Abstract
In this paper, the coal pillar dam body of the underground reservoir in Daliuta coal mine, along with the residual coal and the mine water present in the goaf, were taken as research subjects, and a dynamic simulation experiment device was constructed to simulate the actual process of a coal mine underground reservoir (CMUR). The composition and structure of middling coal during the experiment were determined by X-ray diffraction analysis (XRD) and X-ray fluorescence spectrometry (XRF), while changes in ion content in the mine water were assessed through ion chromatography (IC) and inductively coupled plasma emission spectrometry (ICP-OES). Based on both the composition and structure of coal as well as variations in ion concentrations in water, the interaction mechanism between coal and mine water was explored. The results showed that the water–coal interaction primarily arose from the dissolution of minerals, such as rock salt and gypsum, within coal. Additionally, coal samples in mine water exhibited adsorption and precipitation of metal ions, along with cation exchange reaction. Na+ in mine water predominantly originated from the dissolution of rock salt (sodium chloride) in coal, while Ca2+ and were released through the dissolution of gypsum and other minerals in coal. In the process of the water–coal interaction, Ca2+ in the water body was adsorbed and immobilized by the coal sample, leading to the formation and deposition of CaCO3 on the surface of the coal, thereby increasing the calcite content. These processes collectively contributed to a decrease in the concentration of Ca2+ in the water body. Moreover, the cation exchange reaction occurred between Ca2+ and Mg2+ in mine water and Na+ in the coal sample. The presence of Ca2+ and Mg2+ resulted in their displacement of Na+ within the coal matrix, consequently elevating Na+ concentration in the mine water while reducing both the Ca2+ and Mg2+ concentrations. On this basis, combined with insights from the water–rock interaction, it can be inferred that the adsorption mechanisms involving rocks played a dominant role in the decrease of Ca2+ concentration during the water–rock interactions. Meanwhile, the dissolution processes of minerals both in the water–rock and water–coal interactions predominantly contributed to the increase of Na+ and Cl− concentrations.
1. Introduction
In regions of China abundant in coal resources, water scarcity poses a challenge due to the substantial water requirements of coal mining operations. Consequently, the conservation and efficient utilization of water resources are intricately linked to ensuring sustainable coal extraction practices. In view of the arid climate prevalent in the western mining areas of China, significant quantities of mine water are currently being wasted through evaporation. Scholars have proposed the concept of “coal mine underground reservoir (CMUR)” which utilizes underground goaf for the purification, storage, and utilization of mine water [1,2]. This approach transforms the protection of mine water from a “plugging method” to a “guide storage” [3]. It has been observed that the CMUR can effectively reduce the total hardness, suspended matter, and pollutant concentration in the mine water [4]. The purified mine water obtained through CMUR can be utilized for various purposes including underground water supply [5], thereby achieving the recycling of mine water and effectively addressing issues related to mine water waste and evaporation. Taking the CMUR of Daliuta as an example, the inlet and outlet water samples were detected [6]. The concentrations of Ca2+ and
in the inlet water were 68 mg/L and 367 mg/L, respectively. The concentration of suspended solids ranged from 560 to 2500 mg/L, while the mass concentrations of Fe and Mn ranged between 5.67 and 12.67 mg/L, and 0.24 to 1.35 mg/L, respectively. Following storage in the CMUR, the outlet water exhibited reduced concentrations of Ca2+ (50 mg/L) and (321 mg/L), as well as a decrease in the concentration of suspended solids to a range of 114–182 mg/L. Moreover, there was a decline observed in the mass concentrations of Fe (1.83 mg/L) and Mn (0.06 g/L), with some exceptions noted. However, it is worth mentioning that certain constituents such as Na+ and Cl− showed an increase in the effluent concentrations from the initial values of 210 mg/L and 289 mg/L to the final values of 286 mg/L and 320 mg/L, respectively.
In view of the fluctuations in water quality within and outside CMUR, the Na+ in water can be attributed to the dissolution of albite and other minerals, while
originated from the oxidation of pyrite. The release of Ca2+ and Mg2+ was a result of the dissolution of silicate minerals [7]. The elevated K+ concentration was derived from hydrolysis reactions involving K–feldspar and illite. Additionally, rock mass adsorption led to a decrease in both Mg2+ concentration as well as the adsorption and precipitation of Ca2+. The cation exchange processes between water and rock mass may also involve Ca–Mg and K–Mg exchanges [8]. Dissolved organic matter (DOM) formed complexes with heavy metals, but their complexation with DOM was weakened due to competition for adsorption by rocks [9]. The removal of DOM by coal gangue was attributed to the adsorption and degradation of aluminosilicate minerals, such as kaolinite, muscovite, and chlorite in coal gangue. The adsorption primarily involved chemical adsorption and ion exchange processes. Moreover, the adsorption process exhibited endothermic characteristics. The decrease in temperature would weaken the adsorption capacity of DOM by coal gangue, but the ability of coal gangue to remove aromatic compounds from mine water remained limited [10,11,12].
At present, the research on the purification effect of a CMUR on mine water focuses primarily on the interaction between mine water and rock. However, practical engineering experience revealed that there were not only collapsed rocks in the goaf of CMUR, but also numerous coal pillar dams and residual coal, accounting for more than 80% of the CMUR structure. Furthermore, as a bio-organic rock, coal possesses a more complex structural composition compared to traditional rock formations. Therefore, it is crucial to investigate the interaction mechanism between coal and mine water in a CMUR for clarifying the water purification mechanism.
During the operation of an underground reservoir in the coal mine, the prolonged contact between the mine water and the coal pillar dam, as well as the presence of coal residue in goaf, hinders complex chemical reactions and primarily involves interactions between coal and water. In this paper, the underground reservoir of Daliuta coal mine in Shendong is taken as the research object. Therefore, a dynamic simulation test device for the water–coal interaction is employed to investigate changes in inorganic components within coal using XRF and XRD. The IC and ICP-OES are utilized to examine variations in anions and cations present in mine water. The water–coal interaction mechanism between coal and mine water is explored, thereby providing a scientific foundation for understanding the purification mechanism of mine water within underground reservoirs.
2. Materials and Methods
2.1. Sample Collection
Daliuta coal mine is divided into the 2−2 and 5−2 coal seams. The No.1, No.2, and No.3 reservoirs have been built in the mined 2−2 coal seam, and two water recycling chambers have been built in the goaf of the 5−2 coal seam [13,14]. According to statistics, the total water storage of the three reservoirs is 7.105 million m3, the amount of sewage recharge is 9780 m3/d, and the underground and surface water consumption is 12,420 m3/d [15]. Since the 2−2 coal seam contains three CMURs, the mine water of the inlet and outlet of the No.1 reservoir and the coal of the 5−2 coal seam were collected for experimental research. Na+, K+, Ca2+, Mg2+, Cl−, , , , and other common ions in groundwater were selected to characterize the hydrochemical characteristics of water. The collection of water samples from the inlet and outlet of the CMUR was carried out in accordance with the Chinese standards “HJ494-2009 Water quality–Guidance on sampling techniques” and “HJ495-2009 Water quality–Technical regulation on the design of sampling programmes”.
2.2. Dynamic Simulation
As shown in Figure 1, the mine water was extracted to the higher goaf through a pipeline in the mine. Subsequently, the mine water at the higher goaf flowed downwards to the lower areas via gaps formed by gangue and residual coal. The mine water can realize the natural purification of the mine water by the CMUR through long-distance slow flow. The daily water inflow into the CMUR was significantly smaller than its total water storage capacity, rendering any disturbance effect negligible. Consequently, there existed a prolonged residence time for the mine water with minimal seepage velocity within the CMUR [16,17]. In order to replicate the operational state of the CMUR, a dynamic experimental device was designed in the form of a cuboid with an inlet and an outlet. The peristaltic pump was utilized to connect the inlet and outlet, enabling the long-distance flow of the mine water within the device. By controlling the peristaltic pump parameters, a specific rate of entry for the mine water into the device was achieved, minimizing disturbances while ensuring the continuous and stable flow as well as a slow penetration rate. Pre-crushed coal samples were placed within the simulation test device, followed by addition of 40 L of the mine water. According to the ratio of the total water storage in the CMUR to the experimental water volume, the daily water intake of the experiment was determined to be 55 mL, and the water output was 70 mL. In order to avoid clogging the peristaltic pump, a filter should be filled to filter the coal sample residue when the first orifice of the device is installed with the plastic water tank valve. Valve 2 is used for daily sampling. Samples of the mine water were collected on the 0th, 5th, 10th, 20th, 30th, 40th, and 50th days of the experiment and labeled as numbers 0, 5, 10, 20, 30, 40, and 50. To minimize experimental errors, three sets of parallel water samples were simultaneously collected daily at the same time intervals, and the average of the test water sample results was taken as the basis for subsequent analysis and discussion The dynamic simulation test of water–coal interaction was analyzed as follows:
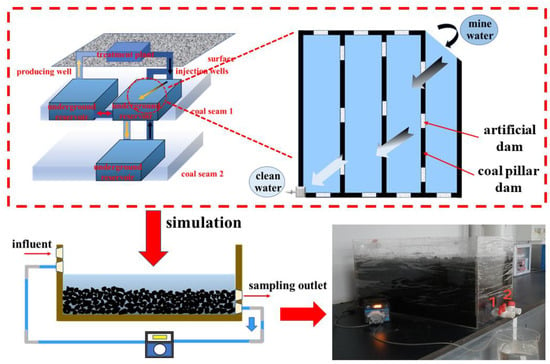
Figure 1.
CMUR principle and experimental device: 1 is a valve for the discharge and circulation of water in the experimental device; 2 is a valve for the sampling of water samples during the experiment.
a. Analyze the variation characteristics of major ions in water and the composition of coal samples.
b. Reveal the interaction mechanism between minerals in coal and mine water, as well as analyze the origin of major anions and cations in the mine water and the mechanism of water–coal interaction.
2.3. Methods to Character the Mine Water and Coal Samples
The mineral composition of coal samples was analyzed by Rigaku’s SmartLab SE X-ray diffractometer (XRD) (Cu target, Kα radiation, step 0.02°, power 40 kV, 150 mA, continuous scanning). The 2θ angle range was 5–80°, and the scanning speed was 4°/min. After ashing, the elemental composition of ash was analyzed by a Shimadzu XRF-1800 X-ray fluorescence spectrometer (XRF).
The contents of Na+, Ca2+, Mg2+, and K+ in the experimental process were determined by ICP-OES with an Optima 8300 inductively coupled plasma emission spectrometer of PerkinElmer. The contents of Cl− and were determined by a Thermo Scientific Dionex Aquion Rfic ion chromatograph (IC). The PerkinElmer Nex ION 300D ionization mass spectrometer was used to determine the content of Fe in water, and the Hanna HI98195 multifunctional and multi-parameter water quality analysis tester was used to determine the pH and TDS of water. The content of
in water was determined using the dual mixed indicator titration method according to the Chinese standard “DZ/T 0064.49-2021 Methods for analysis of groundwater quality. Part 49: Determination of carbonate, bicarbonate ions, hydroxy–Titration”.
3. Results
3.1. Changes of Inorganic Components in Coal under Water–Coal Interaction
The elemental composition of coal samples was determined using XRF on days 0, 5, 15, 30, and 50 to analyze the changes in mineral element content during the water–coal interaction (Figure 2). Si and Al were found to be the most abundant elements in coal, followed by S, Ca, Fe, Na, Mg, and K. After the dynamic simulation experiment, the mass fractions of Si and Ca in coal increased from 38.20% and 12.67% to 49.05% and 15.70%, respectively. Conversely, the mass fractions of Al and Na decreased from 24.98% and 3.17% to 15.29% and 1.56%, respectively, while other elements showed minimal changes. It was determined that these elements typically occurred in the form of minerals listed in Table 1 within coal [18,19].
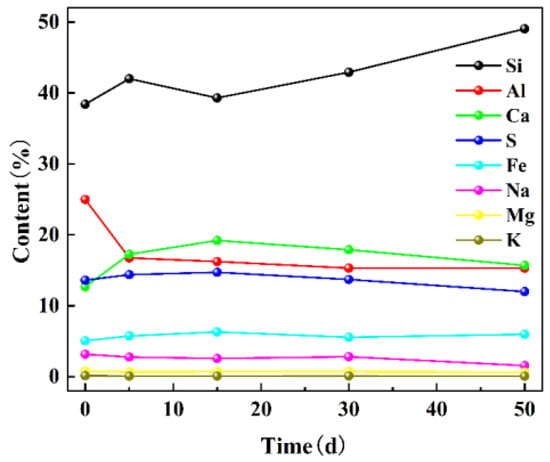
Figure 2.
Changes of mineral elemental content in coal with time.

Table 1.
Occurrence forms of main elements in coal.
With the help of XRD analysis, the mineral morphology of these elements in coal can be revealed. The XRD determination and analysis of coal samples before and after the dynamic simulation experiment are shown in Figure 3, revealing that kaolinite, quartz, calcite, pyrite, and gypsum were the primary constituents of coal. In conjunction with Table 1, Al, Si, and S elements were present in coal as kaolinite, quartz, pyrite, and other minerals, while Ca elements existed in the form of calcite and gypsum minerals. The diffraction peaks of quartz and calcite were intensified, whereas those of kaolinite, pyrite, and gypsum were weakened, indicating an increase in the content of minerals such as quartz and calcite in coal, along with a decrease in the content of kaolinite, pyrite, and gypsum. Overall, XRF and XRD test results corroborated each other, indicating that the increase of Si and Ca content was due to the increase of quartz and calcite, while a decline in kaolinite, pyrite, and gypsum led to a reduction in Al, S, and Ca content.
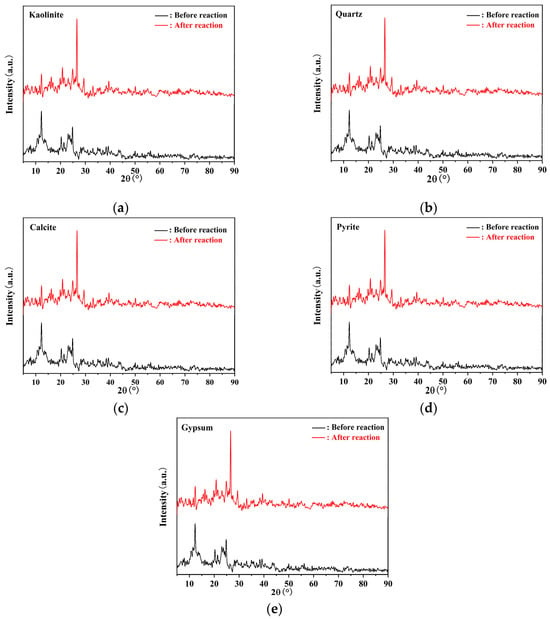
Figure 3.
XRD patterns of coal samples before and after reaction: (a) kaolinite, (b) quartz, (c) calcite, (d) pyrite, (e) gypsum.
3.2. Ion Changes in Mine Water under Water–Coal Interaction
The concentrations of anions and cations in the water samples were measured on the days 0, 5, 10, 20, 30, 40, and 50. Additionally, the pH variations in the mine water during the experiment were monitored, as shown in Figure 4. The cation concentration was Na+ > Ca2+ > Mg2+ > K+, with initial concentrations of 173.9 mg/L, 131.8 mg/L, 10.8 mg/L, and 0.1 mg/L, respectively. As the experiment progressed, the concentration of Na+ gradually increased and reached a final concentration of 373.5 mg/L. The concentration of Ca2+ initially decreased to 79.9 mg/L and then slowly increased to 112.8 mg/L. The concentrations of Mg2+ and K+ fluctuated within the ranges of 5.3–10.8 mg/L and 0.1–0.12 mg/L, respectively. The concentration of anions in the mine water was ranked as > Cl− >
> , with corresponding concentrations of 374.5 mg/L, 256.2 mg/L, 254.0 mg/L, and 209.3 mg/L, respectively. With the extension of the experimental time, the concentrations of both
and Cl− increased to 536.9 mg/L and 499.7 mg/L, respectively. Meanwhile, the concentrations of and
decreased to 209.0 mg/L and 113.1 mg/L, respectively. The initial pH value of the mine water was 7.79, while during the experiment it ranged between 7.89 and 7.47, showing a slight reduction consistent with the actual situation.
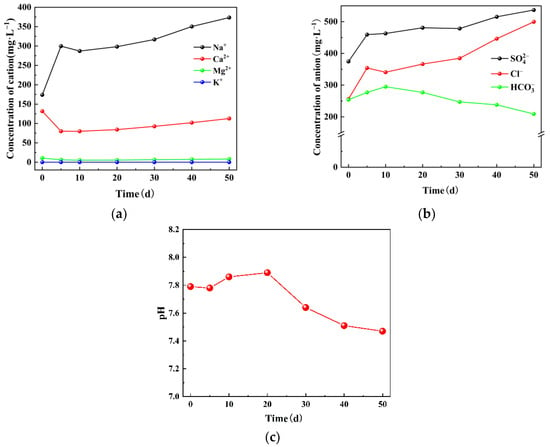
Figure 4.
Change of (a) anion, (b) cation concentration, and (c) pH with time.
Total dissolved solids (TDS) are a comprehensive reflection of the concentration of major ions in water [20]. TDS typically undergo changes due to various factors, such as mineral dissolution in coal. Through the relationship between the main anions and cations in water and TDS, the source of the main ions in mine water can be speculated, which provided a theoretical foundation for subsequent analysis of interaction [21]. In Figure 5a, with the increase of TDS, the concentration of Na+ exhibited an increase, while the concentration of Ca2+ showed a slight decrease and the concentration of Mg2+ remained relatively stable. Notably, a strong linear correlation was observed between Na+ and TDS with a coefficient of R2 value of 0.86711. In Figure 5b, with the increase of TDS, there was a decline in concentrations of and
, accompanied by an elevation in concentrations of and Cl−. However, it is worth mentioning that among these ions, demonstrated the strongest linear correlation with TDS (R2 = 0.87943). The significant correlations observed between and Na+ with TDS suggested potential dissolution processes involving sulfur-containing and sodium-containing minerals during the experimental period.
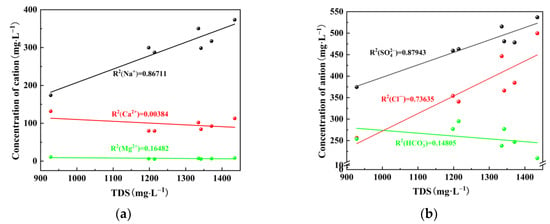
Figure 5.
Relationship between (a) TDS and (b) anion and cation concentration.
3.3. Mechanism Analysis of Water–Coal Interaction
During the experiment, prolonged contact between mine water and coal resulted in alterations to the element content and mineral composition of coal, as well as variations in the concentrations of anions and cations in mine water. Notably, there was a pronounced observed change in Na+ concentration in the mine water. Sodium chloride is a predominant form of Na in coal. If Na+ and Cl− solely originated from the dissolution of sodium salt NaCl under the influence of water–coal interactions, the concentration ratio of Na+ and Cl− should be close to 1 [22,23]. However, the Na+/Cl− concentration ratio for all water samples in the dynamic simulation experiment exceeded the straight line 1 (Figure 6), indicating an excess of Na+ relative to Cl−. This suggested that other sodium-containing minerals in coal also dissolve besides sodium chloride. The content and proportion of water-soluble (inorganic salt form), acid-soluble (organic form), and insoluble (mineral form) materials can be obtained by conducting step-by-step extraction experiments on alkali metals in coal with extractants, such as H2O, NH4Ac, and HCl [24,25].
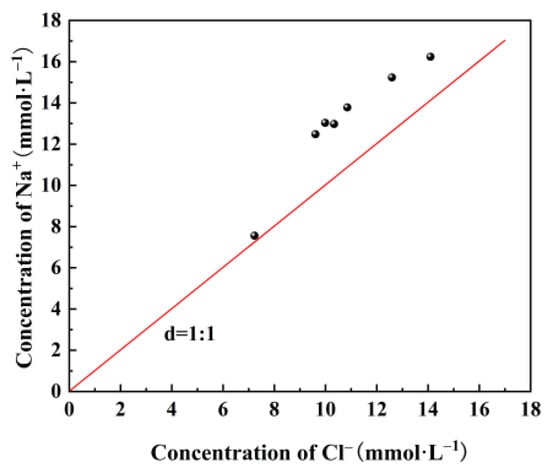
Figure 6.
Concentration ratio of Na+/Cl− in water under the action of water–coal interaction.
The water-soluble sodium in coal primarily consisted of simple free sodium salt (mainly NaCl and Na2SO4, NaHCO3, etc.) and hydrated ions (Na·xH2O) [26,27]. In Figure 4b, the concentration of
increased, followed by an initial increase and subsequent decrease in . Therefore, Na+ in mine water may also be derived from the dissolution of water-soluble compounds such as Na2SO4 and NaHCO3.
A previous study showed that the content of Ca in coal exhibited an initial increase followed by a decrease during the experiment, accompanied by an increase in the content of calcite and a decrease in the content of gypsum. Additionally, the concentration of Ca2+ in mine water decreased first and then increased. Numerous studies have confirmed that clay minerals on coal surfaces generally possess a negative charge, enabling cations such as Ca2+ and Mg2+ to be adsorbed onto the surface of minerals or within crystal interlayers by electrostatic attraction [28,29]. It can be inferred that the adsorption of Ca2+ by coal leads to a reduction in its concentration within mine water. However, with the dissolution of gypsum in coal, as shown in Equation (1), and an increase in adsorbed ions on the surface of coal, the concentration of Ca2+ gradually increased. In the near-neutral solution, the Ca2+ concentration at the interface between water and clay minerals in coal was generally higher than that in water [30]. Therefore, the surface of clay minerals could easily form Ca(OH)2 precipitate. Under the influence of free CO2 in the solution, Ca(OH)2 could easily form CaCO3 precipitate, which deposited on the surface of coal and increased the content of calcite, such as in Equation (2). The formation of Ca(OH)2 by Ca2+ and subsequent precipitation of CaCO3 caused a decrease in Ca2+ content, shifting the reaction equilibrium of Equation (1) to favor gypsum dissolution in the mine water and an increase in calcite content in coal.
In the water–mineral interaction system, alterations in the composition of water led to the replacement of specific ions attached to the mineral surface by exchangeable ions in water [31,32]. The reaction between Ca2+ and Mg2+ in mine water and Na+ in rock can be represented by Equations (3) and (4) [33]. Hence, sodium adsorption ratio (SAR) was employed to assess whether cation exchange occurred between Ca2+ and Mg2+ in the mine water and Na+ in coal, as well as to determine the strength of such reaction. The temporal variation of SAR value was calculated according to SAR value [34], as shown in Figure 7. The SAR value decreased with time, while the cation exchange strength increased. The SAR value exhibited a rapid decline within 5 days, followed by a gradual decrease, indicating a higher rate of the ion exchange reaction in the early stage and a slower rate in the later stage. In the presence of the water–coal interaction, Ca2+ and Mg2+ in water can exchange ion with Na+ in coal, leading to an increase in Na+ concentration in water and a decrease in Ca2+ and Mg2+ concentrations.
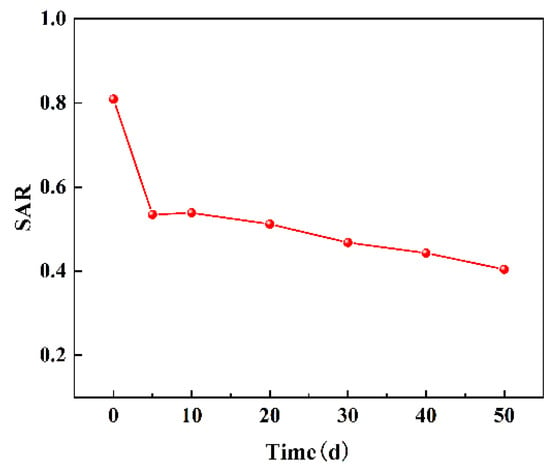
Figure 7.
Changes of sodium adsorption ratio (SAR) with time.
It can be seen from Figure 4 and Figure 5 that the concentration of increased with time and had a good linear correlation with TDS. XRD analysis revealed the presence of pyrite in the coal samples, with its content showing a decreasing trend. Upon complete contact with water, pyrite underwent oxidation to form Fe2+ and , followed by further oxidation of Fe2+ to Fe3+. As the primary oxidant, Fe3+ facilitated the conversion of pyrite to
and H+, as shown in Equations (5)–(7) [35,36]. Although only a small amount of pyrite was oxidized, resulting in limited generation of and H+, this led to a slight decrease in the pH value of mine water that aligns well with the temporal variation observed.
In summary, Na+ in mine water originated from the dissolution and cation exchange of water-soluble sodium (e.g., NaCl) in coal, with the dissolution being more significant than cation exchange. The decrease of Ca2+ concentration was a result of multiple factors, including the dissolution of gypsum and other minerals in coal, as well as the adsorption and precipitation reactions involving Ca2+ by coal and cation exchange. Although the cation exchange reaction led to a reduction in the concentration of Ca2+ in the mine water, its reaction intensity was relatively weak. Therefore, the adsorption and precipitation of Ca2+ by coal played a dominant role in the process of reducing Ca2+ concentration. In addition to the dissolution of gypsum and the oxidation of pyrite, the source of may also originate from the dissolution of water-soluble sodium (e.g., Na2SO4). The content of pyrite remained relatively stable, indicating that the main source of was likely from the dissolution of gypsum and other minerals. By analyzing the changes of inorganic components in coal and the concentration of anions and cations in the mine water under the water–coal interactions, the mechanism of water–coal interactions can be known, as shown in Figure 8, which can be summarized as the dissolution of minerals in coal by mine water, followed by the adsorption and precipitation of ions onto coal minerals and the cation exchange between coal and water.
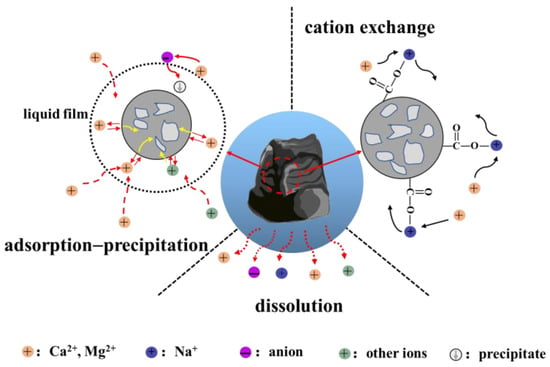
Figure 8.
Water–coal interaction mechanism.
4. Discussion
It can be seen from the above that the CMUR exhibited a discernible purifying effect on mine water, leading to a reduction in suspended solid and pollutant concentrations [4]. The reservoir’s water inlet primarily comprised Na+, Ca2+, Cl−, and
ions. While outlet demonstrates decreased levels of Ca2+ and , there was an increase in Na+ and Cl−. The pH of the water sample remained neutral or slightly alkaline, with effluent quality generally meeting the V-class groundwater standard [6].
A CMUR consists of a coal pillar dam body, residual coal in goaf, and caving rock. Considering the interaction between mine water and rock, several research findings can be summarized as follows:
The presence of Na+ in the mine water resulting from the water–rock interaction was attributed to the dissolution of minerals, such as albite and orthoclase, while Ca2+ and Mg2+ primarily originated from silicate minerals like calcite and dolomite. Cl− arose from the dissolution of rock salt and the mixing of fissure water. The adsorption, precipitation, and ion exchange processes can reduce the concentration of Ca2+ in water. Additionally,
was partially derived from the oxidation of pyrite [7,8,37].
The water–rock and water–coal interactions were investigated with the actual situation of the CMUR to explore the water purification mechanism. As shown in Figure 9, upon storage in the CMUR, the concentration of Na+ and Cl− in the water body increased from 210 mg/L and 289 mg/L to 286 mg/L and 320 mg/L, respectively. Simultaneously, the concentration of Ca2+ and decreased from 68 mg/L and 367 mg/L to 50 mg/L and 321 mg/L, respectively. Furthermore, the concentration of Na+ and Cl− increased from 341 mg/L and 311 mg/L to 473 mg/L and 356 mg/L, respectively, while the concentration of Ca2+ and decreased from 82 mg/L and 330 mg/L to 30 mg/L and 310 mg/L, respectively, under the influence of the water–rock interactions. Moreover, the concentration of Na+, Cl−, and increased from 174 mg/L, 256 mg/L, and 375 mg/L to 373 mg/L, 500 mg/L, and 537 mg/L, respectively, while the concentration of Ca2+ decreased from 132 mg/L to 113 mg/L, under the influence of the water–coal interactions. Due to variations in the sampling time for import and export water samples, as well as the water–rock and water-coal interaction experimental water samples, there existed differences in the initial concentration of ions among these three water bodies. The variation trend of Na+, Cl−, and Ca2+ during the water–rock and water–coal interactions aligned with that observed for ions in the water of CMUR, while the variation trend of under the water–coal interactions contradicted that observed during water–rock interaction and in the water of the CMUR. It can be inferred that the Na+ in the effluent of underground coal reservoirs primarily originates from sodium chloride in coal and the dissolution and ion exchange of sodium chloride in rock. The change in Ca2+ concentration was mainly due to the dissolution of carbonate minerals in rocks, calcium-containing minerals such as gypsum in coal, the adsorption and precipitation of Ca2+ by rocks and coal, and ion exchange. mainly arose from the dissolution of gypsum and the oxidation of a small amount of pyrite, and Cl− was derived from the mixing of fissure water and the dissolution of rock salt. Comparing the impact of the water–rock and water–coal interaction on the purified water of CMUR, it can be concluded that the water–rock interaction primarily contributes to the decrease of
and Ca2+ concentration in the effluent of reservoir. The decrease of Ca2+ concentration was predominantly attributed to rock-mediated adsorption and precipitation processes. Conversely, the dissolution of minerals in the water–rock and water–coal interactions served as the primary mechanism driving an increase in Na+ and Cl− concentration in water.
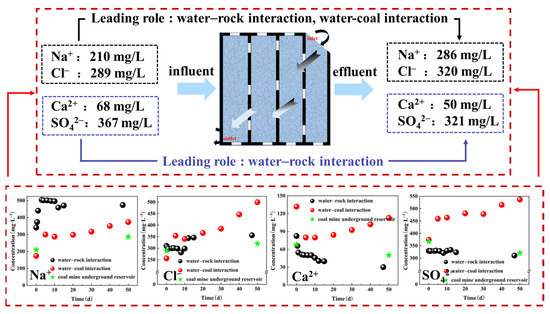
Figure 9.
Water–rock interaction, water–coal interaction, and the actual situation of the CMUR.
5. Conclusions
The interaction mechanism between coal and mine water encompasses the dissolution of minerals in coal by mine water, as well as the adsorption and precipitation of ions from water onto coal surfaces.
The presence of Na+ in the effluent was attributed not only to the dissolution of rock salt but also to the solubilization of water-soluble sodium. Ca2+ originated from both the solubilization of water-soluble calcium and calcium-containing minerals such as gypsum. arose not only from the dissolution of gypsum and a minor oxidation of pyrite but also from the solubilization of water-soluble Na2SO4. The adsorption and sequestration of Ca2+ by coal, along with precipitation-induced formation of CaCO3 on its surface, enhanced calcite content, thereby reducing aqueous concentration levels for Ca2+. Cation exchange reactions between Na+ in the coal mine water and Ca2+/Mg2+ further elevated Na+ concentration while diminishing those for Ca2+ and Mg2+.
Based on this, combined with the mechanism of the water–rock interaction, the increase in Na+ concentration in the effluent of CMUR was attributed to the dissolution and ion exchange of minerals within coal and rock. The mixing of fissure water and the dissolution of rock salt contributed to the rise in Cl− concentration. Meanwhile, the decrease of Ca2+ concentration was due to the adsorption and precipitation of coal and rock as well as ion exchange. The dissolution of minerals during the water–rock and water–coal interaction was identified as the primary cause for the increase of Na+ and Cl− concentrations, while the adsorption and precipitation of Ca2+ by rocks under the water–rock interaction accounted for reduced Ca2+ concentration levels.
Author Contributions
Conceptualization, B.J. and D.L. (Dingcheng Liang); methodology, Z.Z. and D.L. (Dingcheng Liang); validation, Z.C., J.T., M.W. and H.Z.; formal analysis, Z.Z. and D.L. (Dingcheng Liang); investigation, B.J. and Z.Z.; resources, B.J.; data curation, D.L. (Dingcheng Liang); writing—original draft preparation, Z.Z. and D.L. (Deqian Liu); writing—review and editing, D.L. (Dingcheng Liang) and P.L.; supervision, D.L. (Dingcheng Liang) and P.L.; project administration, D.L. (Dingcheng Liang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Fund of State Key Laboratory of Water Resource Protection and Utilization in Coal Mining (Grant No. WPUKFJJ2019-12).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
There are no conflict of interest to declare.
References
- Chi, M.B.; Li, Q.S.; Cao, Z.G.; Fang, J.; Wu, B.Y.; Zhang, Y.; Wei, S.R.; Liu, X.Q.; Yang, Y.M. Evaluation of water resources carrying capacity in ecologically fragile mining areas under the influence of underground reservoirs in coal mines. J. Clean. Prod. 2022, 379, 134449. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, H.; He, X.W.; Guo, S.Q.; Xia, Y.; Zhou, Y.X.; Liu, K.; Yang, S.P. Research progress, problems and prospects of mine water treatment technology and resource utilization in China. Crit. Rev. Environ. Sci. Technol. 2020, 50, 331–383. [Google Scholar] [CrossRef]
- Zhang, C.H.; Luo, B.; Xu, Z.M.; Sun, Y.J.; Feng, L. Research on the capacity of underground reservoirs in coal mines to protect the groundwater resources: A case of Zhangshuanglou coal mine in Xuzhou, China. Water 2023, 15, 1468. [Google Scholar] [CrossRef]
- Jiang, B.B.; Gao, J.; Du, K. Insight into the water–rock interaction process and purification mechanism of mine water in underground reservoir of Daliuta coal mine in China. Environ. Sci. Pollut. Res. 2022, 29, 28538–28551. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.T.; Bai, Q.S. Underground space utilization of coalmines in China; a review of underground water reservoir construction. Tunn. Undergr. Space Technol. 2021, 107, 103657. [Google Scholar] [CrossRef]
- Han, J.M.; Gao, J.; Du, K.; Chen, M.Y.; Jiang, B.B.; Zhang, K. Analysis of hydrochemical characteristics and formation mechanism in coal mine underground reservoir. Coal Sci. Technol. 2020, 48, 223–231. (In Chinese) [Google Scholar]
- Zhang, K.; Gao, J.; Jiang, B.B.; Han, J.M.; Chen, M.Y. Experimental study on the mechanism of water-rock interaction in the coal mine underground reservoir. Chin. J. Coal 2019, 44, 3760–3772. (In Chinese) [Google Scholar]
- Fang, M.Y.; Li, X.Y.; Zhang, G.; Liu, Q.; Tuo, K.Y.; Liu, S.Q. Research on water–rock interaction mechanism in coal mine underground reservoir–taking Daliuta Coal Mine as an example. Coal Sci. Technol. 2022, 50, 236–242. (In Chinese) [Google Scholar]
- Zhang, K.; Gao, J.; Men, D.P.; Zhao, X.Y.; Wu, S.S. Insight into the heavy metal binding properties of dissolved organic matter in mine water affected by water-rock interaction of coal seam goaf. Chemosphere 2021, 265, 129134. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, S.H.; Zhao, L.; Zhang, P.Q.; Wang, S.D.; Sun, C.; Zhang, L. Effect of Darcy flux on the release of dissolved organic matter and nitrogen from coal gangue in a coal mine underground reservoir: Column experiments. Chem. Geol. 2020, 545, 119652. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, C.; Yan, P.X.; Zhang, Q.; Wang, S.D.; Luo, S.H.; Mao, Y.X. Dynamic changes of nitrogen and dissolved organic matter during the transport of mine water in a coal mine underground reservoir: Column experiments. J. Contam. Hydrol. 2019, 223, 103473. [Google Scholar] [CrossRef]
- Zhao, B.H.; Fan, S.; Bian, W.; Jiang, B.B.; Su, C.; Li, J.; Zhu, Y.H.; Li, Y.Q. Study of Dissolved organic matter adsorption removal by coal gangue in high salinity mine water. J. Beijing Univ. Technol. 2022, 48, 989–997. (In Chinese) [Google Scholar]
- Fan, L.M.; Ma, X.D. A review on investigation of water-preserved coal mining in western China. Int. J. Coal Sci. Technol. 2018, 5, 411–416. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, K.; Gao, J.; Yang, Y.M.; Bai, L.; Yan, J.Y. Research on temporal patterns of water–Rock interaction in the coal mine underground reservoir based on the dynamic simulation test. ACS Omega 2023, 8, 13819–13832. [Google Scholar] [CrossRef]
- Chen, S.S. Research on the Key Technology of Water Resources Recycling Utilization in the Underground Goaf Reservoir in Shendong Mining Area; Xi’an University of Science and Technology: Xi’an, China, 2016. (In Chinese) [Google Scholar]
- Qin, W. Study on hydraulic connection and seepage law of goaf groups in coal mine underground reservoir. Geofluids 2022, 2022, 4316878. [Google Scholar] [CrossRef]
- Shi, X.C. Research progress and prospect of underground reservoirs in coal mines. Coal Sci. Technol. 2022, 50, 216–225. (In Chinese) [Google Scholar]
- Finkelman, R.B.; Dai, S.F.; French, D. The importance of minerals in coal as the hosts of chemical elements A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Couch, G. Understanding slagging and fouling in PF combustion. IEA Rep. 1994, 74, 1541–1542. [Google Scholar]
- Yang, J.; Wang, H.; Wang, Q.M.; Zhang, X.Y.; Wang, T.T. Characteristics and sources of typical pollution components in mine water in the border area of Inner Mongolia and Shaanxi. Chin. J. Coal 2023, 48, 1687–1696. (In Chinese) [Google Scholar]
- Chen, S.D.; Tao, S.; Tian, W.G.; Tang, D.Z.; Zhang, B.; Liu, P.C. Hydrogeological control on the accumulation and production of coalbed methane in the Anze Block, southern Qinshui Basin, China. J. Petrol. Sci. Eng. 2021, 198, 108138. [Google Scholar] [CrossRef]
- Magaritz, M.; Nadler, A.; Koyumdjisky, H. The use of Na/Cl ratios to trace solute sources in a semiarid zone. Water Resour. Res. 1981, 17, 602–608. [Google Scholar] [CrossRef]
- Sami, K. Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J. Hydrol. 1992, 139, 27–48. [Google Scholar] [CrossRef]
- Liang, D.C.; Xie, Q.; Zhou, H.B.; Yang, M.S.; Cao, J.Y.; Zhang, J. Catalytic effect of alkali and alkaline earth metals in different occurrence modes in Zhundong coals. Asia–Pac. J. Chem. Eng. 2018, 13, e2190. [Google Scholar] [CrossRef]
- Sun, C.; Wei, X.L.; Kang, R.N.; Bin, F.; Li, S. Intrinsic sodium occurrence in Zhundong coal: Experimental observations and molecular modeling. Fuel 2021, 305, 121491. [Google Scholar] [CrossRef]
- Liang, D.C.; Xie, Q.; Liu, J.C.; Liu, D.Q.; Wan, C.R.; Yang, S. Transformation of alkali and alkaline earth metals during the preparation of activated carbon from Zhundong high-alkali coal. RSC Adv. 2021, 11, 3870–3878. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, X.L.; Li, T.; Li, S. Effect of alkali metals on nitrogen oxide emission: Role of Na and its occurrence in coal. Proc. Combust. Inst. 2021, 38, 5299–5309. [Google Scholar] [CrossRef]
- Susmita, S.G.; Krishna, G.B. Adsorption of metal ions by clays and inorganic solids. RSC Adv. 2014, 4, 28537–28586. [Google Scholar]
- Fang, Z.Y. Research on Mechanism of Purifying Underground Reservoir Water Storage by Collapsed Coal Rock in Goaf of Wanli No.1 Coal Mine; China University of Mining and Technology: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Li, H.L.; Chen, J.; Peng, C.L.; Min, F.F.; Song, S.X. Salt coagulation or flocculation? In situ zeta potential study on ion correlation and slime coating with the presence of clay: A case of coal slurry aggregation. Environ. Res. 2020, 189, 109875. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, C.; Sheikhi, S. Role of ion exchange in the brine-rock interaction systems; a detailed geochemical modeling study. Chem. Geol. 2021, 559, 119992. [Google Scholar] [CrossRef]
- Shi, W.G.; Wang, Q.R.; Danlami, M.S. A novel analytical model of solute transport in a layered aquifer system with mixing processes in the reservoirs. Environ. Sci. Pollut. Res. 2022, 29, 67953–67968. [Google Scholar] [CrossRef]
- Han, J.M. Study on Water-Rock Interaction and Water Purification Mechanism of Coal Mine Underground Reservoir; China University of Mining and Technology: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Liu, X.M.; Zhu, Y.C.; Bennett, J.M.; Wu, L.S.; Li, H. Effects of sodium adsorption ratio and electrolyte concentration on soil saturated hydraulic conductivity. Geoderma 2022, 414, 115772. [Google Scholar] [CrossRef]
- Zhu, J.X.; Xian, H.Y.; Lin, X.J.; Tang, H.M.; Du, R.X.; Yang, Y.P.; Zhu, R.L.; Liang, X.L.; Wei, J.M.; Teng, H.H.; et al. Surface structure-dependent pyrite oxidation in relatively dry and moist air: Implications for the reaction mechanism and sulfur evolution. Geochim. Et Cosmochim. Acta 2018, 228, 259–274. [Google Scholar] [CrossRef]
- Feng, J.L.; Tian, H.; Huang, Y.L.; Ding, Z.Y.; Yin, Z.L. Pyrite oxidation mechanism in aqueous medium. J. Chin. Chem. Soc. 2019, 66, 345–354. [Google Scholar] [CrossRef]
- Gao, J. Study on the Law of Ion Migration and Transformation during Water-Rock Action of Coal Mine Underground Reservoir; China University of Mining and Technology: Beijing, China, 2021. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).