Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review
Abstract
:1. Introduction
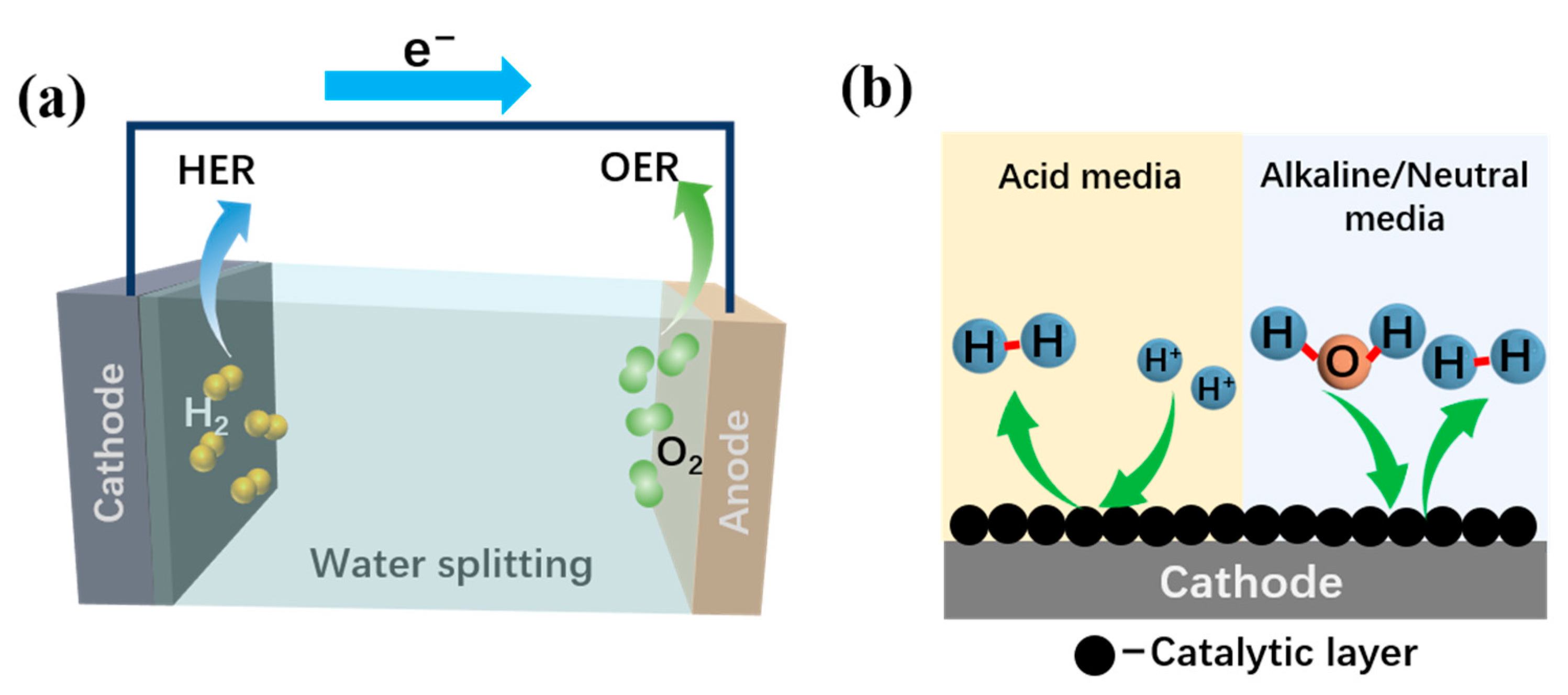
2. Mechanism of Hydrogen Evolution Reaction
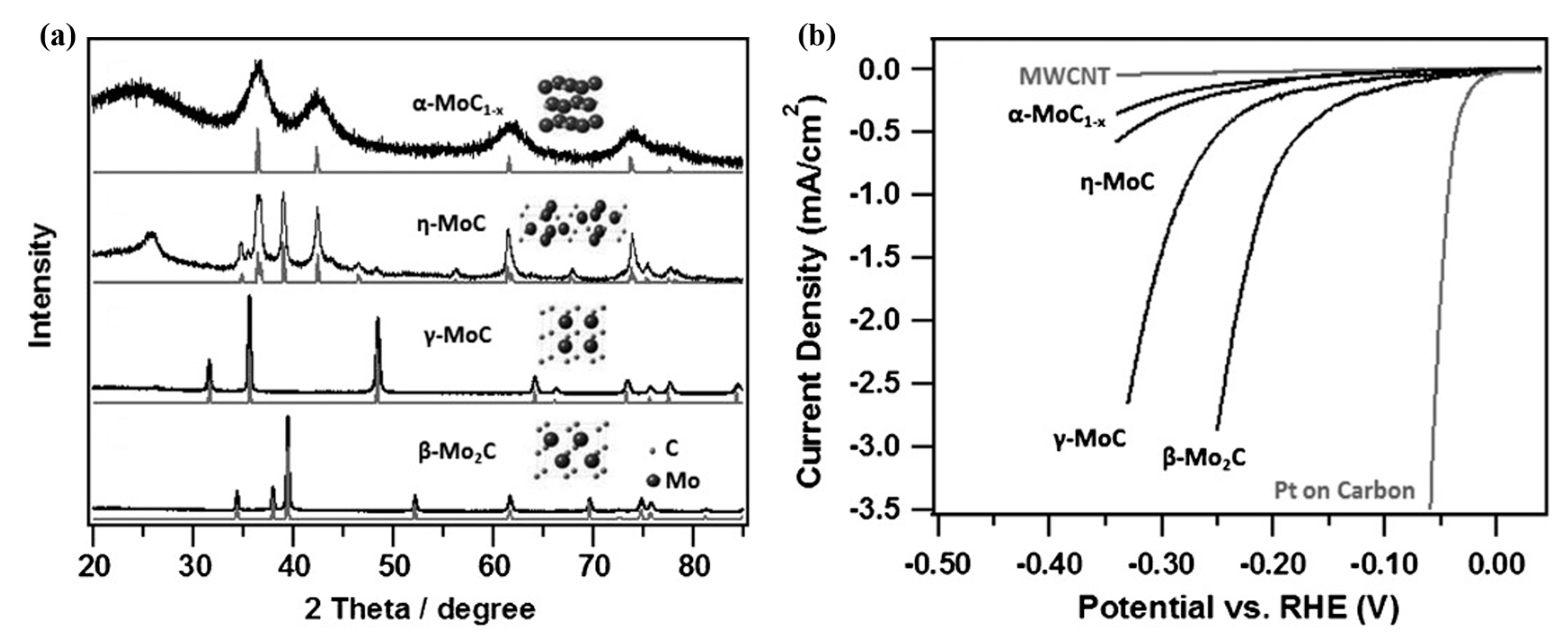
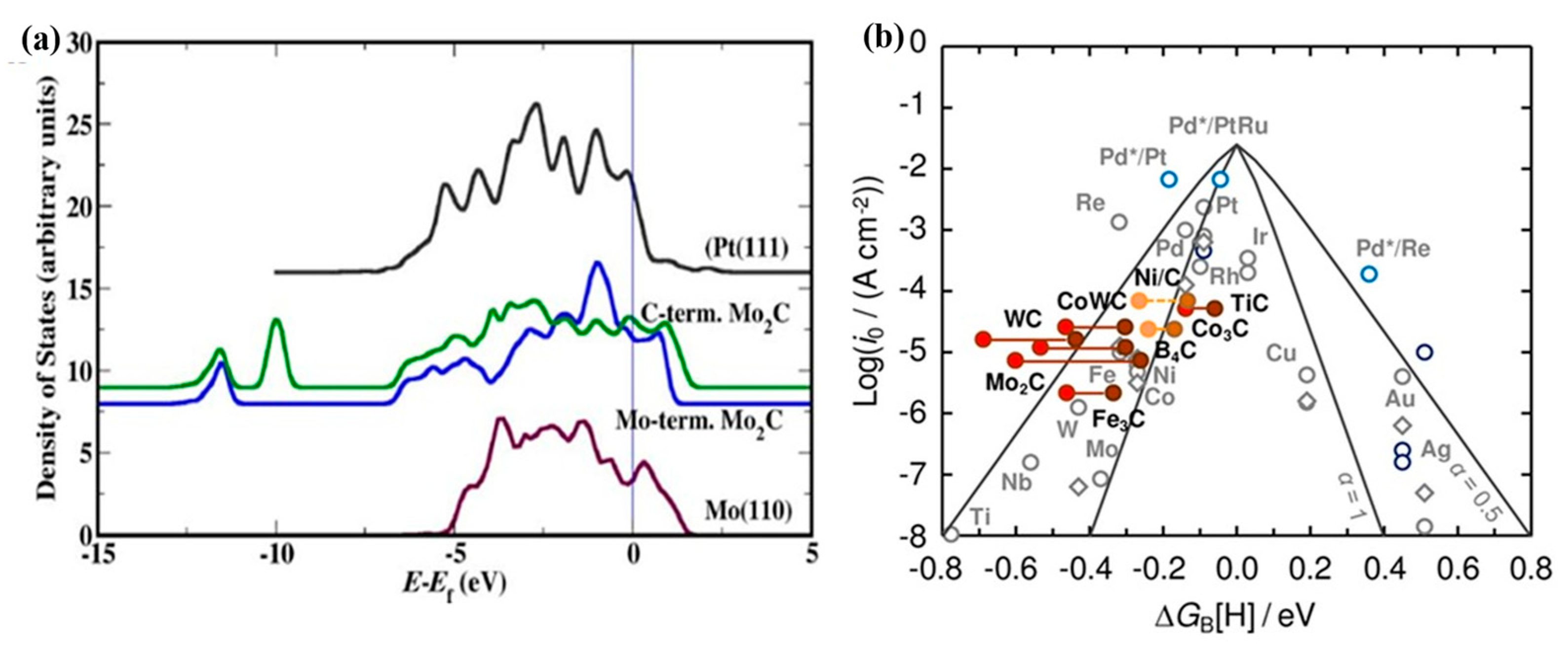
3. Characteristic of Molybdenum Carbide Materials
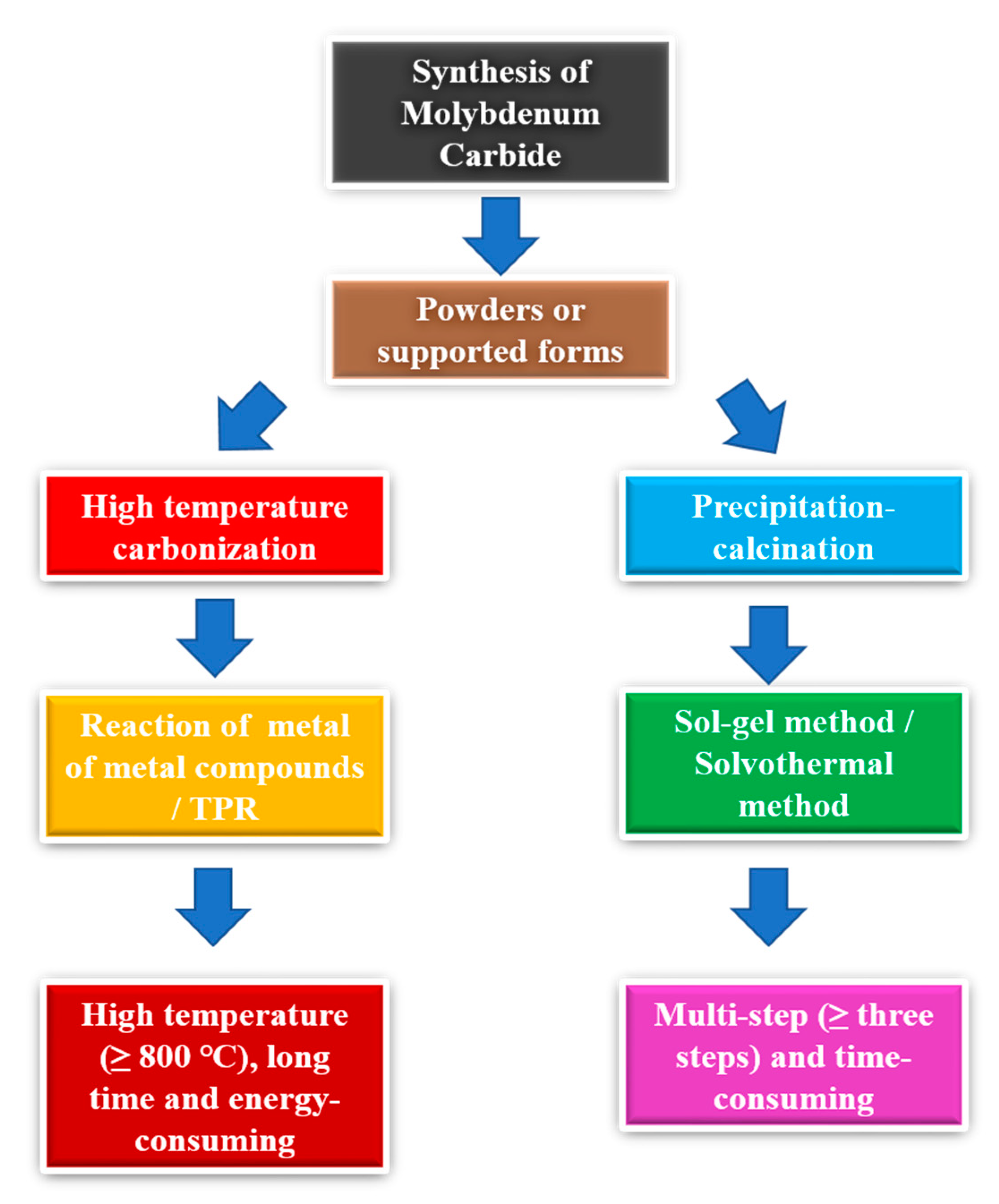
4. Different Synthesis Methods of Molybdenum Carbide Materials
5. The Activity Enhancement of Molybdenum Carbide by Compositing with Carbon Materials
6. Metal doping of Molybdenum Carbide Materials
6.1. Non-Precious Metal Doping of Molybdenum Carbide Materials
6.2. Transition Metal Doping of Molybdenum Carbide Materials
7. Heterophase Structure of Molybdenum Carbide Materials
8. Molybdenum Carbide-Based Catalysts for Full Water Splitting
9. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Xia, C.; Zhu, P.; Jiang, Q.; Pan, Y.; Liang, W.; Stavitski, E.; Alshareef, H.N.; Wang, H. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 2019, 4, 776–785. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guan, D.G.; Yu, B.; Hou, W.; Zheng, B.; Zhang, W.; Chen, Y. Scalable Synthesis of Mo2C/CNT Networks as Highly Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochimica. Acta 2018, 263, 192–200. [Google Scholar] [CrossRef]
- Kou, Z.; Wang, T.; Cai, Y.; Guan, C.; Pu, Z.; Zhu, C.; Hu, Y.; Elshahawy, A.M.; Wang, J.; Mu, S. Ultrafine Molybdenum Carbide Nanocrystals Confined in Carbon Foams Via a Colloid-Confinement Route for Efficient Hydrogen Production. Small Methods 2018, 2, 1700396. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of Renewable Energy Sources in Environmental Protection: A Review. Renew. Sust. Energ. Rev. 2011, 15, 1513–1524. [Google Scholar]
- Chen, W.; Pei, J.; He, C.T.; Wan, J.; Ren, H.; Zhu, Y.; Wang, Y.; Dong, J.; Tian, S.; Cheong, W.C.; et al. Rational Design of Single Molybdenum Atoms Anchored on N-Doped Carbon for Effective Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 16086–16090. [Google Scholar] [CrossRef]
- Das, D.; Santra, S.; Nanda, K.K. In Situ Fabrication of a Nickel/Molybdenum Carbide-Anchored N-Doped Graphene/CNT Hybrid: An Efficient (Pre) Catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Zhang, H.; Zhang, Y.; Liu, L.; Fang, L.; Yang, X.; Gu, X.; Wang, Y. Unique Three-Dimensional Mo2C@MoS2 Heterojunction Nanostructure with S Vacancies as Outstanding All-pH Range Electrocatalyst for Hydrogen Evolution. J. Catal. 2019, 371, 20–26. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.D.; Yuan, L.; Ge, L.; Ding, H.; Wang, D.; Zou, X. Carbon-Protected Bimetallic Carbide Nanoparticles for a Highly Efficient Alkaline Hydrogen Evolution Reaction. Nanoscale 2015, 7, 3130–3136. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, G.X.; Guo, Z. The Intensification Technologies to Water Electrolysis for Hydrogen Production—A Review. Renew. Sust. Energ. Rev. 2014, 29, 573–588. [Google Scholar]
- Armstrong, F.A.; Hirst, J. Reversibility and Efficiency in Electrocatalytic Energy Conversion and Lessons from Enzymes. Proc. Natl. Acad. Sci. USA 2011, 108, 14049–14054. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Gong, Y.; Chen, Y.; Zhang, H.; Wang, Z.; Mao, S.; Xie, L.; Jiang, Z.; Wang, Y. Carbon Vacancy Defect-Activated Pt Cluster for Hydrogen Generation. J. Mater. Chem. A 2019, 7, 15364–15370. [Google Scholar]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar]
- Zhang, J.; Zhang, Q.; Feng, X. Support and interface effects in water-splitting electrocatalysts. Adv. Mater. 2019, 31, 1808167. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Feng, Y.; Jeon, Y.; Guan, B.; Lou, X.W.; Paik, U. Formation of Ni–Co–MoS2 nanoboxes with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 2016, 28, 9006–9011. [Google Scholar] [CrossRef]
- Khiarak, B.N.; Imanparast, S.; Yengejeh, M.M.; Zahraeid, A.A.; Yaghobif, R.; Golmohammad, M. Efficient Water Oxidation Catalyzed by a Graphene Oxide/Copper Electrode, Supported on Carbon Cloth. Russ. J. Electrochem. 2021, 57, 1196–1206. [Google Scholar] [CrossRef]
- Khiarak, B.N.; Golmohammad, M.; Shahraki, M.M.; Simchi, A. Facile synthesis and self-assembling of transition metal phosphide nanosheets to microspheres as a high-performance electrocatalyst for full water splitting. J. Alloys Compd. 2021, 875, 160049. [Google Scholar] [CrossRef]
- Chen, J.; Pan, A.; Zhang, W.; Cao, X.; Lu, R.; Liang, S. Melamine-assisted synthesis of ultrafine Mo2C/Mo2N@N-doped carbon nanofibers for enhanced alkaline hydrogen evolution reaction activity. Sci. China Mater. 2020, 64, 1150–1158. [Google Scholar]
- Wang, J.; Xia, H.; Peng, Z.; Lv, C.; Jin, L.; Zhao, Y.; Huang, Z.; Zhang, C. Graphene Porous Foam Loaded with Molybdenum Carbide Nanoparticulate Electrocatalyst for Effective Hydrogen Generation. ChemSusChem 2016, 9, 855–862. [Google Scholar] [CrossRef]
- Chen, P.; Ouyang, L.; Lang, C.; Zhong, H.; Liu, J.; Wang, H.; Huang, Z.; Zhu, M. All-pH Hydrogen Evolution by Heterophase Molybdenum Carbides Prepared via Mechanochemical Synthesis. ACS Sustain. Chem. Eng. 2023, 11, 3585–3593. [Google Scholar]
- Huang, H.; Yu, C.; Huang, H.; Guo, W.; Zhang, M.; Han, X.; Wei, Q.; Cui, S.; Tan, X.; Qiu, J. Microwave-Assisted Ultrafast Synthesis of Molybdenum Carbide Nanoparticles Grown on Carbon Matrix for Efficient Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900259. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhu, M.; Bao, X.; Xiao, B.; Su, D.; Li, H.; Wang, Y. Molybdenum-Carbide-Modified Nitrogen-Doped Carbon Vesicle Encapsulating Nickel Nanoparticles: A Highly Efficient, Low-Cost Catalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 15753–15759. [Google Scholar] [PubMed]
- Lei, Y.; Wu, X.; Li, S.; Huang, J.; Ng, K.H.; Lai, Y. Noble-metal-free metallic MoC combined with CdS for enhanced visible-light-driven photocatalytic hydrogen evolution. J. Clean. Prod. 2021, 322, 129018. [Google Scholar] [CrossRef]
- Bang, J.; Moon, I.K.; Kim, Y.; Oh, J. Heterostructured Mo2N–Mo2C Nanoparticles Coupled with N-Doped Carbonized Wood to Accelerate the Hydrogen Evolution Reaction. Small Struct. 2023, 4, 2200283. [Google Scholar] [CrossRef]
- Wan, J.; Wu, J.; Gao, X.; Li, T.; Hu, Z.; Yu, H.; Huang, L. Structure Confined Porous Mo2C for Efficient Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1703933. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- Conway, B.; Salomon, M. Electrochemical reaction orders: Applications to the hydrogenand oxygen-evolution reactions. Electrochim. Acta 1964, 9, 1599–1615. [Google Scholar]
- Wu, Y.; Li, G.D.; Liu, Y.; Yang, L.; Lian, X.; Asefa, T.; Zou, X. Overall Water Splitting Catalyzed Efficiently by an Ultrathin Nanosheet-Built, Hollow Ni3S2-Based Electrocatalyst. Adv. Funct. Mater. 2016, 26, 4839–4847. [Google Scholar] [CrossRef]
- Tilak, B.; Chen, C. Generalized analytical expressions for Tafel slope, reaction order and ac impedance for the hydrogen evolution reaction (HER): Mechanism of HER on platinum in alkaline media. J. Appl. Electrochem. 1993, 23, 631–640. [Google Scholar] [CrossRef]
- Chen, L.; Guay, D.; Lasia, A. Kinetics of the hydrogen evolution reaction on RuO2 and IrO2 oxide electrodes in H2SO4 solution: An AC impedance study. J. Electrochem. Soc. 1996, 143, 3576. [Google Scholar] [CrossRef]
- Jakšić, J.; Vojnović, M.; Krstajić, N. Kinetic analysis of hydrogen evolution at Ni-Mo alloy electrodes. Electrochim. Acta 2000, 45, 4151–4158. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.W.; Pan, L.; Zhang, X.; Zou, J.J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: Mechanisms, challenges, and prospective solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the Electrochemistry of the HydrogenEvolution Reaction through Combining Experiment and Theory. Angew. Chem. Int. Edit. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wu, J.; Hardy, W.J.; Loya, P.; Lou, M.; Yang, Y.; Najmaei, S.; Jiang, M.; Qin, F.; Keyshar, K.; et al. Facile Synthesis of Single Crystal Vanadium Disulfide Nanosheets by Chemical Vapor Deposition for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2015, 27, 5605–5609. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Chi, J.Q.; Yang, M.; Chai, Y.M.; Yang, Z.; Wang, L.; Dong, B. Design and Modulation Principles of Molybdenum Carbide-Based Materials for Green Hydrogen Evolution. J. Energy. Chem. 2020, 48, 398–423. [Google Scholar]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple Phases of Molybdenum Carbide as Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 6407–6410. [Google Scholar] [CrossRef]
- Chen, H.; Si, J.; Lyu, S.; Zhang, T.; Li, Z.; Lei, C.; Lei, L.; Yuan, C.; Yang, B.; Gao, L.; et al. Highly Effective Electrochemical Exfoliation of Ultrathin Tantalum Disulfide Nanosheets for Energy-Efficient Hydrogen Evolution Electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 24675–24682. [Google Scholar]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.; Wang, X. Molybdenum carbide-based electrocatalysts for hydrogen evolution reaction. Chem. Eur. J. 2017, 23, 10947–10961. [Google Scholar] [CrossRef]
- Lu, C.; Tranca, D.; Zhang, J.; Hernández, F.N.R.G.; Su, Y.; Zhuang, X.; Zhang, F.; Seifert, G.; Feng, X. Molybdenum Carbide-Embedded Nitrogen-Doped Porous Carbon Nanosheets as Electrocatalysts for Water Splitting in Alkaline Media. ACS Nano 2017, 11, 3933–3942. [Google Scholar] [PubMed]
- Baklanova, O.N.; Vasilevich, A.V.; Lavrenov, A.V.; Drozdov, V.A.; Muromtsev, I.V.; Arbuzov, A.B.; Trenikhin, M.V.; Sigaeva, S.S.; Temerev, V.L.; Gorbunova, O.V.; et al. Molybdenum carbide synthesized by mechanical activation an inert medium. J. Alloys Compd. 2017, 698, 1018–1027. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Wang, S.; Gao, B.; Fan, X.; Xu, S.; Jia, Y.; Zhang, Y.; Gao, Q.; Cao, X.; et al. N-doped molybdenum carbides embedded in porous carbon for efficient hydrogen evolution. Mater. Today Energy 2022, 26, 100992. [Google Scholar] [CrossRef]
- Peng, O.; Hu, Q.; Zhou, X.; Zhang, R.; Du, Y.; Li, M.; Ma, L.; Xi, S.; Fu, W.; Xu, Z.X.; et al. Swinging Hydrogen Evolution to Nitrate Reduction Activity in Molybdenum Carbide by Ruthenium Doping. ACS Catal. 2022, 12, 15045–15055. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, X.; Zhang, Q. Structural transformation of molybdenum carbide with extensive active centers for superior hydrogen evolution. Nano Energy 2022, 98, 107232. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.; Xie, S.; Hua, W.; Zhang, Y.; Ren, N.; Xu, H.; Tang, Y. Synthesis of Nanoporous Molybdenum Carbide Nanowires Based on Organic-Inorganic Hybrid Nanocomposites with Sub-Nanometer Periodic Structures. Chem. Mater. 2009, 21, 5560–5562. [Google Scholar] [CrossRef]
- Wang, H.M.; Wang, X.H.; Zhang, M.H.; Du, X.Y.; Li, W.; Tao, K.Y. Synthesis of Bulk and Supported Molybdenum Carbide by a Single-Step Thermal Carburization Method. Chem. Mater. 2007, 19, 1801–1807. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine Molybdenum Carbide Nanoparticles Composited with Carbon as a Highly Active Hydrogen-Evolution Electrocatalyst. Angew. Chem. Int. Ed. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Lv, C.; Wang, J.; Huang, Q.; Yang, Q.; Huang, Z.; Zhang, C. Facile synthesis of hollow carbon microspheres embedded with molybdenum carbide nanoparticles as an efficient electrocatalyst for hydrogen generation. RSC Adv. 2016, 6, 75870. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Liu, R.; Xia, K.; Xuan, C.; Guo, J.; Lei, W.; Wang, D. Facile preparation of carbon sphere supported molybdenum compounds (P, C and S) as hydrogen evolution electrocatalysts in acid and alkaline electrolytes. Nano Energy 2017, 32, 511–519. [Google Scholar] [CrossRef]
- Du, C.; Huang, H.; Wu, Y.; Wu, S.; Song, W. Ultra-efficient electrocatalytic hydrogen evolution at one-step carbonization generated molybdenum carbide nanosheets/N-doped carbon. Nanoscale 2016, 8, 16251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, W.; Jian, C.; He, X.; Cai, Q.; Liu, W. The intrinsic hydrogen evolution performance of 2D molybdenum carbide. J. Mater. Chem. A 2020, 8, 24204. [Google Scholar]
- Yu, Z.Y.; Duan, Y.; Gao, M.R.; Lang, C.C.; Zheng, Y.R.; Yu, S.H. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973. [Google Scholar] [PubMed]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [PubMed]
- Ma, L.; Ting, L.R.L.; Molinari, V.; Giordano, C.; Yeo, B.S. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the urea glass route. J. Mater. Chem. A 2015, 3, 8361–8368. [Google Scholar] [CrossRef]
- Jia, J.; Xiong, T.; Zhao, L.; Wang, F.; Liu, H.; Hu, R.; Zhou, J.; Zhou, W.; Chen, S. Ultrathin N-Doped Mo2C Nanosheets with Exposed Active Sites as Efficient Electrocatalyst for Hydrogen Evolution Reactions. ACS Nano 2017, 11, 12509–12518. [Google Scholar] [CrossRef]
- Caliskan, S.; Wang, A.; Qin, F.; House, S.D.; Lee, J.K. Molybdenum Carbide-Reduced Graphene Oxide Composites as Electrocatalysts for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 3790–3798. [Google Scholar] [CrossRef]
- Liao, L.; Wang, S.; Xiao, J.; Bian, X.; Zhang, Y.; Scanlon, M.D.; Hu, X.; Tang, Y.; Liu, B.; Giraultb, H.H. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. [Google Scholar] [CrossRef]
- Upadhyay, S.; Pandey, O.P. Synthesis of Mo2C/MoC/C nanocomposite for hydrogen evolution reaction. J. Solid. State Electr. 2022, 26, 559–564. [Google Scholar] [CrossRef]
- Jiang, R.; Pi, L.; Deng, B.; Hu, L.; Liu, X.; Cui, J.; Mao, X.; Wang, D. Electric Field-Driven Interfacial Alloying for in Situ Fabrication of Nano-Mo2C on Carbon Fabric as Cathode toward Efficient Hydrogen Generation. ACS Appl. Mater. Interfaces 2019, 11, 38606–38615. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Q.; Yao, H.; Wang, M.; Wu, P.; Wang, H.; Zhang, L.; Guo, L. Rapid Synthesis of C60-MoC Nanocomposites by Molten Salt Electrolysis for Hydrogen Evolution. J. Electrochem. Soc. 2023, 170, 026503. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Nichelson, A.; Vikraman, D.; Choi, J.H.; Hussain, S.; Ambika, C.; Bose, R.; Alfantazi, A.; Kim, H.S. Recent Advancements in Two-Dimensional Layered Molybdenum and Tungsten Carbide-Based Materials for Efficient Hydrogen Evolution Reactions. Nanomaterials 2022, 12, 3884. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, B.; Gholivand, M.B.; Shamsipur, M.; Amiri, M. Ordered mesoporous carbon/molybdenum carbide nanocomposite with high electrochemical performance asymmetric supercapacitor. J. Alloys Compd. 2022, 905, 164185. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar]
- Wang, H.; Cao, Y.; Sun, C.; Zou, G.; Huang, J.; Kuai, X.; Zhao, J.; Gao, L. Strongly Coupled Molybdenum Carbide on Carbon Sheets as a Bifunctional Electrocatalyst for Overall Water Splitting. ChemSusChem 2017, 10, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Paul, A.; Srivastava, D.N.; Panda, A.B. Porous carbon incorporated β-Mo2C hollow sphere: An efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 21655–21664. [Google Scholar]
- Zhu, J.; Yao, Y.; Chen, Z.; Zhang, A.; Zhou, M.; Guo, J.; Wu, W.D.; Chen, X.D.; Li, Y.; Wu, Z. Controllable Synthesis of Ordered Mesoporous Mo2C@Graphitic Carbon Core–Shell Nanowire Arrays for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 18761–18770. [Google Scholar] [CrossRef]
- Huo, L.; Liu, B.; Zhang, G.; Zhang, J. Universal Strategy to Fabricate a Two-Dimensional Layered Mesoporous Mo2C Electrocatalyst Hybridized on Graphene Sheets with High Activity and Durability for Hydrogen Generation. ACS Appl. Mater. Interfaces 2016, 8, 18107–18118. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, Y.; Liu, C.H.; Li, S.L.; Wang, Y.G.; Dong, L.Z.; Dai, Z.H.; Li, Y.F.; Lan, Y.Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar]
- Mir, R.A.; Upadhyay, S.; Pandey, O.P. A review on recent advances and progress in Mo2C@C: A suitable and stable electrocatalyst for HER. Int. J. Hydrogen Energy 2023, 48, 13044–13067. [Google Scholar]
- Chaitoglou, S.; Giannakopoulou, T.; Tsoutsou, D.; Vavouliotis, A.; Trapalis, C.; Dimoulas, A. Direct versus reverse vertical two-dimensional Mo2C/graphene heterostructures for enhanced hydrogen evolution reaction electrocatalysis. Nanotechnology 2019, 30, 415404. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Pandey, O.P. Review—Synthesis and electrochemical applications of molybdenum carbide: Recent progress and perspectives. J. Electrochem. Soc. 2022, 169, 016511. [Google Scholar]
- Yang, X.J.; Feng, X.J.; Tan, H.Q.; Zang, H.Y.; Wang, X.L.; Wang, Y.H.; Wang, E.B.; Li, Y.G. N-Doped graphene-coated molybdenum carbide nanoparticles as highly efficient electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 3947–3954. [Google Scholar]
- Shi, Z.; Nie, K.; Shao, Z.J.; Gao, B.; Lin, H.; Zhang, H.; Liu, B.; Wang, Y.; Zhang, Y.; Sun, X.; et al. Phosphorus-Mo2C@carbon nanowires toward efficient electrochemical hydrogen evolution: Composition, structural and electronic regulation. Energy Environ. Sci. 2017, 10, 1262–1271. [Google Scholar]
- Wu, Z.; Song, M.; Zhang, Z.; Wang, J.; Liu, X. Various strategies to tune the electrocatalytic performance of molybdenum phosphide supported on reduced graphene oxide for hydrogen evolution reaction. J. Colloid. Interface Sci. 2019, 536, 638–645. [Google Scholar]
- Ji, L.; Wang, J.; Teng, X.; Dong, H.; He, X.; Chen, Z. N, P-Doped Molybdenum Carbide Nanofibers for Efficient Hydrogen Production. ACS Appl. Mater. Interfaces 2018, 10, 14632–14640. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Z.; Li, S.; Li, H.; Chen, J.; Wang, Y.; Huang, Q.; Wang, C.; Karahan, H.E.; Henkelman, G.; et al. Big to Small: Ultrafine Mo2C Particles Derived from Giant Polyoxomolybdate Clusters for Hydrogen Evolution Reaction. Small 2019, 15, 1900358. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Guo, T.; Mu, Z.; Hu, X.; He, K.; Chen, X.; Dravid, V.P.; Wu, Z.; Wang, D. High-Performance MoC Electrocatalyst for Hydrogen Evolution Reaction Enabled by Surface Sulfur Substitution. ACS Appl. Mater. Interfaces 2021, 13, 40705–40712. [Google Scholar]
- Zhu, L.; Zhao, Y.; Yang, W.; Hsu, H.Y.; Peng, P.; Li, F.F. Low-temperature selective synthesis of metastable α-MoC with electrochemical properties: Electrochemical co-reduction of CO2 and MoO3 in molten salts. Chin. Chem. Lett. 2023, 20, 108583. [Google Scholar]
- Wu, Z.Y.; Hu, B.C.; Wu, P.; Liang, H.W.; Yu, Z.L.; Lin, Y.; Zheng, Y.R.; Li, Z.; Yu, S.H. Mo2C nanoparticles embedded within bacterial cellulose-derived 3D N-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Hu, X.; Xie, Z. Surfactant-assisted hydrothermal synthesis of nitrogen doped Mo2C@C composites as highly efficient electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 3702–3710. [Google Scholar]
- Chen, M.; Ma, Y.; Zhou, Y.; Liu, C.; Qin, Y.; Fang, Y.; Guan, G.; Li, X.; Zhang, Z.; Wang, T. Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst. Catalysts 2018, 8, 294. [Google Scholar] [CrossRef]
- Yu, F.; Gao, Y.; Lang, Z.; Ma, Y.; Yin, L.; Du, J.; Tan, H.; Wang, Y.; Li, Y. Electrocatalytic performance of ultrasmall Mo2C affected by different transition metal dopants in hydrogen evolution reaction. Nanoscale 2018, 10, 6080–6087. [Google Scholar]
- Geng, B.; Yan, F.; Liu, L.; Zhu, C.; Li, B.; Chen, Y. Ni/MoC heteronanoparticles encapsulated within nitrogen-doped carbon nanotube arrays as highly efficient self-supported electrodes for overall water splitting. Chem. Eng. J. 2021, 406, 126815. [Google Scholar]
- Zhou, Y.; Huang, W.; Zhang, X.; Wang, M.; Zhang, L.; Shi, J. Ni-Assisted Low Temperature Synthesis of MoCx with Enhanced HER Activity. Chem. Eur. J. 2017, 23, 17029–17036. [Google Scholar] [PubMed]
- Hu, Z.; Huang, J.; Luo, Y.; Liu, M.; Li, X.; Yan, M.; Ye, Z.; Chen, Z.; Feng, Z.; Huang, S. Wrinkled Ni-doped Mo2C coating on carbon fiber paper: An advanced electrocatalyst prepared by molten-salt method for hydrogen evolution reaction. Electrochim. Acta 2019, 319, 293–301. [Google Scholar]
- Ouyang, T.; Chen, A.N.; He, Z.Z.; Liu, Z.Q.; Tong, Y. Rational design of atomically dispersed nickel active sites in β-Mo2C for the hydrogen evolution reaction at all pH values. Chem. Commun. 2018, 54, 9901. [Google Scholar]
- Chen, J.; Jia, J.; Wei, Z.; Li, G.; Yu, J.; Yang, L.; Xiong, T.; Zhou, W.; Tong, Q. Ni and N co-doped MoCx as efficient electrocatalysts for hydrogen evolution reaction at all-pH values. Int. J. Hydrogen Energy 2018, 43, 14301–14309. [Google Scholar]
- Han, W.; Chen, L.; Ma, B.; Wang, J.; Song, W.; Fan, X. Ultra-small Mo2C nanodots encapsulated in nitrogen-doped porous carbon for pH-universal hydrogen evolution: Insights into the synergistic enhancement of HER activity by nitrogen doping and structural defects. J. Mater. Chem. 2019, 7, 4734. [Google Scholar]
- Guo, P.; Wang, M.; Zhang, Y.; Wang, Y.; Xin, X.; Wang, R.; Huang, W.; Li, X. Phase-dependent intermediate adsorption regulation on molybdenum carbides for efficient pH-universal hydrogen evolution. Appl. Surf. Sci. 2023, 625, 157169. [Google Scholar]
- Lin, H.; Shi, Z.; He, S.; Yu, X.; Wang, S.; Gao, Q.; Tang, Y. Heteronanowires of MoC-Mo2C as efficient electrocatalysts for hydrogen evolution reaction. Chem. Sci. 2016, 7, 3399. [Google Scholar] [PubMed]
- Liu, M.; Yang, Y.; Luan, X.; Dai, X.; Zhang, X.; Yong, J.; Qiao, H.; Zhao, H.; Song, W.; Huang, X. Interface-Synergistically Enhanced Acidic, Neutral, and Alkaline Hydrogen Evolution Reaction over Mo2C/MoO2 Heteronanorods. ACS Sustain. Chem. Eng. 2018, 6, 14356–14364. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, B.; Wang, M.; Xiao, X.; Lv, A.; Jiao, S.; Chu, P.K. Dual-phase MoC-Mo2C nanosheets prepared by molten salt electrochemical conversion of CO2 as excellent electrocatalysts for the hydrogen evolution reaction. Nano Energy 2021, 90, 106533. [Google Scholar]
- Liu, W.; Wang, X.; Wang, F.; Du, K.; Zhang, Z.; Guo, Y.; Yin, H.; Wang, D. A durable and pH-universal self-standing MoC-Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12, 6776. [Google Scholar]
- Wu, T.; Zhang, J.; Chen, Y.; Jia, Y.; An, J.; Ge, J.; Wang, M. Simple and Controllable Preparation of Molybdenum Carbides by One-Step Co-Electrolysis of Na2MoO4 and CO2. J. Electrochem. Soc. 2023, 170, 053503. [Google Scholar]
- Zhou, X.; Tian, Y.; Luo, J.; Jin, B.; Wu, Z.; Ning, X.; Zhan, L.; Fan, X.; Zhou, T.; Zhang, S.; et al. MoC Quantum Dots@N-Doped-Carbon for Low-Cost and Efficient Hydrogen Evolution Reaction: From Electrocatalysis to Photocatalysis. Adv. Funct. Mater. 2022, 32, 2201518. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [PubMed]
- Wang, C.; Zhai, P.; Xia, M.; Wu, Y.; Zhang, B.; Li, Z.; Ran, L.; Gao, J.; Zhang, X.; Fan, Z.; et al. Engineering Lattice Oxygen Activation of Iridium Clusters Stabilized on Amorphous Bimetal Borides Array for Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 27126–27134. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Sun, L.; Hou, J. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 5462. [Google Scholar]
- Gong, T.; Zhang, J.; Liu, Y.; Hou, L.; Deng, J.; Yuan, C. Construction of hetero-phase Mo2C-CoO@N-CNFs film as a self-supported Bi-functional catalyst towards overall water splitting. Chem. Eng. J. 2023, 451, 139025. [Google Scholar]
- Wu, Y.; Zhao, Y.; Zhai, P.; Wang, C.; Gao, J.; Sun, L.; Hou, J. Triggering Lattice Oxygen Activation of Single-Atomic Mo Sites Anchored on Ni–Fe Oxyhydroxides Nanoarrays for Electrochemical Water Oxidation. Adv. Mater. 2022, 34, 2202523. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tang, Z.; Zang, W.; Li, Z.; Li, J.; Li, Z.; Cao, L.; Rodriguez, J.S.D.; Mariano, C.O.M.; Xu, H.; et al. Ferromagnetic single-atom spin catalyst for boosting water splitting. Nat. Nanotechnol. 2023, 18, 763–771. [Google Scholar] [PubMed]
- Hou, J.; Zhang, B.; Li, Z.; Cao, S.; Sun, Y.; Wu, Y.; Gao, Z.; Sun, L. Vertically Aligned Oxygenated-CoS2–MoS2 Heteronanosheet Architecture from Polyoxometalate for Efficient and Stable Overall Water Splitting. ACS Catal. 2018, 8, 4612–4621. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Z.; Xu, C.; Jiang, Y.; Ma, X.L.; Cheng, H.M.; Ren, W. Phase transition and in situ construction of lateral heterostructure of 2D superconducting α/β Mo2C with sharp interface by electron beam irradiation. Nanoscale 2017, 9, 7501–7507. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, D.; Hu, Y.; He, J.; Chen, Y.; He, W. Mo2C Nanodots Anchored on N-Doped Porous CNT Microspheres as Electrode for Efficient Li-Ion Storage. Small Methods 2019, 3, 1800287. [Google Scholar]
- Li, T.; Luo, W.; Kitadai, H.; Wang, X.; Ling, X. Probing the Domain Architecture in 2D α-Mo2C via Polarized Raman Spectroscopy. Adv. Mater. 2019, 31, 1807160. [Google Scholar]
- Yu, G.Q.; Yin, W.J.; Li, X.B. Predicted superior hydrogen evolution activities of MoC via surface dopant. Int. J. Hydrogen Energy 2022, 47, 13664–13673. [Google Scholar]
- Meng, S.; Xue, X.; Weng, Y.; Jiang, S.; Li, G.; Sun, Q.; Zhang, Y. Synthesis and characterization of molybdenum carbide catalysts on different carbon supports. Catal. Today 2022, 402, 266–275. [Google Scholar]
- Yu, B.; Huang, A.; Chen, D.; Srinivas, K.; Zhang, X.; Wang, X.; Wang, B.; Ma, F.; Liu, C.; Zhang, W.; et al. In Situ Construction of Mo2C Quantum Dots-Decorated CNT Networks as a Multifunctional Electrocatalyst for Advanced Lithium-Sulfur Batteries. Small 2021, 17, 2100460. [Google Scholar] [CrossRef]



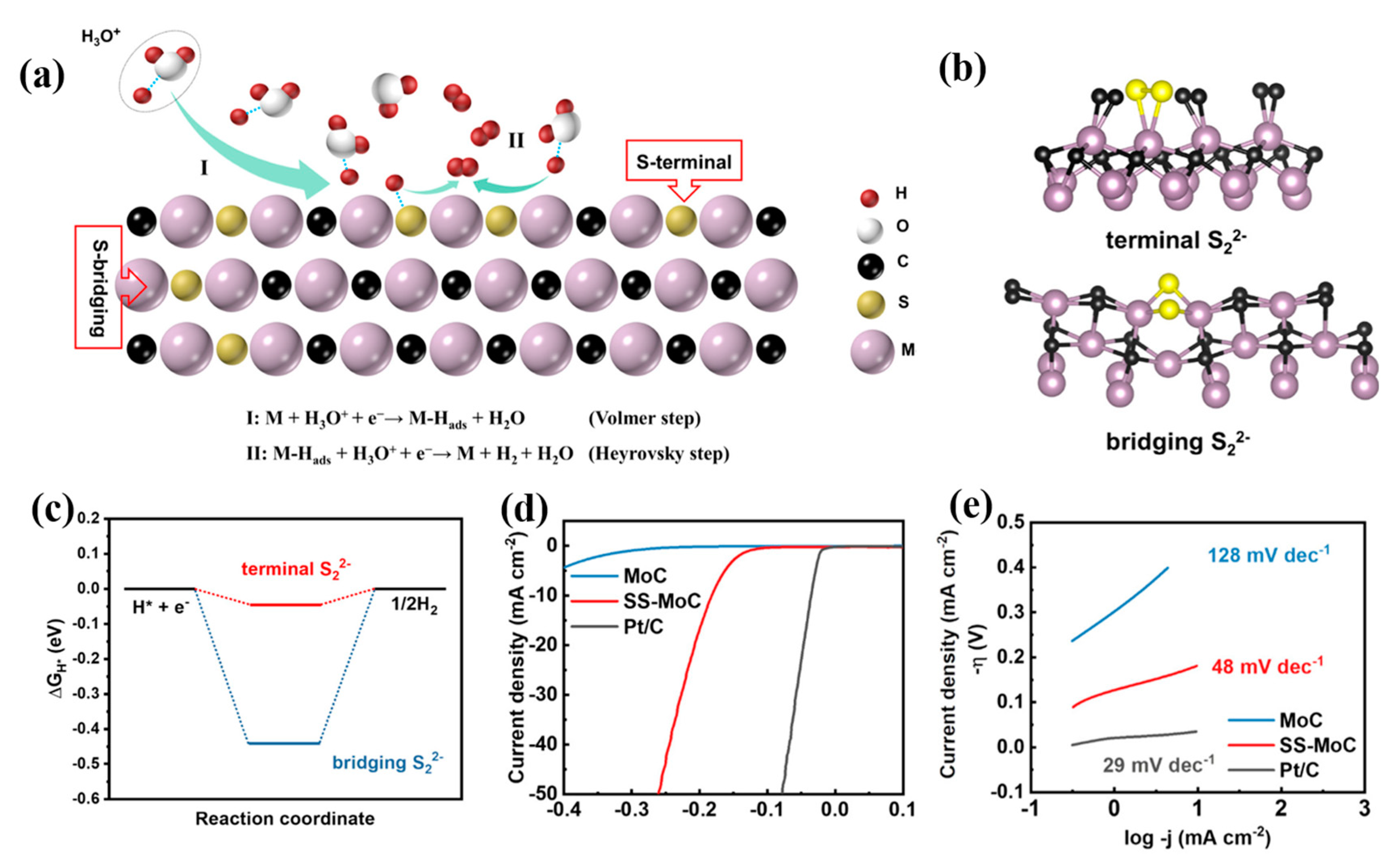

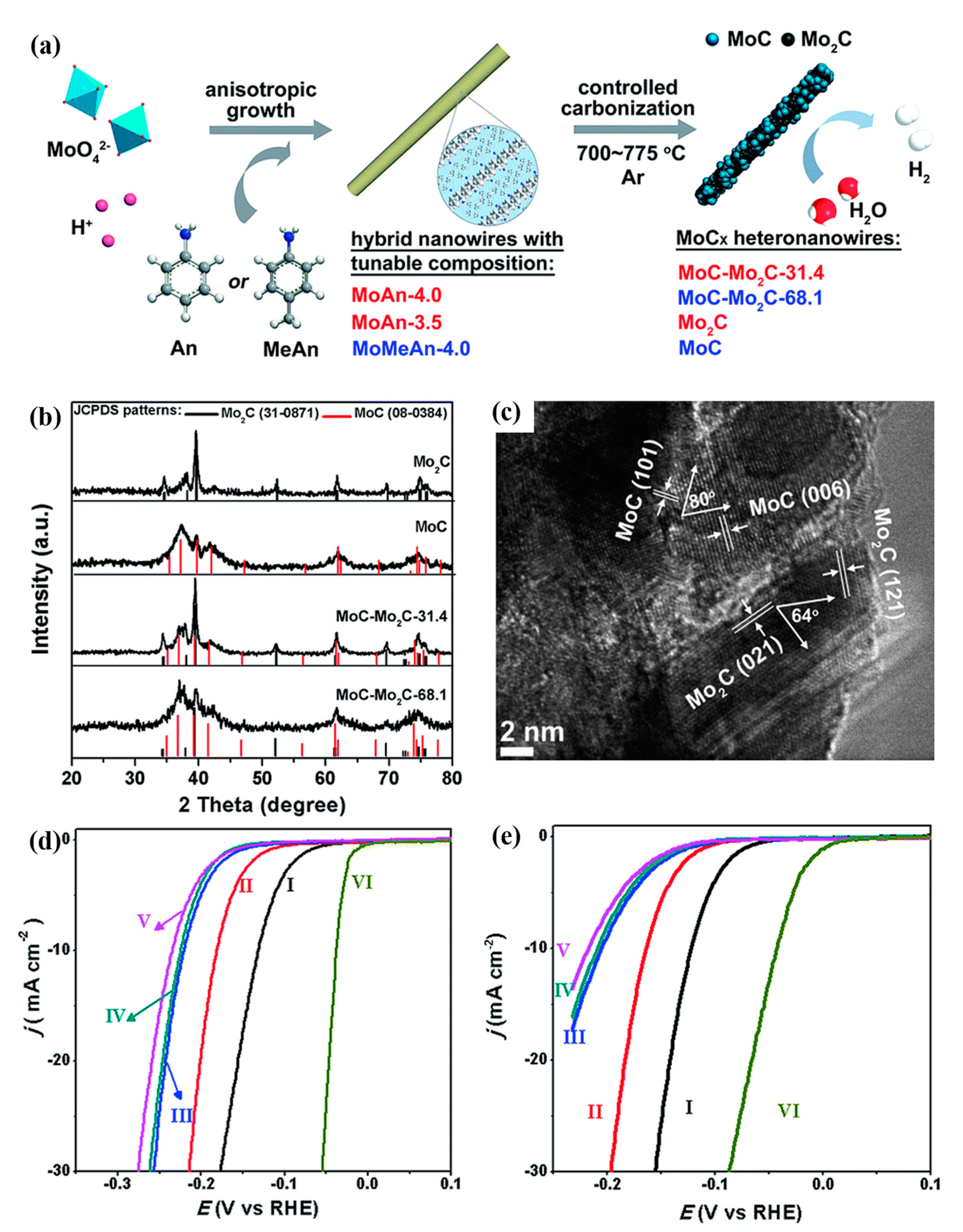
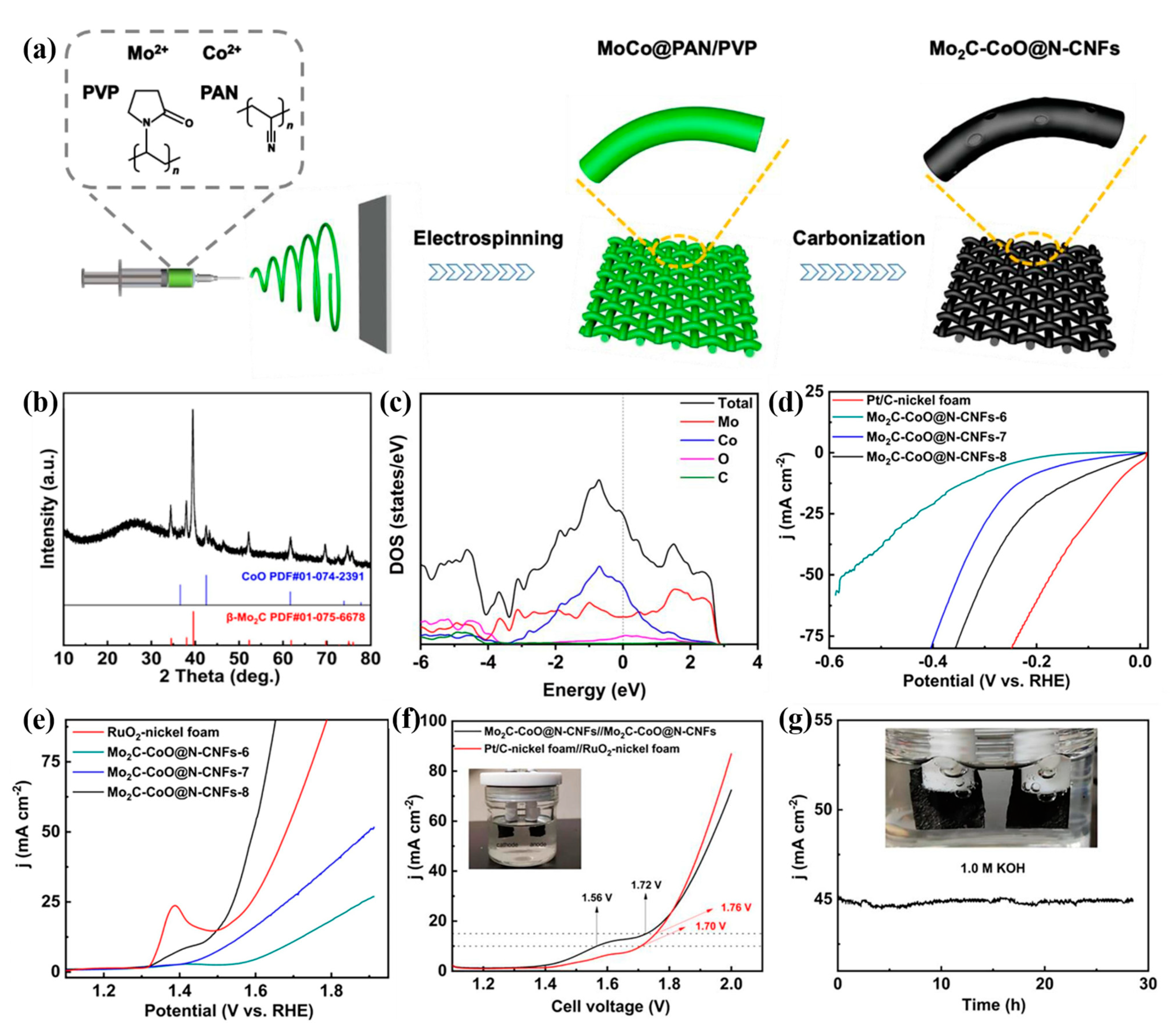
| Sample | Synthetic Method | Precursors | Synthetic Conditions | Electrolyte | η10 | Ref |
|---|---|---|---|---|---|---|
| Mo2C | High-temperature carbonization | (NH4)6Mo7O24·4H2O, dicyanamide | Step 1: calcination at 400 °C for 30 min; step 2: 800 °C in N2 for 6 h | 0.5 M H2SO4 | 205 mV | [48] |
| Mo2C | Precipitation–calcination | Glucose, NaCl and (NH4)6Mo7O24·4H2O | Step 1: stir and preheating at 800 °C in N2; step 2: calcination at 850 °C in 5% H2/95% N2 for 2 h | 1 M KOH | 430 mV | [49] |
| Mo2C-C | Precipitation–calcination | (NH4)6Mo7O24·6H2O, glucose and ethylene glycol | Step 1: stir for 30 min; step 2: heating at 200 °C for 10 h; step 3: calcination at 900 °C in 8%H2/Ar for 2 h | 1 M KOH | 149 mV | [50] |
| Mo2C NS | Precipitation–calcination | Sodium molybdate, cetyltrimethylammonium bromide | Step 1: stir for 2.5 h; calcination at 600 °C for 4 h in N2; step 2: calcination at 900 °C for 2 h in N2 | 1 M KOH | 205 mV | [51] |
| Mo2C | High-temperature carbonization | MoO3 and SiO2/Si | Step 1: chemical vapor deposition in Ar; step 2: carbonization under CH4 and H2; step 3: heated at 800 °C | 1 M KOH | 168 mV | [52] |
| Ni–Mo2C–PC | Precipitation–calcination | Ni(NO3)2·6H2O, Na2MoO4·2H2O and dopamine hydrochloride | Step 1: hydrothermal treatment at 150 °C for 6 h; step 2: stir for 24 h at room temperature; step 3: calcination at 800 °C for 2 h in Ar | 1 M KOH | 179 mV | [53] |
| MoCx | High-temperature carbonization | NENU-5 | Step 1: calcination at 800 °C in Ar for 6 h;step 2: etch with Fe3+ for 2 h | 1 M KOH | 151 mV | [54] |
| 0.5 M H2SO4 | 142 mV | |||||
| Mo2C | High-temperature carbonization | MoCl5 and urea | Step 1: calcination at 800 °C in N2 for 3 h | 0.5 M H2SO4 | 198 mV | [55] |
| N-doped Mo2C | High-temperature carbonization | MoO3 | Step 1: calcination at 900 °C in Ar/H2 for 1 h; step 2: 700 °C in C2H4N4 for 2 h | 0.5 M H2SO4 | 319 mV | [56] |
| Mo2C nanoparticles | High-temperature carbonization | MoCl5, ethanol, and urea | Step 1: irradiation at 50 °C for 10 min; step 2: calcination at 800 °C in N2 for 3 h | 0.5 M H2SO4 | 220 mV | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Jia, Y.; Wang, Q.; Jiang, Z.; Xiao, J.; Guo, L. Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review. Sustainability 2023, 15, 14556. https://doi.org/10.3390/su151914556
Zhou Z, Jia Y, Wang Q, Jiang Z, Xiao J, Guo L. Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review. Sustainability. 2023; 15(19):14556. https://doi.org/10.3390/su151914556
Chicago/Turabian StyleZhou, Zhaoyu, Yongsheng Jia, Qiang Wang, Zhongyu Jiang, Junwu Xiao, and Limin Guo. 2023. "Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review" Sustainability 15, no. 19: 14556. https://doi.org/10.3390/su151914556
APA StyleZhou, Z., Jia, Y., Wang, Q., Jiang, Z., Xiao, J., & Guo, L. (2023). Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review. Sustainability, 15(19), 14556. https://doi.org/10.3390/su151914556