1. Introduction
The feasibility and necessity of the integrated use of minerals results from a combination of different factors: geochemical, technological, economic, and environmental. Geochemical factors, due to the multicomponent chemical and mineral composition of minerals:
Technological factors, due to the availability of a technology that allows them to be subdivided into main mineral components; economic factors, determined with the possibility of obtaining additional profit and improving other technical and economic indicators (saving capital costs, labor resources, reducing land allotment for tailings); environmental factors, due to the fact that waste-free technology of mining and processing of minerals allows for minimizing the impact of mining and its wastes on the environment [
1,
2].
The exploitation of each deposit is accompanied by significant volumes of rock/mineral extraction and the manufacture of required products from them. Overburden from production deposits and tailings from mineral processing are secondary raw materials in the mining industry [
3,
4,
5]. Complete recycling of large volumes of secondary raw materials may not always be feasible in terms of economy and implementation.
The construction industry requires vast quantities of quartz sand. It is used in several applications such as a filler in concrete, different types of building mixtures, and others. The range of sand use in the construction industry involves compliance with a number of requirements for the quality of quartz sand. Moreover, one of the major consumers of quartz sand is the glass industry. Quality requirements for quartz sand are mainly related to the compliance with its particular chemical composition, including a high percentage of SiO
2, a low content of Fe
2O
3, and the absence of clay impurities [
6,
7,
8]. An important criterion for assessing the quality of sand is the fraction size, bulk density, and particle size modulus [
9,
10].
Quartz sands are very common in many countries around the world [
11], Ukraine being no exception. Obviously, the extraction of conditioned quartz sands (with the lowest possible content of impurities) and their close geographic location to consumers are economically beneficial. In practice, construction sand deposits are usually far from the consumer. Therefore, it is advisable to analyze and study locally accessible deposits of natural sands with a polymictic composition [
12]. That being said, the glauconite-bearing sands from the Cenomanian, Oligocene, and Paleogene deserve particular attention. They are quite common in Ukraine, being used as building mineral raw materials and, at the same time, a potential source of glauconite [
13].
Cenomanian sands have a high content of glauconite (from 25 to 70%). Such sands occur beneath 20 m from the surface. Their thickness varies from 1 to 15 m [
13]. In these sands, glauconite occurs in a finely dispersed microaggregate structure, which partially or completely forms its individual fractions. Due to the ferromagnetic properties of glauconite, Cenomanian sands are capable of dry magnetic separation to obtain a glauconite concentrate. The possibility of dry magnetic separation of Oligocene and Paleogene glauconite–quartz sands in Ukraine has not been studied in detail. This issue is of particular relevance in connection with the mass extraction of amber from such sands in the Polissya region of Ukraine. This process is accompanied by the accumulation of a large number of enriched tailings.
A characteristic feature of glauconite-bearing sands from the Eocene–Oligocene series of the Paleogene is their near-surface occurrence in Neogene sections under low-strength Paleogene shelly limestones, and shelly sands and clays. In numerous quarries excavating building sands, glauconite–quartz sands serve as the sole or roof of industrial deposits and are very rarely mineral deposits. They are typically assessed as a low-quality raw material, suitable only for landscaping, backfilling, and landscape design.
As commonly known, glauconite is capable of water absorption, which leads to the increase in its volume [
14,
15]. Due to this property, quartz–glauconite sands are not used as a filler in concrete and building mixtures [
16,
17]. When frozen, such concretes and plasters can quickly collapse due to a significant increase in the volume of glauconite (clay component) [
18]. Mineral–technological knowledge, and technical and economic assessment of glauconite–quartz sands indicate the possibility of creating a highly efficient waste-free technology for their extraction as a raw material for the construction industry. Sands with a glauconite content of 5–20% may be of practical interest.
The physical upgrade of glauconite sands can be achieved during mineral processing operations, e.g., size reduction, sieving, magnetic separation, and flotation [
4,
19]. When glauconite concentrate is extracted from Paleogene glauconite–quartz sands using dry magnetic separation, it is possible to obtain conditioned quartz sands and glauconite concentrates [
5,
20]. The study of the quantitative content of impurities in such sands can significantly expand the scope and possibilities of their industrial use [
21]. In addition, it can help reduce the accumulation of glauconite–quartz sands in dumps, for example, overburden.
Glauconite concentrate obtained in dry magnetic separation can be a valuable product [
22,
23]. It should be noted that due to the low content of glauconite in Paleogene sands, it is impossible to obtain large quantities of this valuable mineral in a short time span. However, the industrial establishment of the process of quartz sand and glauconite separation will allow for the accumulation of glauconite concentrate for local agricultural needs (potassium mineral fertilizer). Moreover, The value of glauconite lies in its sorption properties and, due to the presence of Fe
2+ in the mineral, it has the ability to participate in redox reactions [
24,
25,
26].
The study of the technological feasibility of dry magnetic separation of Paleogene glauconite–quartz sands and the subsequent use of its products in the building and other industries can be a promising research task [
27]. In addition, the organization of non-return closed processes for the processing of Paleogene glauconite–quartz sands will not only allow for a radical solution to the environmental problem (their accumulation), but also promote the economic effect in the building industry when using such sands.
The purpose of this study is as follows:
- (1)
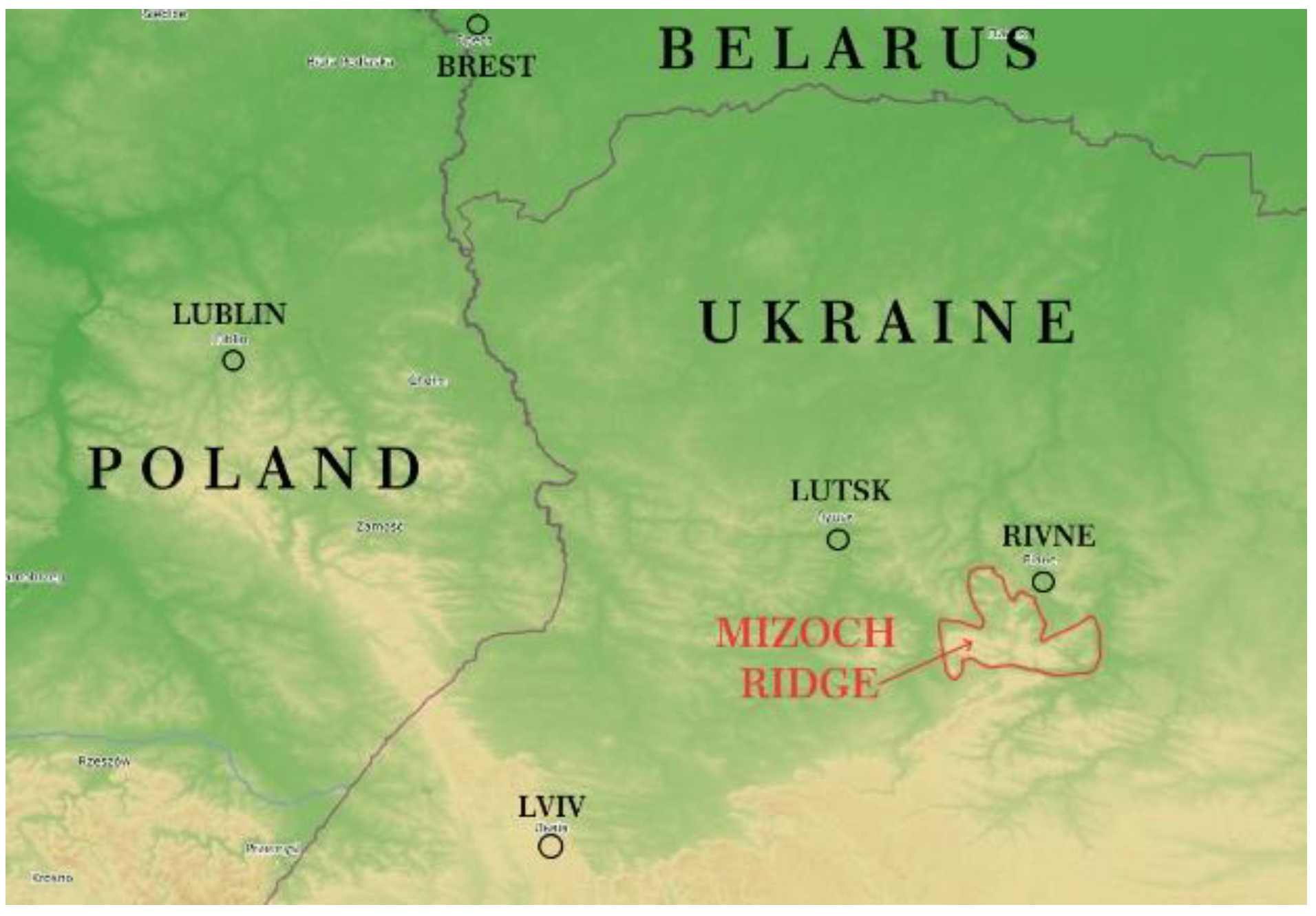
The analysis of the geological conditions of Paleogene glauconite–quartz sand occurrence, which constitute an overburden (on the example of the Dvorovychi deposit, Western Ukraine). Glauconite–quartz sands at the Dvorovychi site (named after the village of Dvorovychi) in the Rivne region became the object of a comprehensive geological and environmental study aimed at solving the presented issues. It is located in the northern part of the Mizoch Ridge, 25 km west of Rivne city (50°33′23.24″ N 25°56′47.40″ E);
- (2)
The determination of the chemical and mineralogical composition of magnetic and nonmagnetic fractions of the studied sands;
- (3)
The assessment of the possibility of using magnetic fractions in environmental technologies, and nonmagnetic fractions in the construction industry and in glass production.
2. Materials and Methods
2.1. Materials
The Mizoch Ridge is a hilly upland in the interfluve of the Goryn and Ikva rivers (
Figure 1) in the southern part of the Rivne region, within the Dubno, Zdolbuniv, and (partially) Ostroh regions of western Ukraine. It extends along the southern margins of the Volhynian Upland in the form of a narrow belt from the east to the west, south of the Mizoch village (hence the name of the ridge); it is located north of the Kremenets mountains. The ridge is 50 km long and 6 to 13 km wide. The area is approximately 22.5 thousand hectares. The highest peaks of the Mizoch Ridge are at 341 m and 342.5 m above sea level, and are located on the north-eastern outskirts of the village of Gorniki in the Dubno region. In some places, cliffs are observed, with slopes rising above the surrounding lowland by 120–150 m.
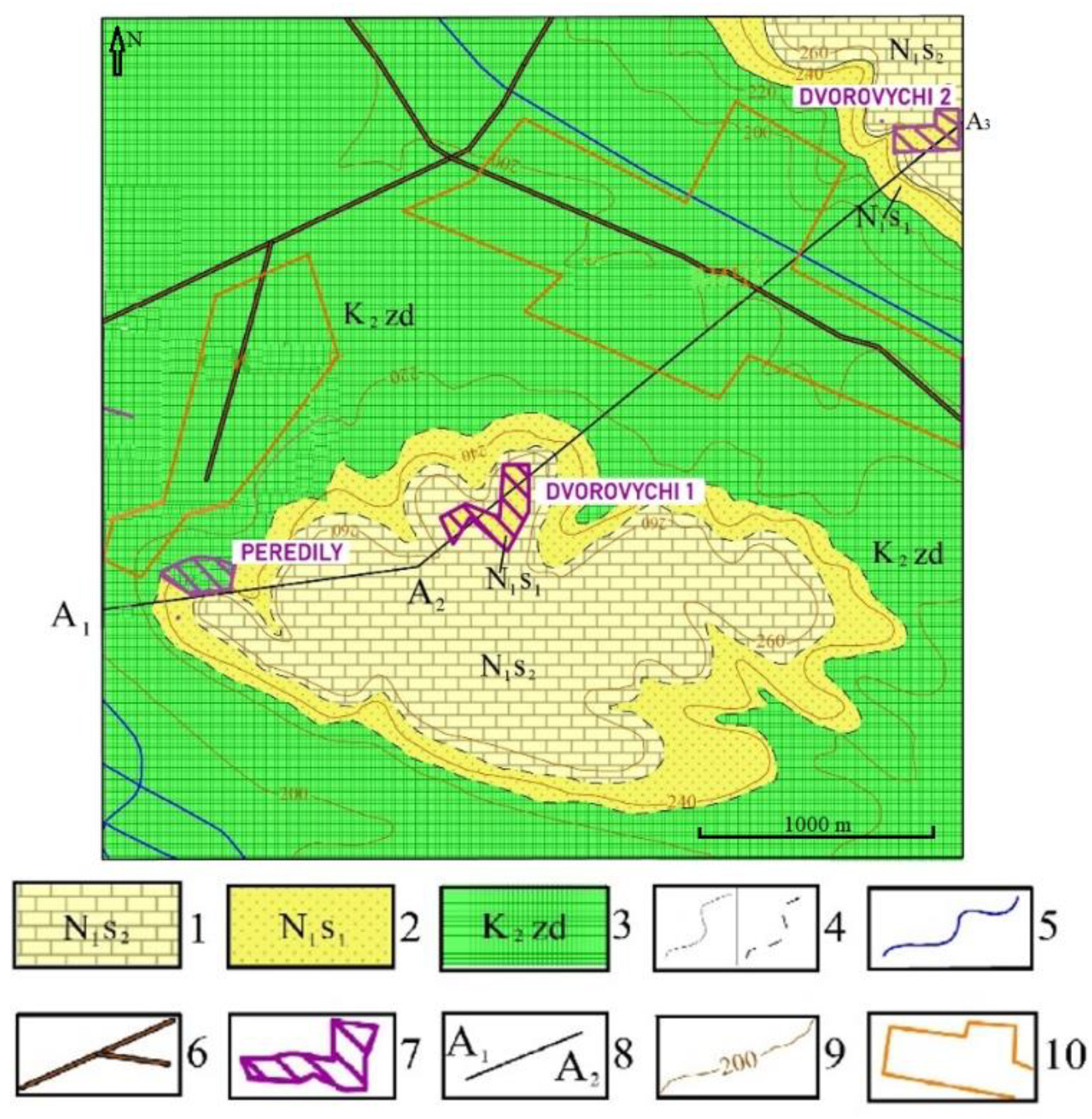
The ridge is composed of oolite-shelly limestones, sands, and clays from the Miocene series of the Neogene, as well as glauconite–quartz sands from the Eocene–Oligocene series of the Paleogene, overlying a thick Cretaceous substratum. The Neogene and Cretaceous deposits are covered by eolian–deluvial loess loams of the Neo-Pleistocene.
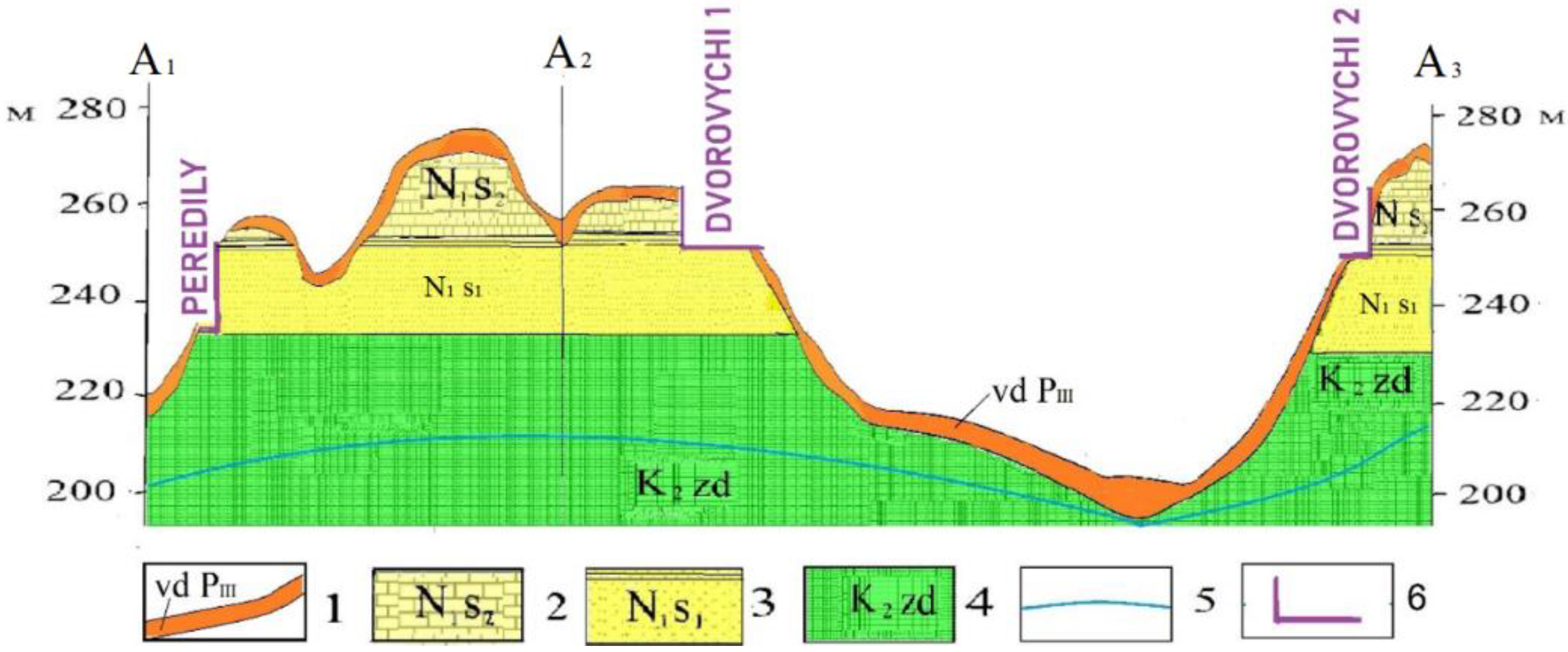
Here, quartz–glauconite sands form a thick (up to 20 m) horizontal layer assigned to the Eocene–Oligocene series of the Paleogene system. It is capped by relatively resistant upper Sarmatian limestones and dense lower Sarmatian clays, covered by eolian–deluvial loess loams (
Figure 2 and
Figure 3).
The remains of Neogene and Paleogene deposits can be observed on elevations from +240 m to +265 m; therefore, they are practically not flooded. The groundwater table occurs much lower within the fissured chalk layer.
The compound chronostratigraphic column in the study area is as follows (from bottom to top):
Cretaceous system, upper part, Zdolbuniv suite (K2zd)—represented by marls and white chalk, distributed around the Dvorovychi sand site under Quaternary deposits at absolute elevations below +230 m.
Paleogene system, Eocene–Oligocene series, Kharkiv Formation (Pe-o hr)—composed of grey-green, glauconite-bearing sands, predominantly glauconite—quartz, often clayey, in places stained with iron hydroxides. The thickness of the succession reaches 20–30 m.
In the lower part of the succession, sands are interbedded with sandy loams. Frequent changes in the grain composition and color of the sands are observed both vertically and laterally. This is clearly seen in the wall of the sand pit near Peredily village, which is located 1.5 km west of the Dvorovychi sand area.
- 3.
Neogene system, Miocene series, Sarmatian stage, lower substage (N1s1)—represented by dense grey-yellow clays, with thicknesses up to 4 m.
- 4.
Neogene system, Miocene series, Sarmatian stage, middle substage (N1s2)—represented by intercalations of light yellow oolitic-shelly limestones, shelly sands, and shelly lags on a sandy substrate. The thickness of the limestone deposits in the Mizoch Ridge reaches 15 m, and within the Dvorovychi sand succession it does not exceed 5 m.
- 5.
Neo-Pleistocene, upper horizon, eolian–deluvial deposits (vd, PIII)—represented by pale-yellow loess loams and loesses, up to 5 m thick in the form of a cover, variably lying at different elevations (from +200 m to +270 m) on the Cretaceous and Neogene strata described above.
With the scientific support of the authors of this work (The National University of Water and Environmental Engineering), geological exploration of the building sands deposit at the Dvorovychi-1 site was carried out in 2021 (State registration number for geological exploration of the subsoil U-20-193/1). The sand complex in the study area was exposed by 16 wells in its full thickness (about 10 m). Analytical studies of the sand samples were carried out by the Central Laboratory of SE “Ukrainian Geological Company”. The authors only studied the upper layers of the sand deposit, in which a high content of glauconite was observed. The uppermost layer of ochre-green limonitized glauconite–quartz sands was about 3 m thick, and the lower layer of grey-green glauconite–quartz sands was 3–4 m thick. The latter was underlain by a layer of light-olive sands with an insignificant content of glauconite. The sand reserves in the Dvorovychi deposit were calculated at the amount of 494.8 thousand m3.
The grain-size composition of the studied sands according to [
28] generally did not meet the requirements as a concrete filler. Due to the content of particles below 0.16 mm (100% of all samples) and partially due to the content of silt and clay particles (6.6% of all samples) in the natural state, they cannot be used for the manufacturing of building materials, products, and structures. However, according to [
29], these sands are suitable for landscaping and reclamation.
The chemical composition of sands within the reserve calculation contours was studied using two group core samples and was characterized by: SiO
2 93.34–94.04 wt.%, a total content of alkali oxides as Na
2O 0.11–0.16 wt.%, and a content of sulfates and sulfites as SO
3 wt 0.02%. The content of natural radionuclides: Ra-226, Th-232, and K-40 did not exceed the norms [
30], which allows for using these sands for all types of constructions without any restrictions.
Glauconite–quartz sands (GQS) and glauconite–quartz sands with limonite (GQS-Lim) were sampled from the wall of the investigated Dvorovychi open pit. The mass of each sample was 10 kg. A general view of the studied quarry and the exposed sands is shown in
Figure 4.
2.2. Methods
Natural glauconite–quartz sands and products of their dry magnetic separation were studied under a petrographic microscope. Samples of the quartz–glauconite sands were studied using an optical microscope (Nikon Eclipse E 600 POL) equipped with a Nikon D5100 digital camera. The magnification of the images of the studied sand samples was 60× under the microscope.
The ability to enrich the studied glauconite–quartz sands was studied in the Laboratory of Physical and Technological Research of the “Prodekologiya” research and production company (Rivne, Ukraine).
Before carrying out dry magnetic separation, samples of the studied sands were dried at 50 °C for 24 h in an oven until complete moisture loss (less than 0.5%). This value of humidity enabled to carry out dry magnetic separation of the sands. This temperature was selected taking into account the fact that preliminary petrographic studies showed the presence of probably ferruginous glauconite. As presented in other reports [
4,
31,
32], glauconite very often contains Fe
2+ in its composition. At high temperatures, Fe
2+ sands can oxidize to Fe
3+ and, as a result, further experimental studies may not provide reliable information.
During preliminary studies, the dried quartz–glauconite sands were quite loose, but contained lumps of polymineral aggregates of glauconite and quartz grains. To disintegrate these lumps, a VD-10 roll crusher (manufactured by LLC, Fantomash, Ukraine) with a working zone width of 100 mm and a roll diameter of 200 mm was used. The gap between the rolls was set to 2 mm. Samples of quartz–glauconite sands contained small amounts of cemented aggregates; therefore, before grinding, the product was sieved on a sieve with a 3 mm mesh. Only the residue retained on a 3 mm sieve was sent for grinding.
The BRS-12/10 laboratory magnetic separator included three stages of magnetic separation with recleaning of the nonmagnetic product in a magnetic field with a higher magnetic induction at each subsequent stage. The laboratory magnetic separator contained a hopper for loading the product (1), a vibrating feeder (2), a drum magnetic separator (3) with a magnetic induction on the working surface of 0.8 Tl and a diameter of 200 mm, two roller magnetic separators (4) and (5) with magnetic induction on the working surfaces at 1.1 and 1.3 Tl and diameters of 120 mm, respectively, the collector of the non-magnetic product (6), and collectors of magnetic products (7). The width of the working area of the magnetic separator is 100 mm. The general view of the magnetic separator is shown in
Figure 5.
The productivity of the magnetic separation process is 0.4 t/h. The speed of rotation of the magnetic separator drum is 60 rpm/min. The nonmagnetic product of the first stage of magnetic separation is introduced through the product pipelines to the working area of the roller magnetic separator at 4.75 rpm.
Abbreviated names of the studied products of dry magnetic separation of GQS and GQS-Lim are presented in
Table 1.
2.3. XRD and XRF Study Samples
The quantitative chemical analysis of the studied samples was determined using the X-ray spectral analysis method with the ARL Advant’X IntelliPower 1200 apparatus (Thermo Fisher Scientific, Waltham, MA, USA). The lithium metaborate–lithium tetraborate flux, containing lithium nitrate as the oxidizing agent, was fused with the sample and then placed in a platinum mold. The resultant disk was in turn analyzed by XRF spectrometry combined with a loss-on-ignition at 950 °C. To determine the final result, both types of data were used.
Mineral phase analysis of the study samples was carried out using the Aeris Minerals PANanalytical diffractometer (Malvern Panalytical, Malvern, UK). Step scan XRD data (8–65° 2θ, 0.022° 2θ step width, 2.1 step/s) were collected for bulk samples at Cu–Kα (λ = 0.15418 nm) radiation. The analysis was carried out by the method of refining the data of powder X-ray diffraction (XRD) according to Rietveld (Rietveld refinement) using Profex-BGMN software.
2.4. Grain Gradation and Coarseness of the Magnetic and Nonmagnetic Fractions
The results of XRF and XRD showed that the magnetic fractions (MF-1 and MF-1-Lim) are mineralogical aggregates, the dominant mineral of which is glauconite. The particle size distribution of MF-1 and MF-1-Lim was determined using a laser diffraction instrument with dry sample dispersion from Sympatec, type HELOS/RODOS. Instrument calibration was verified with a SiC-P600′06 standard from Sympatec. The system uses the Fraunhofer effect in the entire measuring range, from 0.1 to 8750 µm. The benefits of this device include the possibility to analyze particle size on both dry and wet samples—dusts, suspensions, emulsions, and aerosols. The composition of each sample was determined by testing in duplicates. Average values are presented in the results. The advantage of this laser method was the work speed and the repeatability of the results obtained. This method is most often used in research focused on environmental management [
33,
34].
Determination of the particle sizes in the obtained nonmagnetic fractions was carried out in accordance with [
35,
36]. Particle size and coarseness of the nonmagnetic fractions were determined by screen residue analysis. The method was focused on determining the quantitative (mass) distribution of grains of nonmagnetic fractions by their size during sieving.
The grading region and fineness modulus (FM) can be used to express the gradation and the coarseness of sand particles, respectively. Sand with the same fineness modulus may have very different grading regions. Therefore, the particle gradation and the fineness modulus should be considered when developing a recommendation for the use of the obtained nonmagnetic fractions.
The FM of the studied nonmagnetic fractions was determined according to the GOST 8735-88 method. A sample of each investigated nonmagnetic fraction with a mass of 1 kg was sieved through mesh sizes of 2.5 mm, 1.25 mm, 0.63 mm, 0.315 mm, and 0.16 mm. The obtained masses were fixed in percent from 1 kg remaining on the corresponding sieves and the calculation of the modulus of size was carried out according to the following formula:
where Q is the obtained mass of the samples on 5 sieves as a percentage of the total mass of the original sample.
3. Results
3.1. Results of Dry Magnetic Separation of GQS and GQS-Lim
To determine the ability of GQS and GQS-Lim to magnetic separation, dry sieving of GQS and GQS-Lim was carried out. The obtained results are presented in
Table 2. Wet sieving was ineffective for glauconite sand. This is due to the relatively large grain size of glauconite and the large amount of quartz [
23,
27]. In addition, it is believed that dry sieving is more effective for the enrichment of fine fractions of the glauconite grains, with the exception of glauconite–chamosite iron.
According to the results of dry magnetic separation of the studied sands (
Table 2), the content of MF-1 and MF-1-Lim is 5.1 and 2.8%, respectively. These magnetic fractions require detailed investigation to be able to recommend their use as a glauconite concentrate.
3.2. Petrographic Analysis of Bulk Sand Samples and Their Nonmagnetic Fractions
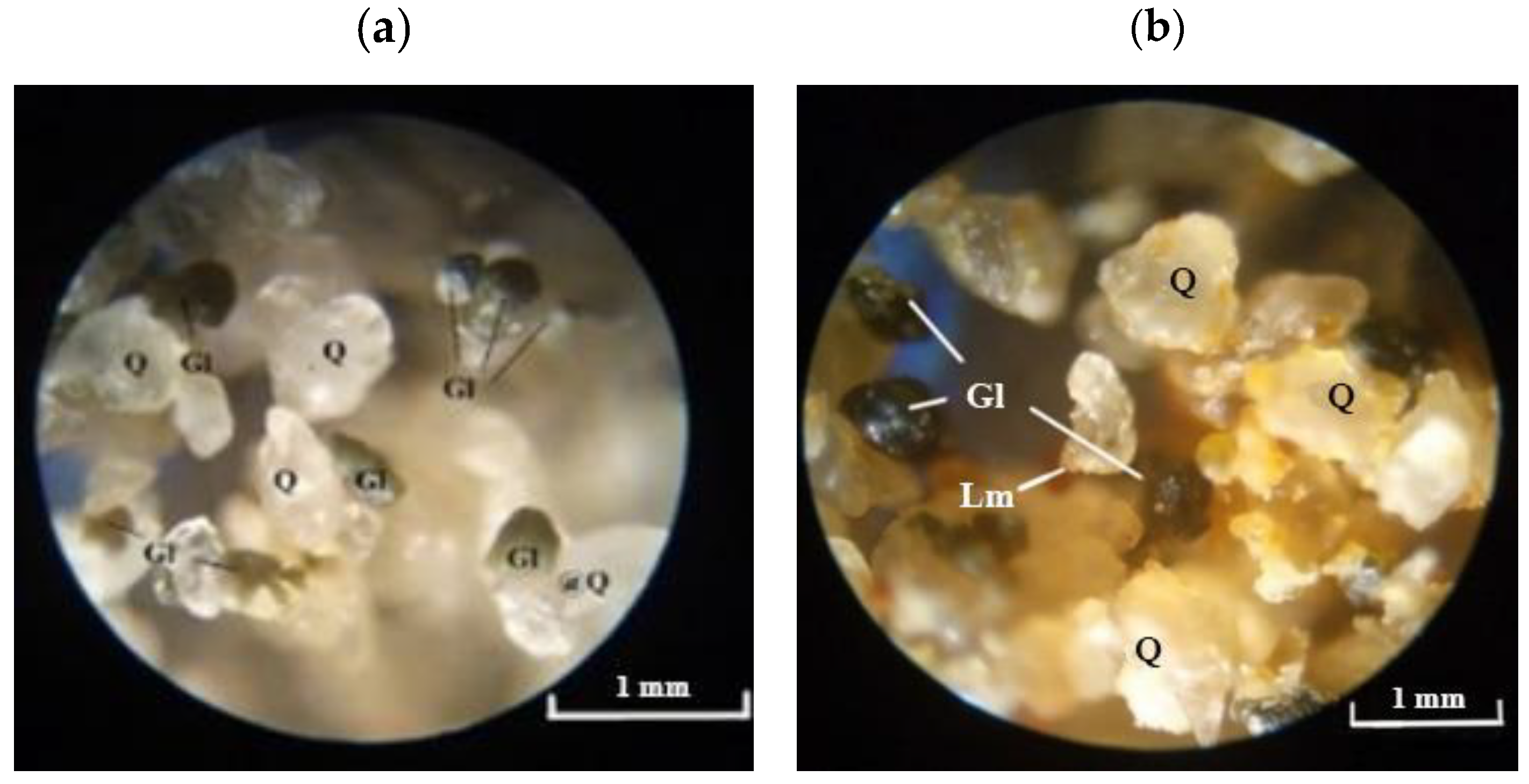
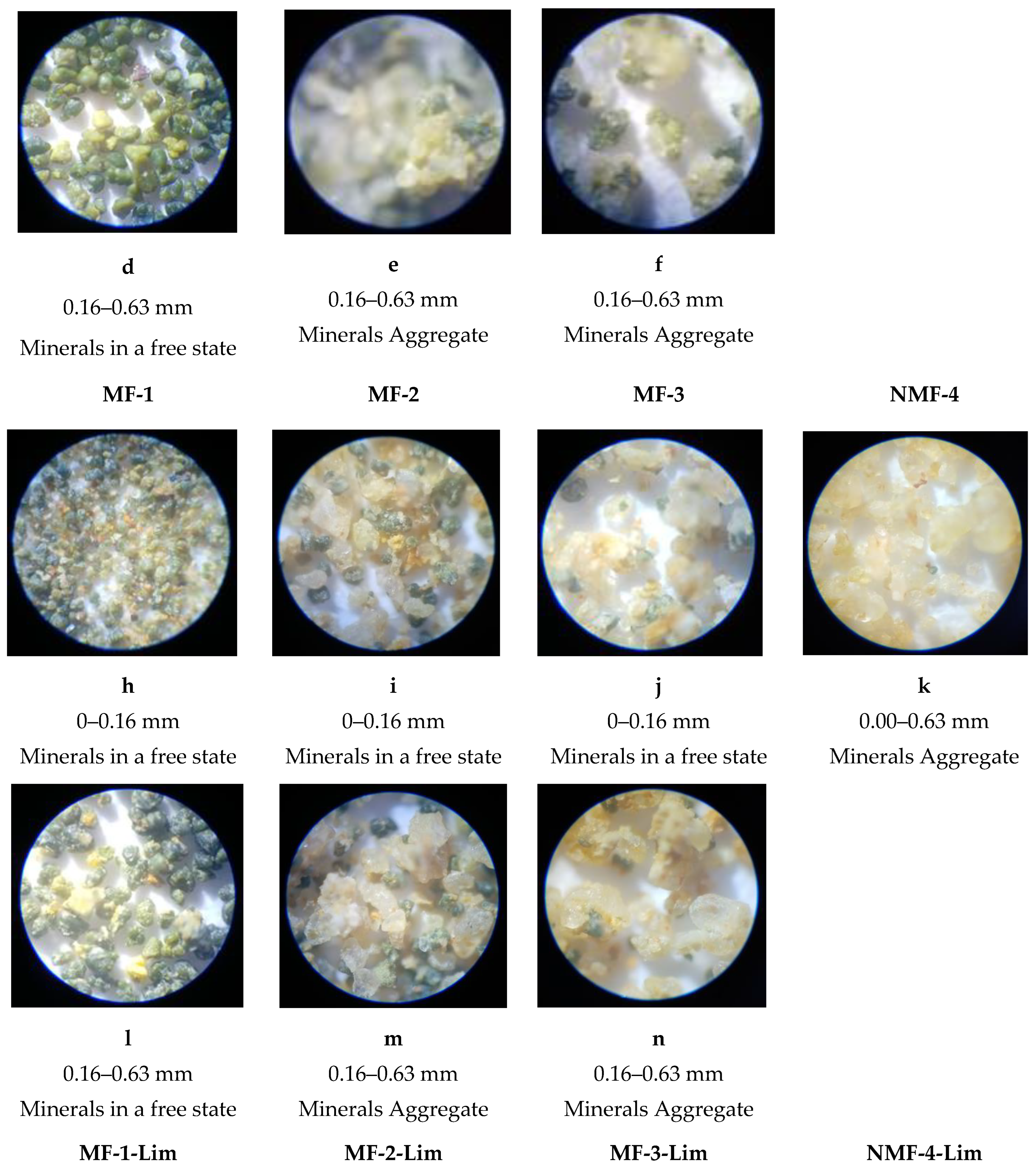
The morphology and content of the glauconite components in the studied sands were examined under a binocular microscope. The photographs are presented in
Figure 6.
Natural GQS green-grey colors are observed under a microscope in reflected light (
Figure 6a). The sands are well sorted, composed of round grains of milky-white translucent quartz ranging 0.2–0.4 mm in size. Dark green and blackish glauconite grains are 0.2–0.3 mm in size. They also have an isometric semicircular shape and some of them are bonded with quartz.
Under the microscope, GQS-Lim grains have a greenish-yellow color (
Figure 6b). They are moderately sorted, composed of different-sized quartz grains up to 0.4 mm in diameter, exhibiting varying degrees of roundness. Quartz grains, the most abundant in the sample, are often angular, elongated, and locally cracked. Accumulations of precipitated iron hydroxides are sometimes observed on them. They give the grains a wax-yellow tint. In addition to quartz grains, intergrown quartz aggregates with a mosaic structure in the form of lumps up to several mm in diameter occur as well. Glauconite grains are dark green, well-rounded, and variable in size. Their size ranges from 0.1 to 0.25 mm. Glauconite also occurs in fine-grained polymineral aggregates (lumps) together with quartz. Individual wax-yellow grains about 0.1 mm in size represent limonite. The remaining opaque minerals appear in trace amounts.
After dry magnetic separation of the studied sands, the obtained magnetic fractions were divided on sieves into 0–0.16 mm and 0.16–0.63 mm fractions.
Figure 7 presents the separated magnetic fractions under a microscope. Magnetic fractions MF-1 and MF-1-Lim, which were subdivided into 0–0.16 mm and 0.16–0.63 mm fractions, represented glauconite mineralogical aggregates without quartz sand. Magnetic fractions MF-2 and MF-2-Lim, the grain size of which was 0.16–0.63 mm, represented mineralogical aggregates consisting of quartz sand and glauconite minerals. The same magnetic fractions, the grain size of which was 0–0.16 mm, were a mixture of free quartz sand and glauconite minerals.
3.3. Chemical and Mineral Analysis of the Analyzed Sands and Their Nonmagnetic Fractions
Comparison of changes in the chemical composition of GQS, GQS-Lim, and the magnetic and nonmagnetic fractions is presented in
Table 3. In all magnetic fractions, an increase in the mass fractions of basic oxides (Al
2O
3, Fe
2O
3, K
2O) and decrease in SiO
2 was observed. In the nonmagnetic fractions NMF-4 and NMF-4-Lim, a decrease in the mass fractions of basic oxides was observed. For example, after GQS separation, the Fe
2O
3 content decreased from 2.43 wt.% to 0.26 wt.%, and the Al
2O
3 content decreased from 4.02 wt.% to 2.01 wt.%.
After dry magnetic separation of GQS-Lim, the content of Fe2O3 decreased from 1.99 wt.% to 0.87 wt.%, and Al2O3 from 5.21 wt.% to 3.77 wt.%. Moreover, in both nonmagnetic fractions, there was a decrease in the mass fraction of TiO2. Due to the extraction of the iron-containing aluminosilicates into magnetic fractions, the obtained nonmagnetic fractions NMF-4 and NMF-4-Lim increased their SiO2 content from 83.60 wt.% to 91.97 wt.%, and 84.10 wt.% to 90.72 wt.%, respectively. The mass fraction of MgO in the nonmagnetic fractions increased slightly compared to the raw material.
MF-2, MF-2-Lim, MF-3, MF-3-Lim, NMF-4, and NMF-4-Lim were characterized by an abundance of quartz sand. As shown
Figure 8, large broad peaks did not occur in the XRD patterns of bulk samples, which indicates the presence of large amounts of quartz sand. In addition to quartz sand, the XRD analysis of the bulk samples showed the presence of glauconite and goethite (limonite) (
Figure 8). Sharp peaks of quartz sand can be seen at 2θ = 21–24°, 26–28°, 35–38°, 50–53°.
The conducted XRF and XRD analyzes showed that MF-1 and MF-1-Lim are mineralogical aggregates. The dominant mineral is glauconite (K<1(Al,Fe3+)(Al,Fe2+,Mg)[Al<1Si<4O10](OH)2) and its content exceeds 70% and 62%, respectively. It is accepted that minerals whose content is below 5% are secondary. The practical use of the magnetic fractions MF-1 and MF-1-Lim, which are glauconite concentrates, depends on the qualitative and quantitative content of the main minerals. Therefore, the main three minerals were selected. The mineralogical aggregates MF-1 and MF-1-Lim include biotite (K(Mg; Fe2+)3(Al; Fe3+)Si3O10(OH; F)2) and quartz sand (SiO2). The content of biotite is 13% and 8%, respectively, and quartz sand is 9% and 15%, respectively.
The XRD results of the investigated magnetic fractions showed that the content of SiO2 (quartz sand) in MF-2 and MF-2-Lim is 92.2% and 91.8%, respectively. In MF-3 and MF-3-Lim, the content of SiO2 (quartz sand) is 92.9% and 92.3%, respectively. Based on the results of the mineral composition, the two nonmagnetic fractions (NMF-4 and NMF-4-Lim) contained 94.8% of quartz sand and 0.8% of glauconite, and 93.1% of quartz sand and 1.9% of glauconite, respectively.
Analysis of the X-ray diffractograms allowed to determine the quantitative and qualitative mineral composition of the studied samples. Accordingly, the numerical values of four coefficients were calculated, i.e., weighted profile residual R
wp, expected profile residual R
exp, chi squared χ
2, and goodness of fit GoF. The results of mineralogical analyses and Rietveld discrepancy values are presented in
Figure 8 and
Table 4.
As can be seen in
Table 4, values of the coefficients that were used to analyze the obtained diffractograms are approximately in the same ranges. Analysis of the calculated coefficients for MF-1 and MF-1-Lim are obtained with good convergence, similarly as for the multiphase samples.
A characteristic feature of the values of coefficients R
wp and R
exp is the large difference between the diffractograms of samples MF-2, MF-2-Lim, MF-3, MF-3-Lim, NMF-4, NMF-4-Lim. In the Rietveld analysis, adaptation of the model to experimental data was performed. As noted in [
37], the value of χ
2 must never be below one, nor must the value of R
wp ever be equal to R
exp. The obtained values of these coefficients correspond to the abovementioned model assessment conditions.
It is traditionally believed that a low value of χ
2 indicates a high probability of the obtained values. However, in practice this is not always the case. High values of χ
2 can occur when the data are collected with very high precision. In this case, small model fit deficiencies can become huge relative to the experimental uncertainty. However, there are also many cases, in which χ
2 ˃ 1 indicates unreliable results. Therefore, to estimate the obtained value of χ
2, it is necessary to take into account the values of coefficients R
exp and R
wp [
37,
38].
It is important to note that a longer count increases the statistical accuracy of the diffraction measurement. Therefore, as the total number of counts collected for the diffraction pattern increases, the value of R
exp decreases. In addition, long calculation very often increases the difference between the R
exp and R
wp values. As a result of the mathematical operation, the value of χ
2 increases and sometimes it can be assumed that the resulting fit is poor. When patterns are measured with a very large number of counts, even minor “imperfections” in the peak shape or peak position may prevent small values of R
wp or χ
2 from being obtained [
37,
39].
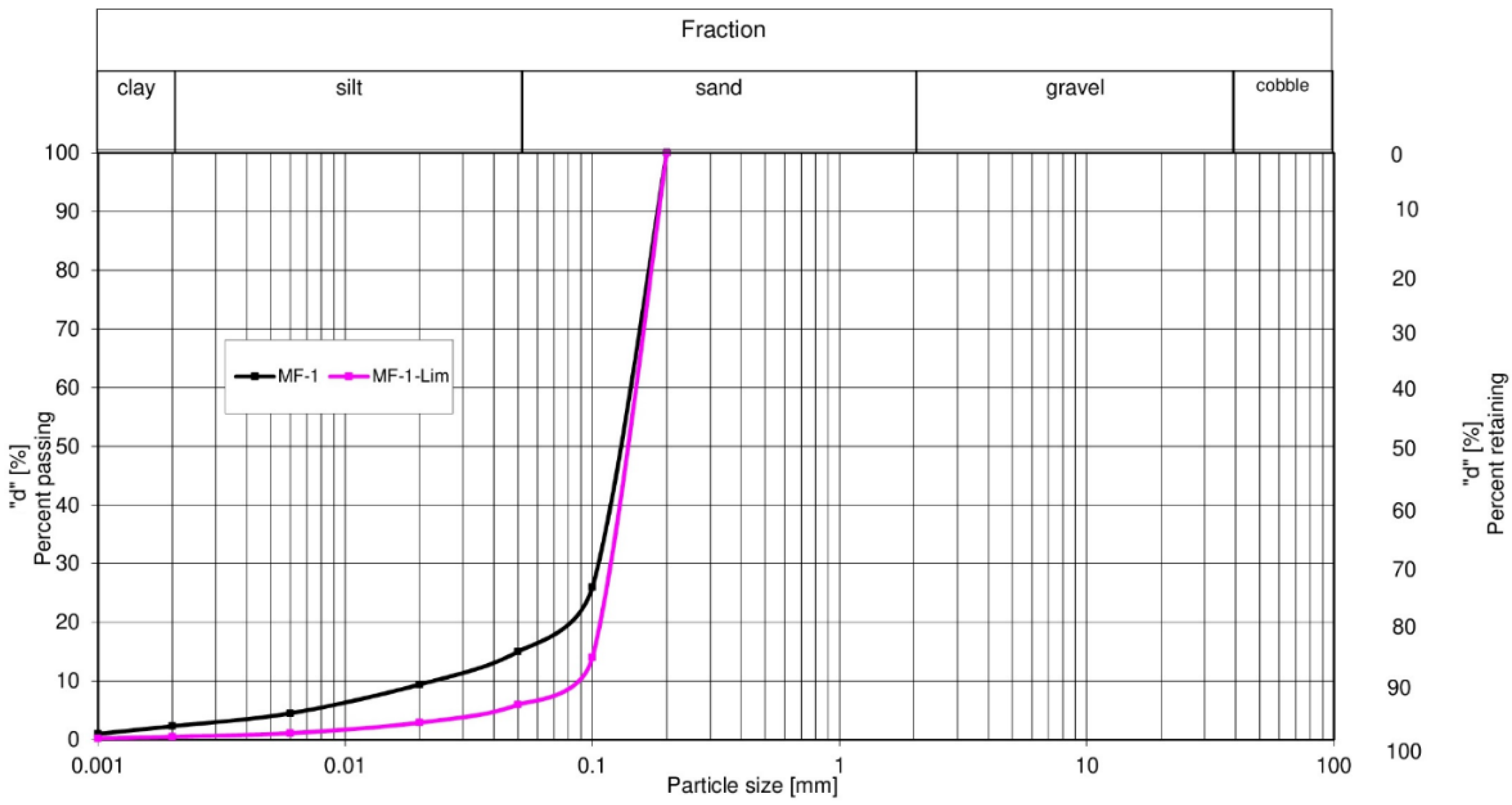
3.4. Results of Determination of the Granulometric Composition of MF-1 and MF-1-Lim by the Optical Method
The spectrometric laser diffraction technique, used in the diagnosis of grain and microchannel stereometry, is currently being developed by many specialized companies. In the presented tests, a modular diffractometer by Sympatec GmbH was used. Different optical modules are used in this devise to determine the granulometric composition. In the current study, the R6T optical unit in the range of 0.5/0.9–175 µm was used. The grain size distribution of the examined resuscitation of the approximation of the explanation using the Rosin–Rammler–Sperling–Bennett (RRSB) function. Based on the new results, the parameter d and the grain uniformity coefficient n in the set were obtained. In order to determine the object of this function by means of a regression function, linear initiated software was used. Characteristics of the RRSB model for each particle size distribution are summarized in
Table 5.
The uniformity coefficient
n characterizes the dispersion (width) of the particle size distribution curve, i.e., the lower is the
n value, the “wider”, more dispersed is the particle size distribution. Both analyzed samples have a similar value of parameter
n (1.98 and 1.82). Therefore, both samples are characterized by a similar grain size distribution. This value indicates that they are relatively unevenly grained and must be sorted for industrial use. The higher the
n value, the greater the uniformity of the ground particles around the statistical average diameter [
40].
Values of the determination coefficients (RRSB d—Table) prove a good fit for the distribution of the actual particle sizes in the studied size range. These assessments relate to the fit of the distributions, which shows that the RRSB model can be used for further analysis, in particular for predicting material extraction in this range.
Figure 9 shows that with similar values of factor
d (characteristic diameter) of the RRSB function for both samples, it can be concluded that they did not differ significantly in terms of the specific surface area
sm. Both the values of “x” in individual classes and the cumulative curves of granularity (
Table 4) show that, according to [
35], the magnetic fraction MF-1 has a high content of small fractions (clay and dust), therefore, according to the classification, it represents silty sand. The magnetic fraction MF-1-Lim belongs to sands. The determined coefficient of uniformity
n for MF-1 and MF-1-Lim was 0.98 and 1.82, respectively. According to [
35], sands are considered heterogeneous if the uniformity coefficient exceeds three, and for clay and dust exceeds five. Thus, the studied magnetic fractions MF-1 and MF-1-Lim are homogeneous [
41].
3.5. Results of Determination of the Granulometric Composition of the Nonmagnetic Fractions NMF-4 and NMF-4-LIM
The grain-size composition of NMF-4 and NMF-4-Lim is shown in
Figure 10. Accordingly, the grain-size distribution of NMF-4 is relatively uniform, and NMF-4-Lim is nonuniform.
The cumulative (total) characteristics of the grain-size composition determined the median diameter of 0.24 mm for the nonmagnetic fraction NMF-4 and 0.48 mm for the nonmagnetic fraction NMF-4-Lim. The main mass fraction of the nonmagnetic fraction NMF-4 (77.15%) is concentrated in the size class −0.315 + 0.16 mm, and the nonmagnetic fraction NMF-4-Lim (56.59%) is contained in the size class −0.63 + 0.315 mm. The mass fractions of the fine silt fractions −0.05 + 0 mm in both nonmagnetic fractions NMF-4 and NMF-4-Lim are 0.57% and 0.58%, respectively. Both samples are concentrated in the size classes −0.8 + 0 mm.
The nonmagnetic fraction NMF-4 is smaller and more homogeneous than the nonmagnetic fraction NMF-4-Lim in terms of grain-size distribution. In the process of magnetic separation from the initial samples of QGS and QGS-Lim, the main part of the fine fractions −0.16 + 0.05 mm and −0.05 + 0 mm was extracted into the magnetic fraction, which contributed to obtaining more homogeneous nonmagnetic fractions.
According to the above procedure (5.4), the fineness modulus (FM) was determined for the nonmagnetic fractions NMF-4 and NMF-4-Lim. It was at 1.10 and 1.85, respectively. In addition, it should be noted that the obtained grain curves for NMF-4 and NMF-4-Lim (
Figure 10) show that such sand does not contain grain fractions larger than 1.25 mm.
4. Discussion
The depth of the occurrence of glauconite-containing sands in the study area (Dvorovychi quarry located in the Mizoch Ridge) are acceptable for the industrial development of sand for construction purposes. The studied glauconite–quartz sands represent the overburden and due to the high content of clay particles cannot be used in the construction industry. Experimental studies of GQS and GQS-Lim showed that they can be subjected to dry magnetic separation to obtain magnetic and nonmagnetic fractions (
Table 2). The main attention was focused on the characteristics of the magnetic fractions MF-1 and MF-1-Lim, as well as the nonmagnetic fractions NMF-1 and NMF-1-Lim.
XRF and XRD analyses showed that MF-1 and MF-1-Lim are mineralogical aggregates. Their dominant mineral is glauconite; its content is its content exceeds 70% and 62%, respectively. These mineral aggregates were sieved into 0–0.16 mm and 0.16–0.63 mm fractions.
Figure 7 shows microscope views of the magnetic fractions MF-1 0–0.16 mm and 0.16–0.63 mm; the fractions clearly differ from one other. In the 0–0.16 mm fraction there are green and yellowish-rusty colored granules, and the percentage content of each group is approximately 50%. The 0.16–0.63 mm fraction has a different percentage ratio-there are more green mineral granules than the yellowish-rusty ones. Analyzing the color of granules of the magnetic fraction MF-1-Lim at sizes 0–0.16 mm and 0.16–0.63 mm, it is possible to characterize the sub-divided granules of glauconite mineral aggregates in approximately the same way.
It is commonly known that the color of minerals is one of the most important diagnostic properties [
42,
43]. The main dye in the mineral world is iron (chromophore), which, depending on the degree of oxidation, structural position and concentration, can give the minerals various shades of brown-brown, yellow, green, red, pink, and even blue (especially at high Fe
2+ contents) [
44,
45].
The obtained magnetic fractions MF-2, MF-3, MF-2-Lim, and MF-3-Lim are mineral aggregates, i.e., they represent the accumulation and fusion of mineral individuals (crystals and grains) of quartz sand and the mineral glauconite. In various fields of science, the term “aggregate” refers to objects that differ in structure, size, and properties. These mineral aggregates, unlike mineral individuals, do not have clear signs of symmetric shapes. Very often, such aggregates can disintegrate into primary (initial) particles under certain external influences (for example, mechanical).
Such mineralogical aggregate is cemented quartz sand and glauconite. One possible application for MF-2, MF-3, MF-2-Lim, and MF-3-Lim may be the building industry. It is known that sands with a high content of clay minerals are unsuitable for use as fillers for concrete. The physical and mechanical properties of concrete depend on the petrographic characteristics, such as mineralogical composition, texture, size, shape, and location of mineral grains, the nature of grain contact and the degree of their cohesion, as well as the degree of deformation of the parent rock [
46,
47]. The mineralogical composition of fillers, i.e., the degree of their change, strongly affects the mechanical behavior and performance characteristics of engineered concrete objects [
48,
49,
50].
The content of quartz sand in MF-2 and MF-3 is 92.2% and 91.8%, respectively, and 92.9% and 92.3% in MF-2-Lim and MF-3-Lim, respectively. It can be theoretically supposed that when mixed with other mineral fillers, the percentage of clay minerals will decrease, and in this way, the use of pure mineral fillers can be reduced. The assumptions described above, based on the results of experimental studies of magnetic fractions MF-2, MF-3, MF-2-Lim, and MF-3-Lim, require additional experimental studies. However, theoretically, it can be assumed that such studies can provide good results.
The obtained fineness modulus of the nonmagnetic fractions NMF-4 and NMF-4-Lim was 1.10 and 1.85, respectively. FM is supposed to specify the proportions of fine and coarse aggregates when designing concrete mixes. The higher the FM value, the coarser the aggregate. Generally, a lower FM results in a larger volume of paste, making the concrete easier to process. However, FM does not define the grading curve and different gradings may have similar FM values. According to this index, following [
28], quartz sands can be used for the production of building materials. Therefore, NMF-4 can be used as sand for the manufacture of silicate stones, bricks, and other pressed products, and plaster mortars for the finishing layer. The resulting nonmagnetic fraction NMF-4-Lim following [
51] can also be used as a material for pressed products, as well as a filler in the manufacture of dry building mixtures.
The results of the chemical composition of the nonmagnetic fractions NMF-4 and NMF-Lim-4 show that they contain an increased content of Fe
2O
3, i.e., 0.26 wt.%, and 0.87 wt.%, respectively. According to the content of Fe
2O
3, following [
29], NMF-4 and NMF-Lim-4 can be attributed to the grade of quartz sand (PK-030-3). They are suitable for the production of glass products from green, brown, olive, and other dark glass colors, as well as opaque glass, foam glass, building materials, components of mixtures for road pavements, squares, airfields, and material for landscaping reclamation.
Examination of the obtained nonmagnetic fractions under a binocular microscope shows that Fe2O3 covers quartzite grains with a thin film. To improve the quality of sands, that is to reduce the content of Fe2O3, the obtained nonmagnetic fractions can be subjected to mechanical operations including washing and rubbing in a pulp with a solid content of 50% and 70%. This technique is often used in wet technologies for the enrichment of quartz sands for the glass industry.
To increase the effect of Fe
2O
3 removal from quartz sand, the addition of solutions of various acids, i.e., oxalic acid [
52], phosphoric acid [
53], and others [
54], is often used. These methods significantly increase the cost of purifying quartz sand, are not always economically justified, and increase the negative impact on the environment.
In the production of glass, cullet is traditionally used along with glass charge components (for example, quartz sand, limestone, dolomite) due to the inherent disadvantage of glass factories and recycling. This component can reduce or improve the quality of transparent glass products depending on the content of Fe2O3 in the cullet added to the glass charge. Usually, charge components with the lowest Fe2O3 content are used to improve the quality of transparent glass products. Cullet is used to reduce the energy intensity of the glass melting process.
It is quite difficult to obtain sand with a quality appropriate for making glass. The use of such sand together with glass from recycling will reduce emissions to the atmosphere, consumption of raw materials, extend the life of glass plant equipment (kilns), and save energy [
55]. Energy costs can be reduced by about 2–3% for every 10% cullet used in the manufacturing process. One ton of carbon dioxide is reduced for every six tons of recycled glass used in the manufacturing process [
56].
Thus, it can be theoretically stated that energy savings in the manufacture of glass (with the involvement of part of the glass from recycling) will partially compensate for the costs associated with dry magnetic separation of glauconite–quartz sands.
5. Conclusions
The development of a building sand deposit is accompanied by the accumulation of significant volumes of secondary raw materials. In our study, these wastes were glauconite–quartz sand and glauconite–quartz sand with limonite. The studied samples were capable of dry magnetic separation. At a magnetic induction of 0.8 Tl, magnetic fractions of 5.1% and 2.8% were obtained, respectively. The results of XRD, XRF, and petrographic analysis showed that the magnetic fractions contained more than 60% of glauconite. A well-known practice of using this mineral is its use in various eco-technologies, for example, for cleaning soils and natural waters. At magnetic inductions of 1.1 and 1.3 Tl, magnetic fractions were obtained in the amount of 10.3%, 3.8% and 10.6%, 6.2%, respectively. The content of quartz sand in them is 92.2% and 91.8%, respectively, and 92.9% and 92.3%, respectively. The low content of sand in such magnetic fractions does not allow recommending their use in construction. Considering the fact that relatively few such fractions are formed, they can be mixed with the conditioned sand. This prevents the accumulation of magnetic fractions during the dry magnetic separation of the overburden rocks of construction sand deposits.
Nonmagnetic fractions were obtained in the amount of 80.7% and 80.4%, respectively. The conducted XRF showed that in each nonmagnetic fraction, the content of SiO2 was 96.9 and 93.7%, and Fe2O3–0.26 and 0.87%, respectively. According to the XRD results, nonmagnetic fractions contained 94.8% and 93.1% of sand, respectively, and 0.8% and 1.9% of glauconite, respectively. The bulk modulus of the nonmagnetic fractions was 1.10 and 1.85, respectively. The grain size classes of the obtained quartz sands were −0.63 + 0 mm and −0.8 + 0 mm, respectively. Current Ukrainian standards allow for the use of the obtained nonmagnetic fractions in construction; as concrete fillers; and in the production of silicate stone, ceramic bricks, building mixtures.
Thus, the use of the studied overburden of the construction sand deposit, after dry magnetic separation, can satisfy the local demand for sand in various industries and use the glauconite concentrate to solve various environmental problems. At the same time, the use of mining waste will reduce their negative impact on the environment.