Macadamia Breeding for Reduced Plant Vigor: Progress and Prospects for Profitable and Sustainable Orchard Systems
Abstract
1. Introduction
2. Manipulation of Tree Vigor for Orchard Profitability and Sustainability
3. Genetic and Environmental Control of Plant Vigor
4. Genotype X Environment Interaction on Plant Vigor
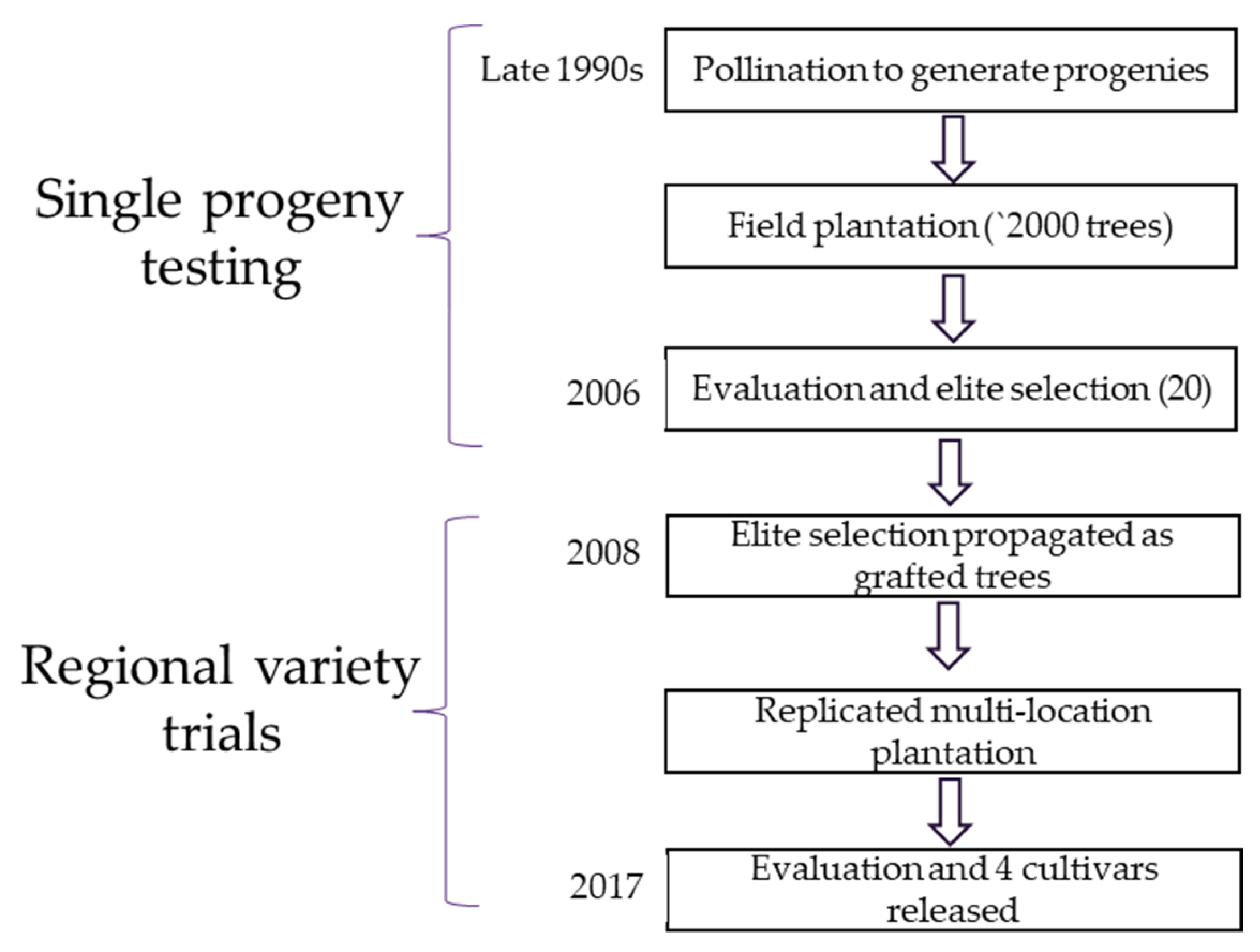
5. Progress of Macadamia Breeding for Reduced Vigor
5.1. Scion Breeding
5.2. Breeding Rootstocks to Improve Orchard Productivity and Efficiency
5.3. Challenges and Opportunities of Low Vigor Rootstock and Scion Breeding
6. Genomics-Assisted Breeding: A Solution to Lengthy Breeding Cycle
6.1. Markers for Genomic Studies
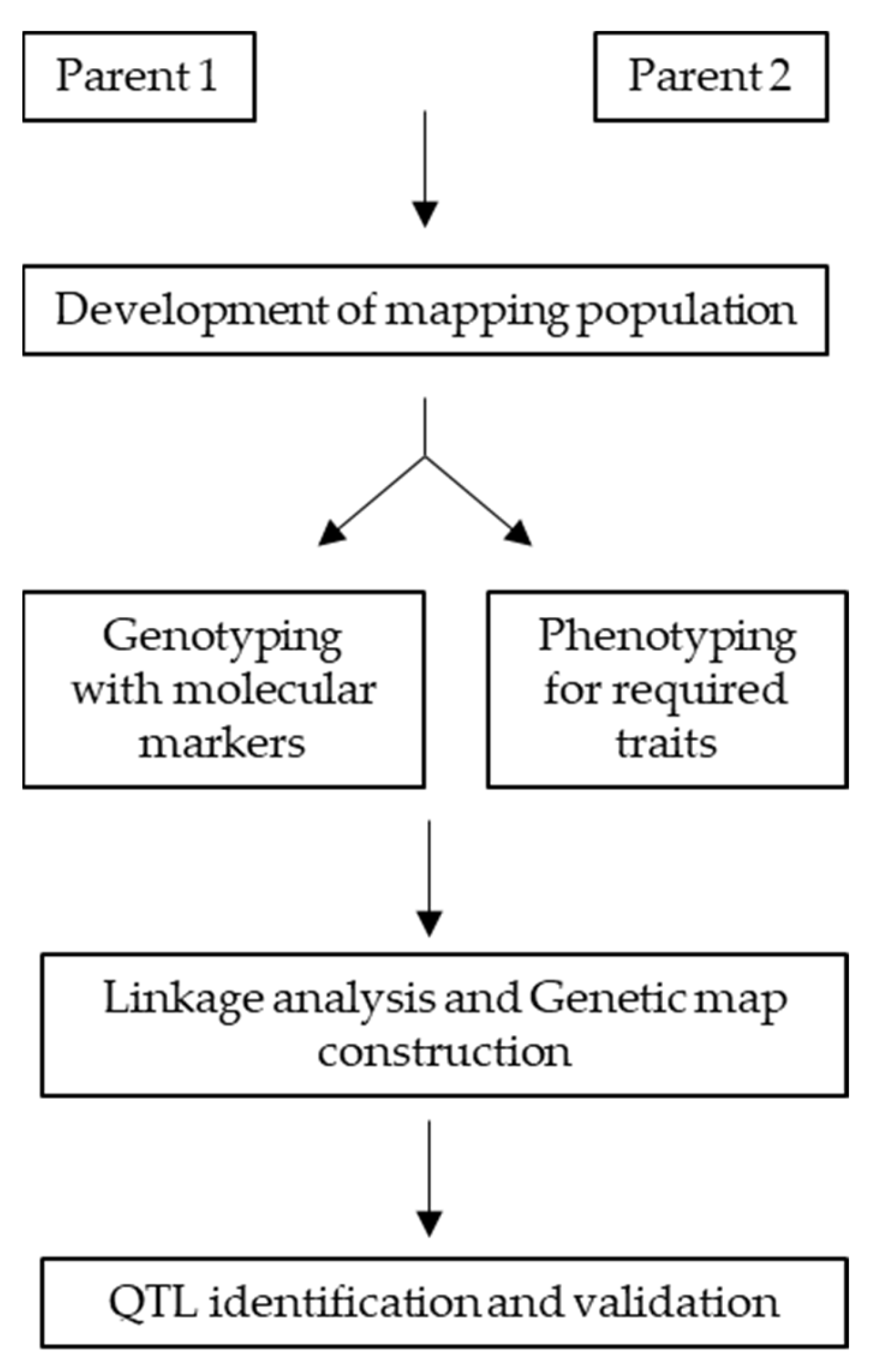
6.2. Marker Discovery via QTL Mapping
6.3. Marker Discovery through Genome-Wide Association Studies (GWAS)
6.4. Marker-Assisted Selection (MAS)
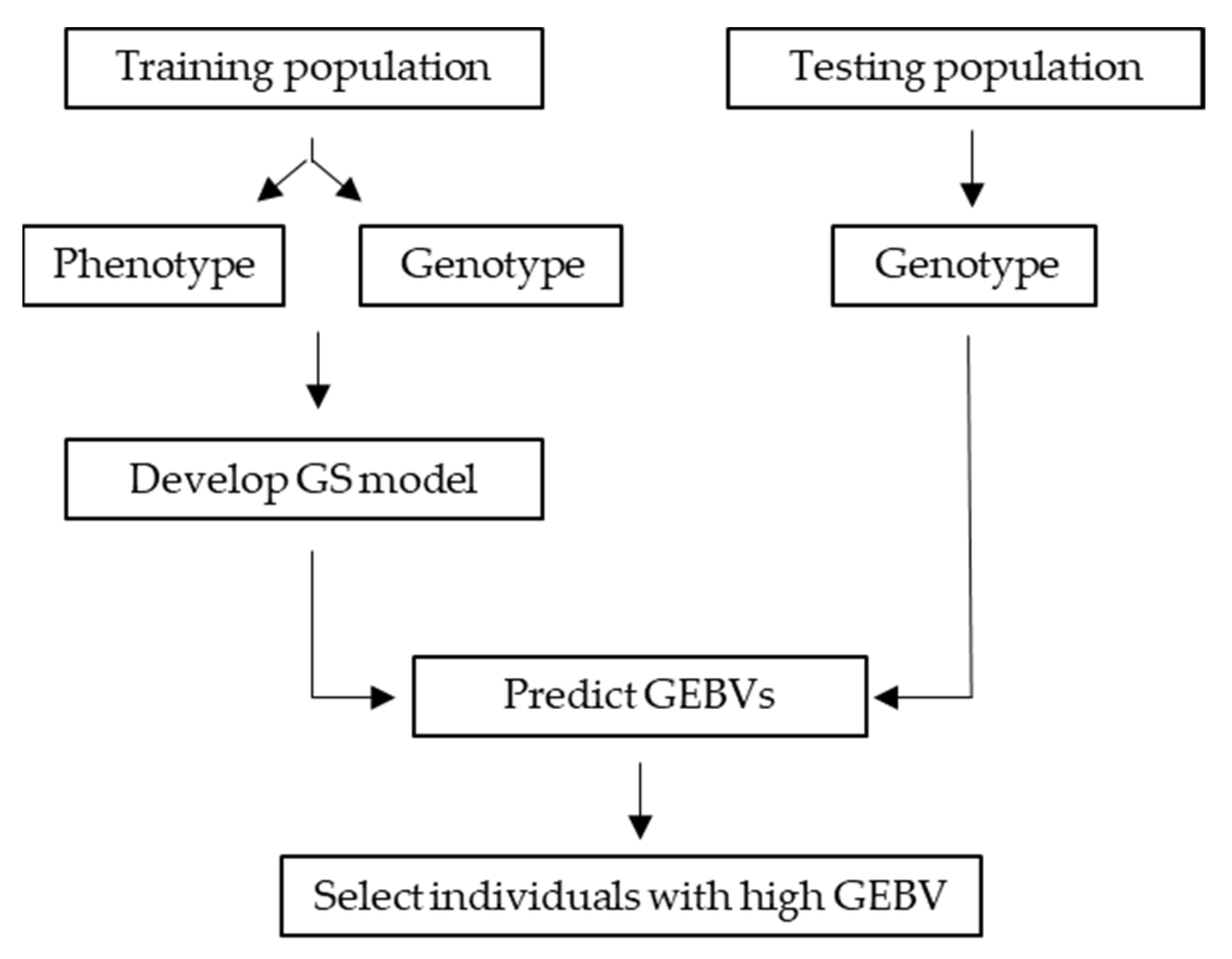
6.5. Genomic Selection (GS)
7. Rapid Phenotyping for Vigor Traits
8. Trait Mining for Low Vigor Trees
| Crop | Traits | Phenotypic Correlations | Source |
|---|---|---|---|
| Walnut (Jugans regia) | Tree vigor and tree height Tree vigor and trunk diameter Tree vigor and branching density | 0.63 0.79 0.58 | [143] |
| Macadamia (Macadamia spp.) | Canopy Volume and TCSA Canopy Volume and internode length Canopy Volume and lateral branching Canopy Volume and branching unit length TC and tree height TC and canopy width TC and canopy length | 0.82 0.47 0.49 0.52 0.76 0.65 0.65 | [36,38] |
| Mango | TCSA and Canopy volume Tree height and canopy volume Branching density and canopy volume Growth unit length and canopy volume | 0.828 0.78 0.466 0.505 | [34] |
| Olive | Tree height and trunk basal diameter | 0.66 | [30] |
9. Rootstocks Affect Scion Growth and Vigor
10. Physiological Traits Associated with Tree Vigor
10.1. Plant Water Potential and Hydraulic Conductivity
10.2. Xylem Anatomy
10.3. Stomatal Conductance, Size, and Density
10.4. Net Photosynthesis and Gas Exchange
10.5. Leaf Area and Leaf Area Index
11. Mechanisms of Rootstock Mediated Vigor Control
11.1. Semi-Incompatibility and Production of Phenolics
11.2. Rootstock-Scion Water Relations
11.3. Molecular Mechanisms (Protein Transport and Differential Gene Expression)
11.4. Aquaporins in Vigor Control Mechanism
11.5. Hormonal Regulation of Vigor
11.5.1. Auxin and Cytokinin
11.5.2. Gibberellin
11.5.3. Abscisic Acid
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardner, C.M.; Peace, C.; Lowe, A.J.; Neal, J.; Pisanu, P.; Powell, M.; Schmidt, A.; Spain, C.; Williams, K. Genetic Resources and Domestication of Macadamia. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 35. [Google Scholar]
- Dahler, J.; Mcconchie, C.; Turnbull, C. Quantification of cyanogenic glycosides in seedlings of three Macadamia (Proteaceae) species. Aust. J. Bot. 1995, 43, 619–628. [Google Scholar] [CrossRef]
- Topp, B.L.; Nock, C.J.; Hardner, C.M.; Alam, M.; O’Connor, K.M. Macadamia (Macadamia spp.) breeding. In Advances in Plant Breeding Strategies: Nut and Beverage Crops; Springer: Cham, Switzerland, 2019; pp. 221–251. [Google Scholar]
- McFadyen, L.; Morris, S.; McConchie, C.; Oldham, M. Effect of hedging and tree removal on productivity of crowding macadamia orchards. Aust. J. Exp. Agric. 2005, 45, 725–730. [Google Scholar] [CrossRef]
- Huett, D. Macadamia physiology review: A canopy light response study and literature review. Aust. J. Agric. Res. 2004, 55, 609–624. [Google Scholar] [CrossRef]
- Webster, T. Dwarfing rootstocks: Past, present and future. Compact. Fruit. Tree 2002, 35, 67–72. [Google Scholar]
- Robinson, T.L. Apple-orchard planting systems. In Apples: Botany, Production and Uses; CAB International: Wallingford, UK, 2003; pp. 345–407. [Google Scholar]
- McFadyen, L.; Morris, S.; Oldham, M.; Huett, D.; Meyers, N.; Wood, J.; McConchie, C. The relationship between orchard crowding, light interception, and productivity in macadamia. Aust. J. Agric. Res. 2004, 55, 1029–1038. [Google Scholar] [CrossRef]
- Costes, E.; Lauri, P.-E.; Regnard, J.-L. Analyzing fruit tree architecture: Implications for tree management and fruit production. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 32, pp. 1–61. [Google Scholar]
- Robinson, T. Advances in apple culture worldwide. Rev. Bras. Frutic. 2011, 33, 37–47. [Google Scholar] [CrossRef]
- Köhne, J.; Kremer-Köhne, S. Yield advantages and control of vegetative growth in a high-density avocado orchard treated with paclobutrazol. In Proceedings of the Second World Avocado Congress, Orange, CA, USA, 21–26 April 1991; pp. 233–235. [Google Scholar]
- Mika, A. Physiological responses of fruit trees to pruning. In Horticultural Reviews; AVI Publishing Company: Westport, CT, USA, 1986; Volume 8, pp. 337–378. [Google Scholar]
- Warner, R.M.; Gitlin, H.M. Effect of hedge pruning on macadamia nut yields. Hawaii Farm. Sci. 1971, 20, 8–12. [Google Scholar]
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Webster, A. Rootstocks for temperate fruit crops: Current uses, future potential and alternative strategies. In ISHS Acta Horticulturae 557, Proceedings of the VII International Symposium on Orchard and Plantation Systems, Nelson, New Zealand, 30 January–5 February 2000; ISHS: Leuven, Belgium, 2001; pp. 25–34. [Google Scholar]
- Rom, R.C.; Carlson, R.F. Rootstocks for Fruit Crops; Wiley: Hoboken, NJ, USA, 1987. [Google Scholar]
- Lauri, P.-E.; Costes, E.; Regnard, J.-L.; Brun, L.; Simon, S.; Monney, P.; Sinoquet, H. Does knowledge on fruit tree architecture and its implications for orchard management improve horticultural sustainability? An overview of recent advances in the apple. In Proceedings of the I International Symposium on Horticulture in Europe 817, Wien, Austria, 17–20 February 2008; pp. 243–250. [Google Scholar]
- Normand, F.; Bello, A.K.P.; Trottier, C.; Lauri, P.-E. Is axis position within tree architecture a determinant of axis morphology, branching, flowering and fruiting? An essay in mango. Ann. Bot. 2009, 103, 1325–1336. [Google Scholar] [CrossRef]
- Thompson, T.; Grauke, L. Pecan tree growth and precocity. J. Am. Soc. Hortic. Sci. 2003, 128, 63–66. [Google Scholar] [CrossRef]
- Vukasovic, S.; Alahmad, S.; Christopher, J.; Snowdon, R.J.; Stahl, A.; Hickey, L.T. Dissecting the Genetics of Early Vigour to Design Drought-Adapted Wheat. Front. Plant Sci. 2021, 12, 754439. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Mahmood, T.; Iqbal, M.S.; Li, H.; Nazir, M.F.; Khalid, S.; Sarfraz, Z.; Hu, D.; Baojun, C.; Geng, X.; Tajo, S.M. Differential seedling growth and tolerance indices reflect drought tolerance in cotton. BMC Plant Biol. 2022, 22, 331. [Google Scholar] [CrossRef]
- Srivastava, R.; Kobayashi, Y.; Koyama, H.; Sahoo, L. Overexpression of cowpea NAC transcription factors promoted growth and stress tolerance by boosting photosynthetic activity in Arabidopsis. Plant Sci. 2022, 319, 111251. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.H. Juvenility and flowering in woody plants: A review. HortScience 1972, 7, 447–455. [Google Scholar] [CrossRef]
- Toft, B.; Alam, M.M.; Wilkie, J.D.; Topp, B.L. Phenotypic Association of Multi-scale Architectural Traits with Canopy Volume and Yield: Moving Toward High-density Systems for Macadamia. HortScience 2019, 54, 596–602. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- León, L.; de la Rosa, R.; Barranco, D.; Rallo, L. Breeding for early bearing in olive. HortScience 2007, 42, 499–502. [Google Scholar] [CrossRef]
- Donadio, L.C.; Lederman, I.E.; Roberto, S.R.; Stucchi, E.S. Dwarfing-canopy and rootstock cultivars for fruit trees. Rev. Bras. Frutic. 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Sadok, I.B.; Moutier, N.; Garcia, G.; Dosba, F.; Grati-Kamoun, N.; Rebai, A.; Khadari, B.; Costes, E. Genetic determinism of the vegetative and reproductive traits in an F1 olive tree progeny. Tree Genet. Genomes 2013, 9, 205–221. [Google Scholar] [CrossRef]
- Chandrababu, R.; Sharma, R. Heritability estimates in almond [Prunus dulcis (Miller) DA Webb]. Sci. Hortic. 1999, 79, 237–243. [Google Scholar] [CrossRef]
- Foster, T.M.; Celton, J.-M.; Chagné, D.; Tustin, D.S.; Gardiner, S.E. Two quantitative trait loci, Dw1 and Dw2, are primarily responsible for rootstock-induced dwarfing in apple. Hortic. Res. 2015, 2, 15001. [Google Scholar] [CrossRef]
- Knäbel, M.; Friend, A.P.; Palmer, J.W.; Diack, R.; Wiedow, C.; Alspach, P.; Deng, C.; Gardiner, S.E.; Tustin, D.S.; Schaffer, R. Genetic control of pear rootstock-induced dwarfing and precocity is linked to a chromosomal region syntenic to the apple Dw1 loci. BMC Plant Biol. 2015, 15, 230. [Google Scholar] [CrossRef]
- Asad, H.U. Improving Mango Breeding Efficiency through Improved Pollen Storage, Fruit Retention and Understanding of the Heritability of Quantitative Tree Architectural Traits. Ph.D. Thesis, James Cook University, Singapore, 2017. [Google Scholar]
- Ding, C.; Hamann, A.; Yang, R.-C.; Brouard, J.S. Genetic parameters of growth and adaptive traits in aspen (Populus tremuloides): Implications for tree breeding in a warming world. PLoS ONE 2020, 15, e0229225. [Google Scholar] [CrossRef]
- Toft, B.D.; Alam, M.; Topp, B. Estimating genetic parameters of architectural and reproductive traits in young macadamia cultivars. Tree Genet. Genomes 2018, 14, 50. [Google Scholar] [CrossRef]
- Hardner, C.M.; Winks, C.W.; Stephenson, R.A.; Gallagher, E.G.; McConchie, C.A. Genetic parameters for yield in macadamia. Euphytica 2002, 125, 255–264. [Google Scholar] [CrossRef]
- Mai, T.T.; Hardner, C.M.; Alam, M.M.; Henry, R.J.; Topp, B.L. Phenotypic Characterisation for Growth and Nut Characteristics Revealed the Extent of Genetic Diversity in Wild Macadamia Germplasm. Agriculture 2021, 11, 680. [Google Scholar] [CrossRef]
- Bridgwater, F.; Stonecypher, R. Genotype x environment interaction: Implications in tree breeding programs. In Proceedings of the 5th North American Forest Biology Workshop edited by CA HOLLIS and AE SQUILLACE, Gainesville, FL, USA, 13–15 March 1978; pp. 46–63. [Google Scholar]
- Boopathi, N.M. Genetic Mapping and Marker Assisted Selection; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wu, H.X.; Matheson, A.C. Genotype by environment interactions in an Australia-wide radiata pine diallel mating experiment: Implications for regionalized breeding. For. Sci. 2005, 51, 29–40. [Google Scholar]
- Segura, V.; Cilas, C.; Costes, E. Dissecting apple tree architecture into genetic, ontogenetic and environmental effects: Mixed linear modelling of repeated spatial and temporal measures. New Phytol. 2008, 178, 302–314. [Google Scholar] [CrossRef]
- Burgueño, J.; Crossa, J.; Cornelius, P.L.; Yang, R.C. Using factor analytic models for joining environments and genotypes without crossover genotype× environment interaction. Crop Sci. 2008, 48, 1291–1305. [Google Scholar] [CrossRef]
- Lindgren, D.; Ying, C. A model integrating seed source adaptation and seed use. New For. 2000, 20, 87–104. [Google Scholar] [CrossRef]
- Hardner, C.M.; Healey, A.L.; Downes, G.; Herberling, M.; Gore, P.L. Improving prediction accuracy and selection of open-pollinated seed-lots in Eucalyptus dunnii Maiden using a multivariate mixed model approach. Ann. For. Sci. 2016, 73, 1035–1046. [Google Scholar] [CrossRef]
- Bally, I.S.; De Faveri, J. Genetic analysis of multiple fruit quality traits in mango across sites and years. Euphytica 2021, 217, 44. [Google Scholar] [CrossRef]
- Koo, Y.; Yeo, J.; Woo, K.; Kim, T. Selection of superior clones by stability analysis of growth performance in Populus davidiana Dode at age 12. Silvae Genet. 2007, 56, 93–100. [Google Scholar] [CrossRef][Green Version]
- Ben Sadok, I.; Martinez, S.; Moutier, N.; Garcia, G.; Leon, L.; Belaj, A.; De La Rosa, R.; Khadari, B.; Costes, E. Plasticity in vegetative growth over contrasted growing sites of an F1 olive tree progeny during its juvenile phase. PLoS ONE 2015, 10, e0127539. [Google Scholar] [CrossRef] [PubMed]
- Hardner, C.; e Silva, J.C.; Williams, E.; Meyers, N.; McConchie, C. Breeding new cultivars for the Australian macadamia industry. HortScience 2019, 54, 621–628. [Google Scholar] [CrossRef]
- Hardner, C. Exploring opportunities for reducing complexity of genotype-by-environment interaction models. Euphytica 2017, 213, 248. [Google Scholar] [CrossRef]
- Peace, C.; Hardner, C.; Brown, A.; O’Connor, K.; Vithanage, V.; Turnbull, C.; Carroll, B. The diversity and origins of macadamia cultivars. WANATCA Yearb. 2002, 26, 19–25. [Google Scholar]
- Jones, K.; Mayer, D. The Australian macadamia industry: The past and the future. S. Afr. Macadamia Growers. Assoc. Yearb. 2009, 17, 98–102. [Google Scholar]
- Hardner, C. Macadamia domestication in Hawaii. Genet. Resour. Crop Evol. 2016, 63, 1411–1430. [Google Scholar] [CrossRef]
- Topp, B.; Hardner, C.; Neal, J.; Kelly, A.; Russell, D.; McConchie, C.; O’Hare, P. Overview of the Australian macadamia industry breeding program. In ISHS Acta Horticulturae 1127, Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): International Symposium on Plant Breeding in Horticulture, Brisbane, Australia, 17–22 August 2014; ISHS: Leuven, Belgium, 2016; pp. 45–50. [Google Scholar]
- Bell, H.; Bell, D.; Winks, C. Macadamia tree breeding and selection program update 1987. In Proceedings of the Second Macadamia Research Workshop, Bangalow, Australia, 15–19 September 1987; pp. 37–48. [Google Scholar]
- Hidden Valley. Available online: https://hvp-macadamias.com/About-Hidden-Valley/ (accessed on 21 June 2023).
- Topp, B. Macadamia Second Generation Breeding and Conservation. 2019. Available online: https://www.horticulture.com.au/globalassets/laserfiche/assets/project-reports/mc14000/mc14000-final-report-complete.pdf (accessed on 21 June 2023).
- Russell, D. Macadamia Regional Variety Trials Series 3, Phase 2. 2018. Available online: https://www.horticulture.com.au/globalassets/laserfiche/assets/project-reports/mc11001/mc11001---final-report-complete.pdf (accessed on 21 June 2023).
- Weibel, A.; Johnson, R.S.; DeJong, T.M. Comparative vegetative growth responses of two peach cultivars grown on size-controlling versus standard rootstocks. J. Am. Soc. Hortic. Sci. 2003, 128, 463–471. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.-R.; Primo-Capella, A.; Forner-Giner, M.A. Influence of rootstock on Citrus tree growth: Effects on photosynthesis and carbohydrate distribution, plant size, yield, fruit quality, and dwarfing genotypes. Plant Growth 2016, 16, 107. [Google Scholar]
- Webster, A. Rootstock and interstock effects on deciduous fruit tree vigour, precocity, and yield productivity. N. Z. J. Crop Hortic. Sci. 1995, 23, 373–382. [Google Scholar] [CrossRef]
- Bowman, K.D.; Faulkner, L.; Kesinger, M. New citrus rootstocks released by USDA 2001–2010: Field performance and nursery characteristics. HortScience 2016, 51, 1208–1214. [Google Scholar] [CrossRef]
- Fazio, G.; Robinson, T.L.; Aldwinckle, H.S. The Geneva apple rootstock breeding program. Plant Breed. Rev. 2015, 39, 379–424. [Google Scholar]
- Gómez Aparisi, J.; Lombarte, P.; Felipe, A.; Carrera, M. First results on the performance of new almond × peach hybrid rootstocks resistant to nematodes on almond growth and cropping. In ISHS Acta Horticulturae 591, Proceedings of the III International Symposium on Pistachios and Almonds, Zaragoza, Spain, Spain, 20–24 May 2001; ISHS: Leuven, Belgium, 2002. [Google Scholar]
- Bally, I.; De Faveri, J. Breeding for low vigour in mango. In ISHS Acta Horticulturae 1183, Proceedings of the XI International Mango Symposium, Darwin, Australia, 28 September–2 October 2015; ISHS: Leuven, Belgium, 2017; pp. 63–68. [Google Scholar]
- Flachowsky, H.; Hanke, M.V.; Peil, A.; Strauss, S.; Fladung, M. A review on transgenic approaches to accelerate breeding of woody plants. Plant Breed. 2009, 128, 217–226. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; Rapoport, H.F.; León, L.; De la Rosa, R. Olive seedling first-flowering position and management. Sci. Hortic. 2010, 124, 74–77. [Google Scholar] [CrossRef]
- Myles, S. Improving fruit and wine: What does genomics have to offer? Trends Genet. 2013, 29, 190–196. [Google Scholar] [CrossRef]
- Iwata, H.; Minamikawa, M.F.; Kajiya-Kanegae, H.; Ishimori, M.; Hayashi, T. Genomics-assisted breeding in fruit trees. Breed. Sci. 2016, 66, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Islam, K.; Latif, M.A. A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int. J. Mol. Sci. 2013, 14, 22499–22528. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Yee, L.K.; Zee, F.T.; Manshardt, R.M. Genetic variability in Macadamia. Genet. Resour. Crop Evol. 1998, 45, 19–32. [Google Scholar] [CrossRef]
- Peace, C.; Allan, P.; Vithanage, V.; Turnbull, C.; Carroll, B. Genetic relationships amongst macadamia varieties grown in South Africa as assessed by RAF markers. S. Afr. J. Plant Soil. 2005, 22, 71–75. [Google Scholar] [CrossRef]
- Machado Neto, N.B.; Moryia, A.T. Variability in Macadamia integrifolia by RAPD markers. Crop Breed. Appl. Biotechnol. 2010, 10, 266–270. [Google Scholar] [CrossRef]
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Hopper, C. Diversity arrays technology: A generic genome profiling technology on open platforms. In Data Production and Analysis in Population Genomics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 67–89. [Google Scholar]
- Alam, M.; Neal, J.; O’Connor, K.; Kilian, A.; Topp, B. Ultra-high-throughput DArTseq-based silicoDArT and SNP markers for genomic studies in macadamia. PLoS ONE 2018, 13, e0203465. [Google Scholar] [CrossRef]
- O’Connor, K.; Powell, M.; Nock, C.; Shapcott, A. Crop to wild gene flow and genetic diversity in a vulnerable Macadamia (Proteaceae) species in New South Wales, Australia. Biol. Conserv. 2015, 191, 504–511. [Google Scholar] [CrossRef]
- Mai, T.; Alam, M.; Hardner, C.; Henry, R.; Topp, B. Genetic Structure of Wild Germplasm of Macadamia: Species Assignment, Diversity and Phylogeographic Relationships. Plants 2020, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef]
- Collard, B.C.; Jahufer, M.; Brouwer, J.; Pang, E. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wing, R.A. Genome mapping in plants. Curr. Opin. Biotechnol. 1993, 4, 142–147. [Google Scholar] [CrossRef]
- Peace, C.P.; Vithanage, V.; Turnbull, C.G.; Carroll, B.J. A genetic map of macadamia based on randomly amplified DNA fingerprinting (RAF) markers. Euphytica 2003, 134, 17–26. [Google Scholar] [CrossRef]
- Langdon, K.S.; King, G.J.; Baten, A.; Mauleon, R.; Bundock, P.C.; Topp, B.L.; Nock, C.J. Maximising recombination across macadamia populations to generate linkage maps for genome anchoring. Sci. Rep. 2020, 10, 5048. [Google Scholar] [CrossRef]
- Risch, N. Genetic linkage: Interpreting LOD scores. Science 1992, 255, 803–805. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Yang, J.; Hu, C.; Hu, H.; Yu, R.; Xia, Z.; Ye, X.; Zhu, J. QTLNetwork: Mapping and visualizing genetic architecture of complex traits in experimental populations. Bioinformatics 2008, 24, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Utz, H.F.; Melchinger, A.E. PLABQTL: A Program for Composite Interval Mapping of QTL; Institut fuer Pflanzenzuechtung, Saatgutforschung und Populationsgenetik, Universitaet Hohenheim: Stuttgart, Germany, 1996. [Google Scholar]
- Joehanes, R.; Nelson, J.C. QGene 4.0, an extensible Java QTL-analysis platform. Bioinformatics 2008, 24, 2788–2789. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, R.R.; Celton, J.; Gardiner, S.; Tustin, D. Genetic markers linked to the dwarfing trait of apple rootstock ‘Malling 9’. J. Am. Soc. Hortic. Sci. 2008, 133, 100–106. [Google Scholar] [CrossRef]
- Conson, A.R.; Taniguti, C.H.; Amadeu, R.R.; Andreotti, I.A.; De Souza, L.M.; Dos Santos, L.H.; Rosa, J.R.; Mantello, C.C.; Da Silva, C.C.; José Scaloppi Junior, E. High-resolution genetic map and QTL analysis of growth-related traits of Hevea brasiliensis cultivated under suboptimal temperature and humidity conditions. Front. Plant Sci. 2018, 9, 1255. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Wünsch, A. Multiple-population QTL mapping of maturity and fruit-quality traits reveals LG4 region as a breeding target in sweet cherry (Prunus avium L.). Hortic. Res. 2020, 7, 127. [Google Scholar] [CrossRef]
- Curtolo, M.; Cristofani-Yaly, M.; Gazaffi, R.; Takita, M.A.; Figueira, A.; Machado, M.A. QTL mapping for fruit quality in Citrus using DArTseq markers. BMC Genom. 2017, 18, 289. [Google Scholar] [CrossRef]
- Khan, M.A.; Korban, S.S. Association mapping in forest trees and fruit crops. J. Exp. Bot. 2012, 63, 4045–4060. [Google Scholar] [CrossRef]
- Hayes, B.; Goddard, M. Genome-wide association and genomic selection in animal breeding. Genome 2010, 53, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Myles, S.; Peiffer, J.; Brown, P.J.; Ersoz, E.S.; Zhang, Z.; Costich, D.E.; Buckler, E.S. Association mapping: Critical considerations shift from genotyping to experimental design. Plant Cell 2009, 21, 2194–2202. [Google Scholar] [CrossRef]
- Kumar, S.; Garrick, D.J.; Bink, M.C.; Whitworth, C.; Chagné, D.; Volz, R.K. Novel genomic approaches unravel genetic architecture of complex traits in apple. BMC Genom. 2013, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Akinsanmi, O.A.; Topp, B.; Drenth, A. Pericarps retained in the tree canopy and stomatal abundance are components of resistance to husk spot caused by Pseudocercospora macadamiae in macadamia. Euphytica 2012, 185, 313–323. [Google Scholar] [CrossRef]
- O’Connor, K.; Hayes, B.; Hardner, C.; Alam, M.; Topp, B. Selecting for nut characteristics in macadamia using a genome-wide association study. HortScience 2019, 54, 629–632. [Google Scholar] [CrossRef]
- O’Connor, K.; Hayes, B.; Topp, B. Prospects for increasing yield in macadamia using component traits and genomics. Tree Genet. Genomes 2018, 14, 7. [Google Scholar] [CrossRef]
- O’Connor, K.; Hayes, B.; Hardner, C.; Nock, C.; Baten, A.; Alam, M.; Henry, R.; Topp, B. Genome-wide association studies for yield component traits in a macadamia breeding population. BMC Genom. 2020, 21, 199. [Google Scholar] [CrossRef]
- McClure, K.A.; Gardner, K.M.; Douglas, G.M.; Song, J.; Forney, C.F.; DeLong, J.; Fan, L.; Du, L.; Toivonen, P.M.; Somers, D.J. A genome-wide association study of apple quality and scab resistance. Plant Genome 2018, 11, 170075. [Google Scholar] [CrossRef]
- Minamikawa, M.F.; Nonaka, K.; Kaminuma, E.; Kajiya-Kanegae, H.; Onogi, A.; Goto, S.; Yoshioka, T.; Imai, A.; Hamada, H.; Hayashi, T. Genome-wide association study and genomic prediction in citrus: Potential of genomics-assisted breeding for fruit quality traits. Sci. Rep. 2017, 7, 4721. [Google Scholar] [CrossRef]
- Imai, A.; Nonaka, K.; Kuniga, T.; Yoshioka, T.; Hayashi, T. Genome-wide association mapping of fruit-quality traits using genotyping-by-sequencing approach in citrus landraces, modern cultivars, and breeding lines in Japan. Tree Genet. Genomes 2018, 14, 24. [Google Scholar] [CrossRef]
- Nishio, S.; Hayashi, T.; Yamamoto, T.; Terakami, S.; Iwata, H.; Imai, A.; Takada, N.; Kato, H.; Saito, T. Bayesian genome-wide association study of nut traits in Japanese chestnut. Mol. Breed. 2018, 38, 99. [Google Scholar] [CrossRef]
- Iwata, H.; Hayashi, T.; Terakami, S.; Takada, N.; Sawamura, Y.; Yamamoto, T. Potential assessment of genome-wide association study and genomic selection in Japanese pear Pyrus pyrifolia. Breed. Sci. 2013, 63, 125–140. [Google Scholar] [CrossRef]
- Da Silva Linge, C.; Cai, L.; Fu, W.; Clark, J.; Worthington, M.; Rawandoozi, Z.; Byrne, D.H.; Gasic, K. Multi-locus genome-wide association studies reveal fruit quality hotspots in peach genome. Front. Plant Sci. 2021, 12, 644799. [Google Scholar] [CrossRef] [PubMed]
- Langridge, P.; Lagudah, E.; Holton, T.; Appels, R.; Sharp, P.; Chalmers, K. Trends in genetic and genome analyses in wheat: A review. Aust. J. Agric. Res. 2001, 52, 1043–1077. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Newell, M.A.; Jannink, J.-L. Genomic selection in plant breeding. In Crop Breeding; Springer: Berlin/Heidelberg, Germany, 2014; pp. 117–130. [Google Scholar]
- Desta, Z.A.; Ortiz, R. Genomic selection: Genome-wide prediction in plant improvement. Trends Plant Sci. 2014, 19, 592–601. [Google Scholar] [CrossRef]
- Howard, R.; Jarquin, D.; Crossa, J. Overview of Genomic Prediction Genomic predictions (GP) Methods and the Associated Assumptions on the Variance of Marker Effect, and on the Architecture of the Target Trait. In Genomic Prediction of Complex Traits: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 139–156. [Google Scholar]
- Montesinos López, O.A.; Montesinos López, A.; Crossa, J. Multivariate Statistical Machine Learning Methods for Genomic Prediction; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Minamikawa, M.F.; Takada, N.; Terakami, S.; Saito, T.; Onogi, A.; Kajiya-Kanegae, H.; Hayashi, T.; Yamamoto, T.; Iwata, H. Genome-wide association study and genomic prediction using parental and breeding populations of Japanese pear (Pyrus pyrifolia Nakai). Sci. Rep. 2018, 8, 11994. [Google Scholar] [CrossRef]
- Heffner, E.L.; Sorrells, M.E.; Jannink, J.-L. Genomic Selection for Crop Improvement; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Topp, B.; Hardner, C.; Kelly, A. Strategies for breeding macadamias in Australia. In ISHS Acta Horticulturae 935, Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on New, Lisbon, Portugal, 22–27 August 2010; ISHS: Leuven, Belgium, 2012; pp. 47–53. [Google Scholar]
- O’Connor, K.M.; Hayes, B.J.; Hardner, C.M.; Alam, M.; Henry, R.J.; Topp, B.L. Genomic selection and genetic gain for nut yield in an Australian macadamia breeding population. BMC Genom. 2021, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Di Guardo, M.; Guerra, W.; Muranty, H.; Patocchi, A.; Costa, F. Prediction of fruit texture with training population optimization for efficient genomic selection in apple. Hortic. Res. 2020, 7, 148. [Google Scholar] [CrossRef]
- Ribeyre, F.; Sounigo, O.; Argout, X.; Cilas, C.; Efombagn, M.I.B.; Denis, M.; Bouvet, J.-M.; Fouet, O.; Lanaud, C. The Genomic Selection of Theobroma cacao: A new strategy of marker assisted selection to improve breeding efficiency and predict useful traits in new populations. In Proceedings of the International Symposium on Cocoa Research, Lima, Peru, 13–17 November 2017. [Google Scholar]
- Rambolarimanana, T.; Ramamonjisoa, L.; Verhaegen, D.; Tsy, J.-M.L.P.; Jacquin, L.; Cao-Hamadou, T.-V.; Makouanzi, G.; Bouvet, J.-M. Performance of multi-trait genomic selection for Eucalyptus robusta breeding program. Tree Genet. Genomes 2018, 14, 71. [Google Scholar] [CrossRef]
- Biscarini, F.; Nazzicari, N.; Bink, M.; Arús, P.; Aranzana, M.J.; Verde, I.; Micali, S.; Pascal, T.; Quilot-Turion, B.; Lambert, P. Genome-enabled predictions for fruit weight and quality from repeated records in European peach progenies. BMC Genom. 2017, 18, 432. [Google Scholar] [CrossRef]
- Fodor, A.; Peros, J.-P.; Launay, A.; Doligez, A.; Berger, G.; Bertrand, Y.; Roques, M.; Beccavin, I.; Le Paslier, M.-C.; Romieu, C. Genome-Wide Association Studies (GWAS) and Genomic Selection (GS) in grape: Evaluation of phenotypic prediction methods using a large panel of diversity. In Proceedings of the XI International Conference on Grapevine Breeding and Genetics, Beijing, China, 28 July–2 August 2014. [Google Scholar]
- Bartholomé, J.; Van Heerwaarden, J.; Isik, F.; Boury, C.; Vidal, M.; Plomion, C.; Bouffier, L. Performance of genomic prediction within and across generations in maritime pine. BMC Genom. 2016, 17, 604. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Baison, J.; Pan, J.; Karlsson, B.; Andersson, B.; Westin, J.; García-Gil, M.R.; Wu, H.X. Accuracy of genomic selection for growth and wood quality traits in two control-pollinated progeny trials using exome capture as the genotyping platform in Norway spruce. BMC Genom. 2018, 19, 946. [Google Scholar] [CrossRef]
- Kwong, Q.B.; Ong, A.L.; Teh, C.K.; Chew, F.T.; Tammi, M.; Mayes, S.; Kulaveerasingam, H.; Yeoh, S.H.; Harikrishna, J.A.; Appleton, D.R. Genomic selection in commercial perennial crops: Applicability and improvement in oil palm (Elaeis guineensis Jacq.). Sci. Rep. 2017, 7, 2872. [Google Scholar] [CrossRef]
- Souza, L.M.; Francisco, F.R.; Gonçalves, P.S.; Scaloppi Junior, E.J.; Le Guen, V.; Fritsche-Neto, R.; Souza, A.P. Genomic selection in rubber tree breeding: A comparison of models and methods for managing G× E interactions. Front. Plant Sci. 2019, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.P.; Resende, M.D.V.d.; Riaz, S.; Walker, M.A. Genome selection in fruit breeding: Application to table grapes. Sci. Agric. 2016, 73, 142–149. [Google Scholar] [CrossRef][Green Version]
- Mir, R.R.; Reynolds, M.; Pinto, F.; Khan, M.A.; Bhat, M.A. High-throughput phenotyping for crop improvement in the genomics era. Plant Sci. 2019, 282, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 107. [Google Scholar] [PubMed]
- Krause, S.; Sanders, T.G.; Mund, J.-P.; Greve, K. UAV-based photogrammetric tree height measurement for intensive forest monitoring. Remote Sens. 2019, 11, 758. [Google Scholar] [CrossRef]
- Dempewolf, J.; Nagol, J.; Hein, S.; Thiel, C.; Zimmermann, R. Measurement of within-season tree height growth in a mixed forest stand using UAV imagery. Forests 2017, 8, 231. [Google Scholar] [CrossRef]
- Atefi, A.; Ge, Y.; Pitla, S.; Schnable, J. Robotic technologies for high-throughput plant phenotyping: Contemporary reviews and future perspectives. Front. Plant Sci. 2021, 12, 611940. [Google Scholar] [CrossRef] [PubMed]
- Nabwire, S.; Suh, H.-K.; Kim, M.S.; Baek, I.; Cho, B.-K. Application of artificial intelligence in phenomics. Sensors 2021, 21, 4363. [Google Scholar] [CrossRef] [PubMed]
- Junker, A.; Muraya, M.M.; Weigelt-Fischer, K.; Arana-Ceballos, F.; Klukas, C.; Melchinger, A.E.; Meyer, R.C.; Riewe, D.; Altmann, T. Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems. Front. Plant Sci. 2015, 5, 770. [Google Scholar] [CrossRef]
- Bally, I.S.E.; Ibell, P.T. Improvement of mango tree architecture. Acta Hortic. 2015, 1075, 59–64. [Google Scholar] [CrossRef]
- King, D.A. Correlations between biomass allocation, relative growth rate and light environment in tropical forest saplings. Funct. Ecol. 1991, 5, 485–492. [Google Scholar] [CrossRef]
- Nesme, T.; Plenet, D.; Hucbourg, B.; Fandos, G.; Lauri, P.-E. A set of vegetative morphological variables to objectively estimate apple (Malus× domestica) tree orchard vigour. Sci. Hortic. 2005, 106, 76–90. [Google Scholar] [CrossRef]
- Srivastava, K.; Kumar, D.; Singh, S.; Sharma, O. Effect of cultivars on tree growth, yield and quality attributes of apple on espalier architecture under high density planting system. J. Hortic. Sci. 2019, 14, 20–25. [Google Scholar] [CrossRef]
- Pearce, S. Studies in the measurement of apple trees. I. The use of trunk girths to estimate tree size. Ann. Rept. E. Malling Res. Sta. 1952, 1951, 101104. [Google Scholar]
- Whiting, M.D.; Lang, G.; Ophardt, D. Rootstock and training system affect sweet cherry growth, yield, and fruit quality. HortScience 2005, 40, 582–586. [Google Scholar] [CrossRef]
- Broeckx, L.S.; Verlinden, M.S.; Vangronsveld, J.; Ceulemans, R. Importance of crown architecture for leaf area index of different Populus genotypes in a high-density plantation. Tree Physiol. 2012, 32, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.D. Vigour mechanisms in dwarfing rootstocks for temperate trees. In Proceedings of Internatinal Symposium on Rootstocks for Deciduous Fruit Tree Species, Zaragoza, Spain, 11–14 June 2002; pp. 29–41. [Google Scholar]
- Solar, A.; Hudina, M.; Stampar, F. Relationship between tree architecture, phenological data and generative development in walnut (Juglans regia L.). In ISHS Acta Horticulturae 544, Proceedings of the IV International Walnut Symposium, Bordeaux, France, 13-16 September 1999; ISHS: Leuven, Belgium, 2001. [Google Scholar]
- Abirami, K.; Singh, R.; Baskaran, V. Studies on the influence of seedling physiological parameters with vigour in some polyembryonic and monoembryonic mango genotypes. Indian. J. Hortic. 2011, 68, 18–23. [Google Scholar]
- Muhammad, S.; Lubna, S. Anatomical studies of stems, roots and leaves of selected citrus rootstock varieties in relation to their vigour. J. Hortic. For. 2010, 2, 87–94. [Google Scholar]
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Forests and Vegetation; Springer: Berlin/Heidelberg, Germany, 1978; pp. 332–385. [Google Scholar]
- Montesinos, Á.; Thorp, G.; Grimplet, J.; Rubio-Cabetas, M.J. Phenotyping Almond Orchards for Architectural Traits Influenced by Rootstock Choice. Horticulturae 2021, 7, 159. [Google Scholar] [CrossRef]
- Hooijdonk, V.; Woolley, D.J.; Warrington, I.J.; Tustin, D.S. Initial alteration of scion architecture by dwarfing apple rootstocks may involve shoot-root-shoot signalling by auxin, gibberellin, and cytokinin. J. Hortic. Sci. Biotechnol. 2010, 85, 59–65. [Google Scholar] [CrossRef]
- Kikuchi, T.; Shiozaki, Y. Apple Canopies as Populations of Branches: A New Concept for Measuring Tree Vigor. In Proceedings of VIII International Symposium on Canopy, Rootstocks and Environmental Physiology in Orchard Systems 732, Budapest, Hungary, 13–18 June 2004; pp. 675–680. [Google Scholar]
- Tworkoski, T.; Miller, S. Rootstock effect on growth of apple scions with different growth habits. Sci. Hortic. 2007, 111, 335–343. [Google Scholar] [CrossRef]
- Neal, J.; Kelly, A.; Hardner, C.; McConchie, C.; Topp, B. Preliminary evaluation of macadamia rootstocks for yield and tree height. In ISHS Acta Horticulturae 1109, Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): International Symposium on Nut Crops, Brisbane, Australia, 17–22 August 2014; ISHS: Leuven, Belgium, 2016; pp. 181–188. [Google Scholar]
- Bassal, M.A. Growth, yield and fruit quality of ‘Marisol’ clementine grown on four rootstocks in Egypt. Sci. Hortic. 2009, 119, 132–137. [Google Scholar] [CrossRef]
- Sitarek, M.; Bartosiewicz, B. Influence of a few seedling rootstocks on the growth, yield and fruit quality of apricot trees. J. Fruit. Ornam. Plant Res. 2011, 19, 81–86. [Google Scholar]
- Tsipouridis, C.; Thomidis, T. Effect of 14 peach rootstocks on the yield, fruit quality, mortality, girth expansion and resistance to frost damages of May Crest peach variety and their susceptibility on Phytophthora citrophthora. Sci. Hortic. 2005, 103, 421–428. [Google Scholar] [CrossRef]
- Santos, A.; Ribeiro, R.; Crespí, A.L. Sweet cherry (Prunus avium) growth is mostly affected by rootstock and much less by budding height. N. Z. J. Crop Hortic. Sci. 2004, 32, 309–318. [Google Scholar] [CrossRef][Green Version]
- Solari, L.I.; Johnson, S.; Dejong, T.M. Hydraulic conductance characteristics of peach (Prunus persica) trees on different rootstocks are related to biomass production and distribution. Tree Physiol. 2006, 26, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Fideghelli, C.; Sartori, A.; Grassi, F. Fruit tree size and architecture. In Proceedings of the XXVI International Horticultural Congress: Genetics and Breeding of Tree Fruits and Nuts 622, Toronto, ON, Canada, 11–17 August 2002; pp. 279–293. [Google Scholar]
- Fazio, G.; Lordan, J.; Francescatto, P.; Robinson, T. Breeding apple rootstocks to match cultural and nutrient requirements of scionvarieties. FRUIT Q. 2018, 26, 25–30. [Google Scholar]
- Osterc, G.; Ljubljana, G.; Spethmann, W. Growth comparison of Prunus rootstocks propagated by stooling, cuttings and in vitro--Part II: Budded Trees. Erwerbsobstbau 2002, 44, 145–152. [Google Scholar]
- Vyvyan, M. Interrelation of scion and rootstock in fruit-trees: I. Weights and relative weights of young trees formed by the reciprocal unions, as scion and rootstock, of three apple rootstock varieties: M. IX, M. IV, and M. XII. Ann. Bot. 1955, 19, 401–423. [Google Scholar] [CrossRef]
- Giménez, C.; Gallardo, M.; Thompson, R.B. Plant–Water Relations. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Olmstead, M.A.; Lang, N.S.; Ewers, F.W.; Owens, S.A. Xylem vessel anatomy of sweet cherries grafted onto dwarfing and nondwarfing rootstocks. J. Am. Soc. Hortic. Sci. 2006, 131, 577–585. [Google Scholar] [CrossRef]
- Sperry, J.S.; Tyree, M.T. Mechanism of Water Stress-Induced Xylem Embolism. Plant Physiol. 1988, 88, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Basile, B.; Marsal, J.; DeJong, T.M. Daily shoot extension growth of peach trees growing on rootstocks that reduce scion growth is related to daily dynamics of stem water potential. Tree Physiol. 2003, 23, 695–704. [Google Scholar] [CrossRef]
- Jupa, R.; Mészáros, M.; Plavcová, L. Linking wood anatomy with growth vigour and susceptibility to alternate bearing in composite apple and pear trees. Plant Biol. 2021, 23, 172–183. [Google Scholar] [CrossRef]
- Cohen, S.; Naor, A. The effect of three rootstocks on water use, canopy conductance and hydraulic parameters of apple trees and predicting canopy from hydraulic conductance. Plant Cell Environ. 2002, 25, 17–28. [Google Scholar] [CrossRef]
- Kamboj, J.; Blake, P.; Quinlan, J.; Webster, A.; Baker, D. Recent advances in studies on the dwarfing mechanism of apple rootstocks. In ISHS Acta Horticulturae 451, Proceedings of the VI International Symposium on Integrated Canopy, Rootstock, Environmental Physiology in Orchard Systems, Wenatchee, WC, USA; Penticton, BC, Canada; ISHS: Leuven, Belgium, 1997; pp. 75–82. [Google Scholar]
- Zorić, L.; Ljubojević, M.; Merkulov, L.; Luković, J.; Ognjanov, V. Anatomical characteristics of cherry rootstocks as possible preselecting tools for prediction of tree vigor. J. Plant Growth Regul. 2012, 31, 320–331. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D.; Singh, A.; Pagliocchini, C. Rootstock–Scion Hydraulic Balance Influenced Scion Vigor and Yield Efficiency of Malus domestica cv. Honeycrisp on Eight Rootstocks. Horticulturae 2021, 7, 99. [Google Scholar] [CrossRef]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; Dejong, T.M. Relationships between xylem vessel characteristics, calculated axial hydraulic conductance and size-controlling capacity of peach rootstocks. Ann. Bot. 2010, 105, 327–331. [Google Scholar] [CrossRef]
- Nardini, A.; Gascó, A.; Raimondo, F.; Gortan, E.; Lo Gullo, M.A.; Caruso, T.; Salleo, S. Is rootstock-induced dwarfing in olive an effect of reduced plant hydraulic efficiency? Tree Physiol. 2006, 26, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Lauri, P.é.; Gorza, O.; Cochard, H.; Martinez, S.; CELTON, J.M.; Ripetti, V.; Lartaud, M.; Bry, X.; Trottier, C.; Costes, E. Genetic determinism of anatomical and hydraulic traits within an apple progeny. Plant Cell Environ. 2011, 34, 1276–1290. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Zach, A.; Schuldt, B.; Brix, S.; Horna, V.; Culmsee, H.; Leuschner, C. Vessel diameter and xylem hydraulic conductivity increase with tree height in tropical rainforest trees in Sulawesi, Indonesia. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 506–512. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Intrigliolo, D.S.; Primo-Millo, E.; Forner-Giner, M.A. Relationships between xylem anatomy, root hydraulic conductivity, leaf/root ratio and transpiration in citrus trees on different rootstocks. Physiol. Plant. 2010, 139, 159–169. [Google Scholar] [CrossRef]
- Tombesi, S.; Almehdi, A.; Dejong, T.M. Phenotyping vigour control capacity of new peach rootstocks by xylem vessel analysis. Sci. Hortic. 2011, 127, 353–357. [Google Scholar] [CrossRef]
- Toft, B.; Alam, M.; Topp, B. Anatomical structure associated with vegetative growth variation in macadamia. Plant Soil. 2019, 444, 343–350. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Li, F.; Cohen, S.; Naor, A.; Shaozong, K.; Erez, A. Studies of canopy structure and water use of apple trees on three rootstocks. Agric. Water Manag. 2002, 55, 1–14. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Rodriguez-Gamir, J.; Martínez-Cuenca, M.-R.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Relationship between hydraulic conductance and citrus dwarfing by the Flying Dragon rootstock (Poncirus trifoliata L. Raft var. monstruosa). Trees 2013, 27, 629–638. [Google Scholar] [CrossRef]
- Beakbane, A.; Majumder, P. A relationship between stomatal density and growth potential in apple rootstocks. J. Hortic. Sci. 1975, 50, 285–289. [Google Scholar] [CrossRef]
- Wakefield, S.; Topp, B.; Alam, M. Crown Position and Rootstock Genotype Influence Leaf Stomatal Density in Macadamia sp. Biol. Life Sci. Forum 2022, 11, 9. [Google Scholar]
- Kalaji, M.H.; Pietkiewicz, S. Some physiological indices to be exploited as a crucial tool in plant breeding. Plant Breed. Seed Sci. 2004, 49, 19–39. [Google Scholar]
- Basile, B.; DeJong, T.M. Control of Fruit Tree Vigor Induced by Dwarfing Rootstocks. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 46, pp. 39–97. [Google Scholar]
- Hayat, F.; Asghar, S.; Yanmin, Z.; Xue, T.; Nawaz, M.A.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Qiu, C. Rootstock Induced Vigour is Associated with Physiological, Biochemical and Molecular Changes in ‘Red Fuji’Apple. Int. J. Agric. Biol. 2020, 24, 1823–1834. [Google Scholar]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of Endogenous Plant Hormones on Physiological and Growth Attributes of Kinnow Mandarin Grafted on Nine Rootstocks. J. Plant Growth Regul. 2021, 41, 1254–1264. [Google Scholar] [CrossRef]
- Steinberg, S.; McFarland, M.; Miller, J. Effect of water stress on stomatal conductance and leaf water relations of leaves along current-year branches of peach. Funct. Plant Biol. 1989, 16, 549–560. [Google Scholar] [CrossRef]
- Poni, S.; Tagliavini, M.; Neri, D.; Scudellari, D.; Toselli, M. Influence of root pruning and water stress on growth and physiological factors of potted apple, grape, peach and pear trees. Sci. Hortic. 1992, 52, 223–236. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Seleznyova, A.N.; Thorp, T.G.; Blattmann, P.; Barnett, A.M.; Lowe, R.G.; Austin, P.T. Vigor-controlling rootstocks affect early shoot growth and leaf area development of kiwifruit. Tree Physiol. 2006, 26, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Hott, C.; Tworkoski, T. Shade effects on growth, flowering and fruit of apple. J. Appl. Hortic. 2015, 17, 101–105. [Google Scholar] [CrossRef]
- Trimble, S. The Importance of Leaf Area Index (LAI) in Environmental and Crop Research; CID-Bioscience: Camas, WA, USA, 2019; Volume 2021. [Google Scholar]
- Anthony, B.; Serra, S.; Musacchi, S. Optimization of Light Interception, Leaf Area and Yield in “WA38”: Comparisons among Training Systems, Rootstocks and Pruning Techniques. Agronomy 2020, 10, 689. [Google Scholar] [CrossRef]
- Otieno, D.O.; Schmidt, M.; Adiku, S.; Tenhunen, J. Physiological and morphological responses to water stress in two Acacia species from contrasting habitats. Tree Physiol. 2005, 25, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, O.; Gogorcena, Y.; Moreno, M.A.; Pinochet, J. Graft compatibility between peach cultivars and Prunus rootstocks. HortScience 2006, 41, 1389–1394. [Google Scholar] [CrossRef]
- Soumelidou, K.; Battey, N.; John, P.; Barnett, J. The anatomy of the developing bud union and its relationship to dwarfing in apple. Ann. Bot. 1994, 74, 605–611. [Google Scholar] [CrossRef]
- Beakbane, A.B. Possible mechanisms of rootstock effect. Ann. Appl. Biol. 1956, 44, 517–521. [Google Scholar] [CrossRef]
- Giulivo, C.; Bergamini, A. Effect of rootstock-scion combination on water balance of apple tree, cv Golden Delicious. In Proceedings of Abstracts. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1399-3054.1986.tb05757.x (accessed on 21 June 2023).
- Atkins, C.A.; Smith, P.M.; Rodriguez-Medina, C. Macromolecules in phloem exudates—A review. Protoplasma 2011, 248, 165–172. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.; Hassan, G.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Li, M.; Harada, T.; Han, Z.; Li, T. Gibberellic acid insensitive mRNA transport in both directions between stock and scion in Malus. Tree Genet. Genomes 2010, 6, 1013–1019. [Google Scholar] [CrossRef]
- Haywood, V.; Yu, T.S.; Huang, N.C.; Lucas, W.J. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 2005, 42, 49–68. [Google Scholar] [CrossRef]
- Foster, T.M.; McAtee, P.A.; Waite, C.N.; Boldingh, H.L.; McGhie, T.K. Apple dwarfing rootstocks exhibit an imbalance in carbohydrate allocation and reduced cell growth and metabolism. Hortic. Res. 2017, 4, 17009. [Google Scholar] [CrossRef] [PubMed]
- Prassinos, C.; Ko, J.H.; Lang, G.; Iezzoni, A.F.; Han, K.H. Rootstock-induced dwarfing in cherries is caused by differential cessation of terminal meristem growth and is triggered by rootstock-specific gene regulation. Tree Physiol. 2009, 29, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.J.; Makalowska, I.; Altman, N.; Fazio, G.; Praul, C.; Maximova, S.N.; Crassweller, R.M.; Travis, J.W.; McNellis, T.W. Rootstock-regulated gene expression patterns in apple tree scions. Tree Genet. Genomes 2010, 6, 57–72. [Google Scholar] [CrossRef]
- Foster, T.M.; Watson, A.E.; van Hooijdonk, B.M.; Schaffer, R.J. Key flowering genes including FT-like genes are upregulated in the vasculature of apple dwarfing rootstocks. Tree Genet. Genomes 2014, 10, 189–202. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Shan, D.; Shi, K.; Wang, L.; Li, Q.; Wang, N.; Zhou, J.; Yao, J.; Xue, Y. Md WRKY 9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase Md DWF 4 expression. New Phytol. 2018, 217, 1086–1098. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, H.; Yu, C.; Ma, L.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock. Acta Physiol. Plant. 2012, 34, 235–244. [Google Scholar] [CrossRef]
- Bianco, R.L.; Rieger, M. Activities of Sucrose and Sorbitol Metabolizing Enzymes in Vegetative Sinks of Peach and Correlation with Sink Growth Rate. J. Am. Soc. Hortic. Sci. 1999, 124, 381–388. [Google Scholar] [CrossRef]
- Xu, D.; Qi, X.; Li, J.; Han, X.; Wang, J.; Jiang, Y.; Tian, Y.; Wang, Y. PzTAC and PzLAZY from a narrow-crown poplar contribute to regulation of branch angles. Plant Physiol. Biochem. 2017, 118, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Suxiao, H.; Yanfen, L.; Jing, L.; Yufen, B.; Qi, C.; Nan, M.; Zhiqin, Z.; Yuncong, Y. GIBBERELLIN INSENSITIVE DWARF1 Plays an Important Role in the Growth Regulation of Dwarf Apple Rootstocks. HortScience Horts 2019, 54, 416–422. [Google Scholar]
- Hollender, C.A.; Hadiarto, T.; Srinivasan, C.; Scorza, R.; Dardick, C. A brachytic dwarfism trait (dw) in peach trees is caused by a nonsense mutation within the gibberellic acid receptor PpeGID1c. New Phytol. 2016, 210, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Underhill, S.J. Differential transcription pathways associated with rootstock-induced dwarfing in breadfruit (Artocarpus altilis) scions. BMC Plant Biol. 2021, 21, 261. [Google Scholar] [CrossRef]
- Maurel, C. Plant aquaporins: Novel functions and regulation properties. FEBS Lett. 2007, 581, 2227–2236. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The Complete Set of Genes Encoding Major Intrinsic Proteins in Arabidopsis Provides a Framework for a New Nomenclature for Major Intrinsic Proteins in Plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef]
- Faize, M.; Fumanal, B.; Luque, F.; Ramírez-Tejero, J.A.; Zou, Z.; Qiao, X.; Faize, L.; Gousset-Dupont, A.; Roeckel-Drevet, P.; Label, P. Genome wild analysis and molecular understanding of the aquaporin diversity in olive trees (Olea europaea L.). Int. J. Mol. Sci. 2020, 21, 4183. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Xin, M.; Ma, F.; Liu, J. Gene-wide analysis of aquaporin gene family in Malus domestica and heterologous expression of the gene MpPIP2; 1 confers drought and salinity tolerance in Arabidposis thaliana. Int. J. Mol. Sci. 2019, 20, 3710. [Google Scholar] [CrossRef]
- Almeida-Rodriguez, A.M.; Cooke, J.E.K.; Yeh, F.; Zwiazek, J.J. Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii × balsamifera clones with different drought resistance strategies. Physiol. Plant. 2010, 140, 321–333. [Google Scholar] [CrossRef]
- Lovisolo, C.; Secchi, F.; Nardini, A.; Salleo, S.; Buffa, R.; Schubert, A. Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance. Physiol. Plant. 2007, 130, 543–551. [Google Scholar] [CrossRef]
- Otto, B.; Kaldenhoff, R. Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 2000, 211, 167–172. [Google Scholar]
- Javot, H.; Maurel, C. The Role of Aquaporins in Root Water Uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. A PIP1 Aquaporin Contributes to Hydrostatic Pressure-Induced Water Transport in Both the Root and Rosette of Arabidopsis. Plant Physiol. 2009, 152, 1418–1430. [Google Scholar] [CrossRef]
- Tyerman, S.; Niemietz, C.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Ancillo, G.; Aparicio, F.; Bordas, M.; Primo-Millo, E.; Forner-Giner, M.Á. Water-deficit tolerance in citrus is mediated by the down regulation of PIP gene expression in the roots. Plant Soil. 2011, 347, 91–104. [Google Scholar] [CrossRef]
- Aloni, R. The Induction of Vascular Tissues by Auxin. In Plant Hormones: Biosynthesis, Signal Transduction, Action; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 485–518. [Google Scholar]
- Jackson, M. Are plant hormones involved in root to shoot communication? Adv. Bot. Res. 1993, 19, 103–187. [Google Scholar]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Sorce, C.; Massai, R.; Picciarelli, P.; Lorenzi, R. Hormonal relationships in xylem sap of grafted and ungrafted Prunus rootstocks. Sci. Hortic. 2002, 93, 333–342. [Google Scholar] [CrossRef]
- Lochard, R.G.; Schneider, G.W. Stock and Scion Growth Relationships and the Dwarfing Mechanism in Apple. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 1981; pp. 315–375. [Google Scholar]
- Hooijdonk, B.; Woolley, D.; Warrington, I.; Tustin, S. Rootstocks modify scion architecture, endogenous hormones, and root growth of newly grafted ‘Royal Gala’apple trees. J. Am. Soc. Hortic. Sci. 2011, 136, 93–102. [Google Scholar] [CrossRef]
- Sorce, C.; Mariotti, L.; Lorenzi, R.; Massai, R. Hormonal factors involved in the control of vigour of grafted peach [Prunus persica (L.) Batsch] trees and hybrid rootstocks. Adv. Hortic. Sci. 2007, 21, 68–74. [Google Scholar]
- Saidha, T.; Goldschmidt, E.; Monselise, S. Endogenous cytokinins from developing ‘Shamouti’orange fruits derived from leafy and leafless inflorescences. Sci. Hortic. 1985, 26, 35–41. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Z.; Zhu, Y.; Li, J. Regulation of the Arabidopsis GSK3-like Kinase BRASSINOSTEROID-INSENSITIVE 2 through Proteasome-Mediated Protein Degradation. Mol. Plant 2008, 1, 338–346. [Google Scholar] [CrossRef]
- Bulley, S.M.; Wilson, F.M.; Hedden, P.; Phillips, A.L.; Croker, S.J.; James, D.J. Modification of gibberellin biosynthesis in the grafted apple scion allows control of tree height independent of the rootstock. Plant Biotechnol. J. 2005, 3, 215–223. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; El Kayal, W.; Prasath, D.; Fernández, H.; Bouzayen, M.; Svircev, A.M.; Jayasankar, S. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. J. Exp. Bot. 2011, 63, 1225–1239. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Lamattina, L. Abscisic acid and nitric oxide modulate cytoskeleton organization, root hair growth and ectopic hair formation in Arabidopsis. Nitric Oxide Biol. Chem. 2018, 80, 89–97. [Google Scholar] [CrossRef]
- Kamboj, J.S.; Browning, G.; Quinlan, J.D.; Blake, P.S.; Baker, D.A. Polar transport of [3H]-IAA in apical shoot segments of different apple rootstocks. J. Hortic. Sci. 1997, 72, 773–780. [Google Scholar] [CrossRef]
- Noda, K.; Okuda, H.; Iwagaki, I. Indole acetic acid and abscisic acid levels in new shoots and fibrous roots of citrus scion-rootstock combinations. Sci. Hortic. 2000, 84, 245–254. [Google Scholar] [CrossRef]
- Moghadam, E.G.; Shabani, Z. The relation of endogenous abscisic acid and indole acetic acid on vigor of some selected dwarf mahaleb (Prunus mahaleb L.) genotypes. J. Hortic. For. 2014, 6, 107–111. [Google Scholar]
- Atkinson, C.; Else, M.; Taylor, L.; Dover, C. Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). J. Exp. Bot. 2003, 54, 1221–1229. [Google Scholar] [PubMed]

| Species | Markers | Traits | QTL | References |
|---|---|---|---|---|
| Apple (Malus domestica) | 520 RAPD markers | Dwarfing trait of apple rootstock ‘M9′ | Dw1 associated with rootstock-induced dwarfing | [90] |
| Apple (Malus domestica) | SSR markers | Dwarfing trait of apple rootstock ‘M9′ | Dw1 and Dw2 associated with rootstock-induced dwarfing | [32] |
| Pear (Pyrus communis) | 710 SNP-based markers | Dwarfing trait of pear rootstock | LG5 synthetic to Dw1 in apple | [33] |
| Rubber (Hevea brasiliensis) | 225 SSRs and 186 SNPs | Stem diameter, tree height, and no. of whorls | 53 significant QTLs | [91] |
| Sweet cherry (Prunus avium) | 842 SNPs | Fruit development time, maturity date, and 5 fruit-quality traits | 18 significant stable QTLs | [92] |
| Sweet Orange (Citrus sinensis) | ~30,000 DArTseq markers | 12 fruit quality and quantity traits | 19 significant QTLs | [93] |
| Species | Population Size | Marker Size | Traits | References |
|---|---|---|---|---|
| Apple (Malus domestica) | 172 accessions | 55,000 SNPs | 11 fruit quality traits and 1 disease resistance | [102] |
| Apple (Malus domestica) | 1200 seedlings | 53 SSRs | Six fruit quality traits | [97] |
| Citrus (Citrus spp.) | 111 varieties and 676 individuals | 1841 SNPs | 17 fruit quality traits | [103] |
| Citrus (Citrus spp.) | 110 accessions | 2309 SNPs | 8 fruit-quality traits | [104] |
| Japanese chestnut (Castanea crenata) | 99 cultivars and selections | 162 SSRs and 741 SNPs | 5 nut traits | [105] |
| Japanese pear (Pyrus pyrifolia) | 76 cultivars | 155 SSRs | 4 fruit quality traits, harvest time, resistance to black spot, spur number and tree vigor | [106] |
| Macadamia (Macadamia spp.) | 281 progenies | 7126 SNPs | 3 yield component traits | [99] |
| Macadamia (Macadamia spp.) | 295 progenies | 4113 SNPs | 7 yield component traits, including trunk circumference | [101] |
| Peach (Prunus persica) | 620 individuals | 4005 SNPs | 3 phenological and 11 fruit quality-related traits | [107] |
| Species | Population Size | Marker Size | Phenotypic Traits | Prediction Accuracy | Reference |
|---|---|---|---|---|---|
| Apple (Malus domestica) | 537 individuals | 8294 SNPs | 12 traits related to fruit texture | 0.64 to 0.81 | [118] |
| Apple (Malus domestica) | 172 accessions | 55,000 SNPs | Harvest date, 8 fruit-quality traits, and scab resistance | 0.08 to 0.72 | [102] |
| Cacao (Theobroma cacao) | 287 individuals | 5000 SNPs | 4 fruit-quality and pathogen-resistance traits | 0.42 to 0.59 | [119] |
| Citrus (Citrus sp.) | 111 varieties and 676 individuals | 1841 SNPs | 17 fruit quality traits | 0.30 to 0.70 | [103] |
| Eucalyptus (Eucalyptus robusta) | 415 individuals | 2919 SNPs | Volume at 49 months, Total lignin content, holo-cellulose content | 0.05 to 0.79 | [120] |
| European peach (Prunus persica) | 1147 individuals | 6076 SNPs | 3 fruit-quality traits | 0.60 to 0.72 (average) | [121] |
| Grapevine (Vitis vinifera) | 3000 individuals | 90,000 SNPs | 4 traits | up to 0.90 | [122] |
| Japanese Chestnut (Castanea crenata) | 99 cultivars | 162 SSRs and 741 SNPs | Nut harvest date, nut weight, pericarp splitting, insect infestation, and specific gravity | 0.60 to 0.84 | [105] |
| Japanese pear (Pyrus pyrifolia Nakai) | 86 varieties and 765 trees from 16 full-sib families | 1506 SNPs | 18 traits | 0.50 to 0.70 (single trait GP) | [114] |
| Macadamia (Macdamia sp.) | 295 full-sib progenies | 4113 SNPs | Nut yield and yield stability | 0.14 to 0.79 | [117] |
| Maritime pine (Pinus pinaster) | 818 individuals | 4436 SNPs | 3 growth traits | 0.70 to 0.85 | [123] |
| Norway spurce (Picea abies L.) | 1370 control-pollinated individuals | 111,765 SNPs | 3 vigor traits | 0.49 to 0.97 | [124] |
| Oil Palm (Elaeis guineensis Jacq.) | 112 individuals | 221 SSRs and 46,933 SNP s | 6 fruit-quality traits | 0.18 to 0.28 (SSR-based models), 0.23 to 0.43 (SNP-based models) | [125] |
| Rubber (Hevea brasiliensis) | 435 individuals | 30,546 SNPs | Diameter and height | 0.59 to 0.75 | [126] |
| Table grape (Vitis vinifera) | 203 individuals | 243 SSR markers | 8 fruit and flower traits | 0.57 to 0.77 | [127] |
| Genes/Proteins | Crops | Findings | Source |
|---|---|---|---|
| PYL4 (Abscisic acid receptor) and Abscisic acid-insensitive mutant 5 (ABI5) | Apple | Upregulated in dwarfing rootstocks | [207] |
| WRKY transcription factor family | Apple | Responsible for dwarfing phenotype in ‘M26′ rootstock of apple | [208] |
| MdAUX1 and MdLAX2 (Auxin influx transporters) | Apple | Down-regulation of these genes, together with an increase in flavonoid concentration, led to reduced auxin movement, which correlated with dwarfing effect of rootstocks. | [204] |
| PIN1 | Apple | Gene expression decreased in trees with dwarfing genotypes used as an inter-stock. | [209] |
| Isopentenyl transferases (IPT) | Apple | IPT3 expression was correlated with plant vigor | [209] |
| Sorbitol dehydrogenase (SDH) | Peach | SDH activity in shoot tips of peach were related to shoot growth rate. | [210] |
| Tiller Angle Control 1 (TAC1) | Peach Prunus Poplar | Gene expression promotes the outer lateral shoot growth. | [211] |
| Gibberellin Insensitive Dwarf 1 c (GID1c) | Apple | The expression of GID1c was comparatively lower in dwarfing rootstock. | [212] |
| 99 transcripts (transcription regulation, brassinosteroid signaling, flavonoid metabolism, and cell-wall biosynthesis) | Sweet cherry | Differentially expressed in dwarfing and semi-vigorous rootstocks | [205] |
| GID1c | Plum | Silencing of GID1c led to a dwarf phenotype | [213] |
| 5049 differentially expressed genes | Breadfruit (Artocarpus altilis) | Upregulation and downregulation of genes in scion stems were associated with rootstock-induced dwarfing | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal Poudel, P.; Cowan, M.; Shaw, L.; De Faveri, J.; Topp, B.; Alam, M. Macadamia Breeding for Reduced Plant Vigor: Progress and Prospects for Profitable and Sustainable Orchard Systems. Sustainability 2023, 15, 14506. https://doi.org/10.3390/su151914506
Dhakal Poudel P, Cowan M, Shaw L, De Faveri J, Topp B, Alam M. Macadamia Breeding for Reduced Plant Vigor: Progress and Prospects for Profitable and Sustainable Orchard Systems. Sustainability. 2023; 15(19):14506. https://doi.org/10.3390/su151914506
Chicago/Turabian StyleDhakal Poudel, Pragya, Max Cowan, Lindsay Shaw, Joanne De Faveri, Bruce Topp, and Mobashwer Alam. 2023. "Macadamia Breeding for Reduced Plant Vigor: Progress and Prospects for Profitable and Sustainable Orchard Systems" Sustainability 15, no. 19: 14506. https://doi.org/10.3390/su151914506
APA StyleDhakal Poudel, P., Cowan, M., Shaw, L., De Faveri, J., Topp, B., & Alam, M. (2023). Macadamia Breeding for Reduced Plant Vigor: Progress and Prospects for Profitable and Sustainable Orchard Systems. Sustainability, 15(19), 14506. https://doi.org/10.3390/su151914506








