The Use of Zebra Mussel (Dreissena polymorpha) as a Sentinel Species for the Microplastic Pollution of Freshwater: The Case of Beyhan Dam Lake, Turkey
Abstract
1. Introduction
2. Materials and Methods
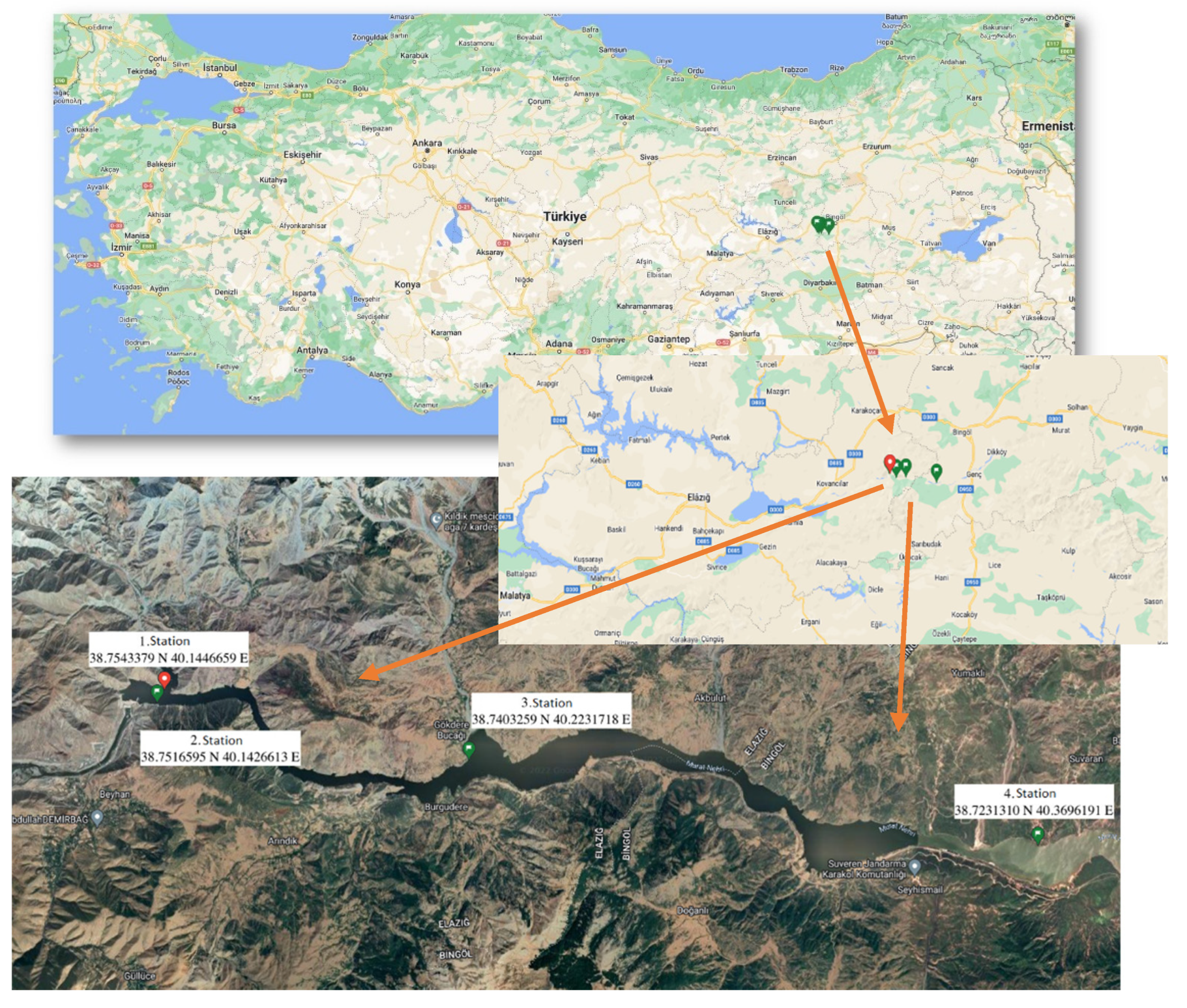
2.1. Study Area
2.2. Sampling
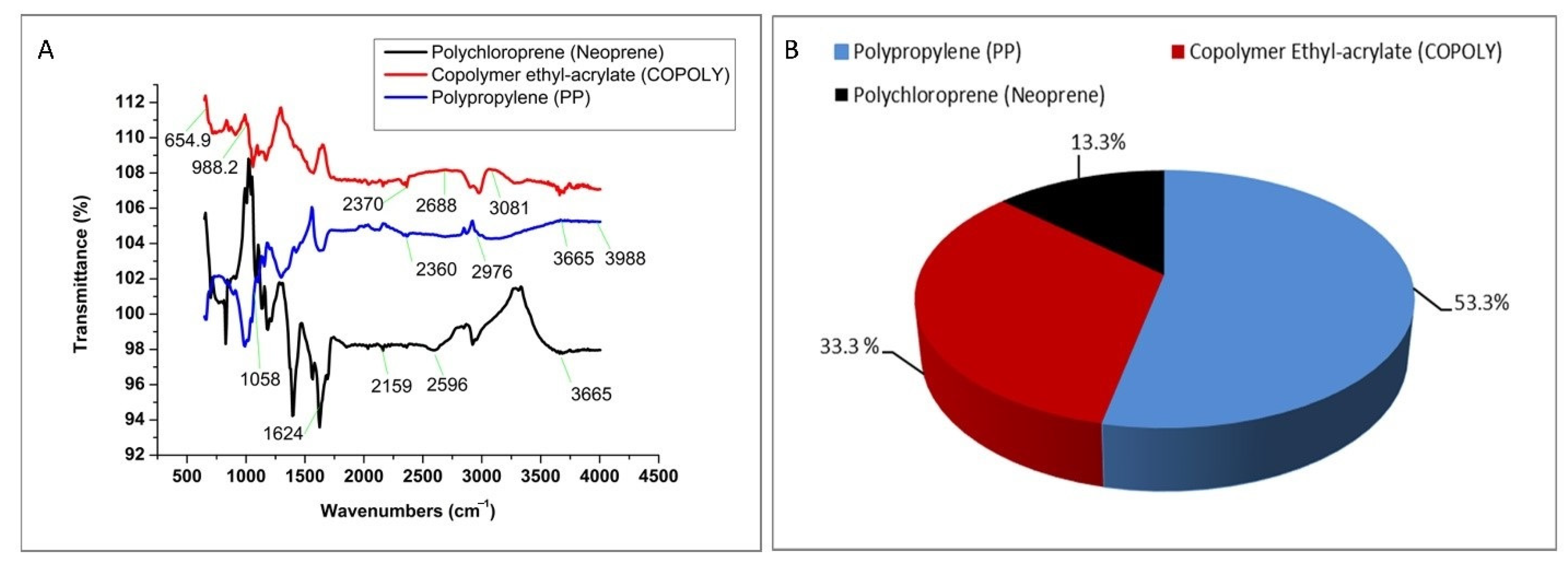
2.3. Observation and Validation of Microplastic
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atamanalp, M.; Kokturk, M.; Kırıcı, M.; Ucar, A.; Kırıcı, M.; Parlak, V.; Aydın, A.; Alak, G. Interaction of Microplastic Presence and Oxidative Stress in Freshwater Fish: A Regional Scale Research, East Anatolia of Türkiye (Erzurum Erzincan Bingöl). Sustainability 2022, 14, 12009. [Google Scholar] [CrossRef]
- Atamanalp, M.; Köktürk, M.; Parlak, V.; Ucar, A.; Arslan, G.; Alak, G. A new record for the presence of microplastics in dominant fish species of the Karasu River Erzurum, Turkey. Environ. Sci. Pollut. Res. 2021, 29, 7866–7876. [Google Scholar] [CrossRef] [PubMed]
- Atamanalp, M.; Köktürk, M.; Uçar, A.; Duyar, H.A.; Özdemir, S.; Parlak, V.; Esenbuğa, N.; Alak, G. Microplastics in tissues (brain, gill, muscle and gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch. Environ. Contam. Toxicol. 2021, 81, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Alak, G.; Uçar, A.; Parlak, V.; Atamanalp, M. Identification, characterisation of microplastic and their effects on aquatic organisms. Chem. Ecol. 2022, 38, 967–987. [Google Scholar] [CrossRef]
- Strungaru, S.A.; Jijie, R.; Nicoara, M.; Plavan, G.-I.; Faggio, C. Micro-(Nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. TrAC-Trends Anal. Chem. 2019, 110, 116–128. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, Z.; Hou, Y.; Lin, S.; Gao, L.; Wang, Z.; Bao, R.; Peng, L. Biodegradable and conventional microplastics posed similar toxicity to marine algae Chlorella vulgaris. Aquat. Toxicol. 2022, 244, 106097. [Google Scholar] [CrossRef]
- Provenza, F.; Rampih, D.; Pignattelli, S.; Pastorino, P.; Barceló, D.; Prearo, M.; Specchiulli, A.; Renzi, M. Mussel watch program for microplastics in the Mediterranean Sea: Identification of biomarkers of exposure using Mytilus galloprovincialis. Ecol. Indic. 2022, 142, 109212. [Google Scholar] [CrossRef]
- Kuşku, H. Biological control of invasive zebra mussel (Dreissena polymorpha) in a freshwater ecosystem through Potamon ibericum. Aquat. Res. 2022, 5, 11–19. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Anselmi, S.; Menconi, V.; Bertoli, M.; Dondo, A.; Pizzul, E.; Renzi, M. Use of the zebra mussel Dreissena polymorpha (Mollusca, Bivalvia) as a bioindicator of microplastics pollution in freshwater ecosystems: A case study from Lake Iseo (North Italy). Water 2021, 13, 434. [Google Scholar] [CrossRef]
- Hoellein, T.; Rovegno, C.; Uhrin, A.V.; Johnson, E.; Herring, C. Microplastics in invasive freshwater mussels (Dreissena sp.): Spatiotemporal variation and occurrence with chemical contaminants. Front. Mar. Sci. 2021, 8, 690401. [Google Scholar] [CrossRef]
- Binelli, A.; Della Torre, C.; Nigro, L.; Riccardi, N.; Magni, S. A realistic approach for the assessment of plastic contamination and its ecotoxicological consequences: A case study in the metropolitan city of Milan (N. Italy). Sci. Total Environ. 2022, 806, 150574. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Bonasoro, F.; Della Torre, C.; Parenti, C.C.; Maggioni, D.; Binelli, A. Plastics and biodegradable plastics: Ecotoxicity comparison between polyvinylchloride and Mater-Bi® micro-debris in a freshwater biological model. Sci. Total Environ. 2020, 720, 137602. [Google Scholar] [CrossRef]
- Berber, S.; Ateş, A.S.; Acar, S. Zebra midyesinin (Dreissena polymorpha’nın (Pallas, 1771)) Türkiye’nin bazı su kaynaklarında yaşayan dar kıskaçlı kerevitler üzerinde ilk olarak gözlenmesi. Su Ürünleri Derg. 2018, 35, 55–61. [Google Scholar]
- Serdar, O.; Yildirim, N.; Tatar, Ş.; Yildirim, N.C. Gadolinyumun Tatlı Su Omurgasızı Dreissena polymorpha Üzerindeki Biyokimyasal Etkileri. Int. J. Pure Appl. Sci. 2021, 7, 229–236. [Google Scholar] [CrossRef]
- Holt, E.A.; Miller, S.W. Bioindicators: Using organisms to measure. Nature 2011, 3, 8–13. [Google Scholar]
- Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.; Yeemin, T.; Zhang, J. Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci. Total Environ. 2021, 781, 146700. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Song, X.; Wu, X.; Song, X.; Zhang, Z. Oil extraction following digestion to separate microplastics from mussels. Chemosphere 2022, 289, 133187. [Google Scholar] [CrossRef] [PubMed]
- Joyce, P.W.; Falkenberg, L.J. Microplastic abundances in co-occurring marine mussels: Species and spatial differences. Reg. Stud. Mar. Sci. 2023, 57, 102730. [Google Scholar] [CrossRef]
- Hurt, R.; O’Reilly, C.M.; Perry, W.L. Microplastic prevalence in two fish species in two US reservoirs. Limnol. Oceanogr. Lett. 2020, 5, 147–153. [Google Scholar] [CrossRef]
- Ta, A.T.; Pupuang, P.; Babel, S.; Wang, L.P. Investigation of microplastic contamination in blood cockles and green mussels from selected aquaculture farms and markets in Thailand. Chemosphere 2022, 303, 134918. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, E.M.; Friberg, N.; Lusher, A.; Jacobsen, D.; Hurley, R.R. Anthropogenically impacted lake catchments in Denmark reveal low microplastic pollution. Environ. Sci. Pollut. Res. 2022, 29, 47726–47739. [Google Scholar] [CrossRef] [PubMed]
- Reguera, P.; Viñas, L.; Gago, J. Microplastics in wild mussels (Mytilus spp.) from the north coast of Spain. Sci. Mar. 2019, 83, 337–347. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Almeshal, W.; Takács, A.; Aradi, L.; Sandil, S.; Dobosy, P.; Záray, G. Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary). Environments 2022, 9, 122. [Google Scholar] [CrossRef]
- Berglund, E.; Fogelberg, V.; Nilsson, P.A.; Hollander, J. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Sci. Total Environ. 2019, 697, 134192. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Pe, E.O.L.; Mashar, A.; Wardiatno, Y. Microplastic distribution and abundance in cimandiri watershed flowing to Palabuhanratu Bay, Sukabumi, West Java, Indonesia. Aquac. Aquar. Conserv. Legis. 2020, 13, 657–668. [Google Scholar]
- Goswami, P.; Vinithkumar, N.V.; Dharani, G. First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar. Pollut. Bull. 2020, 155, 111163. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Jeckel, N.; Wagner, M. Combined effects of polystyrene microplastics and thermal stress on the freshwater mussel Dreissena polymorpha. Sci. Total Environ. 2020, 718, 137253. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, X.; Hu, M.; Fang, J.K.H.; Sokolova, I.M.; Huang, W.; Xu, E.G.; Wang, Y. Is microplastic an oxidative stressor? Evidence from a meta-analysis on bivalves. J. Hazard. Mater. 2022, 423, 127211. [Google Scholar] [CrossRef] [PubMed]
- Atici, A.A. The first evidence of microplastic uptake in natural freshwater mussel, Unio stevenianus from Karasu River, Turkey. Biomarkers 2022, 27, 118–126. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C.; Karaca, S. Fouling assemblage of benthic plastic debris collected from Mersin Bay, NE Levantine coast of Turkey. Mar. Pollut. Bull. 2017, 124, 147–154. [Google Scholar] [CrossRef]
- Dhivert, E.; Phuong, N.N.; Mourier, B.; Grosbois, C.; Gasperi, J. Microplastic trapping in dam reservoirs driven by complex hydrosedimentary processes (Villerest Reservoir, Loire River, France). Water Res. 2022, 225, 119187. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, W.; Hu, Y.; Zhang, J.; Shen, W. Horizontal and vertical distribution of microplastics in dam reservoir after impoundment. Sci. Total Environ. 2022, 832, 154962. [Google Scholar] [CrossRef]
- Yıldız, T.; Yıldız, C. Evaluation of Soma thermal power plant fly ash and polypropylene waste in the production of a new material. Pamukkale Univ. J. Eng. Sci. 2002, 9, 163–169. [Google Scholar]
- Gedik, K.; Eryaşar, A.R. Microplastic pollution profile of Mediterranean mussels (Mytilus galloprovincialis) collected along the Turkish coasts. Chemosphere 2020, 260, 127570. [Google Scholar] [CrossRef]
- Wakkaf, T.; El Zrelli, R.; Kedzierski, M.; Balti, R.; Shaiek, M.; Mansour, L.; Tlig-Zouari, S.; Bruzaud, S.; Rabaoui, L. Microplastics in edible mussels from a southern Mediterranean lagoon: Preliminary results on seawater-mussel transfer and implications for environmental protection and seafood safety. Mar. Pollut. Bull. 2020, 158, 111355. [Google Scholar] [CrossRef]

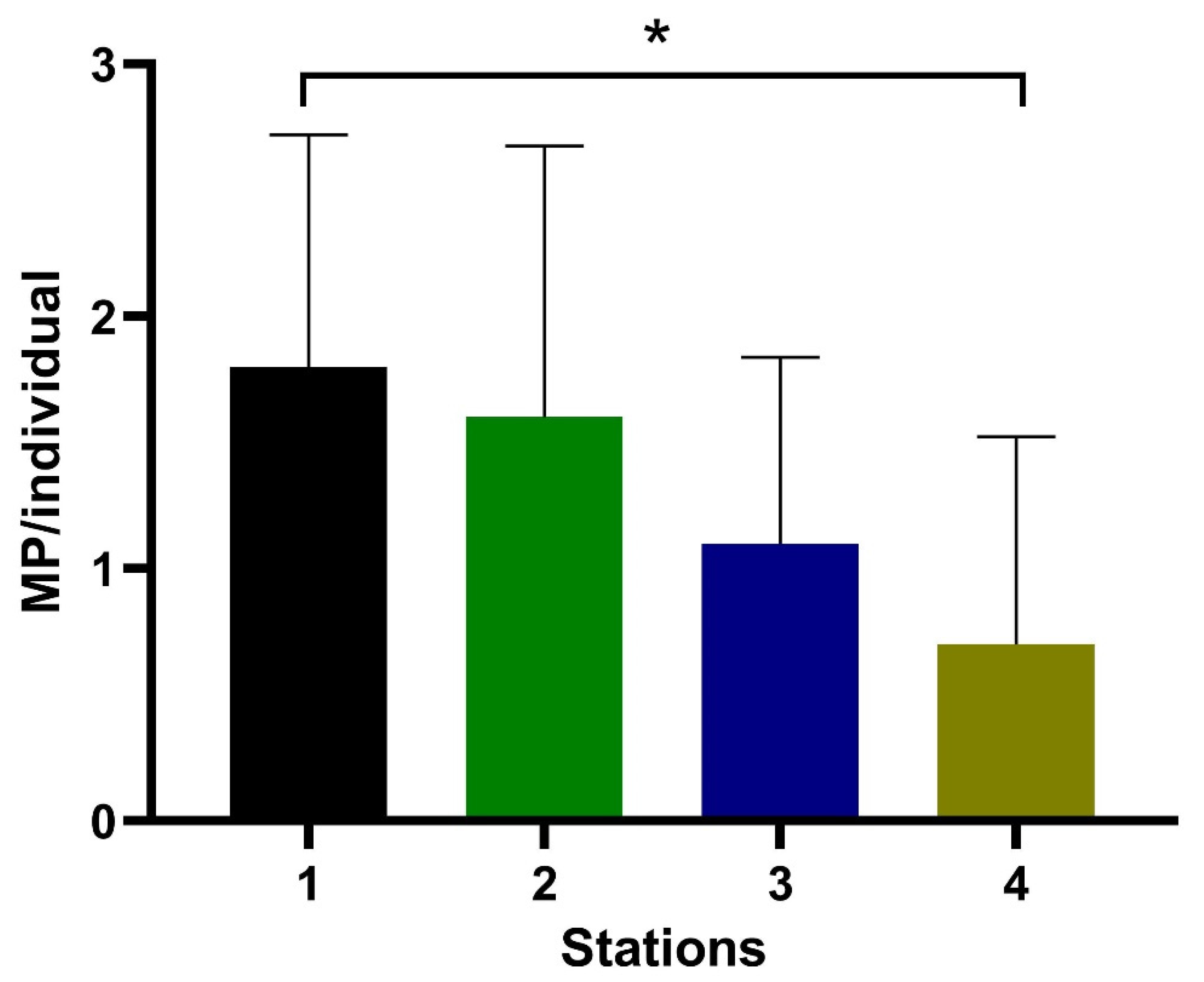
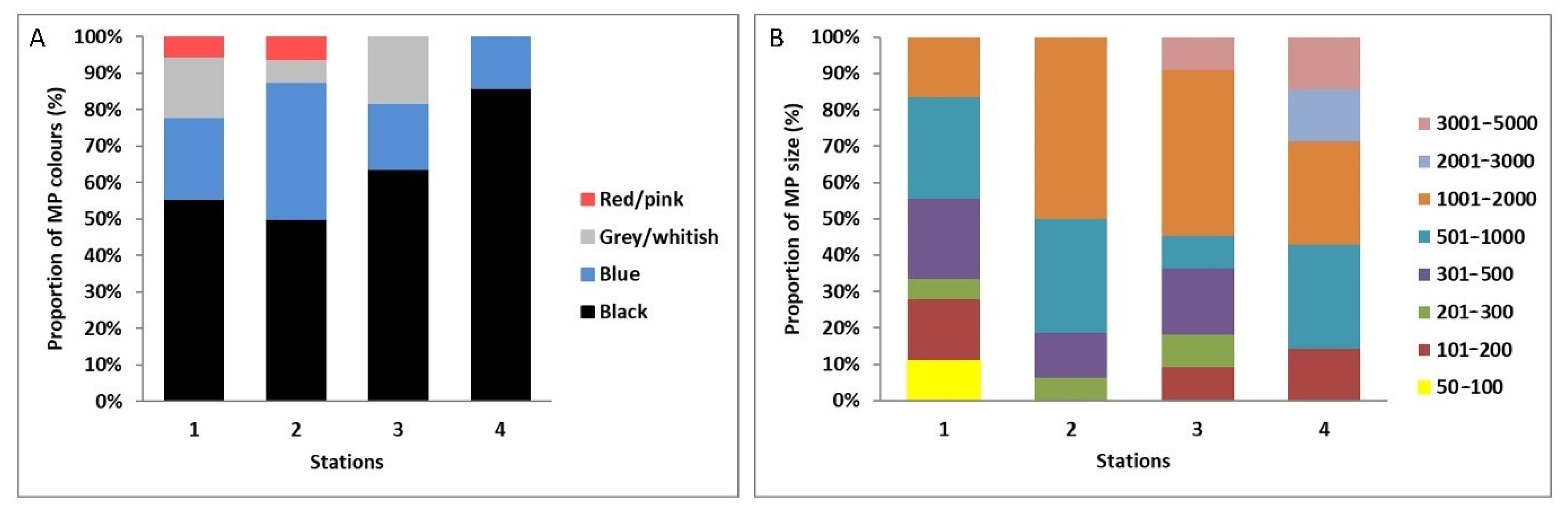
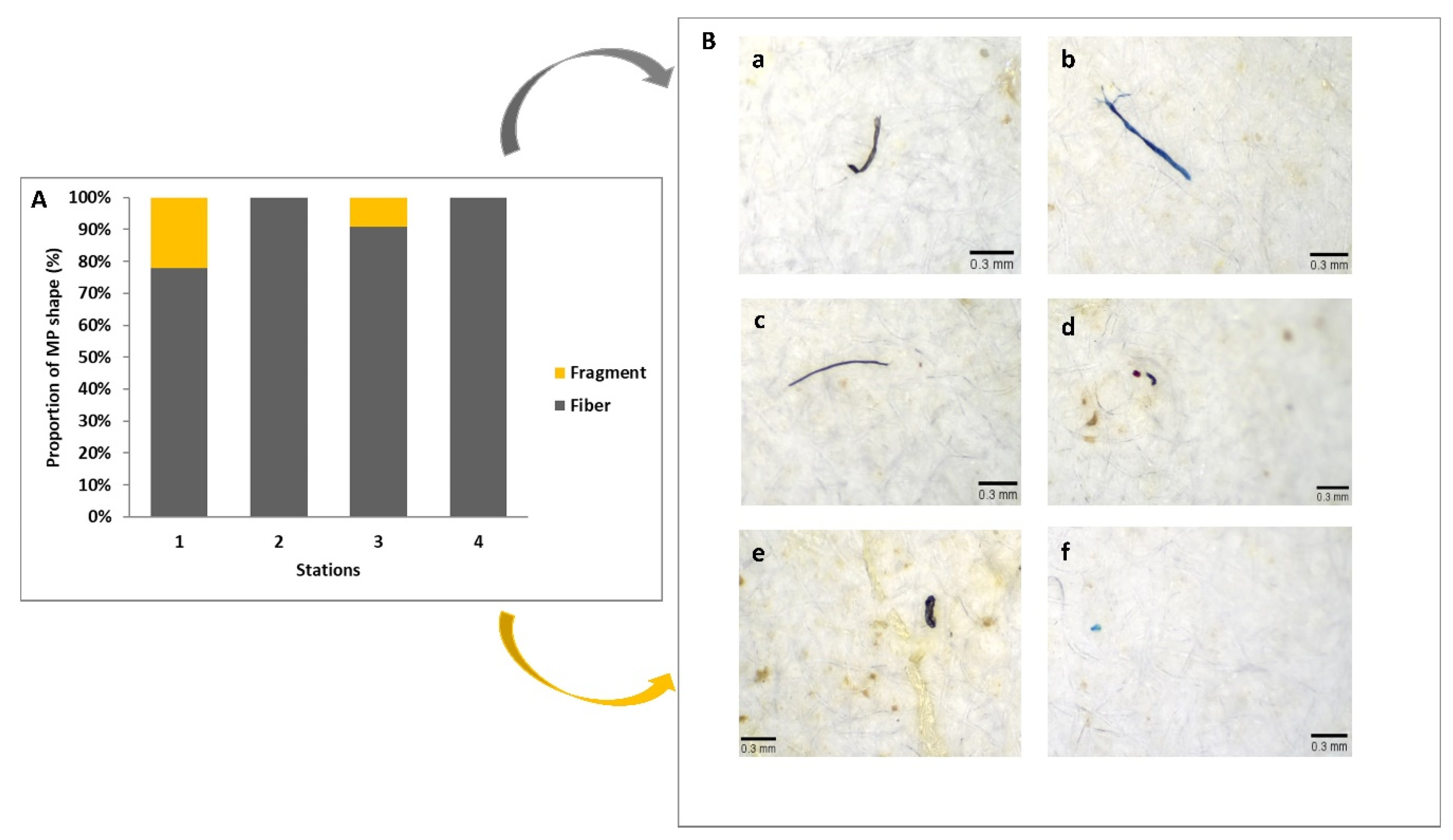
| D. polymorpha | Length (mm) | Tissues Weight (g) | MP/Individual | MP/g | Total MP |
|---|---|---|---|---|---|
| Station 1 | 155.89 ± 6.22 | 0.63 ± 0.21 | 1.80 ± 0.92 | 3.47 ± 2.62 | 18 |
| Station 2 | 154.84 ± 6.45 | 0.55 ± 0.19 | 1.60 ± 1.07 | 2.80 ± 2.17 | 16 |
| Station 3 | 153.92 ± 7.30 | 0.53 ± 0.21 | 1.10 ± 0.74 | 2.41 ± 2.31 | 11 |
| Station 4 | 154.16 ± 7.75 | 0.55 ± 0.24 | 0.70 ± 0.42 | 1.06 ± 0.97 | 7 |
| Length | Tissue Weight | MP/Individual | MP/g | ||
|---|---|---|---|---|---|
| Tissue weight | Pearson Correlation | 0.993 ** | |||
| Sig. (2-tailed) | 0.000 | ||||
| N | 12 | ||||
| MP/individual | Pearson Correlation | 0.890 ** | 0.895 ** | ||
| Sig. (2-tailed) | 0.000 | 0.000 | |||
| N | 12 | 12 | |||
| MP/g | Pearson Correlation | 0.890 ** | 0.895 ** | 0.969 ** | |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | ||
| N | 12 | 12 | 12 | ||
| Total MP | Pearson Correlation | 0.296 | 0.322 | 0.647 * | 0.569 |
| Sig. (2-tailed) | 0.351 | 0.307 | 0.023 | 0.053 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atamanalp, M.; Kokturk, M.; Gündüz, F.; Parlak, V.; Ucar, A.; Alwazeer, D.; Alak, G. The Use of Zebra Mussel (Dreissena polymorpha) as a Sentinel Species for the Microplastic Pollution of Freshwater: The Case of Beyhan Dam Lake, Turkey. Sustainability 2023, 15, 1422. https://doi.org/10.3390/su15021422
Atamanalp M, Kokturk M, Gündüz F, Parlak V, Ucar A, Alwazeer D, Alak G. The Use of Zebra Mussel (Dreissena polymorpha) as a Sentinel Species for the Microplastic Pollution of Freshwater: The Case of Beyhan Dam Lake, Turkey. Sustainability. 2023; 15(2):1422. https://doi.org/10.3390/su15021422
Chicago/Turabian StyleAtamanalp, Muhammed, Mine Kokturk, Fatih Gündüz, Veysel Parlak, Arzu Ucar, Duried Alwazeer, and Gonca Alak. 2023. "The Use of Zebra Mussel (Dreissena polymorpha) as a Sentinel Species for the Microplastic Pollution of Freshwater: The Case of Beyhan Dam Lake, Turkey" Sustainability 15, no. 2: 1422. https://doi.org/10.3390/su15021422
APA StyleAtamanalp, M., Kokturk, M., Gündüz, F., Parlak, V., Ucar, A., Alwazeer, D., & Alak, G. (2023). The Use of Zebra Mussel (Dreissena polymorpha) as a Sentinel Species for the Microplastic Pollution of Freshwater: The Case of Beyhan Dam Lake, Turkey. Sustainability, 15(2), 1422. https://doi.org/10.3390/su15021422









