Interactive Effects of Rising Temperature, Elevated CO2 and Herbivory on the Growth and Stoichiometry of a Submerged Macrophyte Vallisneria natans
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Measuring
2.3. Snail Measuring
2.4. Statistical Analysis
3. Results
3.1. Plant Growth
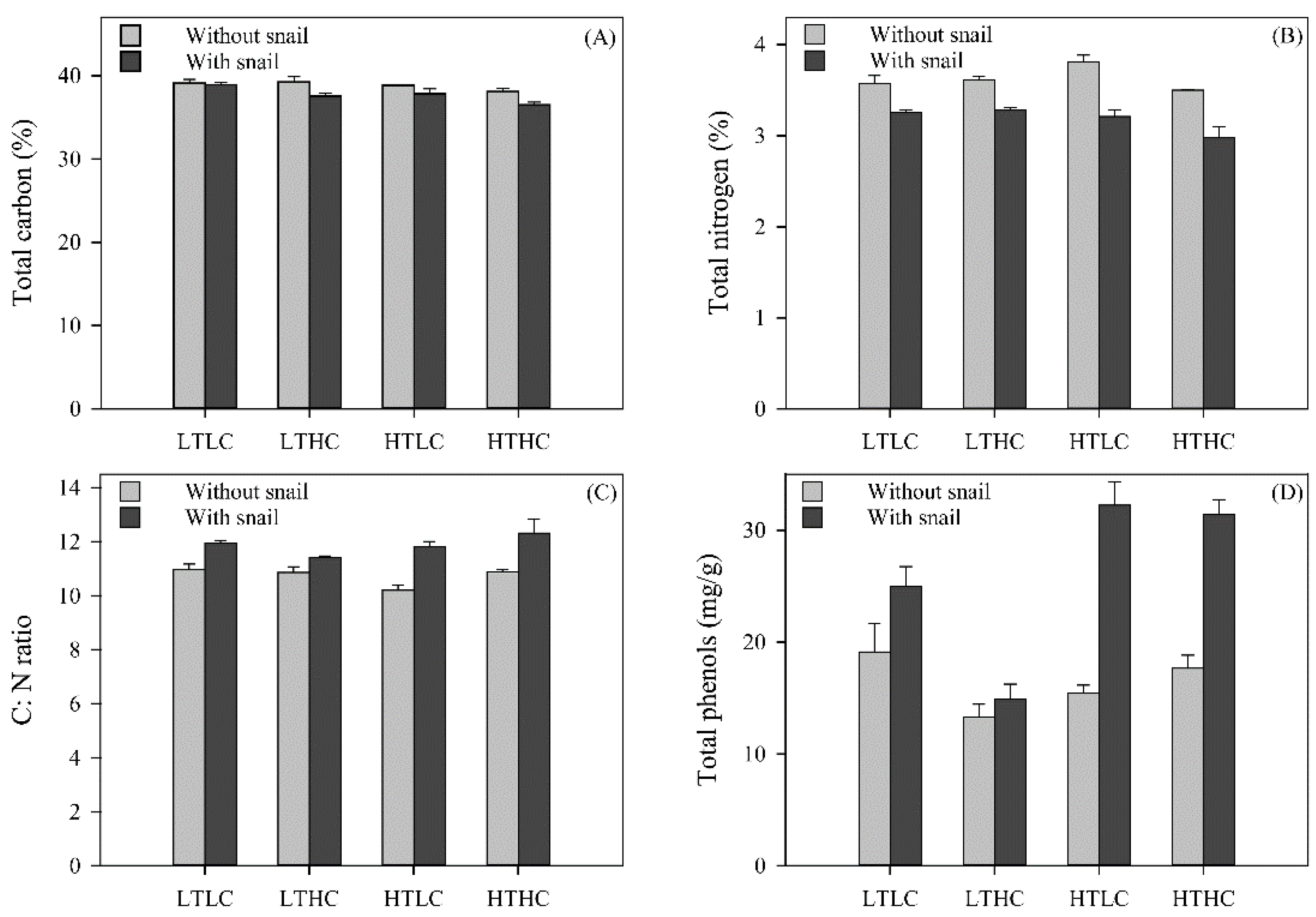
3.2. Plant Chemical Traits
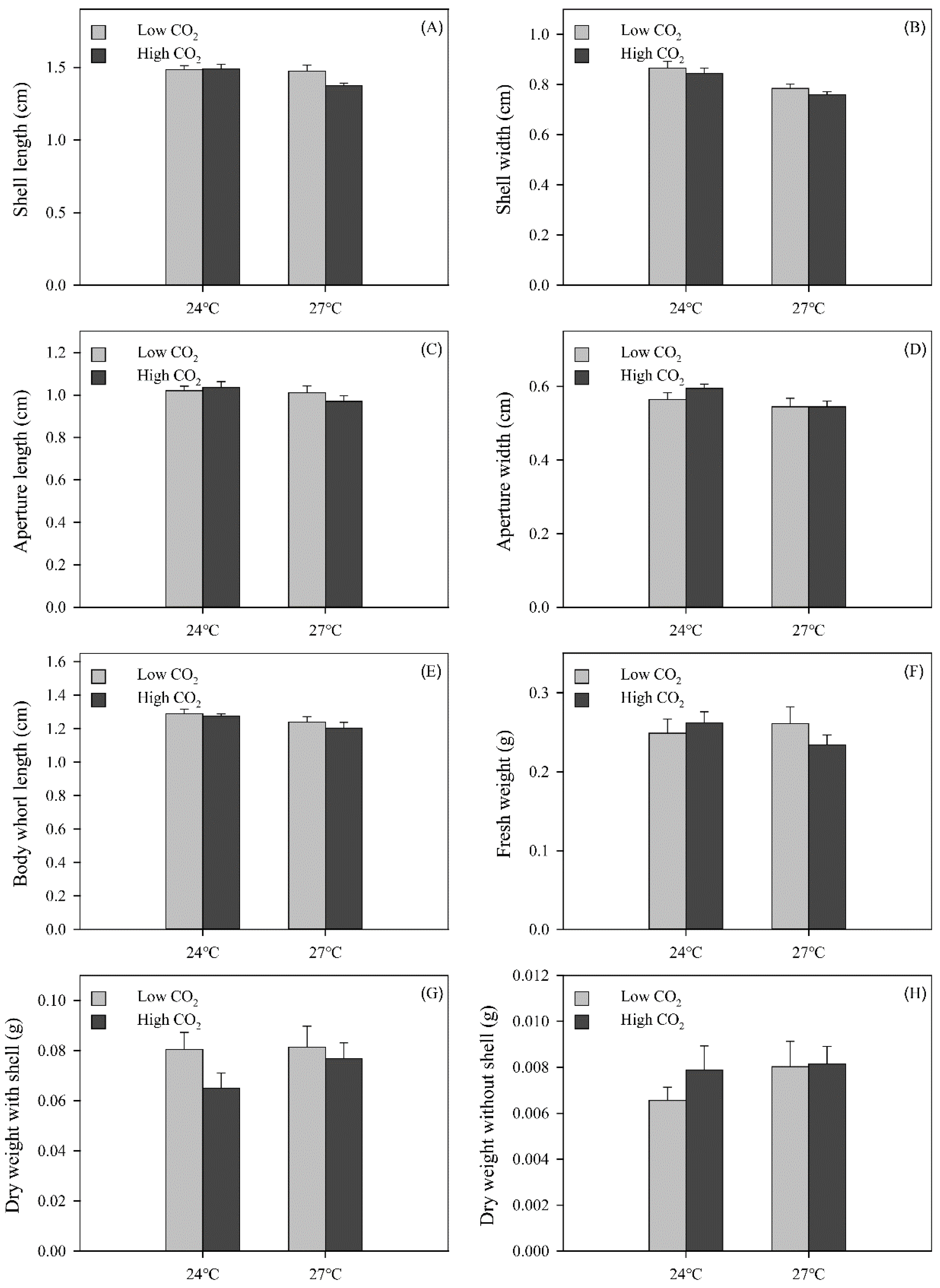
3.3. Snail Growth
3.4. Snail Chemical Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheffer, M. Ecology of Shallow Lakes; Kluwer Academic: Dordrecht, The Netherlands, 2004; ISBN 978-1-4020-2306-4. [Google Scholar]
- O’Hare, M.T.; Aguiar, F.C.; Asaeda, T.; Bakker, E.S.; Chambers, P.A.; Clayton, J.S.; Elger, A.; Ferreira, T.M.; Gross, E.M.; Gunn, I.D.M.; et al. Plants in Aquatic Ecosystems: Current Trends and Future Directions. Hydrobiologia 2018, 812, 1–11. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Lodge, D.M. Effects of Submersed Macrophytes on Ecosystem Processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M. Can Allelopathically Active Submerged Macrophytes Stabilise Clear-Water States in Shallow Lakes? Basic Appl. Ecol. 2008, 9, 422–432. [Google Scholar] [CrossRef]
- Jeppesen, E.; Lauridsen, T.L.; Kairesalo, T.; Perrow, M.R. Impact of Submerged Macrophytes on Fish-Zooplankton Interactions in Lakes. In The Structuring Role of Submerged Macrophytes in Lakes; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Ecological Studies; Springer: New York, NY, USA, 1998; pp. 91–114. ISBN 978-1-4612-0695-8. [Google Scholar]
- Phillips, G.; Willby, N.; Moss, B. Submerged Macrophyte Decline in Shallow Lakes: What Have We Learnt in the Last Forty Years? Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef]
- Short, F.T.; Kosten, S.; Morgan, P.A.; Malone, S.; Moore, G.E. Impacts of Climate Change on Submerged and Emergent Wetland Plants. Aquat. Bot. 2016, 135, 3–17. [Google Scholar] [CrossRef]
- Xing, W.; Wu, H.; Hao, B.; Liu, G. Stoichiometric Characteristics and Responses of Submerged Macrophytes to Eutrophication in Lakes along the Middle and Lower Reaches of the Yangtze River. Ecol. Eng. 2013, 54, 16–21. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The Role of CO2 Capture and Utilization in Mitigating Climate Change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change Summary for Policymakers. In Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1–30. ISBN 978-1-107-05799-9.
- Hasler, C.T.; Butman, D.; Jeffrey, J.D.; Suski, C.D. Freshwater Biota and Rising pCO2? Ecol. Lett. 2016, 19, 98–108. [Google Scholar] [CrossRef]
- California Ocean Protection Council; Phillips, J.; McKinley, G.; Bennington, V.; Bootsma, H.; Pilcher, D.; Sterner, R.; Urban, N. The Potential for CO2-Induced Acidification in Freshwater: A Great Lakes Case Study. Oceanography 2015, 25, 136–145. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Rooney, N.; Kalff, J. Inter-Annual Variation in Submerged Macrophyte Community Biomass and Distribution: The Influence of Temperature and Lake Morphometry. Aquat. Bot. 2000, 68, 321–335. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied Attack: Climate Change and Eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Hosokawa, S. Increasing Temperature Induces Shorter Leaf Life Span in an Aquatic Plant. Oikos 2009, 118, 1158–1163. [Google Scholar] [CrossRef]
- Olesen, B.; Madsen, T.V. Growth and Physiological Acclimation to Temperature and Inorganic Carbon Availability by Two Submerged Aquatic Macrophyte Species, Callitriche cophocarpa and Elodea canadensis. Funct. Ecol. 2000, 14, 252–260. [Google Scholar] [CrossRef]
- Zhang, P.; Blonk, B.A.; Berg, R.F.V.D.; Bakker, E.S. The Effect of Temperature on Herbivory by the Omnivorous Ectotherm Snail Lymnaea stagnalis. Hydrobiologia 2018, 812, 147–155. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Mello, F.T.; Declerck, S.A.J.; Meester, L.D.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of Climate Warming on Lake Fish Community Structure and Potential Effects on Ecosystem Function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global Warming Benefits the Small in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- Lindmark, M.; Huss, M.; Ohlberger, J.; Gårdmark, A. Temperature-Dependent Body Size Effects Determine Population Responses to Climate Warming. Ecol. Lett. 2018, 21, 181–189. [Google Scholar] [CrossRef]
- Hessen, D.O.; Daufresne, M.; Leinaas, H.P. Temperature-size Relations from the Cellular-genomic Perspective. Biol. Rev. Camb. Philos. Soc. 2013, 88, 476–489. [Google Scholar] [CrossRef]
- Yan, X.; Yu, D.; Li, Y. The Effects of Elevated CO2 on Clonal Growth and Nutrient Content of Submerge Plant Vallisneria spinulosa. Chemosphere 2006, 62, 595–601. [Google Scholar] [CrossRef]
- Cao, J.; Ruan, H. Responses of the Submerged Macrophyte Vallisneria natans to Elevated CO2 and Temperature. Aquat. Biol. 2015, 23, 119–127. [Google Scholar] [CrossRef]
- Malheiro, A.C.E.; Jahns, P.; Hussner, A. CO2 Availability Rather than Light and Temperature Determines Growth and Phenotypical Responses in Submerged Myriophyllum aquaticum. Aquat. Bot. 2013, 110, 31–37. [Google Scholar] [CrossRef]
- Vadstrup, M.; Madsen, T.V. Growth Limitation of Submerged Aquatic Macrophytes by Inorganic Carbon. Freshw. Biol. 1995, 34, 411–419. [Google Scholar] [CrossRef]
- Titus, J.E.; Andorfer, J.H. Effects of CO2 Enrichment on Mineral Accumulation and Nitrogen Relations in a Submersed Macrophyte. Freshw. Biol. 1996, 36, 661–671. [Google Scholar] [CrossRef]
- Blinn, D.W.; Sanderson, M.W. Aquatic Insects in Montezuma Well, Arizona, USA: A Travertine Spring Mound with High Alkalinity and Dissolved Carbon Dioxide. Gt. Basin Nat. 1989, 49, 7. [Google Scholar]
- O’Brien, C.D.; Blinn, D.W. The Endemic Spring Snail Pyrgulopsis montezumensis in a High CO2 Environment: Importance of Extreme Chemical Habitats as Refugia. Freshw. Biol. 1999, 42, 225–234. [Google Scholar] [CrossRef]
- Urabe, J.; Togari, J.; Elser, J.J. Stoichiometric Impacts of Increased Carbon Dioxide on a Planktonic Herbivore. Glob. Chang. Biol. 2003, 9, 818–825. [Google Scholar] [CrossRef]
- Bakker, E.S.; Wood, K.A.; Pagès, J.F.; Veen, G.F.C.; Christianen, M.J.A.; Santamaría, L.; Nolet, B.A.; Hilt, S. Herbivory on Freshwater and Marine Macrophytes: A Review and Perspective. Aquat. Bot. 2016, 135, 18–36. [Google Scholar] [CrossRef]
- Chilton, E.W.; Muoneke, M.I. Biology and Management of Grass Carp (Ctenopharyngodon idella, Cyprinidae) for Vegetation Control: A North American Perspective. Rev. Fish Biol. Fish. 2004, 2, 283–320. [Google Scholar] [CrossRef]
- Tomas, F.; Turon, X.; Romero, J. Seasonal and Small-Scale Spatial Variability of Herbivory Pressure on the Temperate Seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2005, 301, 95–107. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Herman, P.M.J.; Bouma, T.J.; Lamers, L.P.M.; van Katwijk, M.M.; van der Heide, T.; Mumby, P.J.; Silliman, B.R.; Engelhard, S.L.; van de Kerk, M.; et al. Habitat Collapse Due to Overgrazing Threatens Turtle Conservation in Marine Protected Areas. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132890. [Google Scholar] [CrossRef]
- Bakker, E.S.; Pagès, J.F.; Arthur, R.; Alcoverro, T. Assessing the Role of Large Herbivores in the Structuring and Functioning of Freshwater and Marine Angiosperm Ecosystems. Ecography 2016, 39, 162–179. [Google Scholar] [CrossRef]
- Elger, A.; Barrat-Segretain, M.-H.; Amoros, C. Plant Palatability and Disturbance Level in Aquatic Habitats: An Experimental Approach Using the Snail Lymnaea stagnalis (L.). Freshw. Biol. 2002, 47, 931–940. [Google Scholar] [CrossRef]
- Elger, A.; Lemoine, D.G. Determinants of Macrophyte Palatability to the Pond Snail Lymnaea stagnalis. Freshw. Biol. 2005, 50, 86–95. [Google Scholar] [CrossRef]
- Fornoff, F.; Gross, E.M. Induced Defense Mechanisms in an Aquatic Angiosperm to Insect Herbivory. Oecologia 2013, 175, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.G.; Barrat-Segretain, M.-H.; Roy, A. Morphological and Chemical Changes Induced by Herbivory in Three Common Aquatic Macrophytes. Int. Rev. Hydrobiol. 2009, 94, 282–289. [Google Scholar] [CrossRef]
- Rosenblatt, A.E.; Schmitz, O.J. Interactive Effects of Multiple Climate Change Variables on Trophic Interactions: A Meta-Analysis. Clim. Chang. Responses 2014, 1, 8. [Google Scholar] [CrossRef]
- Shaw, M.R.; Zavaleta, E.S.; Chiariello, N.R.; Cleland, E.E.; Mooney, H.; Field, C.B. Grassland Responses to Global Environmental Changes Suppressed by Elevated CO2. Science 2002, 298, 1987–1990. [Google Scholar] [CrossRef]
- Luo, Y.; Gerten, D.; le Maire, G.; Parton, W.J.; Weng, E.; Zhou, X.; Keough, C.; Beier, C.; Ciais, P.; Cramer, W.; et al. Modeled Interactive Effects of Precipitation, Temperature, and [CO2] on Ecosystem Carbon and Water Dynamics in Different Climatic Zones. Glob. Chang. Biol. 2008, 14, 1986–1999. [Google Scholar] [CrossRef]
- Rosenblatt, A.E.; Schmitz, O.J. Climate Change, Nutrition, and Bottom-Up and Top-Down Food Web Processes. Trends Ecol. Evol. 2016, 31, 965–975. [Google Scholar] [CrossRef]
- He, L.; Zhu, T.; Wu, Y.; Li, W.; Zhang, H.; Zhang, X.; Cao, T.; Ni, L.; Hilt, S. Littoral Slope, Water Depth and Alternative Response Strategies to Light Attenuation Shape the Distribution of Submerged Macrophytes in a Mesotrophic Lake. Front. Plant Sci. 2019, 10, 169. [Google Scholar] [CrossRef]
- Stift, M.; Michel, E.; Sitnikova, T.Y.; Mamonova, E.Y.; Sherbakov, D.Y. Palaearctic Gastropod Gains a Foothold in the Dominion of Endemics: Range Expansion and Morphological Change of Lymnaea (Radix) auricularia in Lake Baikal. Hydrobiologia 2004, 513, 101–108. [Google Scholar] [CrossRef]
- McCarthy, T.M.; Fisher, W.A. Multiple Predator-avoidance Behaviours of the Freshwater Snail Physella heterostropha pomila: Responses Vary with Risk. Freshw. Biol. 2000, 44, 387–397. [Google Scholar] [CrossRef]
- Xiong, W.; Yu, D.; Wang, Q.; Liu, C.; Wang, L. A Snail Prefers Native over Exotic Freshwater Plants: Implications for the Enemy Release Hypotheses. Freshw. Biol. 2008, 53, 2256–2263. [Google Scholar] [CrossRef]
- Fang, L.; Wong, P.K.; Lin, L.; Lan, C.; Qiu, J.W. Impact of Invasive Apple Snails in Hong Kong on Wetland Macrophytes, Nutrients, Phytoplankton and Filamentous Algae. Freshw. Biol. 2010, 55, 1191–1204. [Google Scholar] [CrossRef]
- Dong, B.-C.; Fu, T.; Luo, F.-L.; Yu, F. Herbivory-Induced Maternal Effects on Growth and Defense Traits in the Clonal Species Alternanthera philoxeroides. Sci. Total Environ. 2017, 605–606, 114–123. [Google Scholar] [CrossRef]
- Ojala, A.; Kankaala, P.; Tulonen, T. Growth Response of Equisetum Fluviatile to Elevated CO2 and Temperature. Environ. Exp. Bot. 2002, 47, 157–171. [Google Scholar] [CrossRef]
- Bianchini, I.; da Cunha-Santino, M.B.; Milan, J.A.M.; Rodrigues, C.J.; Dias, J.H.P. Growth of Hydrilla verticillata (L.f.) Royle under Controlled Conditions. Hydrobiologia 2010, 644, 301–312. [Google Scholar] [CrossRef]
- Cleland, E.E.; Peters, H.A.; Mooney, H.; Field, C.B. Gastropod Herbivory in Response to Elevated CO2 and N Addition Impacts Plant Community Composition. Ecology 2006, 87, 686–694. [Google Scholar] [CrossRef]
- Strauss; Agrawal The Ecology and Evolution of Plant Tolerance to Herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [CrossRef]
- Gassmann, A.J. Effect of Photosynthetic Efficiency and Water Availability on Tolerance of Leaf Removal in Amaranthus hybridus. J. Ecol. 2004, 92, 882–892. [Google Scholar] [CrossRef]
- Cripps, M.; Edwards, G.R.; Bourdôt, G.W.; Saville, D.J.; Hinz, H.L.; Fowler, S.V. Effects of Pasture Competition and Specialist Herbivory on the Performance of Cirsium arvense. Biocontrol Sci. Technol. 2010, 20, 641–656. [Google Scholar] [CrossRef]
- Cargill, S.M.; Jefferies, R.L. The Effects of Grazing by Lesser Snow Geese on the Vegetation of a Sub- Arctic Salt Marsh. J. Appl. Ecol. 1984, 21, 669–686. [Google Scholar] [CrossRef]
- Huang, W.; Siemann, E.; Wheeler, G.S.; Zou, J.; Carrillo, J.; Ding, J. Resource Allocation to Defence and Growth Are Driven by Different Responses to Generalist and Specialist Herbivory in an Invasive Plant. J. Ecol. 2010, 98, 1157–1167. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced Plant Responses to Herbivory. Annu. Rev. Ecol. Evol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Cebrian, J.; Duarte, C.M. Patterns in Leaf Herbivory on Seagrasses. Aquat. Bot. 1998, 60, 67–82. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Jermy, T.; van Loon, J.J.A. Insect-Plant Biology: From Physiology to Evolution; Chapman & Hall: London, UK, 1998; ISBN 978-0-412-58700-9. [Google Scholar]
- Agrawal, A.A.; Weber, M.G. On the Study of Plant Defence and Herbivory Using Comparative Approaches: How Important Are Secondary Plant Compounds. Ecol. Lett. 2015, 18, 985–991. [Google Scholar] [CrossRef]
- Zhang, P.; Grutters, B.M.C.; van Leeuwen, C.H.A.; Xu, J.; Petruzzella, A.; van den Berg, R.F.; Bakker, E.S. Effects of Rising Temperature on the Growth, Stoichiometry, and Palatability of Aquatic Plants. Front. Plant Sci. 2019, 9, 1947. [Google Scholar] [CrossRef]
- Jørgensen, S.E.; Fath, B.D. Encyclopedia of Ecology, 2nd ed; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-444-64130-4. [Google Scholar]
- Lodge, D.M. Herbivory on Freshwater Macrophytes. Aquat. Bot. 1991, 41, 195–224. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R. Carbon/Nutrient Balance of Boreal Plants in Relation to Vertebrate Herbivory. Oikos 1983, 40, 357. [Google Scholar] [CrossRef]
- Barton, A.W.; Hales, B.R.; Waldbusser, G.G.; Langdon, C.; Feely, R.A. The Pacific Oyster, Crassostrea Gigas, Shows Negative Correlation to Naturally Elevated Carbon Dioxide Levels: Implications for Near-term Ocean Acidification Effects. Limnol. Oceanogr. 2012, 57, 698–710. [Google Scholar] [CrossRef]
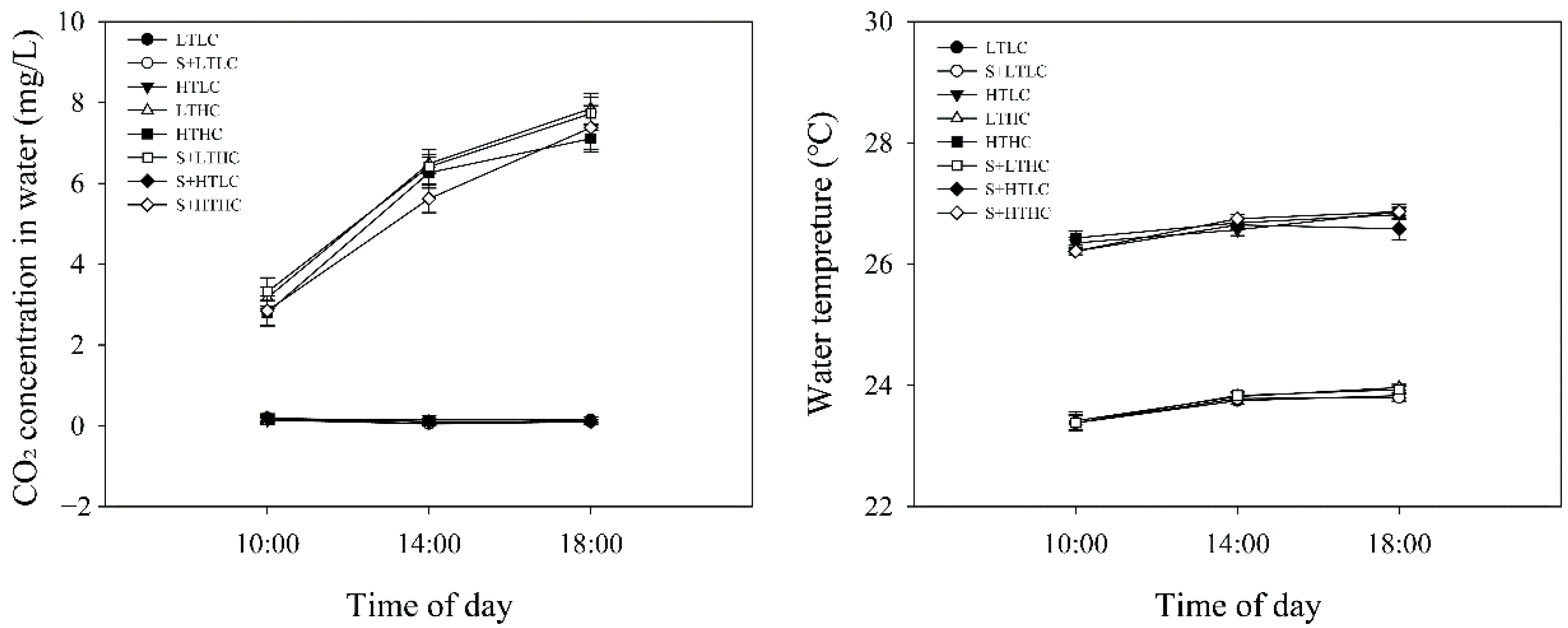


| T (°C) | DO (mg/L) | Cond (μS/cm) | TDS (mg/L) | Sal (‰) | pH | Illumination (Lx) | |
|---|---|---|---|---|---|---|---|
| LTLC | 23.66 ± 0.07 | 4.57 ± 0.03 | 348.82 ± 1.69 | 233.53 ± 1.32 | 0.17 | 7.85 ± 0.03 | 451.2 ± 3.44 |
| S + LTLC | 23.66 ± 0.07 | 4.43 ± 0.03 | 357.07 ± 2.01 | 234.91 ± 0.69 | 0.17 | 7.80 ± 0.03 | 449.33 ± 2.11 |
| LTHC | 23.73 ± 0.08 | 4.46 ± 0.02 | 359.30 ± 2.67 | 234.36 ± 0.93 | 0.17 | 7.41 ± 0.02 | 449.17 ± 4.43 |
| S + LTHC | 23.72 ± 0.08 | 4.39 ± 0.02 | 359.40 ± 2.64 | 234.70 ± 0.86 | 0.17 | 7.39 ± 0.02 | 447.00 ± 4.14 |
| HTLC | 26.59 ± 0.08 | 4.35 ± 0.04 | 365.65 ± 2.81 | 236.36 ± 0.93 | 0.17 | 7.84 ± 0.02 | 445.00 ± 3.14 |
| S + HTLC | 26.48 ± 0.08 | 4.38 ± 0.03 | 361.96 ± 2.04 | 236.15 ± 1.03 | 0.17 | 7.75 ± 0.01 | 458.83 ± 3.50 |
| HTHC | 26.64 ± 0.06 | 4.38 ± 0.02 | 372.32 ± 4.02 | 239.76 ± 1.17 | 0.18 | 7.46 ± 0.02 | 446.17 ± 3.23 |
| S + HTHC | 26.61 ± 0.08 | 4.37 ± 0.03 | 371.59 ± 2.93 | 237.68 ± 1.24 | 0.18 | 7.46 ± 0.02 | 446.67 ± 2.30 |
| Source | d.f. | Snail Herbivory (s) | CO2 Concentration (c) | Temperature (T) | S × C | S × T | C × T | S × C × T | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | p | F | p | F | p | F | p | F | p | F | p | ||
| Leaves number | 1.56 | 43.965 | <0.001 | 5.495 | 0.023 | 73.494 | <0.001 | 13.569 | 0.001 | 5.186 | 0.027 | 11.214 | 0.001 | 4.593 | 0.036 |
| Total biomass | 1.56 | 67.819 | <0.001 | 0.066 | 0.798 | 54.247 | <0.001 | 12.165 | 0.001 | 18.724 | <0.001 | 4.817 | 0.032 | 2.412 | 0.126 |
| Shoot biomass | 1.56 | 62.419 | <0.001 | 0.043 | 0.836 | 53.276 | <0.001 | 13.199 | 0.001 | 16.323 | <0.001 | 4.396 | 0.041 | 2.397 | 0.127 |
| Root biomass | 1.56 | 51.296 | <0.001 | 0.400 | 0.530 | 15.331 | <0.001 | 0.001 | 0.974 | 24.505 | <0.001 | 4.025 | 0.050 | 0.527 | 0.471 |
| Root: shoot ratio | 1.56 | 36.481 | <0.001 | 1.648 | 0.205 | 32.937 | <0.001 | 23.387 | <0.001 | 3.219 | 0.078 | 4.569 | 0.037 | 0.019 | 0.889 |
| Relative growth rate | 1.56 | 116.149 | <0.001 | 3.300 | 0.075 | 65.411 | <0.001 | 17.651 | <0.001 | 8.184 | 0.006 | 13.879 | <0.001 | 3.943 | 0.052 |
| Total carbon | 1.32 | 14.377 | 0.001 | 8.240 | 0.007 | 9.091 | 0.005 | 3.450 | 0.072 | 0.289 | 0.594 | 0.539 | 0.468 | 0.627 | 0.434 |
| Total nitrogen | 1.32 | 88.467 | <0.001 | 6.365 | 0.017 | 1.437 | 0.239 | 0.106 | 0.747 | 6.240 | 0.018 | 10.404 | 0.003 | 0.265 | 0.611 |
| C: N ratio | 1.32 | 46.716 | <0.001 | 0.704 | 0.408 | 0.001 | 0.982 | 0.764 | 0.389 | 4.983 | 0.033 | 7.353 | 0.011 | 0.167 | 0.685 |
| Total phenols | 1.32 | 132.402 | <0.001 | 14.796 | 0.001 | 63.675 | <0.001 | 6.997 | 0.013 | 41.857 | <0.001 | 31.596 | <0.001 | 2.918 | 0.097 |
| Source | d.f. | Temperature (T) | CO2 Concentration (C) | T × C | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Shell length | 1.36 | 4.371 | 0.044 | 2.524 | 0.121 | 3.084 | 0.088 |
| Shell width | 1.36 | 20.763 | <0.001 | 0.831 | 0.368 | 0.017 | 0.897 |
| Aperture length | 1.36 | 1.866 | 0.180 | 0.207 | 0.652 | 1.004 | 0.323 |
| Aperture width | 1.36 | 3.938 | 0.055 | 0.723 | 0.401 | 0.723 | 0.401 |
| Body whorl length | 1.36 | 4.524 | 0.040 | 0.785 | 0.381 | 0.126 | 0.725 |
| Fresh weight | 1.36 | 0.404 | 0.529 | 0.331 | 0.569 | 1.855 | 0.182 |
| Dry weight with shell | 1.36 | 0.831 | 0.368 | 2.804 | 0.157 | 0.624 | 0.435 |
| Dry weight without shell | 1.36 | 0.933 | 0.341 | 0.639 | 0.429 | 0.459 | 0.503 |
| Total carbon | 1.36 | 0.107 | 0.745 | 0.005 | 0.944 | 0.253 | 0.618 |
| Total nitrogen | 1.36 | 0.034 | 0.855 | 0.110 | 0.742 | 1.412 | 0.243 |
| C: N ratio | 1.36 | 2.891 | 0.098 | 2.023 | 0.164 | 4.020 | 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Lv, C.; Miao, T.; Ma, X.; Xia, C. Interactive Effects of Rising Temperature, Elevated CO2 and Herbivory on the Growth and Stoichiometry of a Submerged Macrophyte Vallisneria natans. Sustainability 2023, 15, 1200. https://doi.org/10.3390/su15021200
Zhou C, Lv C, Miao T, Ma X, Xia C. Interactive Effects of Rising Temperature, Elevated CO2 and Herbivory on the Growth and Stoichiometry of a Submerged Macrophyte Vallisneria natans. Sustainability. 2023; 15(2):1200. https://doi.org/10.3390/su15021200
Chicago/Turabian StyleZhou, Chi, Chaochao Lv, Teng Miao, Xufa Ma, and Chengxing Xia. 2023. "Interactive Effects of Rising Temperature, Elevated CO2 and Herbivory on the Growth and Stoichiometry of a Submerged Macrophyte Vallisneria natans" Sustainability 15, no. 2: 1200. https://doi.org/10.3390/su15021200
APA StyleZhou, C., Lv, C., Miao, T., Ma, X., & Xia, C. (2023). Interactive Effects of Rising Temperature, Elevated CO2 and Herbivory on the Growth and Stoichiometry of a Submerged Macrophyte Vallisneria natans. Sustainability, 15(2), 1200. https://doi.org/10.3390/su15021200






