimulator: A mini-review of the little-known plant growth-promoting properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]



| Treatment | AP (mg·kg−1) | CaCl2-P (mg·kg−1) | Phosphorus Fractionation | NO3−-N (mg·kg−1) | NH4+-N (mg·kg−1) | ||
|---|---|---|---|---|---|---|---|
| Al-P (mg·kg−1) | Fe-P (mg·kg−1) | Ca-P (mg·kg−1) | |||||
| CK | 71.53 ± 4.12 bc | 1.59 ± 0.21 b | 237.41 ± 30.84 a | 2338 ± 47.30 a | 51.91 ± 10.56 b | 2.80 ± 0.32 ab | 1.58 ± 0.08 c |
| 2% BC | 81.03 ± 17.48 abc | 1.35 ± 0.00 b | 224.77 ± 23.45 a | 2326 ± 21.53 a | 59.55 ± 3.65 b | 2.16 ± 0.50 b | 1.87 ± 0.23 c |
| 4% BC | 58.03 ± 13.97 c | 1.26 ± 0.15 b | 206.79 ± 20.54 a | 2334 ± 6.09 a | 52.90 ± 8.57 b | 3.13 ± 0.61 ab | 1.68 ± 0.10 c |
| 2% OF | 108.7 ± 12.39 ab | 2.96 ± 0.16 b | 237.53 ± 34.23 a | 2347 ± 30.57 a | 58.07 ± 8.67 b | 3.31 ± 0.57 a | 3.88 ± 0.48 b |
| 4% OF | 107.3 ± 26.61 ab | 5.65 ± 1.72 a | 244.82 ± 18.81 a | 2352 ± 1.89 a | 98.96 ± 15.73 a | 3.41 ± 0.56 a | 8.78 ± 0.79 a |
| Treatment | INV (mg d−1 g−1) | CAT (mL h−1 g−1) | β-GC (μg d−1 g−1) | ACP (mg d−1 g−1) | ALP (mg d−1 g−1) |
|---|---|---|---|---|---|
| CK | 0.25 ± 0.05 a | 8.53 ± 0.87 a | 0.21 ± 0.02 b | 0.47 ± 0.04 a | 0.23 ± 0.00 c |
| 2% BC | 0.28 ± 0.04 a | 8.53 ± 0.87 a | 0.20 ± 0.02 bc | 0.46 ± 0.01 b | 0.23 ± 0.01 c |
| 4% BC | 0.27 ± 0.05 a | 7.53 ± 1.51 a | 0.18 ± 0.02 c | 0.44 ± 0.01 b | 0.23 ± 0.01 c |
| 2% OF | 0.32 ± 0.04 a | 9.04 ± 0.00 a | 0.23 ± 0.03 b | 0.45 ± 0.01 b | 0.39 ± 0.03 b |
| 4% OF | 0.32 ± 0.06 a | 9.04 ± 0.00 a | 0.31 ± 0.01 a | 0.43 ± 0.02 b | 0.46 ± 0.03 a |
| Treatment | Ace | Chao | Coverage | Shannon | Simpson | Sobs |
|---|---|---|---|---|---|---|
| CK | 2804.81 ± 115.52 a | 2761.21 ± 78.1 a | 0.98 ± 0.00) a | 6.27 ± 0.07 a | 0.01 ± 0.00 a | 2348.33 ± 110.18 a |
| 2% BC | 2895.81 ± 224.93 a | 2909.72 ± 197.00 ab | 0.98 ± 0.00 a | 6.39 ± 0.07 a | 0.01 ± 0.00 a | 2506.00 ± 155.47 ab |
| 2% OF | 2634.57 ± 90.91 a | 2612.91 ± 75.35 b | 0.98 ± 0.00 a | 6.12 ± 0.05 b | 0.01 ± 0.00 a | 2184.00 ± 86.88 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Li, X.; Fu, T.; Li, H.; Li, W.; Zhang, Q.; Wang, J.; Chen, B.; Yang, R.; Zhang, B.; et al. Insights into Opposite and Positive Effects of Biochar and Organic Fertilizer on Red Soil Properties and Growth of Pennisetum giganteum. Sustainability 2023, 15, 15142. https://doi.org/10.3390/su152015142
Zhang B, Li X, Fu T, Li H, Li W, Zhang Q, Wang J, Chen B, Yang R, Zhang B, et al. Insights into Opposite and Positive Effects of Biochar and Organic Fertilizer on Red Soil Properties and Growth of Pennisetum giganteum. Sustainability. 2023; 15(20):15142. https://doi.org/10.3390/su152015142
Chicago/Turabian StyleZhang, Bangxi, Xue Li, Tianhong Fu, Hongzhao Li, Wendi Li, Qinyu Zhang, Jie Wang, Bo Chen, Rende Yang, Baige Zhang, and et al. 2023. "Insights into Opposite and Positive Effects of Biochar and Organic Fertilizer on Red Soil Properties and Growth of Pennisetum giganteum" Sustainability 15, no. 20: 15142. https://doi.org/10.3390/su152015142
APA StyleZhang, B., Li, X., Fu, T., Li, H., Li, W., Zhang, Q., Wang, J., Chen, B., Yang, R., Zhang, B., Wang, X., He, X., Chen, H., Zhang, Y., & Peng, Y. (2023). Insights into Opposite and Positive Effects of Biochar and Organic Fertilizer on Red Soil Properties and Growth of Pennisetum giganteum. Sustainability, 15(20), 15142. https://doi.org/10.3390/su152015142









