1. Introduction
Wastewater from medical facilities constitutes a significant part of municipal sewage discharge, because the processes of maintaining hygiene conditions in these facilities causes high water consumption. The high level of suspended substances and chemicals, anionic surfactants and organic substances often exceed the norms regarding the quality of municipal wastewater. The surfactants used for disinfection and washing are the main pollutants in medical wastewater. In recent years, the need to reduce the concentration of chemicals, especially surfactants, in wastewater streams is widely promoted due to more stringent environmental conditions and legal provisions. Surfactants are a group of water-soluble and non-water-soluble detergents. Their task is to remove substances difficult to clean (e.g., oils, blood, inorganic pharmaceuticals). The presence of surfactants in wastewater increases COD and makes the process difficult for quick wastewater treatment—their connection with pollutants is permanent and difficult to break down in the wastewater treatment plant. Excessive concentration of these compounds may also contribute to slowing down the processes of biological wastewater treatment or to partial death of the biological deposit. The most commonly used processes for the purification of this type of wastewater are conventional processes such as coagulation, flotation and chemical oxidation or their combinations [
1,
2].
The conducted research on ozonation of wastewater in order to degrade recalcitrant contaminants does not give satisfactory results [
3,
4]. In the conventional ozonation process, ozone decomposes to oxygen immediately after its production due to a very short half-life in the aquatic environment [
3,
4]. This causes problems in the implementation of the process, such as high cost and the requirement for ozone generation on site. Some of the impurities present in the wastewater are persistent for ozone due to its selectivity in the oxidation of some organic compounds [
3,
4,
5,
6]. For this reason, ozone is often used in combination with other processes and oxidants, such as electrolysis [
5,
6] and H
2O
2 [
7,
8]. These connections increase the efficiency of pollutant degradation. Although the combination of O
3 processes and electrolysis have a large potential for removing contaminants, there are contaminants which in comparison with each of these processes separately, continue to generate problems in the process due to the limited adaptability of ozone generators [
9].
The use of O
3 and H
2O
2 combination in the associated peroxone production process (AOP) has a synergistic effect on the removal of pollutants [
10,
11,
12]. Peroxone is used for cleaning soil, ground water and polluted wastewater with volatile organic compounds, polycyclic aromatic compounds, hydrocarbons, petroleum hydrocarbons, chlorinated solvents and metals, ammunition, diesel oil, methyl tert-butyl ether (MTBE), BTEX (benzene, toluene, ethylbenzene and xylene), trinitrotoluene (TNT) and other soluble components from waste [
13].
Turkay et al. [
14] in their research describe and highlight the advantages of electro-peroxone processes as a combination of two different systems for hydroxyl radical generation (ozonation and electrolysis). The results showed that the combination of ozone and electrolysis with carbon-based cathodes in optimised conditions will lead to higher degradation, removal rates, and efficiencies compared to using ozonation and electrolysis individually. The comparation tests conducted by Donghai et al.l revealed that the coupling of electrolysis and ozonation could synergistically produce hydroxyl radicals (HO•) and the separation of cathodic reactions and anodic oxidations further promoted HO• generation, which was responsible for the enhancement of PABA elimination in the compartmental E-peroxone process [
15].
Electro-peroxone (E-peroxone) is an advanced method of electrochemical wastewater oxidation. In this method, the production of H
2O
2 occurs in controlled conditions, in contrast to the conventional oxidation process. The main advantage of using E-peroxone is the use of graphite cathode to form H
2O
2 from water and oxygen in the gas mixture (O
3 and O
2). Hence, this process is simple in application, cost-effective and safe. The effectiveness of this process was evaluated in the neutralization of oxalic acid [
16], 1,4-dioxane [
17], methylene blue [
18], Orange II [
19] and numerous pharmaceuticals [
20]. All studies concentrated mainly on organic substances and results were highly effective. The innovative factor in the conducted research is the use of E-peroxone technology in the process of neutralizing medical wastewater. This issue has not been deeply described in the literature and the potential for using the above-mentioned technology as an alternative to commonly used solutions is noted.
The purpose of the study was to determine the effect of using E-peroxone on the acceleration of the organic matter biodegradation rate in medical wastewater from healthcare facilities. The biodegradation conditions were controlled over time using COD measurements.
2. Materials and Methods
The sample of wastewater was collected from IBC containers, which were delivered to a waste disposal company located in Poland. Sample (0.1 m3 HDPE container) after collection was stored at 5 °C. After 24 h, conditioning sample was divided into 10 subsamples and subjected to an oxidation process. Before the oxidation process, subsamples were not pretreated. pH of the main sample was measured by an Elmetron CP-511 conductometer with an EPS-1 glass electrode for the measurement of pH in the aqueous solution, in accordance with PN-EN ISO 10523:2012. The initial value of pH was 5.2 units.
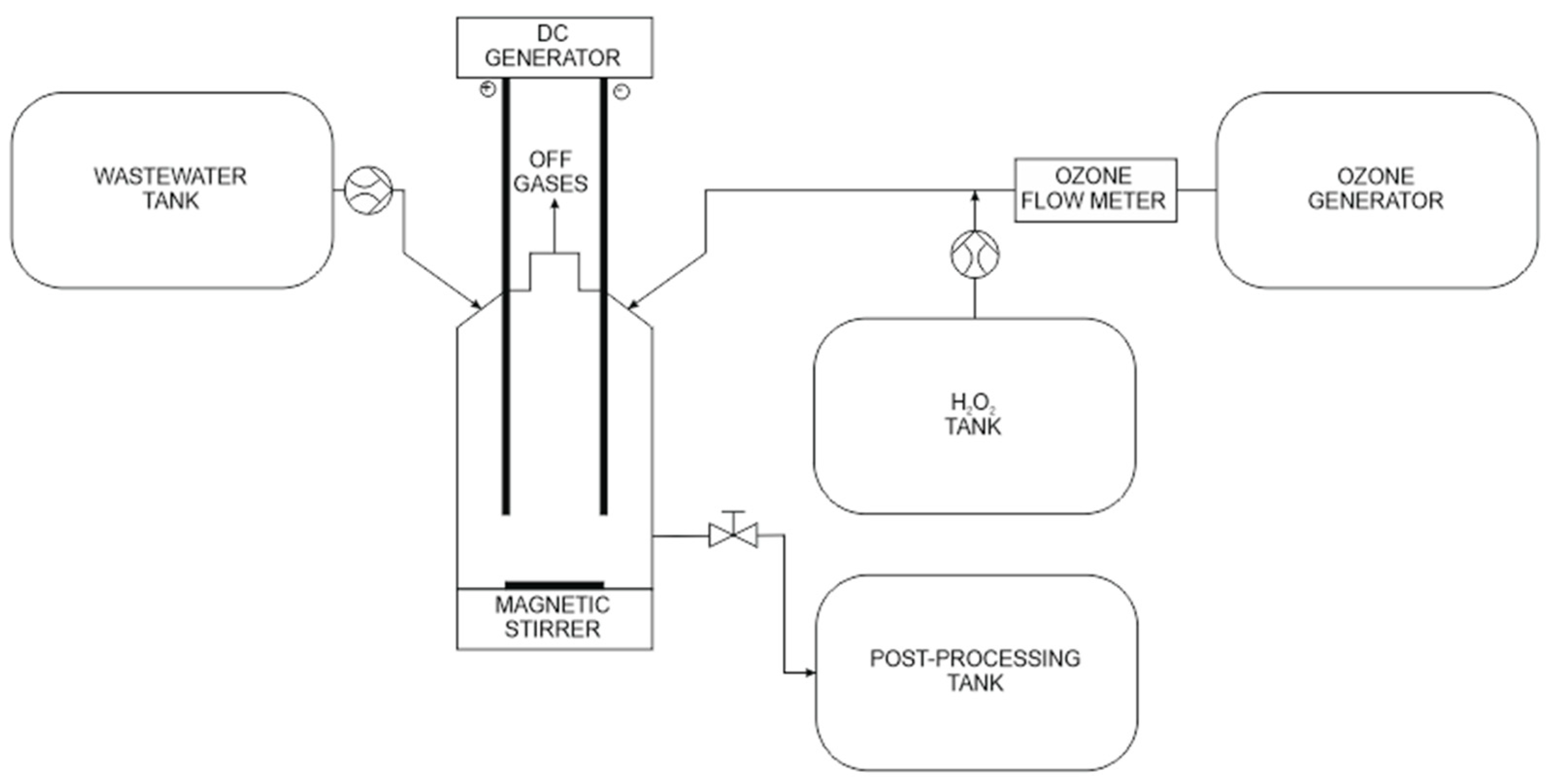
The wastewater oxidation process was carried out in a glass laboratory reactor with a capacity of 2.5 dm
3. The reactor, with the help of electronically controlled dosing systems, was simultaneously treated with wastewater as well as ozone and hydrogen peroxide. The reagents were mixed while dispensing with the use of an electromagnetic stirrer with a constant speed of 1000 rpm. In the process of using E-peroxone, graphite electrodes were additionally mounted to the reactor. The electrodes were supplied with DC current of 500, 1000 and 2000 mA (
Figure 1).
Each oxidation process lasted 10 min. After each minute of the process, 25 cm3 of the COD measurement mixture was automatically collected from the reactor discharge port. For each test, the COD index was determined by means of a miniaturized method using sealed tubes according to PN-ISO 15705:2005, repeating each determination three times. Determination of the chemical oxygen demand consists of the introduction of a specified amount of the chemical oxidant and the necessary catalysts and auxiliary substances to the leachate sample. Then, under strict conditions and time, the oxidation process is conducted. After oxidation, the remaining amount of oxidant is determined. The loss of oxidant, converted into oxygen, is given as COD in mgO2/dm3. The COD was tested in combination with a spectrophotometer (Hach DR-2800). The averaged values of all measurements were assumed for the analysis, after rejecting extreme results (mean value ± 2 times the standard deviation). During testing, the air temperature was maintained at 22 ± 1 °C and air humidity at 55 ± 5%.
Hydrogen peroxide (30%, w/w) was of analytical reagent grade (Merck, Darmstadt, Germany). Ozone was produced in the O3PRO30,7VW generator, equipped with a corona electrode system. Gas ozone concentration and flow (in g/Nm3) was measured by an ultraviolet gas ozone analyzer, Eltech 200. Ozone in the off-gas was measured by Lenntech AQL S200 analyzer.
3. Results
Electro-generation of hydrogen peroxide occurs during ozone presence in the reactor along with wastewater. E-peroxone transforms O
2 gas from O
3 decomposition at graphite cathodes to electro-generate H
2O
2 (Equation (1)) efficiently. Its conjugated base (Equation (4)) may then react with sparged O
3 gas to form hydroxyl radicals and other radical species (Equations (3)–(8)) [
14].
It has been established that the dissociation of H
2O
2 (Equation (4)) increases with increasing pH, as the pKa value of H
2O
2 was 11.6 [
21] and H
2O
2 reacts with O
3 to produce •OH only when present as its conjugated base, •HO
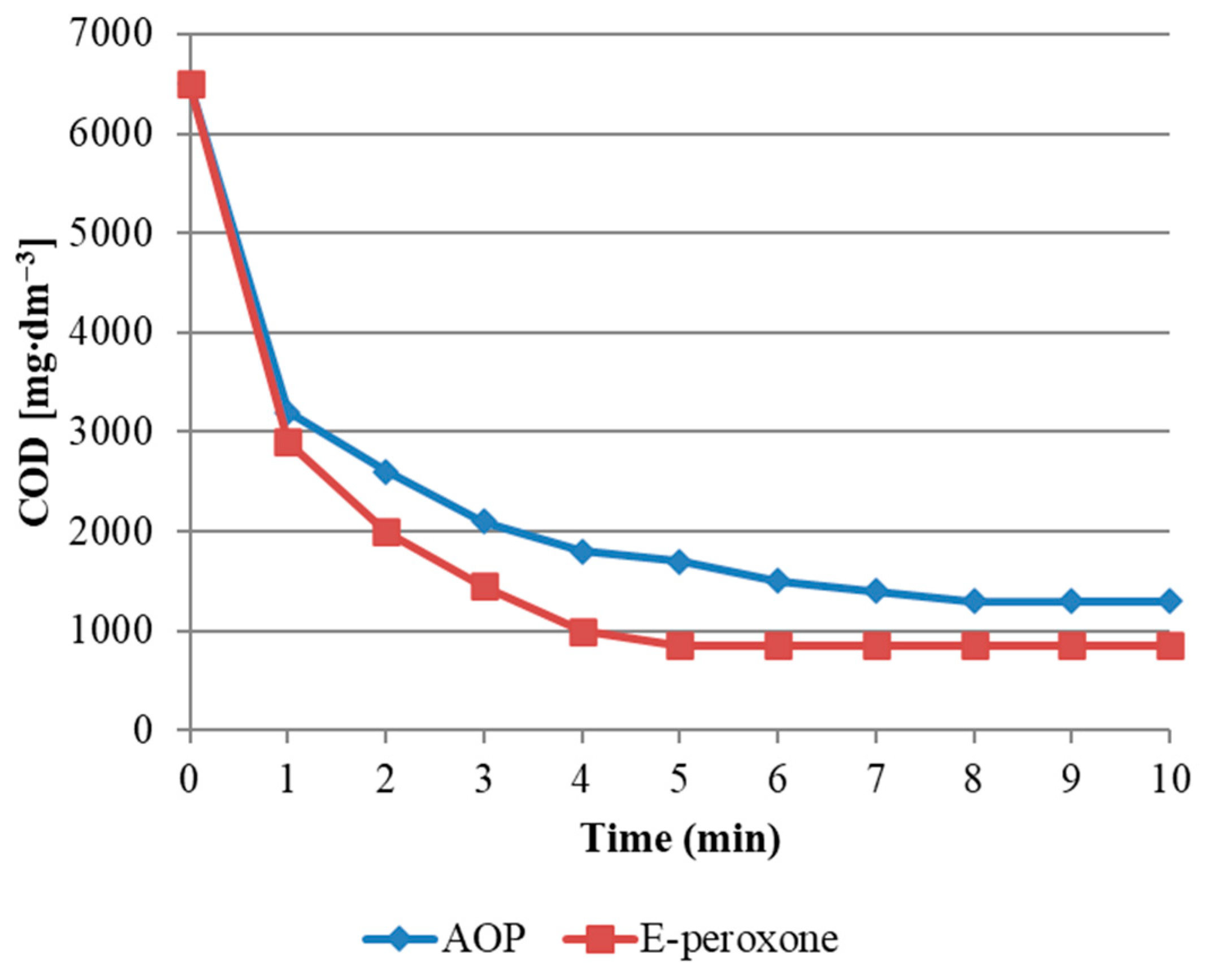
2−.COD reduction in the analyzed wastewater is presented in
Figure 2. For the analyzed oxidation processes, a 30% COD reduction between 2 and 3 min of reaction was obtained. After this period, the AOP process slowed down its speed, and. E-peroxone proceeded at a similar speed. After completion of the reaction at 10 min, both processes obtained similar COD reduction results, which differed by 450 mg/dm
3 COD.
According to
Figure 2, the regulation of the current in the E-peroxone process resulted in a slight improvement in the kinetics of the oxidation reaction. Studies have shown that the use of a current of 500, 1000 and 2000 mA accelerate the COD removal process.
The analysis of the obtained results suggests that the optimal rate of H2O2 formation occurs when using a current of 2000 mA. The use of a higher current intensity applied to the graphite cathode provides faster transformation of the ozone into the hydroxyl radicals in the aqueous environment.
As a result, it is possible to avoid a situation in which the presence of hydrogen peroxide lowers the efficiency of the process, due to the better affinity for hydroxyl radicals than the impurities present in wastewater. This situation is often observed in the AOP process [
10,
11,
22,
23,
24].
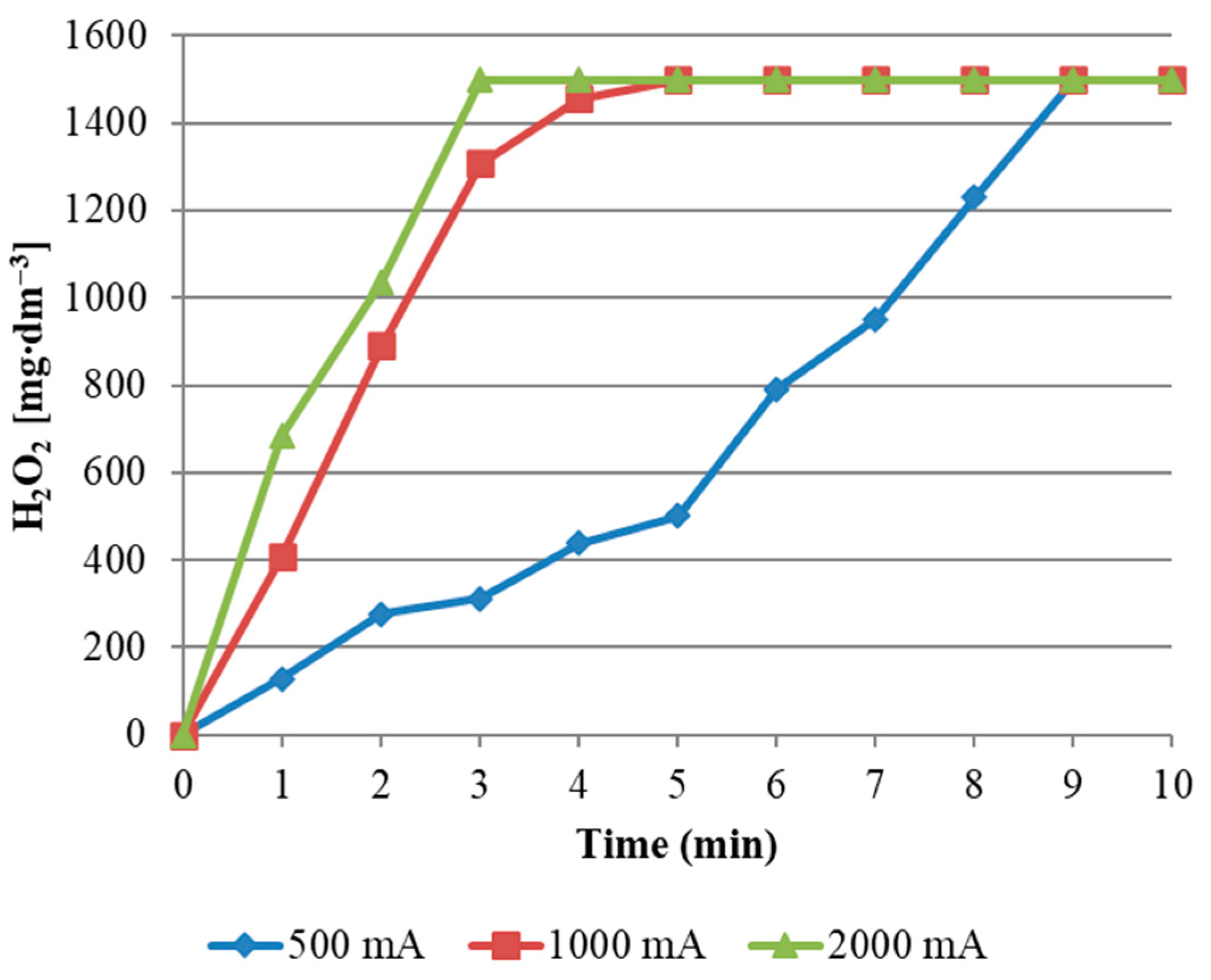
The influence of current intensity on H
2O
2 production in the E-peroxone process is presented in
Figure 3. A rapid increase in H
2O
2 concentration was observed between the beginning of the process and the 5th minute of its duration. At that time, the concentration of hydrogen peroxide was reached at the level of about 1500 mg/dm
3. This value was the maximum concentration for the current intensity in the range of 1000 and 2000 mA. The increase in H
2O
2 concentration was slower when the applied current was reduced to 500 mA. The concentration of hydrogen peroxide generated in the reactor during the use of graphite electrodes reached the equilibrium concentration (~1500 mg/dm
3) after 9 min of the process for all applied values of current intensity.
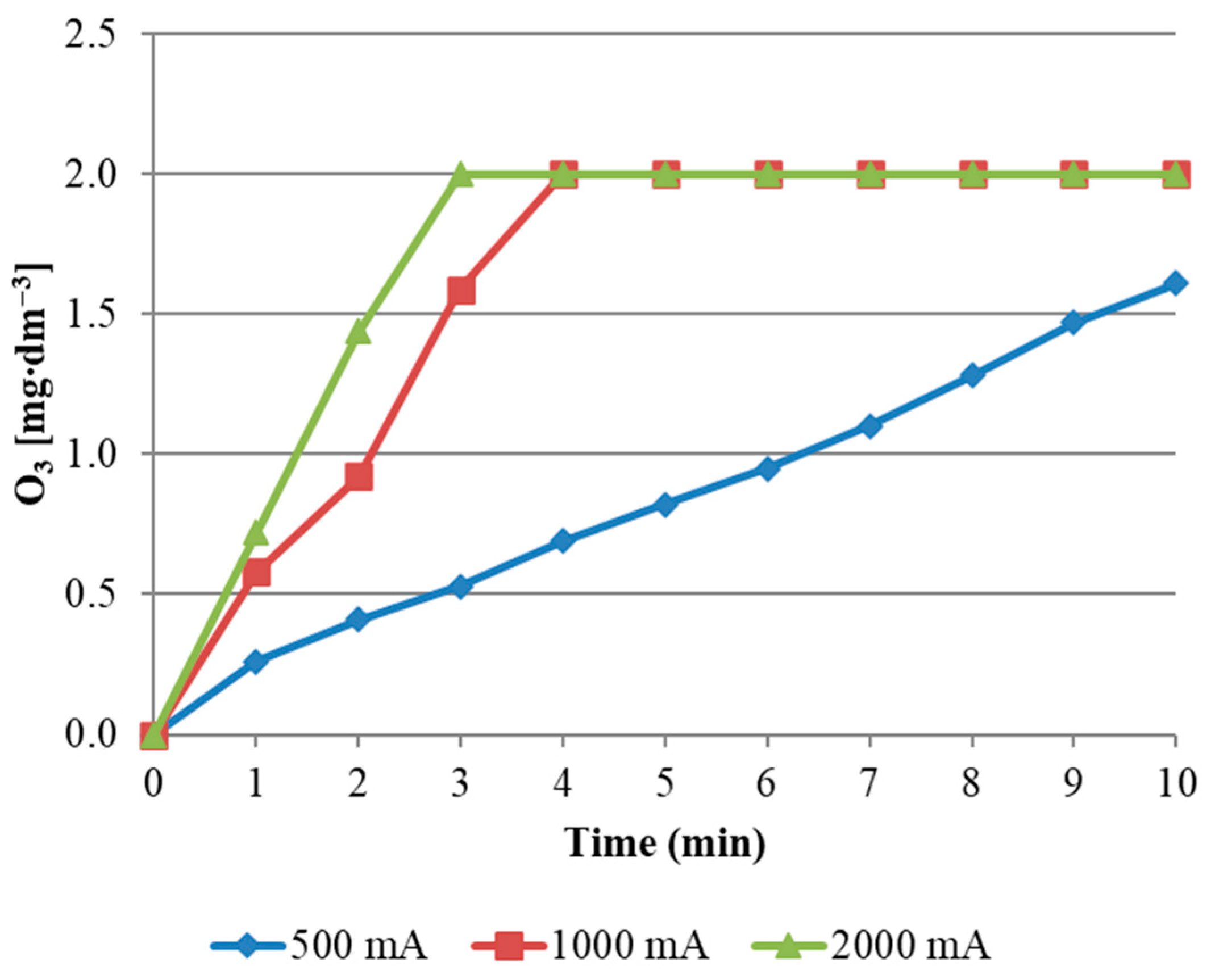
The highest ozone consumption occurred between the beginning of the reaction and the 4th minute of its duration. After this time, the concentration of ozone in the reaction mixture was reached at the level of about 2 mg/dm
3. This value was the maximum concentration for the current intensity in the range of 1000 and 2000 mA. The increase in O
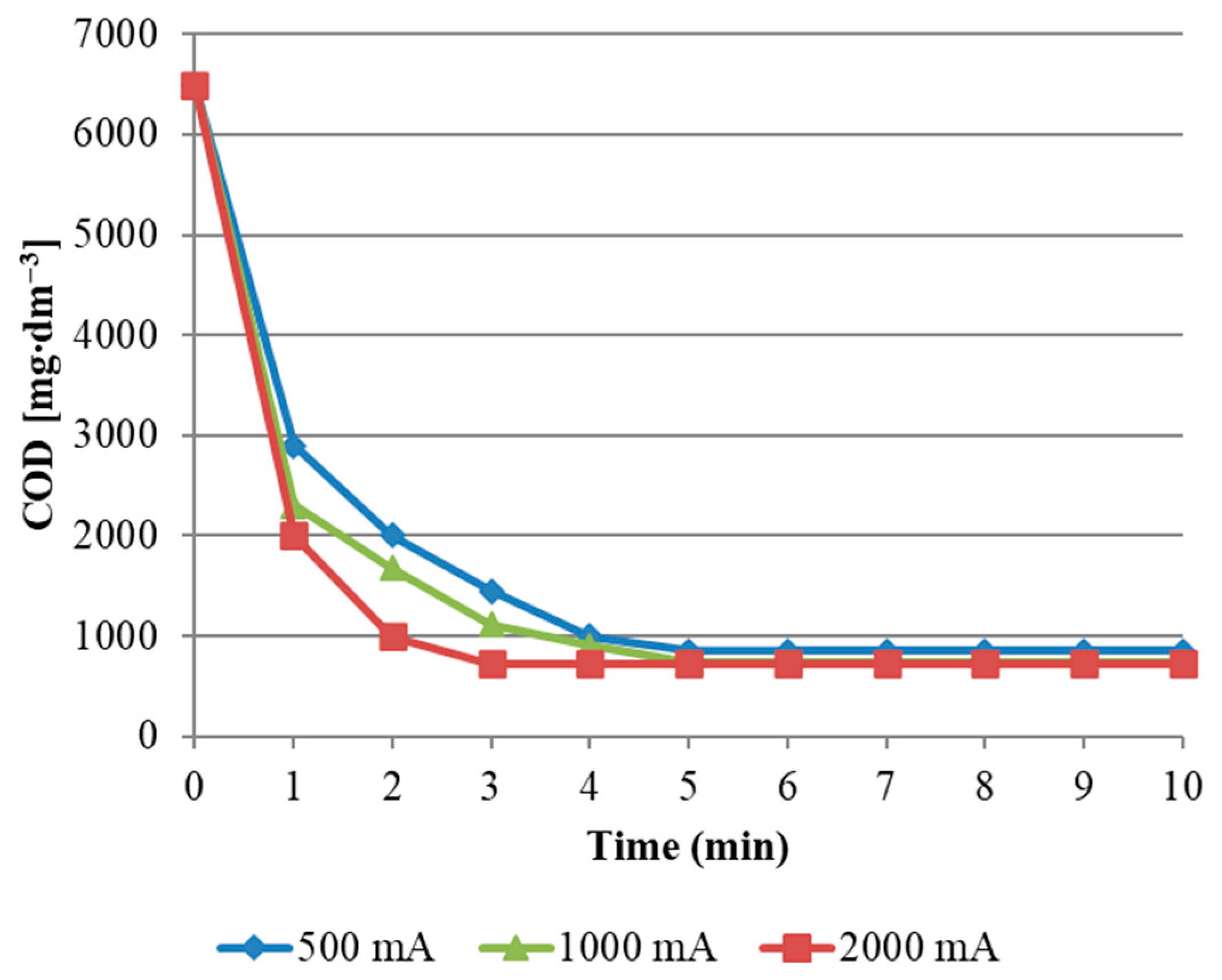
3 concentration was slower when a current of 500 mA was applied. Comparing the effect of the applied current intensity (
Figure 4), it was found that its two-fold increase accelerates the COD reduction.
The conducted experiments also show that after reaching the chemical equilibrium in the reactor (5th minute of the process), the current intensity’s influence on the course of the reaction is balanced. This observation suggests that the optimal current intensity for wastewater treatment is 1000 mA. Comparing the above statements with
Figure 3, it was found that the H
2O
2 formation rate increases with the current applied to the graphite cathode. As a result, faster and more efficient transformation of ozone into hydroxyl radicals in the E-peroxone system is achieved.
During the tests, it was also found that in the 5th minute of the process hydrogen peroxide begins to take over the action of hydroxyl radicals. This phenomenon confirms the stabilization of H
2O
2 concentration and of the content of ozone dissolved in water (
Figure 5). An analogous phenomenon occurs when using the standard AOP process described in [
11]. As can be seen in
Figure 5, the applied current intensity below 1000 mA can provide very different process effects, whereas above this value the effects of the oxidation process are not so diverse. The critical current level may depend on many factors, such as the reaction conditions (O
3 dose, pH, suspension, etc.) and the properties of the organic impurities present in the wastewater to be neutralized.
4. Summary
The article presents the results of research on the wastewater treatment process from a medical facility, using the conventional and electric peroxone systems. The formation of peroxone with the use of electric current is based on the production of hydrogen peroxide on the graphite cathode. The H2O2 generated during the reaction reacts with the ozone dissolved in water, causing the formation of highly reactive hydroxyl radicals. In the E-peroxone process the concentration of H2O2 was maintained at 1500 mg/dm3, with the flow of ozone through the reactor at the amount of 30 dm3∙h−1.
The use of both processes for wastewater treatment has produced positive results. The use of E-peroxone showed 15% better results in COD reduction than conventional peroxone. For analysis of the cost-intensity of the process, the energy consumption for ozone production and reactor functioning was assumed to be 5 kWh/kgCOD; cost of electricity to power 0.25 EUR/kWh; cost of purchasing electrodes, 10 EUR/2pcs; cost of purchasing hydrogen peroxide with a concentration of 35%, 400 EUR/m3; reaction time is 5 min. Based on the above assumptions, the operating cost of the oxidation process using peroxone is on average 2.54 EUR/kgCOD, and 2.62 EUR/kgCOD for the E-peroxone process.
The conducted research confirms the legitimacy of modifying standard oxidation processes that are sufficient to remove most organic pollutants. In practice, before using E-peroxone, it is necessary to consider what kind of wastewater is subject to purification, because its properties will affect the amount of H2O2 that should be generated during neutralization. Considering that the removal of pollutions occurs most intensively in the first minutes of the oxidation process, it is very important to evenly discharge the leachates and discharge the neutralizer from the reactor. It is not recommended to use long reaction times, because excessive concentration of peroxone does not significantly affect the quality of the neutralizer.
E-peroxone was found to be an efficient process for removing wastewater pollutants, the efficiency of the process depending on the type and constituents of the wastewater. Besides, an insufficient amount of O3 and excess amount of H2O2 in the solution may reduce the process efficiency. Therefore, the amount of electro-generated H2O2 and aqueous O3 should be controlled during the process.
The presented study is a preliminary experiment provided at the laboratory scale. Obtained results of E-peroxone usage for medical facilities’ wastewater will be used at a semi-technological scale to explore the real problems of the proposed process along with economic factors.
Author Contributions
Conceptualization, A.B. and M.G.; methodology, A.B.; validation, P.N. and J.T.; resources, P.N.; data curation, K.G.; writing—original draft preparation, A.B.; writing—review and editing, M.G.; visualization, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was Financed by a subsidy from the Ministry of Education and Science for the Hugo Kołłątaj University of Agriculture in Cracow for 2023.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Šostar-Turk, S.; Petrinić, I.; Simonič, M. Laundry wastewater treatment using coagulation and membrane filtration. Resour. Conserv. Recycl. 2005, 44, 185–196. [Google Scholar] [CrossRef]
- Ciabattia, I.; Cesaro, F.; Faralli, L.; Fatarella, E.; Tognotti, F. Demonstration of a treatment system for purification and reuse of laundry wastewater. Desalination 2009, 245, 451–459. [Google Scholar] [CrossRef]
- Punzi, M.; Nilsson, F.; Anbalagan, A.; Svensson, B.M.; Jönsson, K.; Mattiasson, B.; Jonstrup, M. Combined anaerobic–ozonation process for treatment of textile wastewater: Removal of acute toxicity and mutagenicity. J. Hazard. Mater. 2015, 292, 52–60. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef]
- Parsa, J.B.; Golmirzaei, M.; Abbasi, M. Degradation of azo dye CI Acid Red 18 in aqueous solution by ozone-electrolysis process. J. Ind. Eng. Chem. 2014, 20, 689–694. [Google Scholar] [CrossRef]
- Kishimoto, K.; Nakagawa, T.; Asano, M.; Abe, M.; Yamada, M.; Ono, Y. Ozonation combined with electrolysis of 1, 4-dioxane using a two-compartment electrolytic flow cell with solid electrolyte. Water Res. 2008, 42, 379–385. [Google Scholar] [CrossRef]
- Acero, J.L.; Haderlein, S.B.; Schmidt, T.C.; Suter, M.J.F.; Gunten, U. MTBE oxidation by conventional ozonation and the combination ozone/hydrogen peroxide: Efficiency of the processes and bromate formation. Environ. Sci. Technol. 2001, 35, 4252–4259. [Google Scholar] [CrossRef]
- Tachikawa, M.; Yamanaka, K. Synergistic disinfection and removal of biofilms by a sequential two-step treatment with ozone followed by hydrogen peroxide. Water Res. 2014, 64, 94–101. [Google Scholar] [CrossRef]
- Gliniak, M.; Grabowski, Ł.; Wołosiewicz-Głąb, M.; Polek, D. Influence of ozone aeration on toxic metal content and oxygen activity in green waste compost. J. Ecol. Eng. 2017, 18, 90–94. [Google Scholar] [CrossRef]
- Gliniak, M.; Grabowski, Ł.; Polek, D. Ozone aeration impact on the maturation phase in the process of green waste composting, Contemporary Research Trends in Agricultural Engineering. In BIO Web of Conferences; EDP Sciences: Paris, France, 2018; Volume 10, p. 01005. [Google Scholar]
- Gliniak, M.; Lis, A.; Polek, D.; Wołosiewicz-Głąb, M. Advanced oxidation treatment of composting leachate of food solid waste by ozone-hydrogen peroxide. J. Ecol. Eng. 2019, 20, 203–208. [Google Scholar] [CrossRef]
- Merenyi, G.; Lind, J.; Naumov, S.; Sonntag, C.V. Reaction of ozone with hydrogen peroxide (peroxone process): A revision of current mechanistic concepts based on thermokinetic and quantum-chemical considerations. Environ. Sci. Technol. 2010, 44, 3505–3507. [Google Scholar] [CrossRef] [PubMed]
- Prousek, J. Advanced oxidation processes for water treatment. Chemical processes. Chem. List 1996, 90, 229–237. [Google Scholar]
- Turkay, O.; Barışçı, S.; Sillanpää, M. E-peroxone process for the treatment of laundry wastewater: A case study. J. Environ. Chem. Eng. 2017, 5, 4282–4290. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Lu, G.; Lin, Q.; Wei, L.; Zhang, P. Removal of Aqueous Para-Aminobenzoic Acid Using a Compartmental Electro-Peroxone Process. Water 2021, 13, 2961. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, S.; Zhan, J.; Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. Mechanisms of enhanced total organic carbon elimination from oxalic acid solutions by electro-peroxone process. Water Res. 2015, 80, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bakheet, B.; Yuan, S.; Li, X.; Yu, G.; Murayama, S.; Wang, Y. Kinetics and energy efficiency for the degradation of 1, 4-dioxane by electro-peroxone process. J. Hazard. Mater. 2015, 294, 90–98. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.; Wang, Y. Effective degradation of methylene blue by a novel electrochemically driven process. Electrochem. Commun. 2013, 29, 48–51. [Google Scholar] [CrossRef]
- Bakheet, B.; Yuan, S.; Li, Z.; Wang, H.; Zuo, J.; Komarneni, S.; Wang, Y. Electro-peroxone treatment of Orange II dye wastewater. Water Res. 2013, 47, 6234–6243. [Google Scholar] [CrossRef]
- Yao, W.; Wang, X.; Yang, H.; Yu, G.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Removal of pharmaceuticals from secondary effluents by an electro-peroxone process. Water Res. 2016, 88, 826–835. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Q.L.; Zhou, X.J.; Cao, H.O.; Du, J.S.; Yin, R.L.; Ren, N.Q. Enhanced amoxicillin treatment using the electro-peroxone process: Key factors and degradation mechanism. RSC Adv. 2015, 5, 52695–52702. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Demeestere, K.; Díaz-Cruz, M.S.; Barceló, D. Ozonation and peroxone oxidation of benzophenone-3 in water: Effect of operational parameters and identification of intermediate products. Sci. Total Environ. 2013, 443, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, C.; Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2012. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).