Applications of Xerophytophysiology and Signal Transduction in Plant Production—Flower Qualities in Eustoma grandiflorum Were Improved by Sub-Irrigation
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Plant Materials
2.2. Analysis of Leaf Photosynthesis
2.3. Measurement of Leaf Color
2.4. Dry Mass Production
2.5. Measurement of Concentration of Anthocyanins
2.6. Determination of Salicylic Acid Concentration
2.7. PCR Analysis for PAL Gene Expression
2.8. Data Collection for Pressure–Volume Curve Analysis
2.9. Modeling Equation Used in this Paper
- (1)
- (2)
- The incipient plasmolysis or zero-turgor point [9]
- (3)
- The re-compartmentation of the symplastic and apoplastic water
- (4)
- Analysis of osmotic adjustment
- (5)
- The analysis of leaf water retention ability [34]
- (6)
- Analysis of the diurnal opening and closing oscillations
- (7)
- Mathematical modeling for the flower opening oscillation curve
2.10. Statistical Analysis
3. Results
3.1. Sub-Irrigation Improved Leaf Photosynthetic Activities, Plant Growth, and Flower Quality
3.1.1. Photosynthetic Activities
3.1.2. Biomass Production
3.1.3. Leaf Color and Flower Quality
3.2. Increase in Salicylic Acid Concentration and Up-Regulation of PAL Gene
3.2.1. Concentration of Salicylic Acid
3.2.2. Up-Regulation of PAL Gene
3.3. Sub-Irrigation Induced Osmotic Adjustment and Improved Leaf Turgor Potential
3.3.1. Leaf Turgor Potential and Osmotic Adjustment
3.3.2. Cell Water Compartmenting
3.3.3. Osmotic Potential and Relative Water Content at Incipient Plasmolysis
3.4. Leaf Water Retention Ability
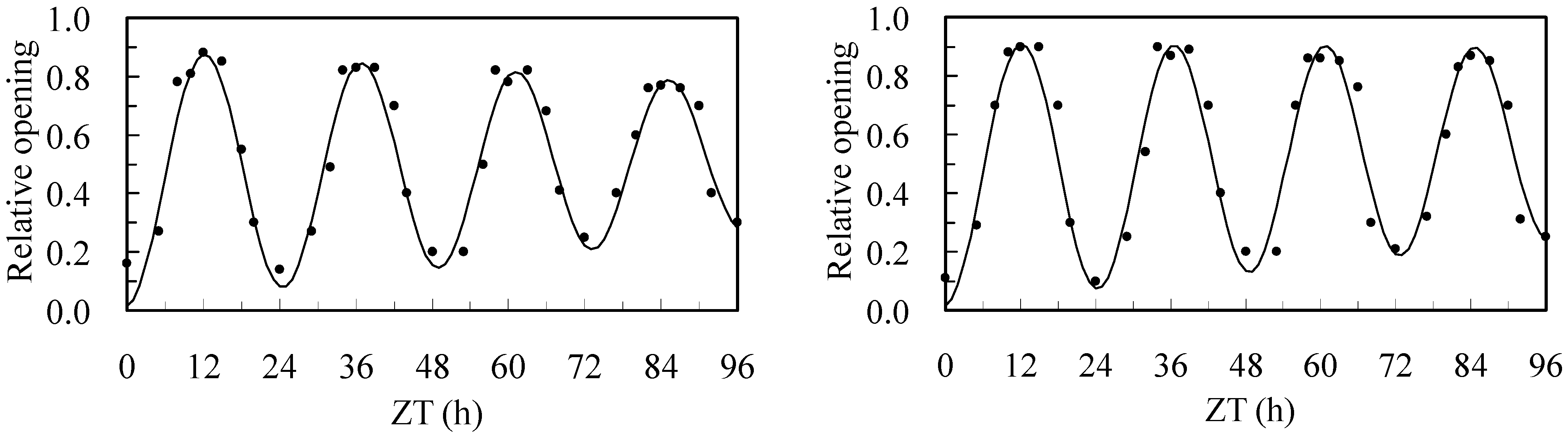
3.5. Flower Opening and Closing Oscillations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.L. Xerophytophysiology in crop production. In Dryland Crop Production—Technology Breakthroughs and Study Cases; Xu, H.L., Ed.; Research Signpost: Kerala, India, 2007; pp. 37–54. [Google Scholar]
- Loveys, B.; Davies, W.J. Physiological approaches to enhance water use efficiency in agriculture: Exploiting plant signaling in novel irrigation practice. In Water Use Efficiency in Plant Biology; Bacon, M., Ed.; Blackwell Publishing: Oxford, UK, 2004; pp. 113–141. [Google Scholar]
- Sahoo, J.P.; Mohapatra, U. Plant Signal Transduction Mechanism against Abiotic and Biotic Stresses. In Advances in Agricultural Biotechnology, 2nd ed.; AkiNik Publications: Delhi, India, 2020; p. 169. [Google Scholar]
- Xu, H.L.; Qin, F.F.; Xu, R.Y.; Wang, F.H.; Li, F.M. Applications of xerophytophysiology in plant production—Sorghum plants improved by exposing the mesocotyl as stimulus. J. Food Agric. 2009, 7, 603–610. [Google Scholar]
- Qin, F.F.; Xu, H.L.; Lü, D.Q.; Takano, T. Modified AnM technique in combination with black and transparent film mulching in peanut production. J. Food Agric. Environ. 2012, 10, 668–674. [Google Scholar]
- Xu, H.L.; Qin, F.F.; Wang, F.H.; Xu, Q.C.; Wang, R.; Shah, S.K.; Zhao, A.H.; Li, F.M. Applications of xerophytophysiology in plant production—Partial root drying improves tomato crops. J. Food Agric. Environ. 2009, 7, 981–988. [Google Scholar]
- Xu, H.L.; Qin, F.F.; Xu, Q.C.; Tan, J.Y.; Liu, G.M. Applications of xerophytophysiology in plant production—The potato crop improved by partial root zone drying of early season but not whole season. Sci. Hortic. 2011, 129, 528–534. [Google Scholar] [CrossRef]
- Xu, H.L.; Qin, F.F.; Tian, C.M.; Wang, R. Applications of xerophytophysiology in plant production—Tomato fruit yield and quality improved by restricted irrigations in soil-based greenhouses. Acta Hortic. 2010, 893, 987–996. [Google Scholar] [CrossRef]
- Xu, H.L.; Qin, F.F.; Xu, Q.C.; Xu, R.Y.; Wang, Y.R.; Wang, R. Applications of xerophytophysiology in plant production: Sub-irrigation improves tomato fruit yield and quality. J. Food Agric. Environ. 2011, 9, 256–263. [Google Scholar]
- Xu, H.L.; Xu, Q.C.; Li, F.L.; Feng, Y.Z.; Qin, F.F.; Fang, W. Applications of xerophytophysiology in plant production—LED blue light as a stimulus improved the tomato crop. Sci. Hortic. 2012, 148, 190–196. [Google Scholar] [CrossRef]
- Su, F.F.; Xu, H.L.; Li, F.L.; Qin, F.F.; Chen, Y.L.; Lü, D.Q.; Hu, L.S.; Li, Y.; Wang, S.P.; Shi, Y.; et al. Applications of xerophytophysiology in plant production: Potato yield increase induced by drying the cut trace of seed tuber blocks. J. Food Agric. Environ. 2014, 12, 255–264. [Google Scholar]
- Yao, J.; Qi, Y.; Li, H.; Shen, Y. Water saving potential and mechanisms of subsurface drip irrigation: A review. Chin. J. Eco Agric. 2021, 29, 1076–1084. [Google Scholar]
- Biel, C.; Savé, R.; Habrouk, A.; Espelta, J.M.; Retana, J. Effects of restricted watering and CO2 enrichment in the morphology and performance after transplanting of nursery-grown Pinus nigra seedlings. Hortic. Sci. 2004, 39, 535–540. [Google Scholar] [CrossRef]
- Xu, H.L.; Qin, F.F.; Xu, Q.C.; Li, F.M. Application of xerophytophysiology in plant production—Growing wheat on ridged bed. J. Food Agric. Environ. 2009, 7, 320–327. [Google Scholar]
- Van Doorn, W.G.; Kamdee, C. Flower opening and closure: An update. J. Exp. Bot. 2014, 65, 5749–5757. [Google Scholar] [CrossRef]
- Bai, J.F.; Kawabata, S. Regulation of diurnal rhythms by direct light stimulation in flower opening and closing in Eustoma grandiflorum. Hort. J. 2015, 84, 148–155. [Google Scholar] [CrossRef]
- Fernie, A.R.; Tohge, T. The genetics of plant metabolism. Annu. Rev. Genet. 2017, 51, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.Q.; Ding, W.S.; Wu, C.; Wang, W.J.; Ye, S.W.; Cai, C.Y.; Hu, X.; Wang, N.N.; Bai, W.Y.; Tang, X.S.; et al. Production of purple Ma bamboo (Dendrocalamus latiflorus Munro) with enhanced drought and cold stress tolerance by engineering anthocyanin biosynthesis. Planta 2021, 254, 50. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; La Rocca, N. Resurrection plants: The puzzle of surviving extreme vegetative desiccation. Crit. Rev. Plant Sci. 2005, 24, 209–225. [Google Scholar] [CrossRef]
- Jiang, C.J.; Yu, Y.B. The research progress (review) of phenylalanine ammonialyase. J. Anhui Agric. Univ. 2001, 28, 425–430. [Google Scholar]
- Zhang, Z.; Sun, C.; Yao, Y.; Mao, Z.; Sun, G.; Dai, Z. Red anthocyanins contents and the relationships with phenylalanine ammonia lyase (PAL) activity, soluble sugar and chlorophyll contents in carmine radish (Raphanus sativus L.). Hort. Sci. 2019, 46, 17–25. [Google Scholar] [CrossRef]
- Medda, S.; Dessena, L.; Maurizio, M. Monitoring of the PAL enzymatic activity and polyphenolic compounds in leaves and fruit of two myrtle cultivars during maturation. Agriculture 2020, 10, 389. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A. Salicylic Acid—A Plant Hormone; Springer Press: Dordrecht, The Netherlands, 2007; p. 410. [Google Scholar]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defense: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Xu, W.; Peng, X.; Han, Q.; Zhu, Y.; Guo, T. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012, 11, 6066–6079. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Qin, F.F.; Xu, R.Y.; Xu, Q.C.; Tian, C.M.; Li, F.M.; Wang, F.H. Photosynthesis in different parts of a wheat plant. J. Food Agric. Environ. 2009, 7, 399–404. [Google Scholar]
- Yang, L.; Li, W.C.; Fu, F.; Qu, J.; Sun, F.; Yu, H.; Zhang, J. Characterization of phenylalanine ammonia-lyase genes facilitating flavonoid biosynthesis from two species of medicinal plant Anoectochilus. PeerJ 2022, 10, e13614. [Google Scholar] [CrossRef]
- Deng, C.H.; Zhang, X.M.; Zhang, J.; Zhu, W.M. Rapid determination of salicylic acid in plant materials by gas chromatography–mass spectrometry. Chromatographia 2003, 58, 225–229. [Google Scholar]
- Haq, K. Restricted Expression in the Phenylalanine Ammonia-Lyase Gene Family in Tomato. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, April 2006. [Google Scholar]
- Turner, N.C. Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Xu, H.L.; Wang, X.J.; Wang, J.H.; Xu, R.Y.; Zhao, A.H. Leaf turgor potential plant growth and photosynthesis in organically fertilized sweet corn. Pedosphere 2004, 14, 165–170. [Google Scholar]
- Patakas, A.A.; Patakas, B. Osmotic adjustment and partitioning of turgor responses to drought in grapevines leaves. Am. J. Enol. Vitic. 1999, 50, 76–80. [Google Scholar] [CrossRef]
- Chang, T.T.; Zhang, Y.J.; Xu, H.L.; Shao, X.H.; Xu, Q.C.; Li, F.L.; Yu, L.N.; Zhang, Z.Y. Osmotic adjustment and up-regulation expression of stress-responsive genes in tomato induced by soil salinity resulted from nitrate fertilization. Int. J. Agric. Biol. Eng. 2018, 11, 126–136. [Google Scholar] [CrossRef]
- Xu, H.L.; Ajiki, N.; Wang, X.J.; Sakakibara, C.; Umemura, H. Water retention in excised leaves of sweet corn grown under organic and chemical fertilizations with or without effective microbe applications. Pedoshpere 1998, 8, 1–8. [Google Scholar]
- Bai, J.F. Regulation of Diurnal Rhythms of Flower Opening and Closure by Light Cycles, Wavelength, and Intensity in Eustoma grandiflorum. Ph.D. Thesis, The University of Tokyo, Tokyo, Japan, 2013. [Google Scholar]
- Xu, H.L.; Xu, Q.C.; Qin, F.F.; Gosselin, A.; Dansereau, B.; Zhang, Y. Photosynthetic oscillation in leaves of tomato and hibiscus under high light intensity. Acta Hort. 2011, 907, 343–347. [Google Scholar] [CrossRef]
- Qin, F.F.; Xu, H.L.; Takano, T. Physiological fundamentals of AnM technique in peanut production—Stomatal oscillations in intact and excised leaves of peanut plants grown with a modified AnM technique. J. Food Agric. Environ. 2012, 10, 653–658. [Google Scholar]
- Knight, H.; Knight, M.R. Abiotic stress signaling pathways: Specificity and crosstalk. Trends Plant Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef]
- Devlin, P.F.; Kay, S.A. Circadian photoperception. Annu. Rev. Physiol. 2001, 63, 677–694. [Google Scholar] [CrossRef]
- Wen, P.F.; Chen, J.Y.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- Anderson, M.D.; Chen, Z.; Klessig, D.F. Possible involvement of lipid peroxidation in salicylic acid-mediated induction of pr-1 gene expression. Phytochemistry 1998, 47, 555–566. [Google Scholar] [CrossRef]
- Yanovsky, M.J.; Kay, K.A. Signaling networks in the plant circadian system. Curr. Opin. Plant Biol. 2001, 4, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Yuan, L.; Yang, X.; Zhang, X.; Wang, L.; Xie, Q.G. Circadian clock in plants: Linking timing to fitness. J. Integr. Plant Biol. 2022, 64, 20. [Google Scholar] [CrossRef] [PubMed]
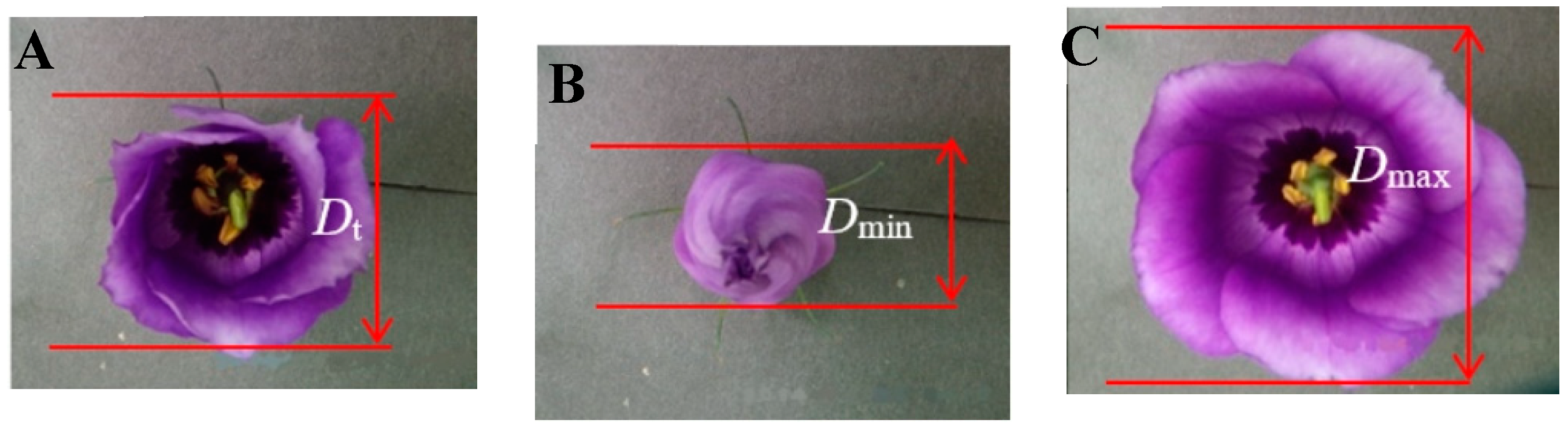
| Abbr. | Unit | Definition |
|---|---|---|
| Photosynthesis–light response curve analysis | ||
| PC | μmol m−2 s−1 | The photosynthetic capacity. |
| PN | μmol m−2 s−1 | The net photosynthetic rate. |
| RD | μmol m−2 s−1 | Dark respiration rate. |
| K | μmol−1 m2 s | Constant proportional to the initial slope and the curviness of the photosynthesis–light response curve. |
| i | μmol m−2 s−1 | Photosynthetic photon flux. |
| YQ | mol mol−1 | The maximum quantum yield, proportional to the initial slope of the photosynthesis–light response curve, calculated as YQ = KPC. |
| Pressure–Volume curve analysis | ||
| Ψ | MPa | Leaf water potential. |
| ΨFT | MPa | Ψ at fully turgid status. |
| π | MPa | Leaf osmotic potential. |
| πs+a | MPa | The average osmotic potential (π) with hypothesis that symplastic solution was diluted by apoplast water. |
| πFT | MPa | π at fully turgid status. |
| πIP | MPa | π at zero turgor or incipient plasmolysis, calculated from the P-V curve at Ψ = 0.99π. |
| ζFT | Relative leaf water content (ζ) at fully turgid status. | |
| ζIP | ζ at the zero-turgor point. | |
| ζap | The water fraction in apoplasm. | |
| ζsym | The water fraction in symplasm. | |
| α | Constant proportional to the slope of the initial part of the P-V curve. | |
| β | Constant proportional to the slope of the second sloping phase of the P-V curve. | |
| CFT | mol m−3 | Concentration of osmotically active substances. |
| cΔCFT | The active increment of CFT compared with control. | |
| Analysis of the transpiration declining curve in excised leaves | ||
| ζsat | Relative leaf water content (ζ) at saturation status. | |
| ζSC | ζ at the time when stomata are completely closed. | |
| t | Time since the drying process started. | |
| α′ | Constant proportional to slope of the initial steep-sloped part of the curve and to the rapid rate of water loss, mainly by stomatal transpiration. | |
| β′ | Constant proportional to the slope of the second gently sloped part and to the rate of water loss by cuticular transpiration when stomata are closed. | |
| τD | Time used to dry up the excised leaf to its relative water content of 10%. | |
| Analysis of flower opening and closing oscillations | ||
| ZT | h | Zone time; in the present study the zone time is Tokyo time. |
| Y | The opening extent relative to the maximum opening of the flower. | |
| YA | The initial amplitude of oscillations. | |
| YR | The residual values of Y at the initial oscillation bottom. | |
| YA96 | The amplitude of oscillations at ZT96. | |
| T | h | The oscillation period. |
| ω | The angular velocity in the sinusoidal function equation. | |
| λ | The coefficient to adjust the change of ω. The value of ω would be larger and thus the oscillation period (T) smaller if λ was positive; if λ was negative, ω would become smaller and thus T larger as time progressed. | |
| τ | h | The time needed to adjust Y to move into the oscillation process. |
| α | The coefficient related to the expansion or decay of the oscillation amplitude (YA), which would be smaller and smaller if λ was negative and larger and larger if λ was positive. | |
| β | The coefficient related to the dynamic change of YR, which would be larger and larger if λ was positive, showing an upward drifting pattern of oscillation rhythm. If λ was negative, the oscillation rhythm shows a downward drifting pattern. | |
| f | h−1 | Oscillation frequency (f = ω/2π = 1/T). |
| T | h | The period of oscillation (T = 2π/ω). T0 and T96 are T at the initial and at ZT96. |
| Irrigation | PC | RD | YQ | Biomass (g pl−1) | R/T | Leaf Color | Antho | SA | PAL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (μmol m−2 s−1) | (mol mol−1) | Shoot | Root | Total | (%) | (SPAD) | (A530 g−1 FW) | (μg kg−1) | |||
| Overhead | 18.6 | 2.7 | 0.0452 | 7.02 | 0.95 | 7.97 | 11.9 | 37.9 | 68.3 | 3.2 | 1.17 |
| Sub | 20.7 | 3.0 | 0.0534 | 8.95 | 1.43 | 10.38 | 13.8 | 41.2 | 79.2 | 44.3 | 7.82 |
| Statistic | * | * | ** | * | ** | ** | * | ** | ** | ** | ** |
| Irrigation | ΨFT | πFT | PFT | πs+a | ΨMD | πMD | PMD | πIP | ζsym | ζapo | α | β | ζIP | CFT | ΔCFT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overhead | −0.27 | −0.88 | 0.61 | −0.57 | −0.65 | −0.99 | 0.34 | −1.11 | 0.26 | 0.74 | 47.6 | 0.98 | 0.842 | 360.8 | 0.0 |
| Sub | −0.28 | −0.96 | 0.68 | −0.63 | −0.63 | −1.08 | 0.43 | −1.19 | 0.31 | 0.69 | 54.3 | 0.99 | 0.821 | 393.6 | 32.8 |
| Statistic | ns | ** | ** | ** | ns | ** | ** | * | ** | ** | * | ns | * | ** | ** |
| Irrigation | ζSC | α | β | τD (103 s) | WRA |
|---|---|---|---|---|---|
| Overhead | 0.721 | 0.648 | 0.122 | 70.6 | 9.74 |
| Sub | 0.702 | 0.699 | 0.101 | 84.1 | 11.33 |
| Statistic | * | * | ** | ** | ** |
| Plot | ω | YA | YA96 | YR | T0 | T96 | λ | τ | α | β |
|---|---|---|---|---|---|---|---|---|---|---|
| Overhead | 0.252 | 0.426 | 0.253 | 0.03 | 24.92 | 24.30 | −0.00454 | 6.2 | −0.000284 | 0.0891 |
| Sub | 0.255 | 0.438 | 0.326 | 0.03 | 24.63 | 24.12 | −0.00284 | 6.1 | −0.000235 | 0.0778 |
| Statistic | * | * | * | ns | * | * | ** | ns | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.-L.; Bai, J.; Kawabata, S.; Chang, T. Applications of Xerophytophysiology and Signal Transduction in Plant Production—Flower Qualities in Eustoma grandiflorum Were Improved by Sub-Irrigation. Sustainability 2023, 15, 1578. https://doi.org/10.3390/su15021578
Xu H-L, Bai J, Kawabata S, Chang T. Applications of Xerophytophysiology and Signal Transduction in Plant Production—Flower Qualities in Eustoma grandiflorum Were Improved by Sub-Irrigation. Sustainability. 2023; 15(2):1578. https://doi.org/10.3390/su15021578
Chicago/Turabian StyleXu, Hui-Lian, Jianfang Bai, Saneyuki Kawabata, and Tingting Chang. 2023. "Applications of Xerophytophysiology and Signal Transduction in Plant Production—Flower Qualities in Eustoma grandiflorum Were Improved by Sub-Irrigation" Sustainability 15, no. 2: 1578. https://doi.org/10.3390/su15021578
APA StyleXu, H.-L., Bai, J., Kawabata, S., & Chang, T. (2023). Applications of Xerophytophysiology and Signal Transduction in Plant Production—Flower Qualities in Eustoma grandiflorum Were Improved by Sub-Irrigation. Sustainability, 15(2), 1578. https://doi.org/10.3390/su15021578







