Diversity in Landscape Management Affects Butterfly Distribution
Abstract
:1. Introduction
2. Objectives and Scope of the Study
- Butterflies show differences in response to land use form.
- Butterflies show differences in area selection according to food preferences and habitat preferences.
- Quantitative occurrence of melliferous vegetation (number of melliferous plant) affects the occurrence of butterflies.
- The stage of succession affects the quantitative occurrence of butterflies and butterfly biodiversity.
3. Materials and Methods
3.1. Field Methods
3.2. Data Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- van Vuuren, D.; Pereira, H.M.; Lodge, D.; Alder, J.; Cumming, G.; Dobson, A.; Wolters, V.; Xenopoulos, M.A. Millennium Ecosystem Assessment. Biodiversity across Scenarios. In Eco-Systems and Human Well-Being: Scenarios; Island Press: Washington, DC, USA, 2005; pp. 375–405. [Google Scholar]
- Aviron, S.; Jeanneret, P.; Schüpbach, B.; Herzog, F. Effects of agri-environmental measures, site and landscape conditions on butterfly diversity of Swiss grassland. Agric. Ecosyst. Environ. 2007, 122, 295–304. [Google Scholar] [CrossRef]
- Herrmann, J.; Buchholz, S.; Theodorou, P. The degree of urbanisation reduces wild bee and butterfly diversity and alters the patterns of flower-visitation in urban dry grasslands. Sci. Rep. 2023, 13, 2702. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K.; Dymitryszyn, I.; Jankiewicz, U.; Kondras, M.; Żyfka-Zagrodzińska, E.; Schwerk, A. Assemblage Characteristics of Butterflies and Carabid Beetles as a Function of Soil Characteristics and Plant Diversity in Differently Managed Fields, Forests and Ecotones: A Case Study in Tuczno Forest District, Poland. Land 2021, 11, 25. [Google Scholar] [CrossRef]
- Koh, L.P. Impacts of land use change on South-east Asian forest butterflies: A review. J. Appl. Ecol. 2007, 44, 703–713. [Google Scholar] [CrossRef]
- Odum, E.P. Podstawy Ekologii; 1997 Wyd; Państwowe Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 1977. [Google Scholar]
- Szyszko, J. Stadia sukcesyjne i ocena wartości przyrodniczych krajobrazu na podstawie taksonów. In Ocena i Wycena Zasobów Przyrodniczych; SGGW: Warszawa, Poland, 2013; pp. 195–202. [Google Scholar]
- Szyszko, J.; Tobolski, K. (Eds.) Podstawy Kompensacji Przyrodniczej; Wydawnictwo WSKSiM: Toruń, Poland, 2010. [Google Scholar]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef]
- Sagwe, R.N.; Muya, S.M.; Maranga, R. Effects of land use patterns on the diversity and conservation status of butterflies in Kisii highlands, Kenya. J. Insect Conserv. 2015, 19, 1119–1127. [Google Scholar] [CrossRef]
- Szyszko, K. Characteristic of occurrence of butterflies (Rhopalocera) on the research object “Krzywda”. In Landscape Architecture and Spatial Planning as the Basic Element in the Protection of Native Species—Modeling of Succession Stages; Warsaw Agricultural University Press: Warsaw, Poland, 2004; pp. 125–133. [Google Scholar]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Homburg, K.; Drees, C.; Boutaud, E.; Nolte, D.; Schuett, W.; Zumstein, P.; Ruschkowski, E.; Assmann, T. Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv. Divers. 2019, 12, 268–277. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Rylke, J.; Szyszko, J. (Eds.) Didactics Trails for Field Classes on Evaluation and Assessment of Natural Resources; Warsaw Agricultural University Press: Warsaw, Poland, 2002; 167p. [Google Scholar]
- Kadlec, T.; Tropek, R.; Konvicka, M. Timed surveys and transect walks as comparable methods for monitoring butterflies in small plots. J. Insect Conserv. 2012, 16, 275–280. [Google Scholar] [CrossRef]
- Turlure, C.; Choutt, J.; Van Dyck, H.; Baguette, M.; Schtickzelle, N. Functional habitat area as a reliable proxy for population size: Case study using two butterfly species of conservation concern. J. Insect Conserv. 2010, 14, 379–388. [Google Scholar] [CrossRef]
- Buszko, J.; Masłowski, J. Motyle Dzienne Polski; Wyd. Koliber: Nowy Sącz, Poland, 2015. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin, Germany, 1964; p. 631. [Google Scholar] [CrossRef]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M.; Paul, W.; Ronikier, M.; Bernacki, L.; Cieślak, E.; Głowacki, Z.; Leda, M.; et al. Flowering Plants and Pteridophytes of Poland. A Checklist. Biodiversity of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; Volume 1, p. 442. [Google Scholar]
- Plewka, T. Określanie klas dominacji w strukturze dominacyjnej zgrupowań owadów, na przykładzie biegaczowatych [Coleoptera: Carabidae]. Wiadomości Entomol. 2007, 26, 225–231. [Google Scholar]
- Szujecki, A. Ekologia Owadów Leśnych; PWN: Warszawa, Poland, 1980; 603p. [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Van Swaay, C.; Dennis, E.B.; Schmucki, R.; Balalaikins, M. The EU Butterfly Indicator for Grassland species: 1990–2017; Technical Report; Butterfly Conservation Europe: Wareham, Dorset, 2019. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K. Motyle dzienne. In Podstawy Kompensacji Przyrodniczej; Szyszko, J., Tobolski, K., Eds.; Wydawnictwo WSKiM: Toruń, Poland, 2010; pp. 238–244. [Google Scholar]
- Jugovic, J.; Grando, M.; Genov, T. Microhabitat selection of Aporia crataegi (Lepidoptera: Pieridae) larvae in a traditionally managed landscape. J. Insect Conserv. 2017, 21, 307–318. [Google Scholar] [CrossRef]
- Mazurkiewicz, A.; Tumialis, D.; Pezowicz, E.; Skrzecz, I.; Błażejczyk, G. Sensitivity of Pieris brassicae, P. napi and P. rapae (Lepidoptera: Pieridae) larvae to native strains of Steinernema feltiae (Filipjev, 1934). J. Plant Dis. Prot. 2017, 124, 521–524. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R. Plant Glucosinolate Content and Host-Plant Preference and Suitability in the Small White Butterfly (Lepidoptera: Pieridae) and Comparison with Another Specialist Lepidopteran. Plants 2023, 12, 2148. [Google Scholar] [CrossRef]
- Jesus, J.G.F. The life cycle of the little known and endangered endemic Madeiran Brimstone Butterfly Gonepteryx maderensis Felder, 1862 (Pieridae). Nota lepid. 2009, 32, 145–157. [Google Scholar]
- Wiklund, C.; Ahrberg, C. Host Plants, Nectar Source Plants, and Habitat Selection of Males and Females of Anthocharis cardamines (Lepidoptera). Oikos 1978, 31, 169–183. [Google Scholar] [CrossRef]
- Dempster, J.P. The role of larval food resources and adult movement in the population dynamics of the orange-tip butterfly (Anthocharis cardamines). Oecologia 1997, 111, 549–556. [Google Scholar] [CrossRef]
- Kurze, S.; Heinken, T.; Fartmann, T. Nitrogen enrichment in host plants increases the mortality of common Lepidoptera species. Oecologia 2018, 188, 1227–1237. [Google Scholar] [CrossRef]
- Schneider, C.; Dover, J.; Fry, G.L.A. Movement of two grassland butterflies in the same habitat network: The role of adult resources and size of the study area. Ecol. Entomol. 2003, 28, 219–227. [Google Scholar] [CrossRef]
- Toivonen, M.; Peltonen, A.; Herzon, I.; Heliölä, J.; Leikola, N.; Kuussaari, M. High cover of forest increases the abundance of most grassland butterflies in boreal farmland. Insect Conserv. Divers. 2017, 10, 321–330. [Google Scholar] [CrossRef]
- Rodriguez, J.; Jordano, D.; Haeger, J.F. Spatial Heterogeneity in a Butterfly—Host Plant Interaction. J. Anim. Ecol. 1994, 63, 31–38. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Early succession of butterfly and plant communities on set-aside fields. Oecologia 1997, 109, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K. Effects of larval diet on myrmecophilous qualities of Polyommatus icarus caterpillars (Lepidoptera: Lycaenidae). Oecologia 1990, 83, 284–287. [Google Scholar] [CrossRef]
- Wiesen, B.; Krug, E.; Fiedler, K.; Wray, V.; Proksch, P. Sequestration of host-plant-derived flavonoids by lycaenid butterfly Polyommatus icarus. J. Chem. Ecol. 1994, 20, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Andersen, D.C. Butterfly (Papilionoidea and Hesperioidea) assemblages associated with natural, exotic, and restored riparian habitats along the lower Colorado River, USA. Regul. Rivers Res. Manag. 1999, 15, 485–504. [Google Scholar] [CrossRef]
- Young, A.M. Some observations on the natural history and behavior of the Camberwell Beauty (Mourning Cloak) butterfly Nymphalis antiopa (Linnaeus) (Lepidoptera: Nymphalidae) in the United States. Entomol. Gaz. 1980, 31, 7–18. [Google Scholar]
- Pullin, A.S. Influence of the food plant, Urtica dioica, on larval development, feeding efficiences, and voltinism of a specialist insect, Inachis io. Ecography 1986, 9, 72–78. [Google Scholar] [CrossRef]
- Janz, N.; Nylin, S. The role of female search behaviour in determining host plant range in plant feeding insects: A test of the information processing hypothesis. Proc. R. Soc. B 1997, 264, 701–707. [Google Scholar] [CrossRef]
- Blackford, M.J.P.; Dinan, L. The Effects of Ingested 20-Hydroxyecdysone on the Larvae of Aglais urticae, Inachis io, Cynthia cardui (Lepidoptera: Nymphalidae) and Tyria jacobaeae (Lepidoptera: Arctiidae). J. Insect Physiol. 1997, 43, 315–327. [Google Scholar] [CrossRef]
- Stefanescu, C. The nature of migration in the red admiral butterfly Vanessa atalanta: Evidence from the population ecology in its southern range. Ecol. Entomol. 2008, 26, 525–536. [Google Scholar] [CrossRef]
- Janz, N. The Relationship Between Habitat Selection and Preference for Adult and Larval Food Resources in the Polyphagous Butterfly Vanessa cardui (Lepidoptera: Nymphalidae). J. Insect Behav. 2005, 18, 767–780. [Google Scholar] [CrossRef]
- Wilson, A. Flavonoid pigments in marbled white butterfly (Melanargia galathea) are dependent on flavonoid content of larval diet. J. Chem. Ecol. 1985, 11, 1161–1179. [Google Scholar] [CrossRef]
- Karlsson, B.; Wiklund, C. Butterfly life history and temperature adaptations; dry open habitats select for increased fecundity and longevity. J. Anim. Ecol. 2004, 74, 99–104. [Google Scholar] [CrossRef]
- Elligsen, H.; Beinlich, B.; Plachter, H. Effects of large-scale cattle grazing on populations of Coenonympha glycerion and Lasiommata megera (Lepidoptera:Satyridae). J. Insect Conserv. 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Ellis, S.; Wainwright, D.; Dennis, E.B.; Bourn, N.A.D.; Bulman, C.R.; Hobson, R.; Jones, R.; Middlebrook, I.; Plackett, J.; Smith, R.G.; et al. Are habitat changes driving the decline of the UK’s most threatened butterfly: The High Brown Fritillary Argynnis adippe (Lepidoptera: Nymphalidae)? J. Insect Conserv. 2019, 23, 351–367. [Google Scholar] [CrossRef]
- Hanski, I.; Kuussaari, M.; Nieminen, M. Metapopulation Structure and Migration in the Butterfly Melitaea Cinxia. Ecology 1994, 75, 747–762. [Google Scholar] [CrossRef]
- Saastamoinen, M.; van Nouhuys, S.; Nieminen, M.; O’Hara, B.; Suomi, J. Development and survival of a specialist herbivore, Melitaea cinxia, on host plants producing high and low concentrations of iridoid glycosides. Ann. Zool. Fenn. 2007, 44, 70–80. [Google Scholar]
- Nylin, S.; Janz, N. Host plant preferences in the comma butterfly (Polygonia c-album): Do parents and offspring agree? Écoscience 2016, 3, 285–289. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Freitak, D.; Janz, N.; Söderlind, L.; Vogel, H.; Nylin, S. Phylogenetic relatedness and host plant growth form influence gene expression of the polyphagous comma butterfly (Polygonia c-album). BMC Genom. 2009, 10, 506. [Google Scholar] [CrossRef]
- Harding, J.; Jacob, M. Addition of Small Skipper butterfly (Thymelicus sylvestris) to the Irish List and notes on the Essex Skipper (Thymelicus lineola) (Lepidoptera: Hesperiidae). Ir. Nat. J. 2013, 32, 142–144. [Google Scholar]
- Dooley, C.A.; Bonsall, M.B.; Brereton, T.; Oliver, T. Spatial variation in the magnitude and functional form of density-dependent processes on the large skipper butterfly Ochlodes sylvanus. Ecol. Entomol. 2013, 38, 608–616. [Google Scholar] [CrossRef]
- Thomas, J.A.; Thomas, C.D.; Simcox, D.J.; Clarke, R.T. Ecology and Declining Status of the Silver-Spotted Skipper Butterfly (Hesperia comma) in Britain. J. Appl. Ecol. 1986, 23, 365–380. [Google Scholar] [CrossRef]
- Rabiej, M. Statystyka z Programem Statistica; Wyd. Helion: Gliwice, Poland, 2012. [Google Scholar]
- Ter Braak, C.J.F. CANOCO—A FORTRAN Program. for Canonical Community Ordination by [Par-tial][Detrended][Canonical] Correspondence Analysis, Principal Components Analysis and Redundancy Analysis (Version 2.1); DLO Agricultural Mathematics Group: Wageningen, The Netherland, 1987; p. 95. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; p. 499. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 268. [Google Scholar] [CrossRef]
- Trojan, P. Nowe perspektywy w badaniach entomofaunistycznych. Wiad. Entomol. 1998, 17, 137–155. [Google Scholar]
- Ekroos, J.; Heliölä, J.; Kuussaari, M. Homogenization of lepidopteran communities in intensively cultivated agricultural landscapes. J. Appl. Ecol. 2010, 47, 459–467. [Google Scholar] [CrossRef]
- Zingg, S.; Grenz, J.; Humbert, J.-Y. Landscape-scale effects of land use intensity on birds and butterflies. Agric. Ecosyst. Environ. 2018, 267, 119–128. [Google Scholar] [CrossRef]
- Zingg, S.; Ritschard, E.; Arlettaz, R.; Humbert, J.-Y. Increasing the proportion and quality of land under agri-environment schemes promotes birds and butterflies at the landscape scale. Biol. Conserv. 2019, 231, 39–48. [Google Scholar] [CrossRef]
- Tiple, A.; Khurad, A.; Dennis, R. Butterfly diversity in relation to a human-impact gradient on an Indian university campus. Nota Lepidopterol. 2007, 30, 179–188. [Google Scholar]
- Toltman, T.; Lewington, R. Motyle Polski I Europy; Wydawnictwo: Influence: Dąbrowa Górnicza, Poland, 2007. [Google Scholar]
- Sielezniew, M.; Stankiewicz, A.M. Ekologiczne, prawne i praktyczne aspekty ochrony motyli w Polsce na przykładzie modraszków Maculinea spp. (Lepidoptera: Lycaenidae). Wiad. Entomol. 2006, 25 (Suppl. S2), 179–188. [Google Scholar]
- Bonari, G.; Fajmon, K.; Malenovský, I.; Zelený, D.; Holuša, J.; Jongepierová, I.; Kočárek, P.; Konvička, O.; Uřičář, J.; Chytrý, M. Management of semi-natural grasslands benefiting both plant and insect diversity: The importance of heterogeneity and tradition. Agric. Ecosyst. Environ. 2017, 246, 243–252. [Google Scholar] [CrossRef]
- Szyszko-Podgórska, K. Characteristics of the butterflies on various forms of land uses. Environ. Prot. Nat. Resour. 2019, 30, 15–22. [Google Scholar] [CrossRef]
- Weibull, A.-C.; Östman, Ö.; Granqvist, Å. Species richness in agroecosystems: The effect of landscape, habitat and farm management. Biodivers. Conserv. 2003, 12, 1335–1355. [Google Scholar] [CrossRef]
- Purtauf, T.; Dauber, J.; Wolters, V. Carabid communities in the spatio-temporal mosaic of a rural landscape. Landsc. Urban Plan. 2004, 67, 185–193. [Google Scholar] [CrossRef]
- Hendrickx, F.; Maelfait, J.-P.; van Wingerden, W.; Schweiger, O.; Speelmans, M.; Aviron, S.; Augenstein, I.; Billeter, R.; Bailey, D.; Bukacek, R. How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. J. Appl. Ecol. 2007, 44, 340–351. [Google Scholar] [CrossRef]
- Ryszkowski, L.; Karg, J.; Kujawa, K.; Gołdyn, H.; Arczyńska-Chudy, E. Influence of Landscape Mosaic Structure on Diversity of Wild Plant and Animal Communities in Agricultural Landscapes of Poland. In Landscape Ecology in Agroecosystems Management; Ryszkowski, L., Ed.; CRC Press: Boca Raton, FL, USA; New York, NY, USA; Washington, DC, USA, 2001; pp. 185–217. [Google Scholar] [CrossRef]
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Ouin, A.; Aviron, S.; Dover, J.; Burel, F. Complementation/supplementation of resources for butterflies in agricultural landscapes. Agric. Ecosyst. Environ. 2004, 103, 473–479. [Google Scholar] [CrossRef]
- Szabó, A.; Ernst, L.; Gallé, R.; Batáry, P. Grassland type and presence of management shape butterfly functional diversity in agricultural and forested landscapes. Glob. Ecol. Conserv. 2022, 35, e02096. [Google Scholar] [CrossRef]
- Betzholtz, P.; Pettersson, L.B.; Ryrholm, N.; Franzén, M. With that diet, you will go far: Trait-based analysis reveals a link between rapid range expansion and a nitrogen-favoured diet. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122305. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Fontana, P.; Battisti, A.; Gaston, K.J. Agricultural management, vegetation traits and landscape drive orthopteran and butterfly diversity in a grassland–forest mosaic: A multi-scale approach. Insect Conserv. Divers. 2009, 2, 213–220. [Google Scholar] [CrossRef]
- Swengel, A.B. Effects of Management on Butterfly Abundance in Tallgrass Prairie and Pine Barrens. Biol. Conserv. 1998, 83, 77–89. [Google Scholar] [CrossRef]
- Majewska, A.A.; Sims, S.; Wenger, S.J.; Davis, A.K.; Altizer, S. Do characteristics of pollinator-friendly gardens predict the diversity, abundance, and reproduction of butterflies? Insect Conserv. Divers. 2018, 11, 370–382. [Google Scholar] [CrossRef]
- Wix, N.; Reich, M.; Schaarschmidt, F. Butterfly richness and abundance in flower strips and field margins: The role of local habitat quality and landscape context. Heliyon 2019, 5, e01636. [Google Scholar] [CrossRef] [PubMed]
- Rusterholz, H.-P.; Erhardt, A. Can nectar properties explain sex-specific flower preferences in the Adonis Blue butterfly Lysandra bellargus? Ecol. Entomol. 2000, 25, 81–90. [Google Scholar] [CrossRef]
- Chmielewski, M.W.; Naya, S.; Borghi, M.; Cortese, J.; Fernie, A.R.; Swartz, M.T.; Zografou, K.; Sewall, B.J.; Spigler, R.B. Phenology and foraging bias contribute to sex-specific foraging patterns in the rare declining butterfly Argynnis idalia idalia. Ecol. Evol. 2023, 13, e10287. [Google Scholar] [CrossRef]
- Smith, G.P.; Gardner, J.; Gibbs, J.; Griswold, T.; Hauser, M.; Yanega, D.; Ponisio, L.C. Sex-associated differences in the network roles of pollinators. Ecosphere 2021, 12, e03863. [Google Scholar] [CrossRef]
- Kishi, S.; Kakutani, T. Male visitors may decrease modularity in flower–Visitor networks. Front. Ecol. Evol. 2020, 8, 124. [Google Scholar] [CrossRef]
- Morris, M.G. The effects of structure and its dynamics on the ecology and conservation of arthropods in British grasslands. Biol. Conserv. 2000, 95, 129–142. [Google Scholar] [CrossRef]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Szyszko, J.; Schwerk, A.; Malczyk, J. Animals as an indicator of carbon sequestration and valuable landscapes. ZooKeys 2011, 100, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, W.; Schmid, B. Conservation of arthropod diversity in montane wetlands: Effect of altitude, habitat quality and habitat fragmentation on butterflies and grasshoppers. J. Appl. Ecol. 1999, 36, 363–373. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Butterfly community structure in fragmented habitats. Ecol. Lett. 2000, 3, 449–456. [Google Scholar] [CrossRef]
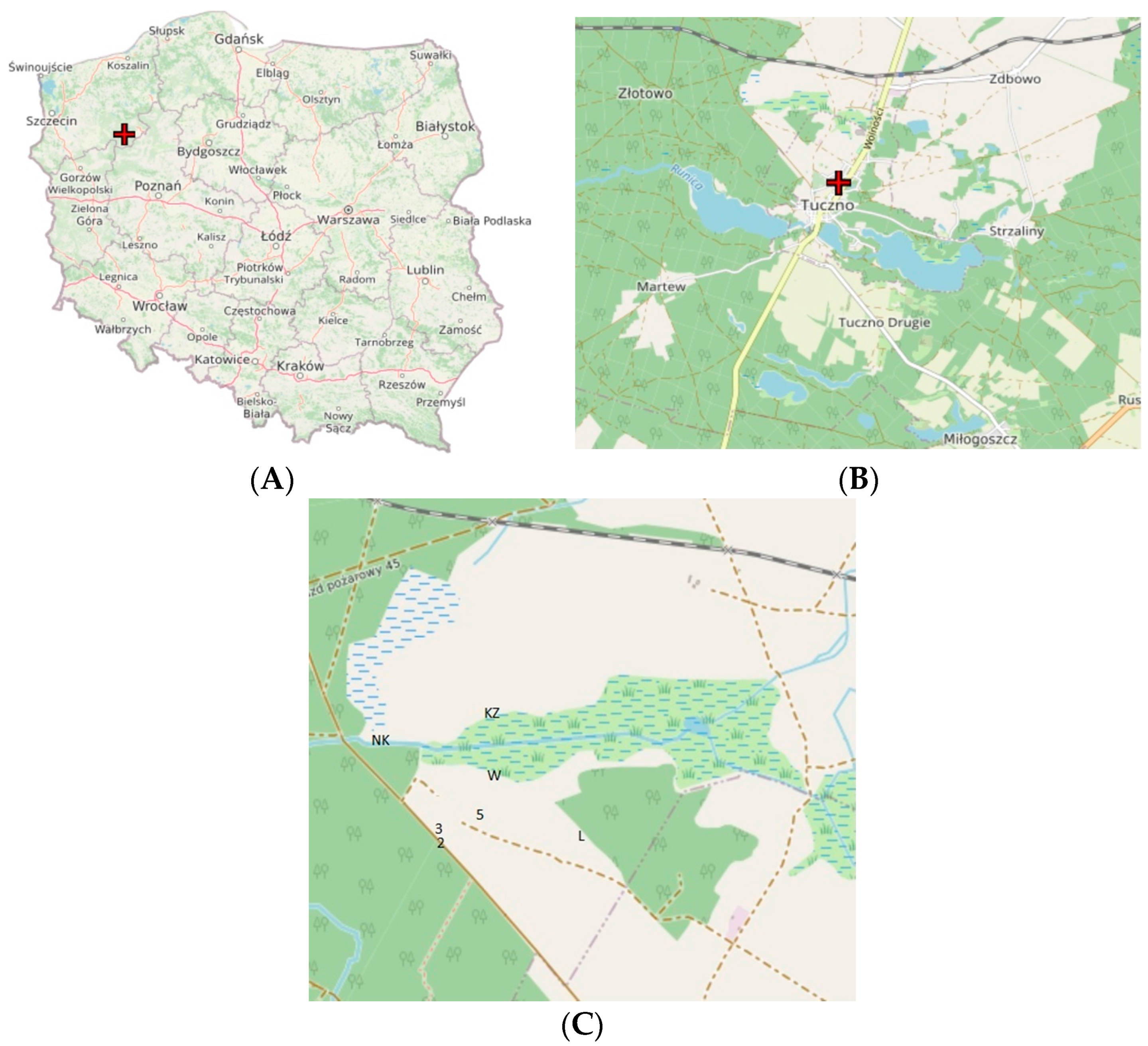
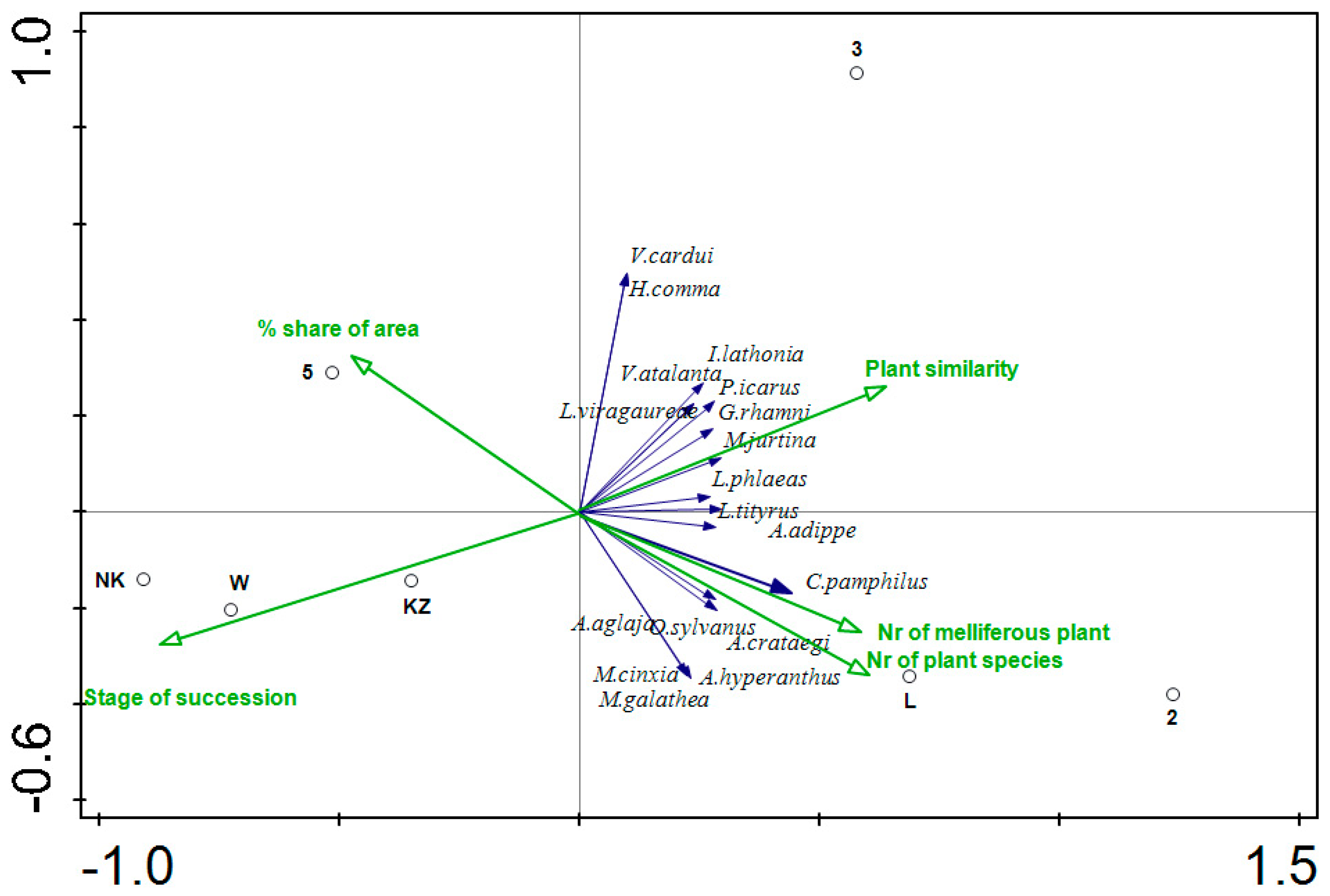
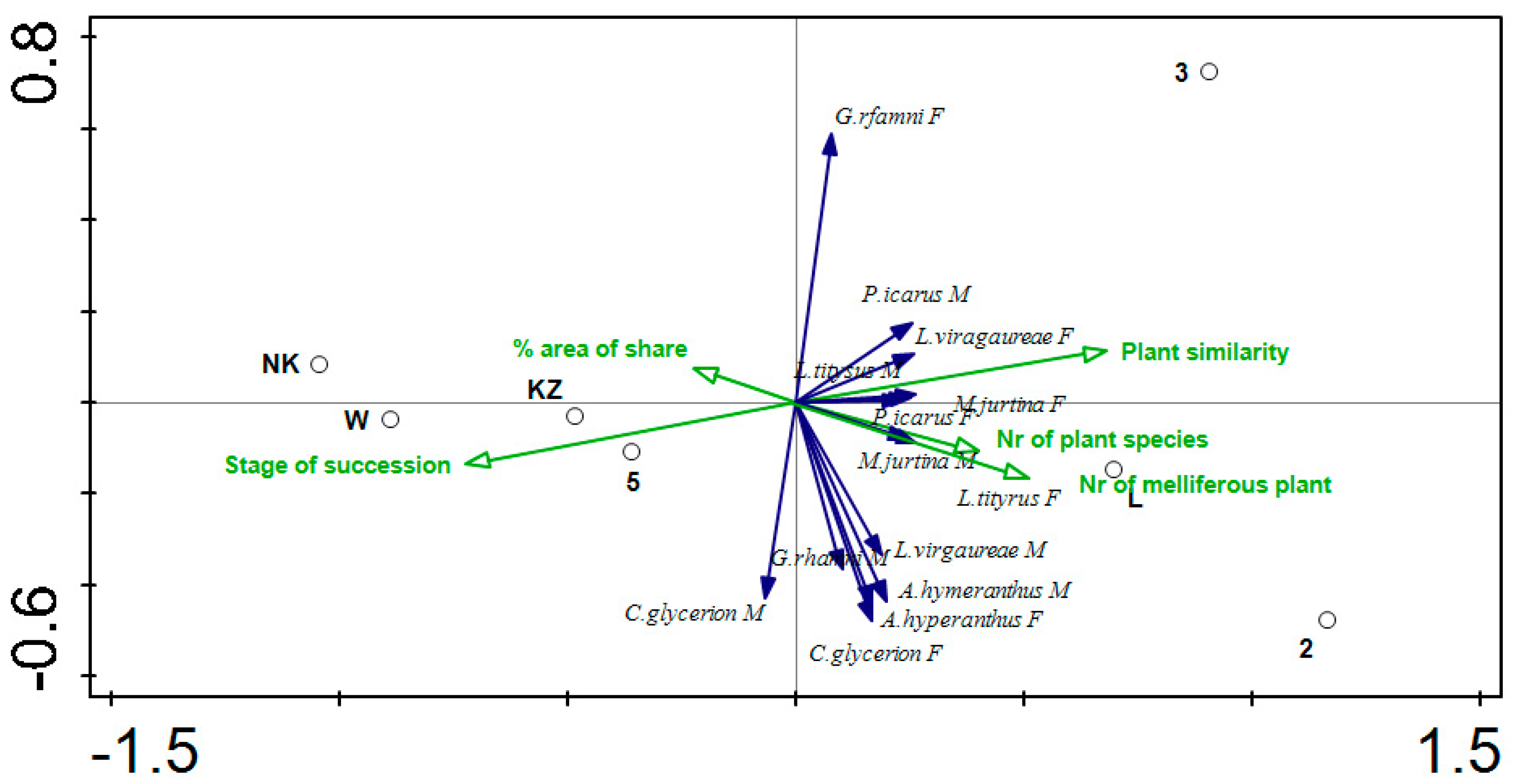
| Transect No. | Type | Description | Area (ha) | Plant Cover (%) | Dominant Plant Species | Bray–Curtis Plant Similarity | Stage of Succession | No. of Plant Species | No. of Melliferous Plant Species |
|---|---|---|---|---|---|---|---|---|---|
| 2 | Fallow land | Mown post-agricultural land with biomass not removed | 3.2 | 1.86 | Anthoxanthum odoratum, Pleurozium schreberi, Holcus lanatus, Deschampsia flexuosa | 0.503 ± 0.100 | 2 | 50 | 25 |
| 3 | Fallow land | Mown post-agricultural land with biomass removed | 5.7 | 3.31 | Anthoxanthum odoratum, Hieracium pilosella, Festuca rubra, Armeria elongata | 0.599 ± 0.088 | 1 | 44 | 23 |
| 5 | Fallow land | Unmown post-agricultural land | 10.8 | 6.28 | Anthoxanthum odoratum, Pleurozium schreberi, Deschampsia flexuosa, Phleum pretense | 0.511 ± 0.094 | 3 | 44 | 26 |
| W | Ecotone | Ecotone marshland -fallow | 2.5 | 1.45 | Agrostis capillaris, Arrhenatherum elatius, Festuca rubra, Phalaris arundinacea | 0.366 ± 0.157 | 4 | 39 | 21 |
| L | Ecotone | Ecotone forest -fallow | 0.9 | 0.52 | Sarothamnus scoparius, Anthoxanthum odoratum, Pinus silvestris, Agrostis capillaris | 0.550 ± 0.101 | 4 | 66 | 40 |
| NK | Meadow | Unmown meadow | 6.8 | 3.95 | Festuca rubra, Pleurozium schreberi, Arrhenatherum elatius, Agrostis capillaris | 0.400 ± 0.188 | 3 | 42 | 28 |
| KZ | Meadow | Mown meadow | 2.6 | 1.51 | Agrostis capillaris, Arrhenatherum elatius, Anthoxanthum odoratum, Dactylis glomerata | 0.474 ± 0.099 | 2 | 54 | 25 |
| Butterfly Species | Transect | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | L | W | KZ | NK | Sum | Fi | |
| Aporia crataegi (Linnaeus, 1758) | 57 | 6 | 0 | 15 | 0 | 3 | 3 | 84 | 71.43 |
| Pieris brassicae (Linnaeus, 1758) | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 | 14.29 |
| P.rapae (Linnaeus, 1758) | 12 | 12 | 6 | 15 | 15 | 12 | 3 | 75 | 100 |
| P. napi (Linnaeus, 1758) | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 14.29 |
| Gonepteryx rhamni (Linnaeus, 1758) | 21 | 24 | 0 | 3 | 3 | 0 | 3 | 54 | 71.43 |
| Anthocharis cardamines (Linnaeus, 1758) | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 14.29 |
| Lycaena phlaeas (Linnaeus, 1761) | 12 | 9 | 6 | 24 | 0 | 3 | 0 | 54 | 71.43 |
| L. virgaureae (Linnaeus, 1758) | 222 | 228 | 72 | 141 | 24 | 15 | 6 | 708 | 100 |
| L.tityrus (Poda, 1761) | 144 | 99 | 9 | 90 | 21 | 54 | 18 | 435 | 100 |
| Cyaniris semiargus (Rottemburg, 1775) | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 9 | 14.29 |
| Polyommatus icarus (Rottemburg, 1775) | 63 | 96 | 6 | 15 | 0 | 9 | 0 | 189 | 71.43 |
| Nymphalis antiopa (Linnaeus, 1758) | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 6 | 28.57 |
| Inachis io (Linnaeus, 1758) | 0 | 6 | 0 | 0 | 6 | 0 | 0 | 12 | 28.57 |
| Vanessa atalantha (Linnaeus, 1758) | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 6 | 28.57 |
| Vanessa cardui (Linnaeus, 1758) | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 14.29 |
| Aglais urticae (Linnaeus, 1758) | 9 | 18 | 3 | 0 | 12 | 6 | 0 | 48 | 71.43 |
| Issoria lathonia (Linnaeus, 1758) | 48 | 192 | 15 | 78 | 12 | 15 | 9 | 369 | 100 |
| Melanargia galathea (Linnaeus, 1758) | 138 | 12 | 9 | 24 | 24 | 60 | 18 | 285 | 100 |
| Maniola jurtina (Linnaeus, 1758) | 222 | 180 | 54 | 195 | 9 | 39 | 9 | 708 | 100 |
| Aphantopus hyperanthus (Linnaeus 1758) | 24 | 0 | 0 | 21 | 3 | 0 | 0 | 48 | 42.86 |
| Coenonympha pamphilus (Linnaeus, 1758) | 369 | 48 | 6 | 135 | 18 | 42 | 15 | 633 | 100 |
| C. glycerion (Borkhausen, 1788) | 15 | 0 | 9 | 3 | 3 | 6 | 12 | 48 | 85.71 |
| Argynnis adippe (Denis and Schiffermüller, 1775) | 18 | 6 | 0 | 3 | 0 | 0 | 0 | 27 | 42.86 |
| Argynnis aglaja (Linnaeus, 1758) | 3 | 0 | 3 | 9 | 0 | 0 | 0 | 15 | 42.86 |
| Melitaea cinxia (Linnaeus, 1758) | 21 | 0 | 0 | 33 | 3 | 0 | 0 | 57 | 42.86 |
| Polygonia c-album (Linnaeus, 1758) | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 14.29 |
| Thymelicus lineola (Ochsenheimer, 1808) | 9 | 0 | 0 | 15 | 30 | 15 | 6 | 75 | 71.43 |
| T. sylvestris (Poda, 1761) | 135 | 9 | 33 | 21 | 12 | 24 | 6 | 240 | 100 |
| Ochlodes sylvanus (Esper, 1777) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 14.29 |
| Hesperia comma (Linnaeus, 1758) | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 14.29 |
| Number of specimens | 1551 | 957 | 231 | 849 | 210 | 306 | 108 | 4212 | |
| Number of species | 22 | 19 | 13 | 20 | 17 | 15 | 12 | 30 | |
| 2 | 3 | 5 | L | W | KZ | NK | |
|---|---|---|---|---|---|---|---|
| the number of specimens | 1551 | 957 | 231 | 849 | 210 | 306 | 108 |
| the number of species | 22 | 19 | 13 | 20 | 17 | 15 | 12 |
| H | −0.538 | −0.356 | −0.118 | −0.345 | −0.121 | −0.156 | −0.067 |
| Transect | eR I | R II | sR III | sD IV | D V | eDVI |
|---|---|---|---|---|---|---|
| 2 | 6 | 7 | 3 | 6 | 0 | 0 |
| 3 | 7 | 6 | 3 | 3 | 0 | 0 |
| 5 | 9 | 1 | 3 | 0 | 0 | 0 |
| L | 6 | 9 | 2 | 3 | 0 | 0 |
| W | 8 | 9 | 0 | 0 | 0 | 0 |
| KZ | 6 | 5 | 4 | 0 | 0 | 0 |
| NK | 8 | 4 | 0 | 0 | 0 | 0 |
| average number of species | 7.14 | 5.86 | 2.14 | 1.71 | 0 | 0 |
| Butterfly Species | Habitat | Host Plant | Ie | It | Literature |
|---|---|---|---|---|---|
| Aporia crataegi (L.) | Deciduous forests, ruderal areas, meadows, orchards, agricultural fields | Family Rosaceae: Crataegus monogyna,Prunus, Sorbus,Frangula | m | m | [27] |
| Pieris brassicae (L.) | Ruderal areas, forest edges, scrub vegetation, roadside vegetation, dry and wet meadows, mixed forests | Family Brassicaceae: Brassicae, Sinapsis arvensis, Raphanus raphanistrum, Tropaeolum majus | u | p | [28] |
| P.rapae (L.) | Ruderal areas, forest edges, roadside vegetation, scrub vegetation, dry and wet meadows, gardens and recreational areas, parks | Family Brassicaceae: Brassicae, Lepidium, Arabis, Alliaria petiolata | u | o | [28,29] |
| P. napi (L.) | Forest edges, ruderal areas, scrub vegetation, mixed forests, roadside vegetation, dry and wet meadows, gardens and recreational areas, parks | Thlaspi arvense, Thlaspi alpestre, Alliaria petiolata, Brassica campestris, Brassica rapa, Brassica napus, Brassica oleracea, Raphanus raphanistrum, Raphanus sativus, Armoracia rusticana, Rorippa islandica, Cardamine amara, Arabis alpina, Hesperis matronalis, Berteroa incana, Reseda odorata, Tropaeolum majus, Calendula officinalis, Cardamine leucantha, Cardamine niponica, Rorippa isbandica, Rorippa sylvestris [3]. | u | o | [28] |
| Gonepteryx rhamni (L.) | Forest edges, mixed forests ruderal areas, scrub vegetation, dry and wet meadows, roadside vegetation, gardens and recreational areas | Family Rhamnaceae: Rhamnus cathartica, Frangula alnus | m | o | [30] |
| Anthocharis cardamines (L.) | Forest edges, forest roads, forest glades and cutting areas, wet meadows, scrub vegetation, roadside vegetation, parks | Family Brassicaceae: Cardamine pratensis L., Alliaria petiolata, Arabis glabra (L.) Bernh., Sisymbrium officinale (L.) Scop. | h | o | [31,32] |
| Lycaena phlaeas (L.) | Field borders, fallow lands, forest glades | Rumex acetosella L., Rumex acetosa L. Polygonum L. | m | m | [33] |
| L. virgaureae (L.) | Forest edges, scrub vegetation, fallow lands and natural areas, dry and wet meadows, roadside vegetation | Rumex spp. | m | m | [34,35] |
| L.tityrus (Poda) | Forest edges, scrub vegetation, roadside vegetation, dry and wet meadows, ruderal areas | Rumex acetosella, Rumex acetosa L., | m | m | [33] |
| Cyaniris semiargus (Rott.) | Forest edges, ruderal areas, dry and wet meadows, scrub vegetation, forest glades, fallow lands | Trifolium pratense L., Trifolium medium (L.) Lasius niger | m | m | [36,37] |
| Polyommatus icarus (Rott.) | Forest edges, ruderal areas, roadside vegetation, dry and wet meadows, forest glades, fallow lands | Family Fabaceae Trifolium pratense L., Trifolium medium (L.), Medicago L. Lotus L., Ononis L. | m | o | [38,39] |
| Nymphalis antiopa (L.) | Mixed forests, forest edges, forest pathways; sometimes: meadows and scrub vegetation | Salix spp., Populus spp., Betula spp., Ulmus spp. | m | o | [40,41] |
| Inachis io (L.) | Forest edges and glades, gardens, orchards, wastelands, pastures | Urtica dioica L. | u | m | [42,43,44] |
| Vanessa atalanta (L.) | Forest edges, dry and wet meadows, fallow lands, ruderal areas, roadside vegetation, scrub vegetation, deciduous forests, parks, gardens | Urtica dioica L. | u | m | [45] |
| Vanessa cardui (L.) | Ruderal areas, parks, field borders, fallow lands, roadside vegetation | Cirsium Mill., Urtica dioica L., Carduus L., Onopordum L. | m | p | [44,46] |
| Aglais urticae (L.) | Sunny forest glades, meadows, gardens, ruderal areas | Urtica dioica L. | x | m | [44] |
| Issoria lathonia (L.) | Dry or sandy habitats, fallow lands, ruderal areas, roadside vegetation | Viola arvensis | m | m | [37] |
| Melanargia galathea (L.) | Forest edges, ruderal areas, scrub vegetation, dry and moderately wet meadows, railway embankments | Family Poaceae, Gramineae | m | o | [47] |
| Maniola jurtina (L.) | Ruderal areas, scrub vegetation, dry and wet meadows, forest edges, roadside vegetation, railway embankments | Lolium perenne, Festuca rubra L., Poa pratensis L. | m | p | [34] |
| Aphantopus hyperantus (L.) | Ruderal areas, scrub vegetation, forest grasslands, dry and wet meadows, roadside vegetation, roadside vegetation, railway embankments | Brachypodium, Dactylis, Festuca L., Bromus, Poa pratensis, Carex, Agrostis, Holcus | m | p | [48] |
| Coenonympha pamphilus (L.) | Forest edges, ruderal areas, scrub vegetation, dry and wet meadows, roadside vegetation | Family Poaceae: Festuca spp., Poa spp., Agrotis spp. | u | p | [33,48] |
| C. glycerion (Borkh.) | Forest edges, ruderal areas, scrub vegetation, dry and wet meadows, roadside vegetation, grasslands with trees and shrubs | Molinia L., Festuca L. | m | p | [35,49] |
| Argynnis adippe (D and S) | Sunny forest glades, coppices, clearcutting areas, railway embankments | Viola canina L., Viola odorata L., Viola hirta L. | m | o | [35,50] |
| A. aglaja (L.) | Wild-flower meadows, scrub vegetation, peat meadows, railway embankments, forest edges, fallow lands and ruderal areas, dry and wet meadows | Family Violaceae | m | o | [35] |
| Melitaea cinxia (L.) | Forest glades, extensively used pastures, wild-flower meadows | Plantago L. | m | o | [51,52] |
| Polygonia c-album (L.) | Ruderal areas, gardens, forest glades and roads | Urtica dioica, Humulus lupulus, Ulmus glabra, Salix caprea L. | m | p | [53,54] |
| Thymelicus lineola (Ochs.) | Meadows, gardens and parks, forest glades and edges | Family Poaceae | m | o | [35,55] |
| T. sylvestris (Poda) | Forest edges, ruderal areas, scrub vegetation, dry and wet meadows, roadside vegetation | Holcus, Dactylis glomerata | m | o | [55] |
| Ochlodes Sylvanus (Esper) | Meadows, forest glades, forests | Family Poaceae | m | o | [56] |
| Hesperia comma (L.) | Xerothermic grasslands, fallow lands, forest edges and glades | Festuca ovina, Lolium perenne L., Corynephorus canescens L. | m | m | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszko-Podgórska, K.; Dymitryszyn, I.; Kondras, M. Diversity in Landscape Management Affects Butterfly Distribution. Sustainability 2023, 15, 14775. https://doi.org/10.3390/su152014775
Szyszko-Podgórska K, Dymitryszyn I, Kondras M. Diversity in Landscape Management Affects Butterfly Distribution. Sustainability. 2023; 15(20):14775. https://doi.org/10.3390/su152014775
Chicago/Turabian StyleSzyszko-Podgórska, Katarzyna, Izabela Dymitryszyn, and Marek Kondras. 2023. "Diversity in Landscape Management Affects Butterfly Distribution" Sustainability 15, no. 20: 14775. https://doi.org/10.3390/su152014775
APA StyleSzyszko-Podgórska, K., Dymitryszyn, I., & Kondras, M. (2023). Diversity in Landscape Management Affects Butterfly Distribution. Sustainability, 15(20), 14775. https://doi.org/10.3390/su152014775






