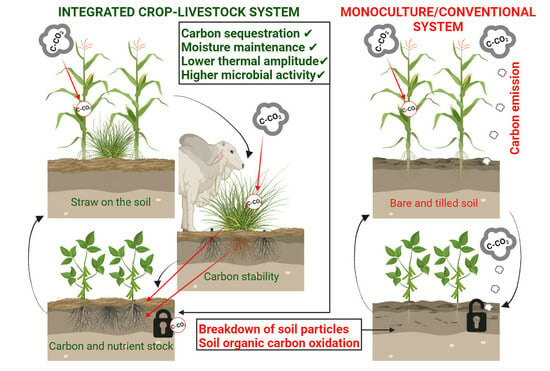
Accumulated Carbon Fractions in Tropical Sandy Soils and Their Effects on Fertility and Grain Yield in an Integrated Crop–Livestock System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site and Local Climatic Conditions
2.2. Experimental Design and Treatments
2.3. Sowing, Management, and Evaluation of Corn and Intercropped Forage
2.4. Soybean Sowing and Evaluation of Soybean Yield and Soil Moisture and Temperature
2.5. Soil Sampling and Enzymatic Activity Analysis
2.6. Soil Sampling and Chemical, Physical, and SOM Fractionation Analyses
2.7. Statistical Analyses
3. Results
3.1. Changes in Soil Physical and Chemical Properties
3.2. Changes in Grain Yield, Temperature, Moisture, and Enzymatic Activity in the Soil
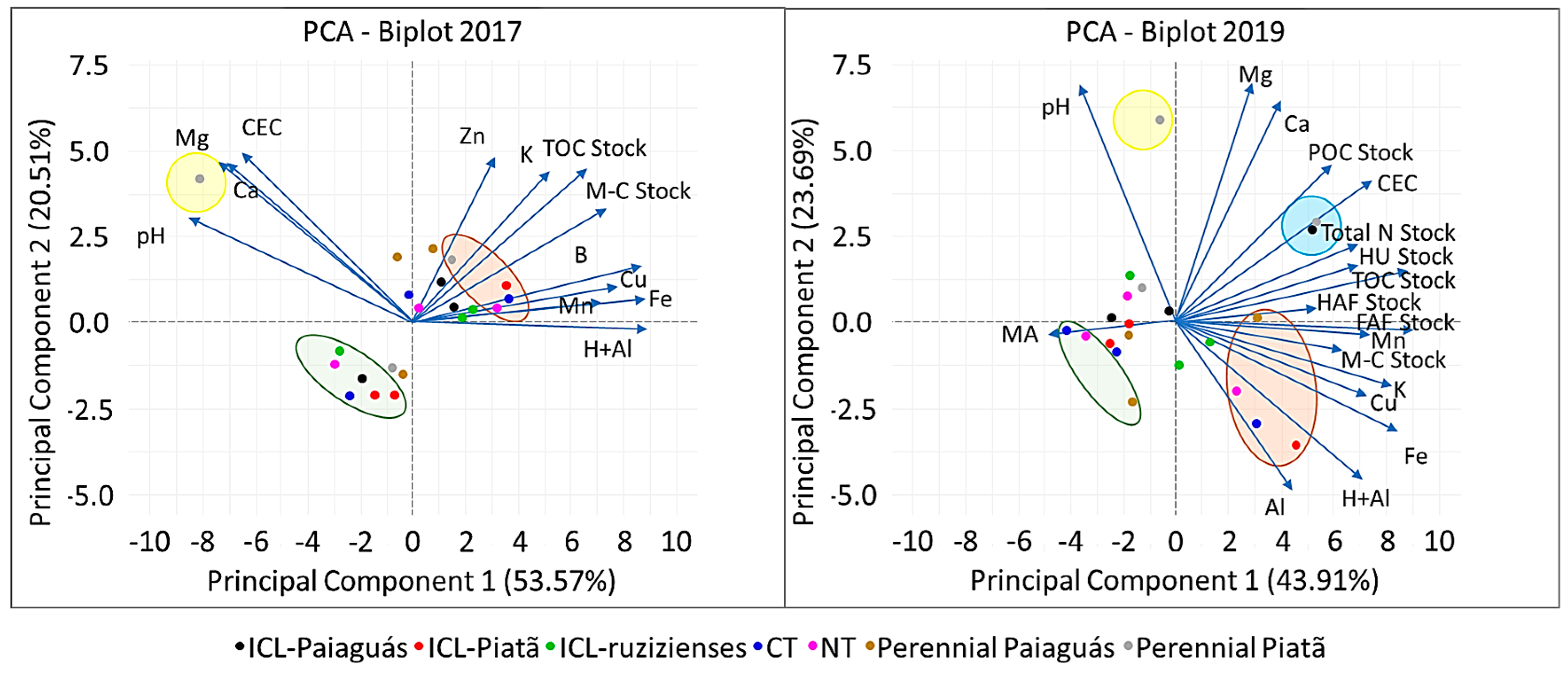
3.3. Relationships and Correlations between Management Types and Physical and Chemical Soil Properties
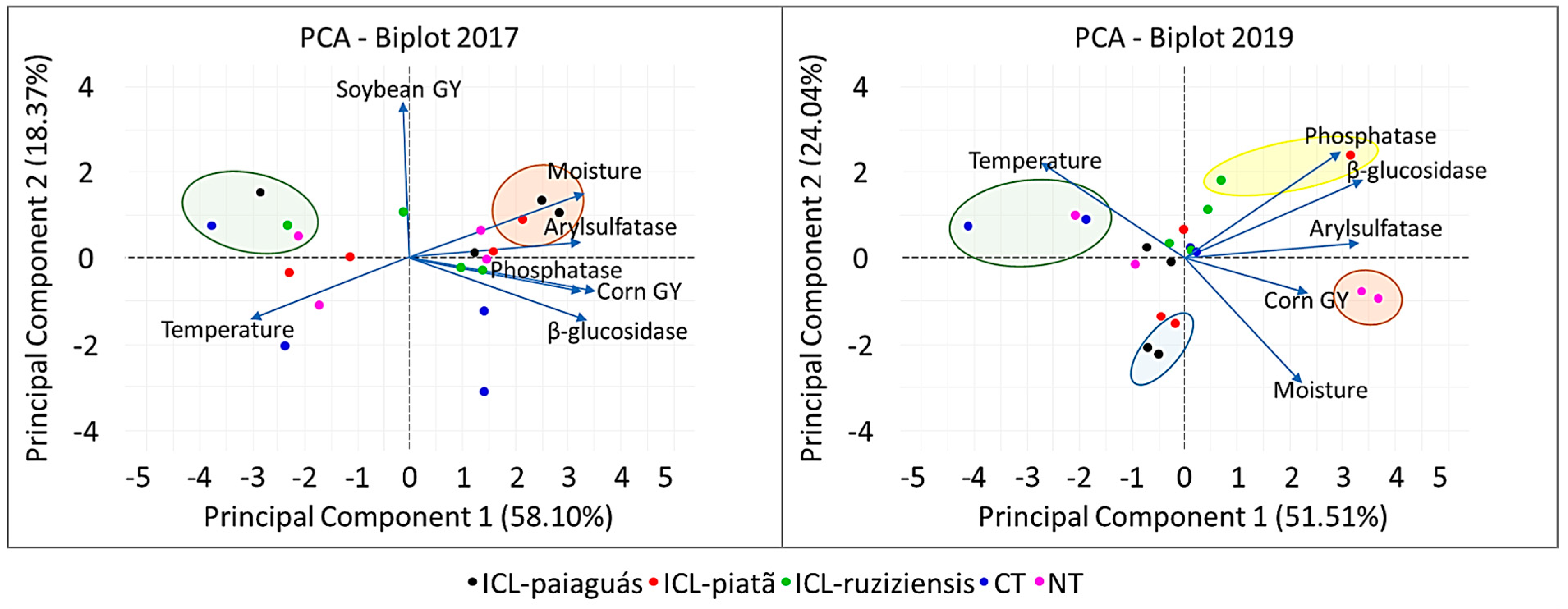
3.4. Correlations between Management, Grain Yield, and Soil Temperature, Moisture, and Enzymatic Activity
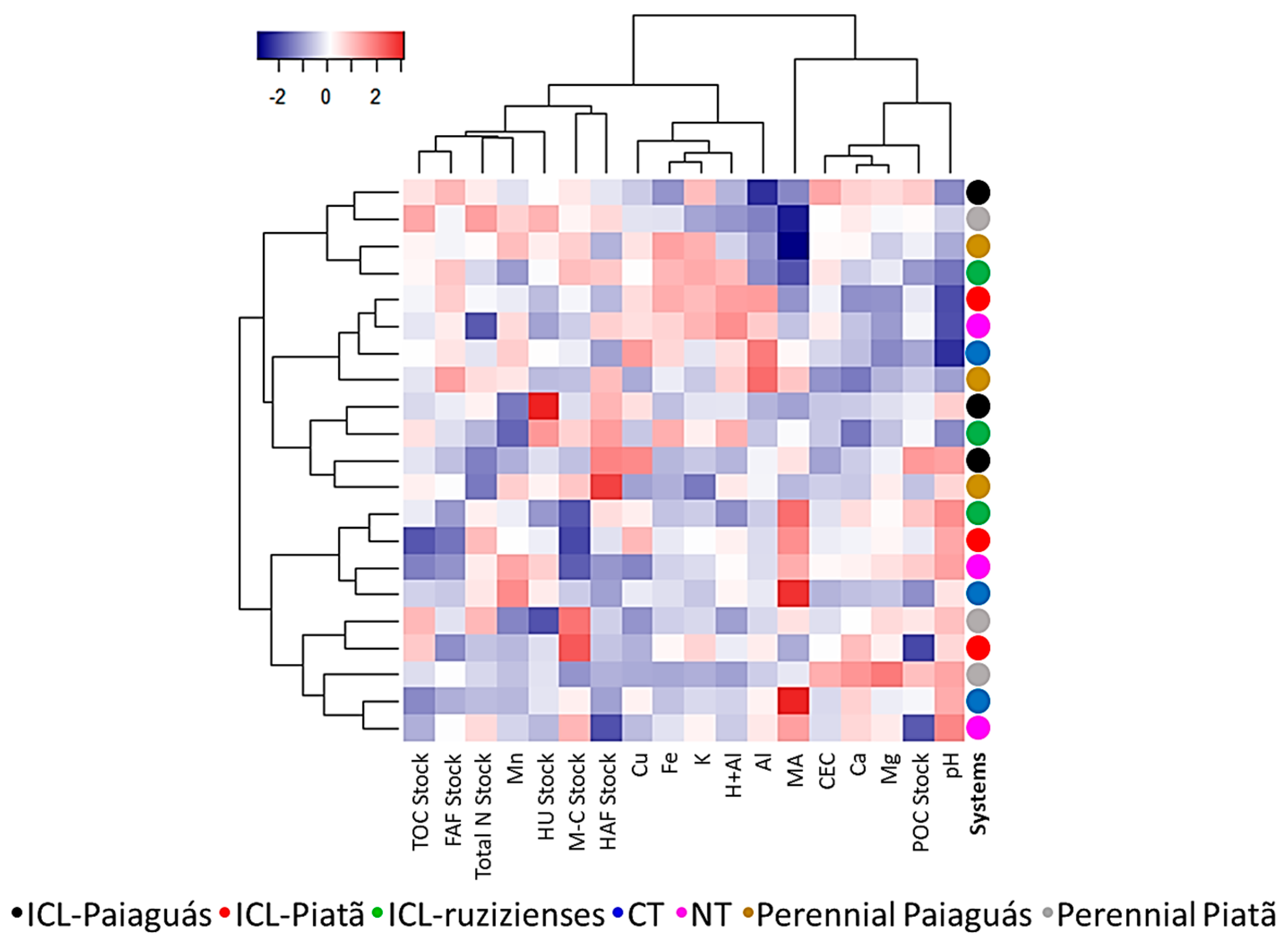
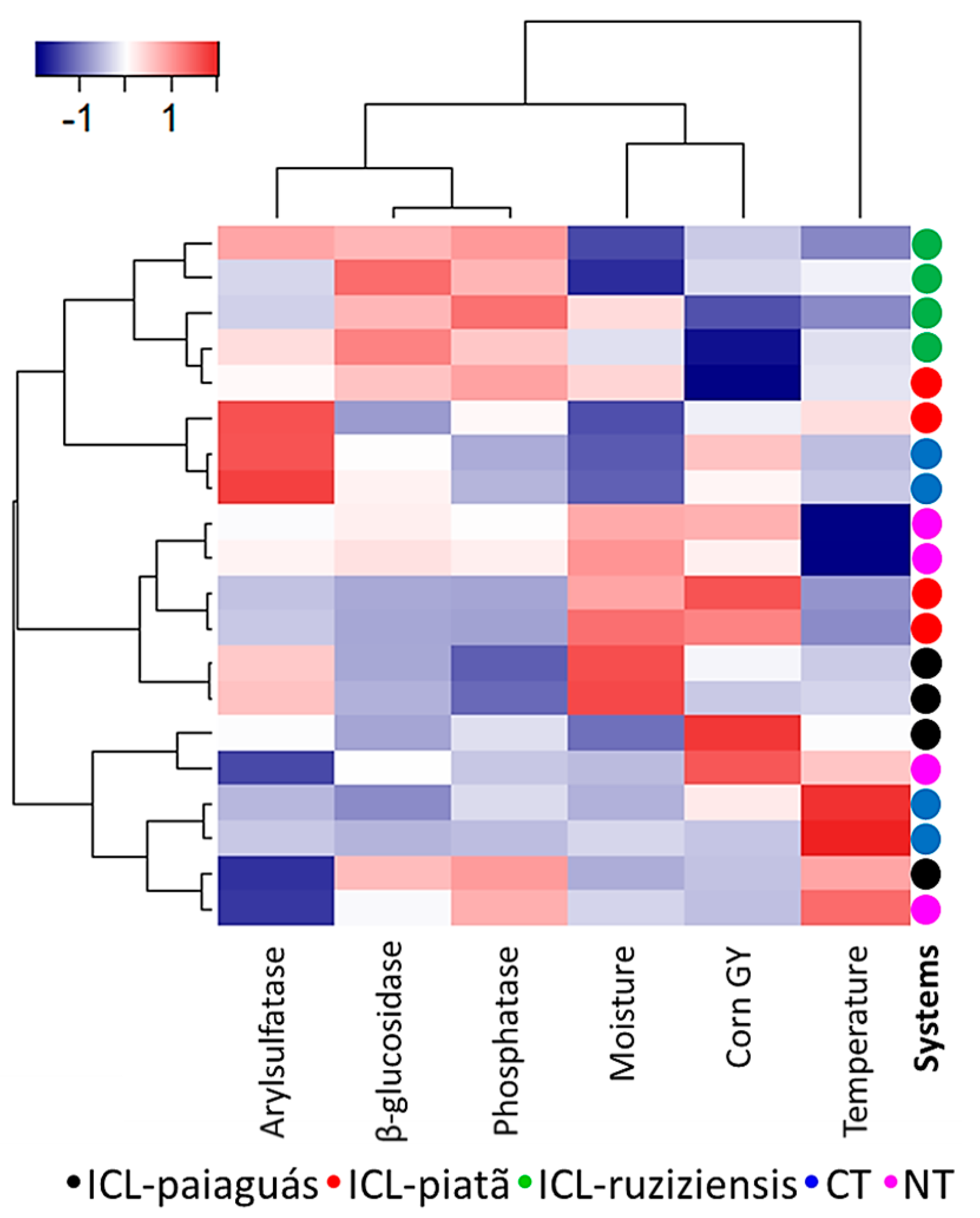
3.5. Similarities and Dissimilarities in Parameters According to Land-Use Type
4. Discussion
4.1. Relationships between Management and Soil Physical and Chemical Properties
4.2. Relationships among Management, Grain Yield, and Soil Temperature, Moisture, and Enzymatic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wachiye, S.; Merbold, L.; Vesala, T.; Rinne, J.; Räsänen, M.; Leitner, S.; Pellikka, P. Soil Greenhouse Gas Emissions under Different Land-Use Types in Savanna Ecosystems of Kenya. Biogeosciences 2020, 17, 2149–2167. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change Climate Change (IPCC) 2022: Mitigation of Climate Change. Working Group III Contribution to the IPCC Sixth Assessment Report (AR6). Available online: https://report.ipcc.ch/ar6wg3/pdf/IPCC_AR6_WGIII_FinalDraft_FullReport.pdf (accessed on 11 August 2023).
- Chaudhary, A.; Gustafson, D.; Mathys, A. Multi-Indicator Sustainability Assessment of Global Food Systems. Nat. Commun. 2018, 9, 848. [Google Scholar] [CrossRef]
- Dos Santos, F.C.; de Albuquerque Filho, M.R.; Vilela, L.; Ferreira, G.B.; Santana Carvalho, M.d.C.; Viana, J.H.M. Decomposição e Liberação de Macronutrientes Da Palhada de Milho e Braquiária, Sob Integração Lavoura-Pecuária No Cerrado Baiano. Rev. Bras. Cienc. Solo 2014, 38, 1855–1861. [Google Scholar] [CrossRef]
- Assmann, J.M.; Martins, A.P.; Anghinoni, I.; de Oliveira Denardin, L.G.; de Holanda Nichel, G.; de Andrade Costa, S.E.V.G.; Pereira e Silva, R.A.; Balerini, F.; de Faccio Carvalho, P.C.; Franzluebbers, A.J. Phosphorus and Potassium Cycling in a Long-Term No-till Integrated Soybean-Beef Cattle Production System under Different Grazing Intensities Insubtropics. Nutr. Cycl. Agroecosyst. 2017, 108, 21–33. [Google Scholar] [CrossRef]
- Damian, J.M.; Firmano, R.F.; Cherubin, M.R.; Pavinato, P.S.; de Marches Soares, T.; Paustian, K.; Cerri, C.E.P. Changes in Soil Phosphorus Pool Induced by Pastureland Intensification and Diversification in Brazil. Sci. Total Environ. 2020, 703, 135463. [Google Scholar] [CrossRef]
- Damian, J.M.; Matos, E.; Pedreira, B.C.; de Carcio Carvalho, P.C.; Souza, A.J.; Andreote, F.D.; Premazzi, L.M.; Cerri, C.E.P. Pastureland Intensification and Diversification in Brazil Mediate Soil Bacterial Community Structure Changes and Soil C Accumulation. Appl. Soil Ecol. 2021, 160, 103858. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Fostering the Future with Forage: The Case for Pasture Crop Rotations. Better Crops with Plant Food 2017. Available online: http://www.ipni.net/publication/bettercrops.nsf/issue/BC-2017-4 (accessed on 4 September 2023).
- Assmann, J.M.; Anghinoni, I.; Martins, A.P.; de Andrade Costa, S.E.V.G.; Cecagno, D.; Carlos, F.S.; de Faccio Carvalho, P.C. Soil Carbon and Nitrogen Stocks and Fractions in a Long-Term Integrated Crop–Livestock System under No-Tillage in Southern Brazil. Agric. Ecosyst. Environ. 2014, 190, 52–59. [Google Scholar] [CrossRef]
- Donagemma, G.K.; Luiz de Freitas, P.; Balieiro, F.C.; Fontana, A.; Spera, S.T.; Lumbreras, J.F.; Viana, J.H.M.; de Araújo Filho, J.C.; dos Santos, F.C.; de Albuquerque, M.R.; et al. Characterization, Agricultural Potential, and Perspectives for the Management of Light Soils in Brazil. Pesqui. Agropecu. Bras. 2016, 51, 1003–1020. [Google Scholar] [CrossRef]
- Salton, J.C.; Mercante, F.M.; Tomazi, M.; Zanatta, J.A.; Concenço, G.; Silva, W.M.; Retore, M. Integrated Crop-Livestock System in Tropical Brazil: Toward a Sustainable Production System. Agric. Ecosyst. Environ. 2014, 190, 70–79. [Google Scholar] [CrossRef]
- Sulc, R.M.; Franzluebbers, A.J. Exploring Integrated Crop–Livestock Systems in Different Ecoregions of the United States. Eur. J. Agron. 2014, 57, 21–30. [Google Scholar] [CrossRef]
- Kragt, M.E.; Pannell, D.J.; Robertson, M.J.; Thamo, T. Assessing Costs of Soil Carbon Sequestration by Crop-Livestock Farmers in Western Australia. Agric. Syst. 2012, 112, 27–37. [Google Scholar] [CrossRef]
- Tavanti, R.F.R.; Montanari, R.; Panosso, A.R.; La Scala, N.; Chiquitelli Neto, M.; da Silva Freddi, O.; Paz González, A.; de Carvalho, M.A.C.; Soares, M.B.; Tavanti, T.R.; et al. What Is the Impact of Pasture Reform on Organic Carbon Compartments and CO2 Emissions in the Brazilian Cerrado? Catena Amst. 2020, 194, 104702. [Google Scholar] [CrossRef]
- Colazo, J.C.; Herrero, J.D.; Sager, R.; Guzmán, M.L.; Zaman, M. Contribution of Integrated Crop Livestock Systems to Climate Smart Agriculture in Argentina. Land 2022, 11, 2060. [Google Scholar] [CrossRef]
- Caetano, J.O.; de Melo Benites, V.; Silva, G.P.; da Silva, I.R.; de Assis, R.L.; Cargnelutti Filho, A. Dinâmica Da Matéria Orgânica de Um Neossolo Quartzarênico de Cerrado Convertido Para Cultivo Em Sucessão de Soja e Milheto. Rev. Bras. Cienc. Solo 2013, 37, 1245–1255. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; de Lima, C.L.R.; Kunde, R.J.; dos Santos Carvalho, J.; Abeijon, L.M.; Pillon, C.N. Agregação e Proteção Física Da Matéria Orgânica Em Planossolo Háplico Sob Diferentes Sistemas de Manejo. Biosci. J. 2012, 28, 54–63. [Google Scholar]
- Guardiola, M.D.E.; Vale Júnior, J.F.; da Silva, E.E.; Rossi, C.Q.; Pereira, M.G. Chemical and Physical Fractions of Soil Organic Matter under Various Management Regimes in Roraima, Brazil. Semin. Cienc. Agrar. 2017, 38, 2419. [Google Scholar] [CrossRef]
- Locatelli, J.L.; Santos, R.S.; Cherubin, M.R.; Cerri, C.E.P. Changes in Soil Organic Matter Fractions Induced by Cropland and Pasture Expansion in Brazil’s New Agricultural Frontier. Geoderma Reg. 2022, 28, e00474. [Google Scholar] [CrossRef]
- Loss, A.; Pereira, M.G.; Perin, A.; Beutler, S.J.; Anjos, L.H.C. Oxidizable Carbon and Humic Substances in Rotation Systems with Brachiaria/Livestock and Pearl Millet/No Livestock in the Brazilian Cerrado. Span. J. Agric. Res. 2013, 11, 217–231. [Google Scholar] [CrossRef]
- Rosset, J.S.; do Carmo Lana, M.; Pereira, M.G.; Schiavo, J.A.; Rampim, L.; Sarto, M.V.M. Frações Químicas e Oxidáveis da Matéria Orgânica Do Solo Sob Diferentes Sistemas de Manejo, Em Latossolo Vermelho. Pesqui. Agropecu. Bras. 2016, 51, 1529–1538. [Google Scholar] [CrossRef]
- Steverson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Willey: New York, NY, USA, 1994. [Google Scholar]
- Castro, G.S.A.; Crusciol, C.A.C.; Calonego, J.C.; Rosolem, C.A. Management Impacts on Soil Organic Matter of Tropical Soils. Vadose Zone J. 2015, 14, vzj2014.07.0093. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, R.; Marchini, F.; Saviozzi, A. Soil Organic Matter Characteristics, Biochemical Activity and Antioxidant Capacity in Mediterranean Land Use Systems. Soil Tillage Res. 2012, 120, 8–14. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G.; Mamo, T. Soil Aggregation, and Total and Particulate Organic Matter Following Conversion of Native Forests to Continuous Cultivation in Ethiopia. Soil Tillage Res. 2007, 94, 101–108. [Google Scholar] [CrossRef]
- Piccolo, A. The Supramolecular Structure of Humic Substances: A Novel Understanding of Humus Chemistry and Implications in Soil Science. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2002; Volume 75, pp. 57–134. ISBN 0120007932. [Google Scholar]
- Marinari, S.; Liburdi, K.; Corradini, D.; Grego, S. Reversed-Phase High Performance Liquid Chromatographic Profile of Organic Fractions Extracted by Solvents with Different Polarity as a Tool to Evaluate the Hydrophobic Character of Soil under Different Management. Soil Tillage Res. 2010, 109, 36–40. [Google Scholar] [CrossRef]
- Costa, S.E.V.G.A.; Souza, E.D.; Anghinoni, I.; Carvalho, P.C.F.; Martins, A.P.; Kunrath, T.R.; Cecagno, D.; Balerini, F. Impact of an Integrated No-till Crop-Livestock System on Phosphorus Distribution, Availability and Stock. Agric. Ecosyst. Environ. 2014, 190, 43–51. [Google Scholar] [CrossRef]
- Wendling, B.; Jucksch, I.; Mendonça, E.S.; Alvarenga, R.C. Organic-Matter Pools of Soil under Pines and Annual Cultures. Commun. Soil Sci. Plant Anal. 2010, 41, 1707–1722. [Google Scholar] [CrossRef]
- Jamala, G.Y.; Oke, D.O. Humic Substances and Mineral-Associated Soil Organic Carbon as Influenced By Land Use in Southeastern Adamawa State, Nigeria. J. Environ. Sci. 2013, 6, 59–70. [Google Scholar] [CrossRef]
- Sone, J.S.; Sanches de Oliveira, P.T.; Zamboni, P.A.P.; Vieira, N.O.M.; Carvalho, G.A.; Macedo, M.C.M.; Romeiro de Araujo, A.; Montagner, D.B.; Sobrinho, T.A. Effects of Long-Term Crop-Livestock-Forestry Systems on Soil Erosion and Water Infiltration in a Brazilian Cerrado Site. Sustainability 2019, 11, 5339. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Brasilia, Brazil, 2018; Volume 1. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2017; ISBN 9788570357717. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia (INMET). Climatologia e Histórico de Previsão do Tempo Em Caiuá. Available online: https://www.climatempo.com.br/climatologia/2248/caiua-sp (accessed on 11 August 2023).
- National Aeronautics and Space Administration (NASA). Giovanni—The Bridge between Data and Science. Available online: https://giovanni.gsfc.nasa.gov/giovanni/ (accessed on 11 August 2023).
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico/Fundação IAC: Campinas, Brazil, 2001. [Google Scholar]
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo, 1st ed.; IAC: Campinas, Brazil, 1997. [Google Scholar]
- Jakelaitis, A.; Silva, A.A.; Ferreira, L.R.; Silva, A.F.; Pereira, J.L.; Viana, R.G. Efeitos de Herbicidas No Consórcio de Milho Com Brachiaria Brizantha. Planta Daninha 2005, 23, 69–78. [Google Scholar] [CrossRef]
- Ceccon, G.; Matoso, A.O.; Neto Neto, A.L.; Palombo, L. Uso de Herbicidas No Consórcio de Milho Safrinha Com Brachiaria Ruziziensis. Planta Daninha 2010, 28, 359–364. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Montagner, D.B.; Barbosa, R.A.; do Valle, C.B.; Nantes, N.N. Animal Performance and Sward Characteristics of Two Cultivars of Brachiaria Brizantha (BRS Paiaguás and BRS Piatã). Rev. Bras. Zootec. 2016, 45, 85–92. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; Volume 2, pp. 775–833. ISBN 9780891188650. [Google Scholar]
- De Carvalho Mendes, I.; de Souza, L.M.; de Sousa, D.M.G.; de Castro Lopes, A.A.; dos Reis Junior, F.B.; Lacerda, M.P.C.; Malaquias, J.V. Critical Limits for Microbial Indicators in Tropical Oxisols at Post-Harvest: The FERTBIO Soil Sample Concept. Appl. Soil Ecol. 2019, 139, 85–93. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Swift, R.S. Organic Matter Characterization. In Methods of Soil Analysis; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; American Society of Agronomy Soil Science Society: Madison, WI, USA, 1996; pp. 1011–1020. [Google Scholar]
- Benites, V.M.; Madari, B.; Machado, P.L.O.A. Extração e Fracionamento Quantitativo de Substâncias Húmicas Do Solo: Um Procedimento Simplificado de Baixo Custo; Embrapa Solos: Rio de Janeiro, Brazil, 2003. [Google Scholar]
- Bayer, C.; Mielniczuk, J.; Amado, T.J.C.; Martin-Neto, L.; Fernandes, S.V. Organic Matter Storage in a Sandy Clay Loam Acrisol Affected by Tillage and Cropping Systems in Southern Brazil. Soil Tillage Res. 2000, 54, 101–109. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Cerri, C.E.P.; Feigl, B.J.; Píccolo, M.C.; Godinho, V.P.; Cerri, C.C. Carbon Sequestration in Agricultural Soils in the Cerrado Region of the Brazilian Amazon. Soil Tillage Res. 2009, 103, 342–349. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Varimax Criterion for Analytic Rotation in Factor Analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Savegnago, R.P.; Caetano, S.L.; Ramos, S.B.; Nascimento, G.B.; Schmidt, G.S.; Ledur, M.C.; Munari, D.P. Estimates of Genetic Parameters, and Cluster and Principal Components Analyses of Breeding Values Related to Egg Production Traits in a White Leghorn Population. Poult. Sci. 2011, 90, 2174–2188. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento Boletim (CONAB). Das Safras de Grãos. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos?start=20 (accessed on 11 August 2023).
- Reichert, J.M.; Suzuki, L.E.A.S.; Reinert, D.J.; Horn, R.; Håkansson, I. Reference Bulk Density and Critical Degree-of-Compactness for No-till Crop Production in Subtropical Highly Weathered Soils. Soil Tillage Res. 2009, 102, 242–254. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Fabian, A.J.; Pereira, M.G. Alterações Dos Atributos Físicos de Um Latossolo Vermelho Submetido a Diferentes Sistemas de Manejo. Ciência Agrotecnol. 2011, 35, 437–445. [Google Scholar] [CrossRef]
- Silva, J.R.; Silva, D.J.; Gava, C.A.T.; Tavares de Oliveira, T.C.; Conceição de Freitas, M.d.S. Carbon in Humic Fractions of Organic Matter in Soil Treated with Organic Composts under Mango Cultivation. Rev. Bras. Cienc. Solo 2016, 40, e0150095. [Google Scholar] [CrossRef]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic Acid Behavior in Soils—Misconceptions and Knowledge Gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and Transformations of Micronutrients in Agricultural Soils as Influenced by Organic Matter Build-up: A Review. Environ. Sustain. Indic. 2019, 1–2, 100007. [Google Scholar] [CrossRef]
- Franchini, J.C.; Hoffmann-Campo, C.B.; Torres, E.; Miyazawa, M.; Pavan, M.A. Organic Composition of Green Manure During Growth and Its Effect on Cation Mobilization in an Acid Oxisol. Commun. Soil Sci. Plant Anal. 2003, 34, 2045–2058. [Google Scholar] [CrossRef]
- Pegoraro, R.F.; da Silva, I.R.; Novais, R.F.; de Sa Mendonça, E.; de Oliveira Gebrim, F.; Moreira, F.F. Fluxo Difusivo e Biodisponibilidade de Zinco, Cobre, Ferro e Manganês No Solo: Influência Da Calagem, Textura Do Solo e Resíduos Vegetais. Rev. Bras. Cienc. Solo 2006, 30, 859–868. [Google Scholar] [CrossRef]
- Vitti, G.C.; Trevisan, T. Manejo de Macro e Micronutrientes Para a Alta Produtividade Da Soja. In Soja: Tecnologia da Produção; Câmara, G.M.S., Ed.; Potafos: Piracicaba, Brazil, 2000; pp. 383–422. [Google Scholar]
- Van Raij, B. Fertilidade Do Solo e Manejo de Nutrientes; International Plant Nutrition Institute: Piracicaba, Brazil, 2011. [Google Scholar]
- Lopes, A.S.; Guilherme, L.R.G. A Career Perspective on Soil Management in the Cerrado Region of Brazil. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2016; Volume 137, pp. 1–72. ISBN 9780128046920. [Google Scholar]
- Meurer, E.J. Fundamentos de Química Do Solo, 3rd ed.; Evangraf: Porto Alegre, Brazil, 2006. [Google Scholar]
- Islabão, G.O.; Pinto, M.A.B.; Selau, L.P.R.; Vahl, L.C.; Timm, L.C. Characterization of Soil Chemical Properties of Strawberry Fields Using Principal Component Analysis. Rev. Bras. Cienc. Solo. 2013, 37, 168–176. [Google Scholar] [CrossRef]
- Camargo, F.A.O.; Silva, L.S.; Gianello, C.; Tedesco, M.J. Nitrogênio Orgânico Do Solo. In Fundamentos da Matéria Orgânica do Solo: Ecossistemas Tropicais e Subtropicais; Santos, G.A., Silva, L.S., Canellas, L.P., Camargo, F.A.O., Eds.; Metrópole: Porto Alegre, Brazil, 2008; pp. 87–99. [Google Scholar]
- Purwanto, B.H.; Alam, S. Impact of Intensive Agricultural Management on Carbon and Nitrogen Dynamics in the Humid Tropics. Soil Sci. Plant Nutr. 2020, 66, 50–59. [Google Scholar] [CrossRef]
- Cenciani, K.; dos Freitas, S.S.; Critter, S.A.M.; Airoldi, C. Microbial Enzymatic Activity and Thermal Effect in a Tropical Soil Treated with Organic Materials. Sci. Agric. 2008, 65, 674–680. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Gałązka, A.; Grządziel, J.; Stuczyński, T. Impact of Water Stress on Microbial Community and Activity in Sandy and Loamy Soils. Agronomy 2020, 10, 1429. [Google Scholar] [CrossRef]
- De Souza Gallo, A.; dos Santos Araujo, T.; dos Santos Araujo, F.; dos Santos, L.C.; de Franca Guimarães, N.; da Silva, R.F. Biomassa e Atividade Microbiana Em Solo Cultivado Com Milho Consorciado Com Leguminosas de Cobertura. Rev. Ciências Agrárias 2019, 42, 347–357. [Google Scholar] [CrossRef]
- Bonetti, J.D.A.; Anghinoni, I.; Zulpo, L. Temperatura e Umidade Do Solo Em Sistema de Integração Soja-Bovinos de Corte Com Diferentes Manejos Da Altura Do Pasto. Sci. Agrar. 2017, 18, 11. [Google Scholar] [CrossRef]
- Mendes, I.C.; Sousa, D.M.G.; Reis Junior, F.B.; Lopes, A.A.C.; Souza, L.M.; Chaer, G.M. Bioanálise de Solo: Aspectos Teóricos e Práticos. In Tópicos em Ciência do Solo; Severino, E.C., Morais, M.F., Paula, A.M., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2019. [Google Scholar]
- Xie, X.; Liao, M.; Yang, J.; Chai, J.; Fang, S.; Wang, R. Influence of Root-Exudates Concentration on Pyrene Degradation and Soil Microbial Characteristics in Pyrene Contaminated Soil. Chemosphere 2012, 88, 1190–1195. [Google Scholar] [CrossRef]
- Balota, E.L.; Nogueira, M.A.; Mende, I.C.; Hungria, M.; Fagotti, D.S.L.; Melo, G.M.P.; Souza, R.C.; Melo, W.J. Enzimas e Seu Papel Na Qualidade Do Solo. In Tópicos em Ciência do Solo; Araújo, A.P., Ed.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2013; pp. 189–250. [Google Scholar]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Gebremikael, M.T.; Wu, H.; Cai, D.; Wang, B.; Li, B.; Zhang, J.; Li, Y.; Xi, J. Response of Soil Organic Carbon Fractions, Microbial Community Composition and Carbon Mineralization to High-Input Fertilizer Practices under an Intensive Agricultural System. PLoS ONE 2018, 13, e0195144. [Google Scholar] [CrossRef]
- Bogati, K.A.; Golińska, P.; Sewerniak, P.; Burkowska-But, A.; Walczak, M. Deciphering the Impact of Induced Drought in Agriculture Soils: Changes in Microbial Community Structure, Enzymatic and Metabolic Diversity. Agronomy 2023, 13, 1417. [Google Scholar] [CrossRef]
- De Jesus Franco, A.; da Silva, A.P.V.; Souza, A.B.S.; Oliveira, R.L.; Batista, É.R.; de Souza, E.D.; Silva, A.O.; Carneiro, M.A.C. Plant Diversity in Integrated Crop-Livestock Systems Increases the Soil Enzymatic Activity in the Short Term. Pesqui. Agropecu. Trop. 2020, 50, e64026. [Google Scholar] [CrossRef]
- Sarto, M.V.M.; Borges, W.L.B.; Sarto, J.R.W.; Pires, C.A.B.; Rice, C.W.; Rosolem, C.A. Soil Microbial Community and Activity in a Tropical Integrated Crop-Livestock System. Appl. Soil Ecol. 2020, 145, 103350. [Google Scholar] [CrossRef]
- Mendes, I.C.; Martinhão Gomes Sousa, D.; Dario Dantas, O.; Alves Castro Lopes, A.; Bueno Reis Junior, F.; Ines Oliveira, M.; Chaer, G.M. Soil Quality and Grain Yield: A Win–Win Combination in Clayey Tropical Oxisols. Geoderma 2021, 388, 114880. [Google Scholar] [CrossRef]
- Ding, W.; Yu, H.; Cai, Z.; Han, F.; Xu, Z. Responses of Soil Respiration to N Fertilization in a Loamy Soil under Maize Cultivation. Geoderma 2010, 155, 381–389. [Google Scholar] [CrossRef]
- Tominaga, T.T.; Cássaro, F.A.M.; Bacchi, O.O.S.; Reichardt, K.; Oliveira, J.C.M.; Timm, L.C. Variability of Soil Water Content and Bulk Density in a Sugarcane Field. Soil Res. 2002, 40, 604. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Ferraudo, A.S.; Panosso, A.R.; da Silva Bicalho, E.; Teixeira, D.D.B.; de Andrade Barbosa, M.; Tsai, S.M.; Borges, B.M.F.; de Souza Cannavan, F.; de Souza, J.A.M.; et al. Effects of Burned and Unburned Sugarcane Harvesting Systems on Soil CO2 Emission and Soil Physical, Chemical, and Microbiological Attributes. Catena Amst. 2021, 196, 104903. [Google Scholar] [CrossRef]
- De Asevedo Soares, D.; Modesto, V.C.; Nakao, A.H.; Soares, W.R.; Freitas, L.A.; Dickmann, L.; Pascoaloto, I.M.; Andreotti, M. Soybean Yield and Nutrition Grown on the Straw of Grain Sorghum Inoculated with Azospirillum Brasilense and Intercropped with BRS Paiaguás Grass. Plants 2023, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Agricultura (MAPA). Zoneamento Agrícola. Available online: https://www.gov.br/agricultura/pt-br/assuntos/riscos-seguro/programa-nacional-de-zoneamento-agricola-de-risco-climatico/zoneamento-agricola (accessed on 11 August 2023).
- Evangelista, C.R.; Partelli, F.L.; de Ferreira, E.P.; Correchel, V. Atividade Enzimática Do Solo Sob Sistema de Produção Orgânica e Convencional Na Cultura da Cana-de-Açúcar Em Goiás. Semin. Cienc. Agrar. 2012, 33, 1251–1262. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Roper, M.M.; Thompson, J.; Pratley, J.E.; Kirkegaard, J. Harnessing the Benefits of Soil Biology in Conservation Agriculture. In Australian Agriculture in 2020: From Conservation o Automation; Pratley, J., Kirkegaard, J., Eds.; Charles Sturt University: Wagga Wagga, NSW, Australia, 2020; pp. 237–253. [Google Scholar]
- Barbieri, M.; Dossim, M.F.; Nora, D.D.; dos Santos, W.B.; Bevilacqua, C.B.; de Andrade, N.; Boeni, M.; Deuschle, D.; Jacques, R.J.S.; Antoniolli, Z.I. Ensaio Sobre a Bioatividade Do Solo Sob Plantio Direto Em Sucessão e Rotação de Culturas de Inverno e Verão. Rev. Ciências Agrárias 2019, 42, 122–134. [Google Scholar] [CrossRef]
- Guangming, L.; Xuechen, Z.; Xiuping, W.; Hongbo, S.; Jingsong, Y.; Xiangping, W. Soil Enzymes as Indicators of Saline Soil Fertility under Various Soil Amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Li, J.; Niu, L.; Zhang, Q.; Di, H.; Hao, J. Impacts of Long-Term Lack of Potassium Fertilization on Different Forms of Soil Potassium and Crop Yields on the North China Plains. J. Soils Sediments 2017, 17, 1607–1617. [Google Scholar] [CrossRef]

| Parameter | Minimum | Mean | Maximum | SD | CV | Minimum | Mean | Maximum | SD | CV |
|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2019 | |||||||||
| FAF stock (t ha−1) | - | - | - | - | - | 1.0 | 1.5 | 2.2 | 0.37 | 25 |
| HAF stock (t ha−1) | - | - | - | - | - | 0.5 | 1.2 | 1.8 | 0.36 | 31 |
| HU stock (t ha−1) | - | - | - | - | - | 3.0 | 5.1 | 8.1 | 1.23 | 24 |
| Total N stock (t ha−1) | - | - | - | - | - | 1.0 | 1.4 | 2.1 | 0.26 | 19 |
| POC stock (t ha−1) | - | - | - | - | - | 2.2 | 4.0 | 5.9 | 1.01 | 25 |
| MA (m−3 m−3) | - | - | - | - | - | 0.1 | 0.2 | 0.3 | 0.04 | 24 |
| Al (mmolc dm−3) | - | - | - | - | - | 0.1 | 0.3 | 2.0 | 0.64 | 125 |
| TOC stock (t ha−1) | 11.09 | 12.7 | 14.85 | 1.07 | 8 | 10.6 | 13.7 | 18.6 | 1.85 | 14 |
| M-C stock (t ha−1) | 6.46 | 9.10 | 11.68 | 1.50 | 17 | 5.9 | 9.7 | 13.6 | 2.5 | 26 |
| pH (CaCl2) | 5.10 | 5.63 | 6.90 | 0.43 | 8 | 4.8 | 5.4 | 6.2 | 0.33 | 6 |
| K (mmolc dm−3) | 1.30 | 2.90 | 5.90 | 1.35 | 46 | 1.3 | 2.8 | 5.0 | 1.06 | 38 |
| Ca (mmolc dm−3) | 16.00 | 30.71 | 162.00 | 30.55 | 99 | 8.0 | 14.3 | 26.0 | 4.23 | 30 |
| Mg (mmolc dm−3) | 6.00 | 13.57 | 62.00 | 11.57 | 85 | 5.0 | 8.7 | 21.0 | 3.68 | 42 |
| H+Al (mmolc dm−3) | 10.00 | 16.57 | 22.00 | 3.20 | 19 | 11.0 | 15.6 | 25.0 | 3.61 | 23 |
| CEC (mmolc dm−3) | 41.30 | 63.55 | 235.60 | 40.18 | 63 | 30.9 | 41.4 | 59.9 | 8.24 | 20 |
| Cu (mmolc dm−3) | 0.30 | 0.58 | 0.80 | 0.15 | 26 | 0.3 | 0.5 | 0.8 | 0.15 | 33 |
| Fe (mmolc dm−3) | 2.00 | 6.62 | 11.00 | 2.52 | 38 | 6.0 | 13.4 | 29.0 | 6.54 | 49 |
| Mn (mmolc dm−3) | 4.80 | 8.80 | 13.70 | 2.64 | 30 | 4.2 | 7.5 | 12.7 | 2.64 | 35 |
| B (mmolc dm−3) | 0.08 | 0.17 | 0.34 | 0.07 | 39 | - | - | - | - | - |
| Zn (mmolc dm−3) | 0.40 | 1.04 | 1.90 | 0.38 | 36 | - | - | - | - | - |
| Parameter | Minimum | Mean | Maximum | SD | CV | Minimum | Mean | Maximum | SD | CV |
|---|---|---|---|---|---|---|---|---|---|---|
| 2017/18 | 2018/19 | |||||||||
| Soybean GY (kg ha−1) | 2442 | 3185 | 3499 | 273 | 9 | - | - | - | - | - |
| Corn GY (kg ha−1) | 3979 | 5708 | 7274 | 947 | 17 | 1173 | 1857 | 2705 | 410 | 22 |
| Temperature (°C) | 30.5 | 34.0 | 41.9 | 3.3 | 10 | 29.0 | 32.7 | 41.0 | 2.3 | 10 |
| Moisture (%) | 4.5 | 6.8 | 8.7 | 1.2 | 17 | 4.7 | 7.3 | 11.9 | 2.1 | 29 |
| β-Glucosidase * | 63.0 | 76.4 | 90.9 | 8.6 | 11 | 62.3 | 96.9 | 139.3 | 20.9 | 22 |
| Phosphatase * | 141.4 | 319.8 | 454.5 | 83.0 | 26 | 23.0 | 50.6 | 92.5 | 17.8 | 35 |
| Arylsulfatase * | 12.2 | 28.6 | 57.1 | 12.4 | 43 | 11.6 | 24.3 | 50.8 | 11.9 | 39 |
| ----------2017---------- | ----------2019---------- | |||
|---|---|---|---|---|
| PCA | PC1 | PC2 | PC1 | PC2 |
| Eigenvalue | 6.96 | 2.67 | 7.90 | 4.26 |
| Explained variance (%) | 53.57 | 20.51 | 43.91 | 23.69 |
| Cumulative variance (%) | 53.57 | 74.08 | 43.91 | 67.60 |
| Parameters 1 | Correlation 2 | |||
| FAF stock | - | - | 0.90 | −0.03 |
| HAF stock | - | - | 0.53 | 0.05 |
| HU stock | - | - | 0.70 | 0.22 |
| Total N stock | - | - | 0.69 | 0.29 |
| POC stock | - | - | 0.59 | 0.59 |
| MA | - | - | −0.48 | −0.05 |
| Al | 0.44 | −0.64 | ||
| TOC stock | 0.67 | 0.59 | 0.88 | 0.19 |
| M-C stock | 0.74 | 0.44 | 0.64 | −0.11 |
| pH | −0.85 | 0.40 | −0.36 | 0.91 |
| K | 0.52 | 0.58 | 0.82 | −0.24 |
| Ca | −0.69 | 0.60 | 0.40 | 0.84 |
| Mg | −0.73 | 0.62 | 0.29 | 0.91 |
| H+Al | 0.89 | −0.02 | 0.70 | −0.60 |
| CEC | −0.65 | 0.65 | 0.75 | 0.54 |
| Cu | 0.87 | 0.22 | 0.73 | −0.28 |
| Fe | 0.88 | −0.09 | 0.85 | −0.42 |
| Mn | 0.73 | 0.07 | 0.73 | −0.05 |
| B | 0.78 | 0.15 | - | - |
| Zn | 0.31 | 0.63 | - | - |
| PCA | PC1 | PC2 | PC1 | PC2 |
|---|---|---|---|---|
| ----------2017---------- | ----------2019---------- | |||
| Eigenvalue | 4.07 | 1.29 | 3.09 | 1.44 |
| Explained variance (%) | 58.10 | 18.37 | 51.51 | 24.04 |
| Cumulative variance (%) | 58.10 | 76.48 | 51.51 | 75.55 |
| Parameter 1 | Correlation 2 | |||
| Corn grain yield | 0.88 | −0.20 | 0.59 | −0.21 |
| Soybean grain yield | −0.02 | 0.90 | - | - |
| Temperature 3 | −0.75 | −0.36 | −0.68 | 0.55 |
| Moisture 1 | 0.83 | 0.37 | 0.56 | −0.72 |
| β-Glucosidase | 0.84 | −0.36 | 0.85 | 0.44 |
| Phosphatase | 0.82 | −0.19 | 0.75 | 0.61 |
| Arylsulfatase | 0.81 | 0.09 | 0.82 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, D.d.A.; Sekiya, B.M.S.; Modesto, V.C.; Nakao, A.H.; Freitas, L.A.; Souza, I.M.D.d.; da Luz, J.H.S.; Galindo, F.S.; Lupatini, G.C.; Pavan Mateus, G.; et al. Accumulated Carbon Fractions in Tropical Sandy Soils and Their Effects on Fertility and Grain Yield in an Integrated Crop–Livestock System. Sustainability 2023, 15, 13829. https://doi.org/10.3390/su151813829
Soares DdA, Sekiya BMS, Modesto VC, Nakao AH, Freitas LA, Souza IMDd, da Luz JHS, Galindo FS, Lupatini GC, Pavan Mateus G, et al. Accumulated Carbon Fractions in Tropical Sandy Soils and Their Effects on Fertility and Grain Yield in an Integrated Crop–Livestock System. Sustainability. 2023; 15(18):13829. https://doi.org/10.3390/su151813829
Chicago/Turabian StyleSoares, Deyvison de Asevedo, Bianca Midori Souza Sekiya, Viviane Cristina Modesto, Allan Hisashi Nakao, Leandro Alves Freitas, Isabela Malaquias Dalto de Souza, João Henrique Silva da Luz, Fernando Shintate Galindo, Gelci Carlos Lupatini, Gustavo Pavan Mateus, and et al. 2023. "Accumulated Carbon Fractions in Tropical Sandy Soils and Their Effects on Fertility and Grain Yield in an Integrated Crop–Livestock System" Sustainability 15, no. 18: 13829. https://doi.org/10.3390/su151813829
APA StyleSoares, D. d. A., Sekiya, B. M. S., Modesto, V. C., Nakao, A. H., Freitas, L. A., Souza, I. M. D. d., da Luz, J. H. S., Galindo, F. S., Lupatini, G. C., Pavan Mateus, G., Bonini, C. d. S. B., Andrighetto, C., Mendes, I. d. C., Pavinato, P. S., & Andreotti, M. (2023). Accumulated Carbon Fractions in Tropical Sandy Soils and Their Effects on Fertility and Grain Yield in an Integrated Crop–Livestock System. Sustainability, 15(18), 13829. https://doi.org/10.3390/su151813829