Advancement of Abiotic Stresses for Microalgal Lipid Production and Its Bioprospecting into Sustainable Biofuels
Abstract
1. Introduction
2. Microalgal Species and Biorefinery for Sustainable Biofuel Feedstock
3. Microalgae Cultivation Systems
3.1. Autotrophic Cultivation
3.2. Heterotrophic Cultivation
3.3. Mixotrophic Cultivation
3.4. Algal Turf Scrubber and Hybride Systems
4. Microalgae Harvesting Techniques
5. Approaches to Stimulate Lipid Production through Abiotic Stresses
5.1. Effect of Carbon Dioxide
5.2. Effect of Nitrogen Starvation
5.3. Effect of Light Intensity and Wavelength
5.4. Effect of Temperature
5.5. Effect of Salinity
5.6. Effect of pH
5.7. Effect of Metal
5.8. Effect of Sulfur Starvation
5.9. Effect of Phytohormones
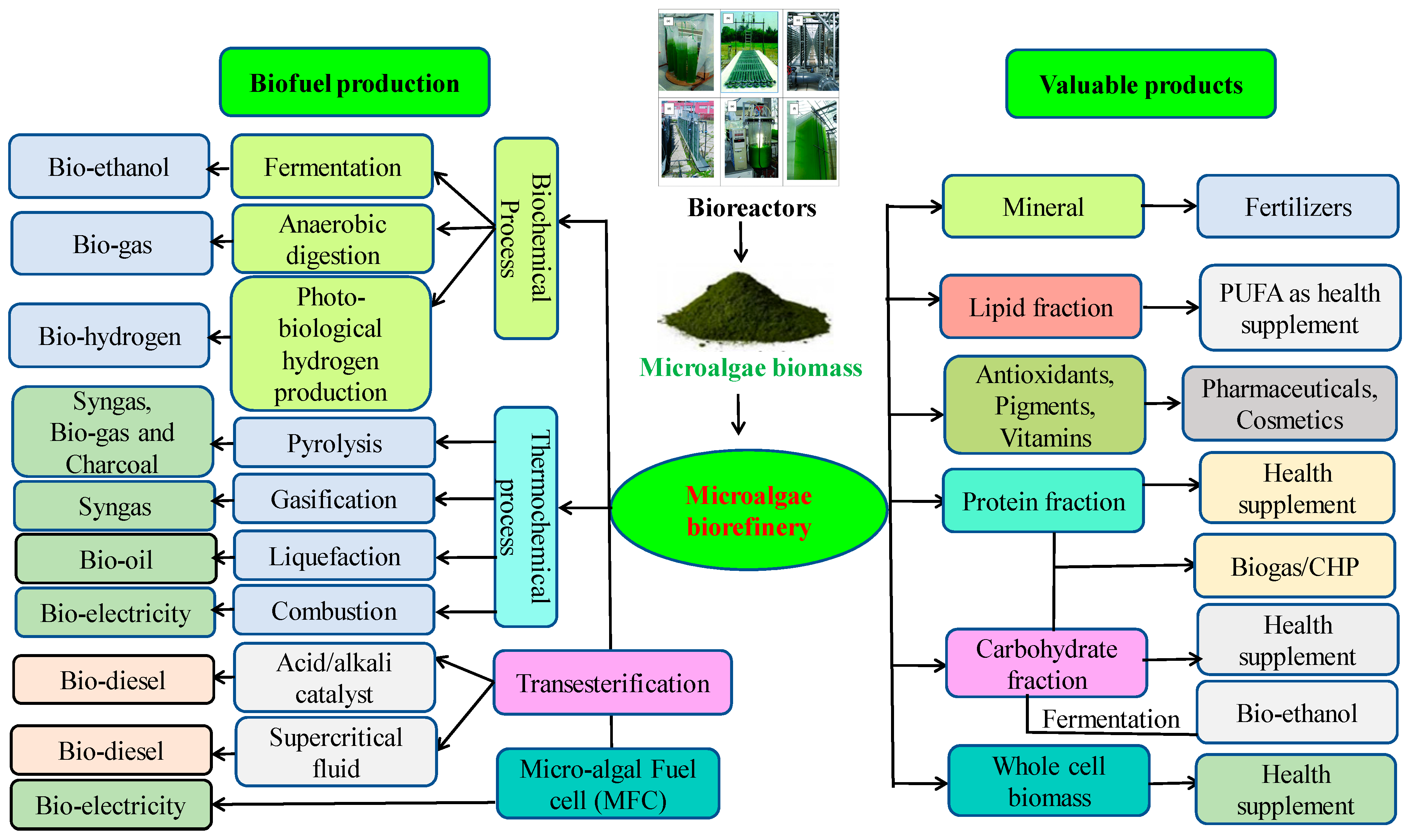
6. Microalgal Biorefinery for Biofuels Production
6.1. Biodiesel
6.2. Bio-Oil
6.3. Biobutanol
6.4. Bioethanol
6.5. Biogas
6.6. Biohydrogen
6.7. Bioelectricity
7. Abiotic Stress Induced Genes Responsible for Lipid Production
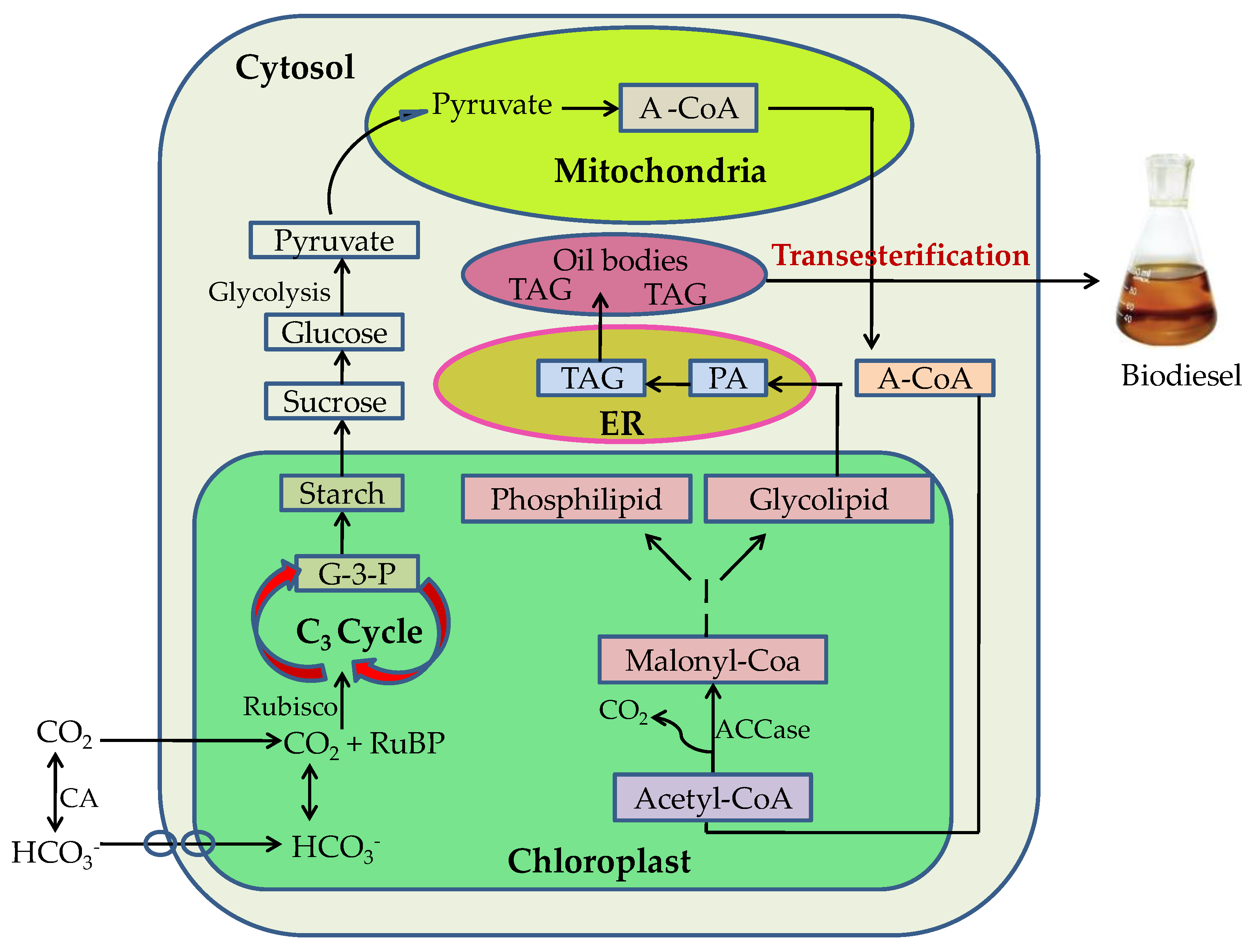
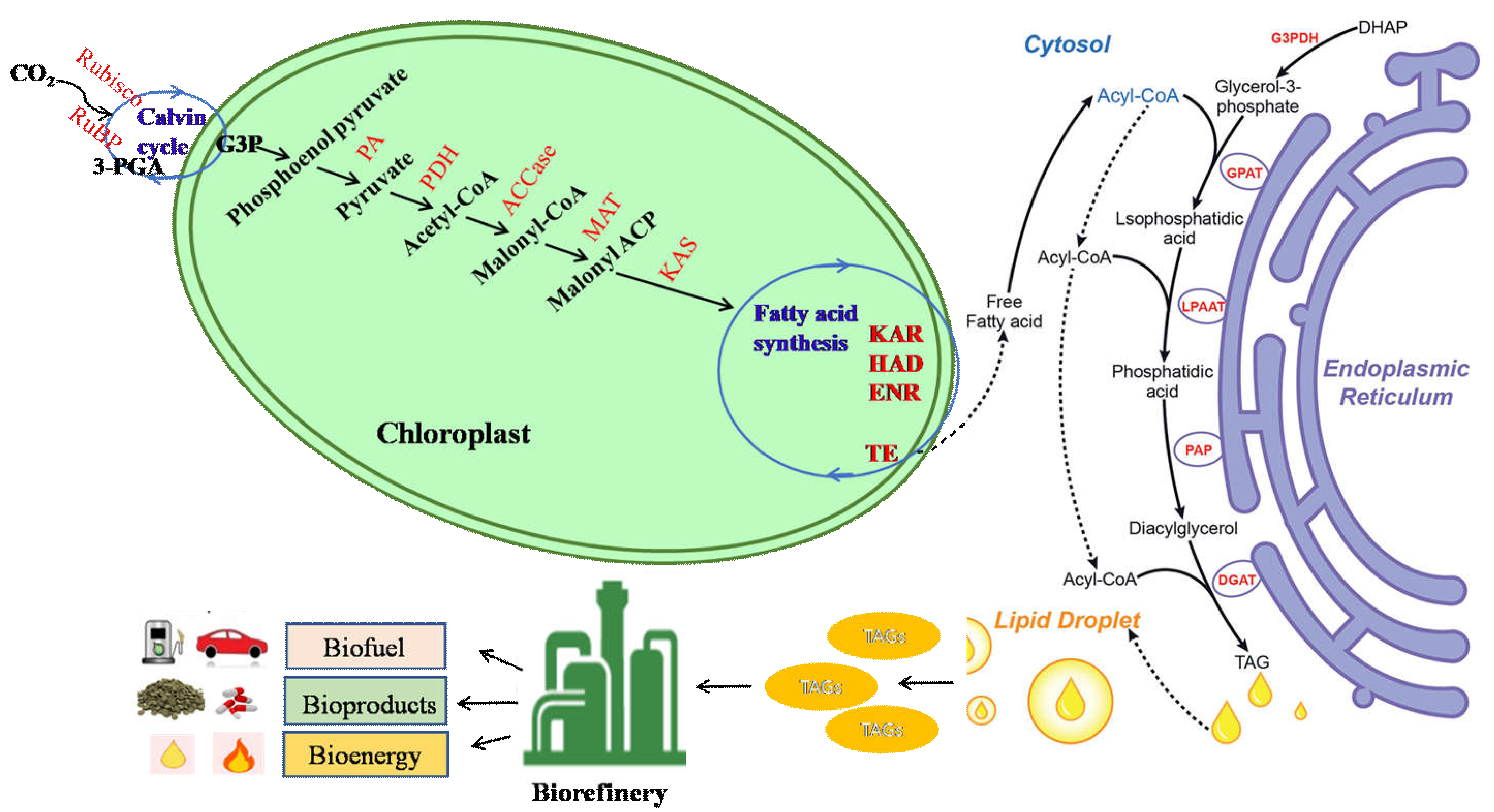
8. Cell Membrane Lipid and Triglycerides Accumulated in Lipid Droplets (LDs) in the Cells by Abiotic Stress for Biodiesel Production
9. Software Tools for Microalgae Biorefinery
10. Challenges of Microalgal Biofuel Production
11. Future Perspective
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Cooke, P.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel 2012, 97, 822–831. [Google Scholar] [CrossRef]
- Ahamed, T.S.; Brindhadevi, K.; Krishnan, R.; Phuong, T.N.; Alharbi, S.A.; Chinnathambi, A.; Mathimani, T. In vivo detection of triacylglycerols through Nile red staining and quantification of fatty acids in hyper lipid producer Nannochloropsis sp. cultured under adequate nitrogen and deficient nitrogen condition. Fuel 2022, 322, 124179. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Hoang, A.T.; Nižetić, S.; Balasubramanian, D.; Kamaraj, S.; Pandian, P.L.; Sirohi, R.; Nguyen, P.Q.P.; Nguyen, X.P. Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 2022, 329, 125362. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Govarthanan, M.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, J.R.; et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Al-Arjani, A.B.F.; Abd_Allah, E.F.; Rodríguez Dorantes, A.; Gupta, R.K. Physiological and biochemical responses of bicarbonate supplementation on biomass and lipid content of green algae Scenedesmus sp. BHU1 isolated from wastewater for renewable biofuel feedstock. Front. Microbiol. 2022, 13, 839800. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, O.N.; Bhunia, B.; Bandyopadhyay, T.K.; Oinam, G. Strategies for improved induction of lipid in Leptolyngbya sp. BTA 287 for biodiesel production. Fuel 2019, 256, 115896. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Tiwari, O.N.; Ray, R.N.; Bandyopadhyay, T.K.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017, 79, 372–389. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Pinto, L.R.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pandey, A.K.; Sirohi, R.; Hoang, A.T.; Kim, S.H. Recent advances in computational fluid dynamics (CFD) modelling of photobioreactors: Design and applications. Bioresour. Technol. 2022, 350, 126920. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.A.; Shahid, A.; Malik, S.; Wang, N.; Javed, M.R.; Haider, M.N.; Verma, P.; Ashraf, M.U.F.; Habib, N.; Syafiuddin, A.; et al. Advances in developing metabolically engineered microbial platforms to produce fourth-generation biofuels and high-value biochemicals. Bioresour. Technol. 2021, 337, 125510. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Karpagam, R.; Renukadevi, P.; Jawaharraj, K.; Varalakshmi, P. Biomass enhancement and bioconversion of brown marine microalgal lipid using heterogeneous catalysts mediated transesterification from biowaste derived biochar and bio nanoparticle. Fuel 2019, 255, 115789. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Yasin, N.H.M. Microalgae acclimatization in industrial wastewater and its effect on growth and primary metabolite composition. Algal Res. 2021, 53, 102163. [Google Scholar] [CrossRef]
- Bibi, F.; Ali, M.I.; Ahmad, M.; Bokhari, A.; Khoo, K.S.; Zafar, M.; Asif, S.; Mubashir, M.; Han, N.; Show, P.L. Production of lipids biosynthesis from Tetradesmus nygaardii microalgae as a feedstock for biodiesel production. Fuel 2022, 326, 124985. [Google Scholar] [CrossRef]
- Ali, S.S.; Mastropetros, S.G.; Schagerl, M.; Sakarika, M.; Elsamahy, T.; El-Sheekh, M.; Sun, J.; Kornaros, M. Recent advances in wastewater microalgae-based biofuels production: A state-of-the-art review. Energy Rep. 2022, 8, 13253–13280. [Google Scholar] [CrossRef]
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Patel, P.; Patel, B.; Vekaria, E.; Shah, M. Biophysical economics and management of biodiesel, a harbinger of clean and sustainable energy. Int. J. Energy Water Resour. 2020, 4, 411–423. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Obulisamy, P.K.; Verma, P. Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour. Technol. 2021, 320, 124301. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated approach for wastewater treatment and biofuel production in microalgae biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Salama, E.S.; Abou-Shanab, R.A.; Kim, J.R.; Lee, S.; Kim, S.H.; Oh, S.E.; Kim, H.C.; Roh, H.S.; Jeon, B.H. The effects of salinity on the growth and biochemical properties of Chlamydomonas mexicana GU732420 cultivated in municipal wastewater. Environ. Technol. Innov. 2014, 35, 1491–1498. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Mathimani, T.; Uma, L.; Prabaharan, D. Formulation of low-cost seawater medium for high cell density and high lipid content of Chlorella vulgaris BDUG 91771 using central composite design in biodiesel perspective. J. Clean. Prod. 2018, 198, 575–586. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Alodaini, H.A.; Hatamleh, A.A.; Santoyo, G.; Kumar, A.; Gupta, R.K. Impact of dehydration on the physiochemical properties of Nostoc calcicola BOT1 and its untargeted metabolic profiling through UHPLC-HRMS. Front. Plant Sci. 2023, 14, 1147390. [Google Scholar] [CrossRef]
- Cheng, D.; He, Q. Assessment of environmental stresses for enhanced microalgal biofuel production–an overview. Front. Energy Res. 2014, 2, 26. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Ocampo, D.; Vargas, G.J.; Sáez, A.A. Trends on CO2 capture with microalgae: A bibliometric analysis. Molecules 2022, 27, 4669. [Google Scholar] [CrossRef]
- Park, S.; Nguyen, T.H.T.; Jin, E. Improving lipid production by strain development in microalgae: Strategies, challenges and perspectives. Bioresour. Technol. 2019, 292, 121953. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Pandey, A.; Larroche, C.; Madamwar, D. Algal Green Energy–R&D and technological perspectives for biodiesel production. Renew. Sustain. Energy Rev. 2018, 82, 2946–2969. [Google Scholar]
- Seo, J.Y.; Jeon, H.J.; Kim, J.W.; Lee, J.; Oh, Y.K.; Ahn, C.W.; Lee, J.W. Simulated-sunlight-driven cell lysis of magnetophoretically separated microalgae using ZnFe2O4 octahedrons. Ind. Eng. Chem. Res. 2018, 57, 1655–1661. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef] [PubMed]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef]
- Khandelwal, A.; Vijay, A.; Dixit, A.; Chhabra, M. Microbial fuel cell powered by lipid extracted algae: A promising system for algal lipids and power generation. Bioresour. Technol. 2018, 247, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Park, J.; Kim, K.; Lee, L.S.; Seo, J.Y.; Oh, Y.K.; Kim, Y.J.; Ryou, M.H.; Lee, Y.M.; Lee, K. Recycling oil-extracted microalgal biomass residues into nano/micro hierarchical Sn/C composite anode materials for lithium-ion batteries. Electrochim. Acta 2017, 250, 59–67. [Google Scholar] [CrossRef]
- Beacham, T.A.; Macia, V.M.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/3 following EMS and UV induced mutagenesis. Biotechnol. Rep. 2015, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Gao, K.; Addy, M.; Li, D.; Zhang, R.C.; Lu, Q.; Ma, Y.W.; Cheng, Y.L.; Chen, P.; Liu, Y.H.; et al. Cultivation of Chlorella vulgaris on anaerobically digested swine manure with daily recycling of the post-harvest culture broth. Bioresour. Technol. 2018, 247, 716–723. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef]
- Takeshita, T.; Ivanov, I.N.; Oshima, K.; Ishii, K.; Kawamoto, H.; Ota, S.; Yamazaki, T.; Hirata, A.; Kazama, Y.; Abe, T.; et al. Comparison of lipid productivity of Parachlorella kessleri heavy-ion beam irradiation mutant PK4 in laboratory and 150-L mass bioreactor, identification and characterization of its genetic variation. Algal Res. 2018, 35, 416–426. [Google Scholar] [CrossRef]
- Yu, N.; Dieu, L.T.J.; Harvey, S.; Lee, D.Y. Optimization of process configuration and strain selection for microalgae-based biodiesel production. Bioresour. Technol. 2015, 193, 25–34. [Google Scholar] [CrossRef]
- Feng, P.; Deng, Z.; Hu, Z.; Wang, Z.; Fan, L. Characterization of Chlorococcum pamirum as a potential biodiesel feedstock. Bioresour. Technol. 2014, 162, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, M.; Wang, C. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour. Technol. 2013, 147, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Karpagam, R.; Raj, K.J.; Ashokkumar, B.; Varalakshmi, P. Characterization and fatty acid profiling in two fresh water microalgae for biodiesel production: Lipid enhancement methods and media optimization using response surface methodology. Bioresour. Technol. 2015, 188, 177–184. [Google Scholar] [CrossRef]
- Mao, X.; Wu, T.; Sun, D.; Zhang, Z.; Chen, F. Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour. Technol. 2018, 249, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Geng, Y.; Li, Y. Enhanced lipid production in Chlorella pyrenoidosa by continuous culture. Bioresour. Technol. 2014, 161, 297–303. [Google Scholar] [CrossRef]
- Maity, J.P.; Bundschuh, J.; Chen, C.Y.; Bhattacharya, P. Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives—A mini review. Energy 2014, 78, 104–113. [Google Scholar] [CrossRef]
- Wiencke, C.; Clayton, M.N.; Gómez, I.; Iken, K.; Lüder, U.H.; Amsler, C.D.; Karsten, U.; Hanelt, D.; Bischof, K.; Dunton, K. Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev. Environ. Sci. Biotechnol. 2007, 6, 95–126. [Google Scholar] [CrossRef]
- Kuo, C.M.; Lin, T.H.; Yang, Y.C.; Zhang, W.X.; Lai, J.T.; Wu, H.T.; Chang, J.S.; Lin, C.S. Ability of an alkali-tolerant mutant strain of the microalga Chlorella sp. AT1 to capture carbon dioxide for increasing carbon dioxide utilization efficiency. Bioresour. Technol. 2017, 244, 243–251. [Google Scholar] [CrossRef]
- Abid, A.; Saidane, F.; Hamdi, M. Feasibility of carbon dioxide sequestration by Spongiochloris sp. microalgae during petroleum wastewater treatment in airlift bioreactor. Bioresour. Technol. 2017, 234, 297–302. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Patel, A.K.; Hong, M.E.; Chang, W.S.; Sim, S.J. Microalgae Bioenergy with Carbon Capture and Storage (BECCS): An emerging sustainable bioprocess for reduced CO2 emission and biofuel production. Bioresour. Technol. Rep. 2019, 7, 100270. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Algae Products Market by Type (Lipids, Carotenoids, Carrageenan, Alginate, Algal Protein), Facility Type, Form (Liquid, Solid), Source (Brown Algae, Green Algae, Red Algae, Blue-green Algae), and Region—Global Forecast to 2026; Market Survey Report No. FB 6208, Algae Products Market; Markets and Markets: Pune, India, 2021.
- Scarcelli, P.G.; Ruas, G.; Lopez-Serna, R.; Serejo, M.L.; Blanco, S.; Boncz, M.Á.; Muñoz, R. Integration of algae-based sewage treatment with anaerobic digestion of the bacterial-algal biomass and biogas upgrading. Bioresour. Technol. 2021, 340, 125552. [Google Scholar] [CrossRef] [PubMed]
- Khammee, P.; Ramaraj, R.; Whangchai, N.; Bhuyar, P.; Unpaprom, Y. The immobilization of yeast for fermentation of macroalgae Rhizoclonium sp. for efficient conversion into bioethanol. Biomass Convers. Biorefin. 2021, 11, 827–835. [Google Scholar] [CrossRef]
- Monlau, F.; Suarez-Alvarez, S.; Lallement, A.; Vaca-Medina, G.; Giacinti, G.; Munarriz, M.; Urreta, I.; Raynaud, C.; Ferrer, C.; Castañón, S. A cascade biorefinery for the valorization of microalgal biomass: Biodiesel, biogas, fertilizers and high valuable compounds. Algal Res. 2021, 59, 102433. [Google Scholar] [CrossRef]
- Nagarajan, D.; Dong, C.D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Biohydrogen production from microalgae-Major bottlenecks and future research perspectives. Biotechnol. J. 2021, 16, 2000124. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int. J. Hydrogen Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Razzak, S.A.; Ali, S.A.M.; Hossain, M.M.; deLasa, H. Biological CO2 fixation with production of microalgae in wastewater–a review. Renew. Sustain. Energy Rev. 2017, 76, 379–390. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Hamelin, J.; Bonnafous, A.; Steyer, J.P. CO2 addition to increase biomass production and control microalgae species in high-rate algal ponds treating wastewater. J. CO2 Util. 2018, 28, 292–298. [Google Scholar] [CrossRef]
- Saha, S.K.; Murray, P. Exploitation of microalgae species for nutraceutical purposes: Cultivation aspects. Fermentation 2018, 4, 46. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Deruyck, B.; Nguyen, K.H.T.; Decaestecker, E.; Muylaert, K. Modeling the impact of rotifer contamination on microalgal production in open pond, photobioreactor and thin layer cultivation systems. Algal Res. 2019, 38, 101398. [Google Scholar] [CrossRef]
- Kannan, D.C.; Venkat, D. An open outdoor algal growth system of improved productivity for biofuel production. J. Chem. Technol. Biotechnol. 2019, 94, 222–235. [Google Scholar] [CrossRef]
- Acién, F.G.; Molina, E.; Reis, A.; Torzillo, G.; Zittelli, G.C.; Sepúlveda, C.; Masojídek, J. Photobioreactors for the Production of Microalgae. In Microalgae-Based Biofuels and Bioproducts; Woodhead Publishing: Sawston, UK, 2017; pp. 1–44. [Google Scholar]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Optimisation of tray drier microalgae dewatering techniques using response surface methodology. Energies 2018, 11, 2327. [Google Scholar] [CrossRef]
- Mazumdar, N.; Novis, P.M.; Visnovsky, G.; Gostomski, P.A. Effect of nutrients on the growth of a new alpine strain of Haematococcus (Chlorophyceae) from New Zealand. Phycological Res. 2019, 67, 21–27. [Google Scholar] [CrossRef]
- Mantzorou, A.; Ververidis, F. Microalgal biofilms: A further step over current microalgal cultivation techniques. Sci. Total Environ. 2019, 651, 3187–3201. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Su, C.H.; Yu, Y.K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crops Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Zuccaro, G.; Yousuf, A.; Pollio, A.; Steyer, J.P. Microalgae Cultivation Systems. In Microalgae Cultivation for Biofuels Production; Academic Press: Cambridge, MA, USA, 2020; pp. 11–29. [Google Scholar]
- Meng, T.K.; Kassim, M.A.; Cheirsilp, B. Mixotrophic cultivation: Biomass and biochemical biosynthesis for biofuel production. In Microalgae Cultivation for Biofuels Production; Academic Press: Cambridge, MA, USA, 2020; pp. 51–67. [Google Scholar]
- Judd, S.J.; Al Momani, F.A.O.; Znad, H.; Al Ketife, A.M.D. The cost benefit of algal technology for combined CO2 mitigation and nutrient abatement. Renew. Sustain. Energy Rev. 2017, 71, 379–387. [Google Scholar] [CrossRef]
- Sipaúba-Tavares, L.H.; Segali, A.M.D.L.; Berchielli-Morais, F.A.; Scardoeli-Truzzi, B. Development of low-cost culture media for Ankistrodesmus gracilis based on inorganic fertilizer and macrophyte. Acta Limnol. Bras. 2017, 29. [Google Scholar] [CrossRef][Green Version]
- Sundar Rajan, P.; Gopinath, K.P.; Greetham, D.; Antonysamy, A.J. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J. Clean. Prod. 2019, 210, 445–458. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Raeesossadati, M.J.; Ahmadzadeh, H.; McHenry, M.P.; Moheimani, N.R. CO2 bioremediation by microalgae in photobioreactors: Impacts of biomass and CO2 concentrations, light, and temperature. Algal Res. 2014, 6, 78–85. [Google Scholar] [CrossRef]
- Lv, J.M.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Cabanelas, I.T.D.; dos Santos, J.N.; Nascimento, M.A.; Sousa, L.; Sansone, G. Biodiesel yields and fuel quality as criteria for algal-feedstock selection: Effects of CO2-supplementation and nutrient levels in cultures. Algal Res. 2015, 8, 53–60. [Google Scholar] [CrossRef]
- Moreno, R.; Aita, G.M.; Madsen, L.; Gutierrez, D.L.; Yao, S.; Hurlburt, B.; Brashear, S. Identification of naturally isolated Southern Louisiana’s algal strains and the effect of higher CO2 content on fatty acid profiles for biodiesel production. J. Chem. Technol. Biotechnol. 2013, 88, 948–957. [Google Scholar] [CrossRef]
- Bibi, F.; Jamal, A.; Huang, Z.; Urynowicz, M.; Ali, M.I. Advancement and role of abiotic stresses in microalgae biorefinery with a focus on lipid production. Fuel 2022, 316, 123192. [Google Scholar] [CrossRef]
- Sulochana, S.B.; Arumugam, M. Targeted metabolomic and biochemical changes during nitrogen stress mediated lipid accumulation in Scenedesmus quadricauda CASA CC202. Front. Bioeng. Biotechnol. 2020, 8, 585632. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. Regulatory mechanisms of lipid biosynthesis in microalgae. Biol. Rev. 2021, 96, 2373–2391. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.W.; Hong, J.W.; Do, J.M.; Na, H.; Kim, J.J.; Park, S.I.; Kim, Y.S.; Kim, I.S.; Yoon, H.S. Nitrogen deficiency-dependent abiotic stress enhances carotenoid production in indigenous green microalga Scenedesmus rubescens KNUA042, for use as a potential resource of high value products. Sustainability 2020, 12, 5445. [Google Scholar] [CrossRef]
- Shtaida, N.; Khozin, G.I.; Boussiba, S. The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. Photosynth. Res. 2015, 125, 407–422. [Google Scholar] [CrossRef]
- Nagappan, S.; Devendran, S.; Tsai, P.C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics integrated with transcriptomics and proteomics: Evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process Biochem. 2020, 91, 1–14. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and morphological changes triggered by nitrogen stress in the oleaginous microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Pei, H.; Mu, R.; Ma, G.; Wu, D.; Han, Q. Characterization and optimization of endogenous lipid accumulation in Chlorella vulgaris SDEC-3M ability to rapidly accumulate lipid for reversing nightly lipid loss. Biotechnol. Biofuels 2019, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Gao, L.; Zhao, W.; Chen, W.; Li, M. Effects of nitrogen forms and supply mode on lipid production of microalga Scenedesmus obliquus. Energies 2020, 13, 697. [Google Scholar] [CrossRef]
- Shi, T.Q.; Wang, L.R.; Zhang, Z.X.; Sun, X.M.; Huang, H. Stresses as first-line tools for enhancing lipid and carotenoid production in microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Napolitano, G.E. The relationship of lipids with light and chlorophyll measurements in freshwater algae and periphyton 1. J. Phycol. 1994, 30, 943–950. [Google Scholar] [CrossRef]
- Gris, B.; Morosinotto, T.; Giacometti, G.M.; Bertucco, A.; Sforza, E. Cultivation of Scenedesmus obliquus in photobioreactors: Effects of light intensities and light–dark cycles on growth, productivity, and biochemical composition. Appl. Biochem. Biotechnol. 2014, 172, 2377–2389. [Google Scholar] [CrossRef]
- Gim, G.H.; Ryu, J.; Kim, M.J.; Kim, P.I.; Kim, S.W. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J. Ind. Microbiol. 2016, 43, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Severes, A.; Hegde, S.; D’Souza, L.; Hegde, S. Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae-based biofuels. J. Photochem. Photobiol. B Biol. 2017, 170, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Helamieh, M.; Gebhardt, A.; Reich, M.; Kuhn, F.; Kerner, M.; Kümmerer, K. Growth and fatty acid composition of Acutodesmus obliquus under different light spectra and temperatures. Lipids 2021, 56, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Helamieh, M.; Reich, M.; Bory, S.; Rohne, P.; Riebesell, U.; Kerner, M.; Kümmerer, K. Blue-green light is required for a maximized fatty acid unsaturation and pigment concentration in the microalga Acutodesmus obliquus. Lipids 2022, 57, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kumaran, R.S.; Jeon, H.J.; Song, H.J.; Yang, Y.H.; Lee, S.H.; Song, K.G.; Kim, K.J.; Singh, V.; Kim, H.J. LED light stress induced biomass and fatty acid production in microalgal biosystem, Acutodesmus obliquus. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 245–253. [Google Scholar] [CrossRef]
- Shih, S.C.; Mufti, N.S.; Chamberlain, M.D.; Kim, J.; Wheeler, A.R. A droplet-based screen for wavelength-dependent lipid production in algae. Energy Environ. Sci. 2014, 7, 2366–2375. [Google Scholar] [CrossRef]
- Seo, Y.H.; Lee, Y.; Jeon, D.Y.; Han, J.I. Enhancing the light utilization efficiency of microalgae using organic dyes. Bioresour. Technol. 2015, 181, 355–359. [Google Scholar] [CrossRef]
- Seo, Y.H.; Cho, C.; Lee, J.Y.; Han, J.I. Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation. Bioresour. Technol. 2014, 173, 193–197. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Al-Qasmi, M.; Raut, N.; Talebi, S.; Al-Rajhi, S.; Al-Barwani, T. A review of effect of light on microalgae growth. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012; Volume 2, pp. 1–7. [Google Scholar]
- Brown, M.R.; Dunstan, G.A.; Norwood, S.J.; Miller, K.A. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana 1. J. Phycol. 1996, 32, 64–73. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Trivedi, K.; George, B.; Maurya, R.; Ghosh, A.; Mishra, S. Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour. Technol. 2015, 180, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Wang, Y.; He, B.; Sun, Z.; Chen, Y.F. Chemically enhanced lipid production from microalgae under low sub-optimal temperature. Algal Res. 2016, 16, 20–27. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K.; Chao, T.M. Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J. Appl. Phycol. 1997, 9, 451–457. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (bacillariophyceae) 1. J. Phycol. 2004, 40, 651–654. [Google Scholar] [CrossRef]
- Takagi, M.; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323. [Google Scholar] [CrossRef]
- Duan, X.; Ren, G.Y.; Liu, L.L.; Zhu, W.X. Salt-induced osmotic stress for lipid overproduction in batch culture of Chlorella vulgaris. Afr. J. Biotechnol. 2012, 11, 7072–7078. [Google Scholar]
- Cao, J.; Yuan, H.; Li, B.; Yang, J. Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour. Technol. 2014, 152, 177–184. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gupta, R.K. Salinity-Induced Physiochemical Alterations to Enhance Lipid Content in Oleaginous Microalgae Scenedesmus sp. BHU1 via Two-Stage Cultivation for Biodiesel Feedstock. Microorganisms 2023, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Bartley, M.L.; Boeing, W.J.; Dungan, B.N.; Holguin, F.O.; Schaub, T. pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J. Appl. Phycol. 2014, 26, 1431–1437. [Google Scholar] [CrossRef]
- Sang, M.; Wang, M.; Liu, J.; Zhang, C.; Li, A. Effects of temperature, salinity, light intensity, and pH on the eicosapentaenoic acid production of Pinguiococcus pyrenoidosus. J. Ocean Univ. China 2012, 11, 181–186. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Jin, X.; Xia, J.; Rosenberg, J.N.; Yu, G.; Nie, Z.; Oyler, G.A.; Betenbaugh, M.J. The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2014, 98, 9473–9481. [Google Scholar] [CrossRef]
- Sibi, G.; Anuraag, T.S.; Bafila, G. Copper stress on cellular contents and fatty acid profiles in Chlorella species. Online J. Biol. Sci. 2014, 14, 209–217. [Google Scholar] [CrossRef]
- Li, X.; Přibyl, P.; Bišová, K.; Kawano, S.; Cepák, V.; Zachleder, V.; Čížková, M.; Brányiková, I.; Vítová, M. The microalga Parachlorella kessleri––A novel highly efficient lipid producer. Biotechnol. Bioeng. 2013, 110, 97–107. [Google Scholar] [CrossRef]
- Rocchetta, I.; Mazzuca, M.; Conforti, V.; Ruiz, L.; Balzaretti, V.; de Molina, M.D.C.R. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ. Pollut. 2006, 141, 353–358. [Google Scholar] [CrossRef]
- Mao, X.; Lao, Y.; Sun, H.; Li, X.; Yu, J.; Chen, F. Time-resolved transcriptome analysis during transitions of sulfur nutritional status provides insight into triacylglycerol (TAG) and astaxanthin accumulation in the green alga Chromochloris zofingiensis. Biotechnol. Biofuels. 2020, 13, 128. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, H.; Xu, N.; Li, A. Effects of sulfur limitation on nitrogen and sulfur uptake and lipid accumulation in Scenedesmus acuminatus. J. Appl. Phycol. 2021, 33, 301–311. [Google Scholar] [CrossRef]
- Wu, T.; Yu, L.; Zhang, Y.; Liu, J. Characterization of fatty acid desaturases reveals stress-induced synthesis of C18 unsaturated fatty acids enriched in triacylglycerol in the oleaginous alga Chromochloris zofingiensis. Biotechnol. Biofuels. 2021, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, X.; Wang, Q. Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front. Plant Sci. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Dao, G.H.; Wu, G.X.; Wang, X.X.; Zhuang, L.L.; Zhang, T.Y.; Hu, H.Y. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour. Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jain, D.; Agarwal, P.; Singh, R.P. Auxin and cytokinin synergism augmenting biomass and lipid production in microalgae Desmodesmus sp. JS07. Process Biochem. 2020, 95, 223–234. [Google Scholar] [CrossRef]
- Bhuyar, P.; Yusoff, M.M.; Rahim, M.H.A.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Effect of plant hormones on the production of biomass and lipid extraction for biodiesel production from microalgae Chlorella sp. J. Microbiol. Biotechnol. Food Sci. 2021, 9, 671–674. [Google Scholar] [CrossRef]
- Che, R.; Huang, L.; Xu, J.W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Effect of fulvic acid induction on the physiology, metabolism, and lipid biosynthesis-related gene transcription of Monoraphidium sp. FXY-10. Bioresour. Technol. 2017, 227, 324–334. [Google Scholar] [CrossRef]
- Udayan, A.; Sabapathy, H.; Arumugam, M. Stress hormones mediated lipid accumulation and modulation of specific fatty acids in Nannochloropsis oceanica CASA CC201. Bioresour. Technol. 2020, 310, 123437. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M.; Kamizela, T.; Kowalczyk, M.; Kalaji, H.M.; Bąba, W. Inexpensive and universal growth media for biomass production of microalgae. Glob. Nest. J. 2019, 21, 82–89. [Google Scholar]
- Ananthi, V.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. Impact of abiotic factors on biodiesel production by microalgae. Fuel 2021, 284, 118962. [Google Scholar] [CrossRef]
- Smachetti, M.E.S.; Coronel, C.D.; Salerno, G.L.; Curatti, L. Sucrose-to-ethanol microalgae-based platform using seawater. Algal Res. 2020, 45, 101733. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Circular bioeconomy: An introduction. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–23. [Google Scholar]
- Behera, B.; Paramasivan, B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour. Technol. 2022, 351, 127038. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Tripathi, S.; Poluri, K.M. Microalgal-based bioenergy: Strategies, prospects, and sustainability. Energy Fuel 2022, 36, 14584–14612. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Gupta, V.K. Recent Progress and Future Perspectives for Zero Agriculture Waste Technologies: Pineapple Waste as a Case Study. Sustainability 2023, 15, 3575. [Google Scholar] [CrossRef]
- Kuppan, P.; Sudharsanam, A.; Venkateswarlu, K.; Megharaj, M. Solar technology—Closed loop synergy facilitates low-carbon circular bioeconomy in microalgal wastewater treatment. NPJ Clean Water 2023, 6, 43. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans–biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as Tools for Bio-Circular-Green Economy: Zero-waste Approaches for Sustainable Production and Biorefineries of Microalgal Biomass. Bioresour. Technol. 2023, 387, 129620. [Google Scholar] [CrossRef]
- Idenyi, J.N.; Eya, J.C.; Nwankwegu, A.S.; Nwoba, E.G. Aquaculture sustainability through alternative dietary ingredients: Microalgal value-added products. Eng. Microbiol. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other nutrients from Haematococcus pluvialis—Multifunctional applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef]
- Patel, A.; Krikigianni, E.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprocessing of volatile fatty acids by oleaginous freshwater microalgae and their potential for biofuel and protein production. J. Chem. Eng. 2022, 438, 135529. [Google Scholar] [CrossRef]
- Callejón, M.J.J.; Medina, A.R.; Sánchez, M.D.M.; Moreno, P.A.G.; López, E.N.; Cerdán, L.E.; Grima, E.M. Supercritical fluid extraction and pressurized liquid extraction processes applied to eicosapentaenoic acid-rich polar lipid recovery from the microalga Nannochloropsis sp. Algal Res. 2022, 61, 102586. [Google Scholar] [CrossRef]
- Oliver, L.; Fernández-de-Castro, L.; Dietrich, T.; Villaran, M.C.; Barrio, R.J. Production of docosahexaenoic acid and odd-chain fatty acids by microalgae Schizochytrium limacinum grown on waste-derived volatile fatty acids. Appl. Sci. 2022, 12, 3976. [Google Scholar] [CrossRef]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A realistic scenario on microalgae-based biodiesel production: Third generation biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lee, M.H.; Leong, Y.K.; Chang, J.S.; Lee, D.J. Biodiesel production from heterotrophic oleaginous microalga Thraustochytrium sp. BM2 with enhanced lipid accumulation using crude glycerol as alternative carbon source. Bioresour. Technol. 2020, 306, 123113. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E. Application of microalgae biomass for biodiesel fuel production. Energies 2022, 15, 4178. [Google Scholar] [CrossRef]
- Toro-Trochez, J.L.; Carrillo-Pedraza, E.S.; Bustos-Martínez, D.; García-Mateos, F.J.; Ruiz-Rosas, R.R.; Rodríguez-Mirasol, J.; Cordero, T. Thermogravimetric characterization and pyrolysis of soybean hulls. Bioresour. Technol. Rep. 2019, 6, 183–189. [Google Scholar] [CrossRef]
- Sun, K.; Li, Q.; Zhang, L.; Shao, Y.; Zhang, Z.; Zhang, S.; Liu, Q.; Wang, Y.; Hu, X. Impacts of water-organic solvents on polymerization of the sugars and furans in bio-oil. Bioresour. Technol. Rep. 2020, 10, 100419. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Arvindnarayan, S.; Prabhu, K.K.S.; Shobana, S.; Pasupathy, A.; Dharmaraja, J.; Kumar, G. Potential assessment of micro algal lipids: A renewable source of energy. J. Energy Inst. 2017, 90, 431–440. [Google Scholar] [CrossRef]
- Kurji, H.; Valera-Medina, A.; Runyon, J.; Giles, A.; Pugh, D.; Marsh, R.; Cerone, N.; Zimbardi, F.; Valerio, V. Combustion characteristics of biodiesel saturated with pyrolysis oil for power generation in gas turbines. Renew. Energy 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Vivek, N.; Nair, L.M.; Mohan, B.; Nair, S.C.; Sindhu, R.; Pandey, A.; Shurpali, N.; Binod, P. Bio-butanol production from rice straw–recent trends, possibilities, and challenges. Bioresour. Technol. Rep. 2019, 7, 100224. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Yeong, T.K.; Jiao, K.; Zeng, X.; Lin, L.; Pan, S.; Danquah, M.K. Microalgae for biobutanol production–Technology evaluation and value proposition. Algal Res. 2018, 31, 367–376. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of carbon capture technology: Microalgal biorefinery concept and state-of-the-art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.Q.; Chang, J.S.; Ren, N.Q. Butanol production using carbohydrate-enriched Chlorella vulgaris as feedstock. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2014; Volume 830, pp. 122–125. [Google Scholar]
- Kao, W.C.; Lin, D.S.; Cheng, C.L.; Chen, B.Y.; Lin, C.Y.; Chang, J.S. Enhancing butanol production with Clostridium pasteurianum CH4 using sequential glucose–glycerol addition and simultaneous dual-substrate cultivation strategies. Bioresour. Technol. 2013, 135, 324–330. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, V.; Salam, Z.; Tiwari, O.N.; Chinnasamy, S.; Mohammed, S.; Ani, F.N. An integrated approach for biodiesel and bioethanol production from Scenedesmus bijugatus cultivated in a vertical tubular photobioreactor. Energy Convers. Manag. 2015, 101, 778–786. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- Abo-Hashesh, M.; Wang, R.; Hallenbeck, P.C. Metabolic engineering in dark fermentative hydrogen production; theory and practice. Bioresour. Technol. 2011, 102, 8414–8422. [Google Scholar] [CrossRef] [PubMed]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Maciel Filho, R. Potential of algal biofuel production in a hybrid photobioreactor. Chem. Eng. Sci. 2017, 171, 282–292. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Savakis, P.; Hellingwerf, K.J. Engineering cyanobacteria for direct biofuel production from CO2. Curr. Opin. Biotechnol. 2015, 33, 8–14. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Daroch, M.; Geng, S.; Wang, G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Bose, A.; O’Shea, R.; Lin, R.; Murphy, J.D. A perspective on novel cascading algal biomethane biorefinery systems. Bioresour. Technol. 2020, 304, 123027. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel production and phosphorus recovery through an integrated treatment of agro-industrial waste. Sustainability 2018, 11, 52. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Passos, F.; Romero-Güiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Marín, D.; Posadas, E.; Cano, P.; Pérez, V.; Blanco, S.; Lebrero, R.; Muñoz, R. Seasonal variation of biogas upgrading coupled with digestate treatment in an outdoors pilot scale algal-bacterial photobioreactor. Bioresour. Technol. 2018, 263, 58–66. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Serejo, M.L.; Blanco, S.; Pérez, R.; Lebrero, R.; Muñoz, R. Photosynthetic biogas upgrading to bio-methane: Boosting nutrient recovery via biomass productivity control. Algal Res. 2016, 17, 46–52. [Google Scholar] [CrossRef]
- Meier, L.; Barros, P.; Torres, A.; Vilchez, C.; Jeison, D. Photosynthetic biogas upgrading using microalgae: Effect of light/dark photoperiod. Renew. Energy 2017, 106, 17–23. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Alcántara, C.; Noyola, A.; Muñoz, R.; González-Sánchez, A. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: Influence of the illumination regime. Sci. Total Environ. 2017, 592, 419–425. [Google Scholar] [CrossRef]
- Prandini, J.M.; Da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, M.; Liang, C.; Chui, C.; Wang, N.; Shi, J.; Liu, L. Multivariate insights into enhanced biogas production in thermophilic dry anaerobic co-digestion of food waste with kitchen waste or garden waste: Process properties, microbial communities and metagenomic analyses. Bioresour. Technol. 2022, 361, 127684. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Ma, Y.W.; Feng, S.X.; Zhang, Y.X.; Kou, H.J.; Sun, Y. The synergistic effect of chemical oxidation and microbial activity on improving volatile fatty acids (VFAs) production during the animal wastewater anaerobic digestion process treated with persulfate/biochar. Sci. Total Environ. 2023, 857, 159276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, B.; Yao, F.; He, L.; Chen, F.; Ma, Y.; Shu, X.; Hou, K.; Wang, D.; Li, X. Biogas production from anaerobic co-digestion of waste activated sludge: Co-substrates and influencing parameters. Rev. Environ. Sci. Biotechnol. 2019, 18, 771–793. [Google Scholar] [CrossRef]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of cell wall degrading enzymes to improve the recovery of lipids from Chlorella sorokiniana. Chem. Eng. J. 2019, 377, 120325. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Marques, P.A.; Ferreira, A.F.; Dias, A.P.; Pinheiro, H.M.; Reis, A.; Gouveia, L. Scenedesmus obliquus mediated brewery wastewater remediation and CO2 biofixation for green energy purposes. J. Clean. Prod. 2017, 165, 1316–1327. [Google Scholar] [CrossRef]
- Giraldo-Calderón, N.D.; Romo-Buchelly, R.J.; Arbeláez-Pérez, A.A.; Echeverri-Hincapié, D.; Atehortúa-Garcés, L. Microalgae biorefineries: Applications and emerging technologies. Dyna 2018, 85, 219–233. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, I.; Kumar, A.; Gupta, R.K. Bioprospecting and Mechanisms of Cyanobacterial Hydrogen Production and Recent Development for Its Enhancement as a Clean Energy. In Cyanobacterial Biotechnology in the 21st Century; Springer Publishing: Berlin/Heidelberg, Germany, 2023; pp. 107–131. [Google Scholar]
- Singh, R.P.; Yadav, P.; Gupta, R.K. Modern strategy of cyanobacterial biohydrogen production and current approaches toward its enhancement. In Expanding Horizon of Cyanobacterial Biology; Elsevier Publishing: Amsterdam, The Netherlands, 2022; pp. 219–238. [Google Scholar]
- Rahman, S.N.A.; Masdar, M.S.; Rosli, M.I.; Majlan, E.H.; Husaini, T.; Kamarudin, S.K.; Daud, W.R.W. Overview biohydrogen technologies and application in fuel cell technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kabra, A.N.; Kim, J.R.; Jeon, B.H. Photoheterotrophic microalgal hydrogen production using acetate-and butyrate-rich wastewater effluent. Energy 2014, 78, 887–894. [Google Scholar] [CrossRef]
- Papazi, A.; Pappas, I.; Kotzabasis, K. Combinational system for biodegradation of olive oil mill wastewater phenolics and high yield of bio-hydrogen production. J. Biotechnol. 2019, 306, 47–53. [Google Scholar] [CrossRef]
- Vargas, S.R.; Santos, P.V.D.; Giraldi, L.A.; Zaiat, M.; Calijuri, M.D.C. Anaerobic phototrophic processes of hydrogen production by different strains of microalgae Chlamydomonas sp. FEMS Microbiol. Lett. 2018, 365, fny073. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Neethu, B.; Ghangrekar, M.M. Microbial carbon capture cell: Advanced bio-electrochemical system for wastewater treatment, electricity generation and algal biomass production. In Application of Microalgae in Wastewater Treatment; Volume 2: Biorefinery Approaches of Wastewater Treatment; Springer: Cham, Switzerland, 2019; pp. 317–338. [Google Scholar]
- Chandrasekhar, K.; Mohan, S.V. Induced catabolic bio-electrohydrolysis of complex food waste by regulating external resistance for enhancing acidogenic biohydrogen production. Bioresour. Technol. 2014, 165, 372–382. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Sharma, N.; Rautela, I.; Nanda, M.; Arora, N.; Singh, A.; Chauhan, P.K. Microalgae fuel cell for wastewater treatment: Recent advances and challenges. J. Water Process. Eng. 2020, 38, 101549. [Google Scholar] [CrossRef]
- Deval, A.S.; Parikh, H.A.; Kadier, A.; Chandrasekhar, K.; Bhagwat, A.M.; Dikshit, A.K. Sequential microbial activities mediated bioelectricity production from distillery wastewater using bio-electrochemical system with simultaneous waste remediation. Int. J. Hydrogen Energy 2017, 42, 1130–1141. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, S.P.; Datta, S.; Taraphder, S.; Panda, S.; Saikhom, R.; Laishram, M.; Swain, D.P.; Nanotkar, R.Y. Microbial fuel cell (MFC): Recent advancement and its application. Int. J. Pure. App. Biosci. 2017, 5, 911–923. [Google Scholar]
- Lee, D.J.; Chang, J.S.; Lai, J.Y. Microalgae–microbial fuel cell: A mini review. Bioresour. Technol. 2015, 198, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Arana, T.J.; Gude, V.G. A microbial desalination process with microalgae biocathode using sodium bicarbonate as an inorganic carbon source. Int. Biodeterior. Biodegrad. 2018, 130, 91–97. [Google Scholar] [CrossRef]
- Behl, K.; SeshaCharan, P.; Joshi, M.; Sharma, M.; Mathur, A.; Kareya, M.S.; Jutur, P.P.; Bhatnagar, A.; Nigam, S. Multifaceted applications of isolated microalgae Chlamydomonas sp. TRC-1 in wastewater remediation, lipid production and bioelectricity generation. Bioresour. Technol. 2020, 304, 122993. [Google Scholar] [CrossRef] [PubMed]
- Juang, D.F.; Lee, C.H.; Hsueh, S.C. Comparison of electrogenic capabilities of microbial fuel cell with different light power on algae grown cathode. Bioresour. Technol. 2012, 123, 23–29. [Google Scholar] [CrossRef]
- Hou, Q.; Cheng, J.; Nie, C.; Pei, H.; Jiang, L.; Zhang, L.; Yang, Z. Features of Golenkinia sp. and microbial fuel cells used for the treatment of anaerobically digested effluent from kitchen waste at different dilutions. Bioresour. Technol. 2017, 240, 130–136. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Jayabalan, T.; Muthukumar, K. Simultaneous bioenergy generation and carbon dioxide sequestration from food wastewater using algae microbial fuel cell. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 2913–2921. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: Nutrient, organics removal and bioenergy production. Chem. Eng. J. 2018, 332, 277–285. [Google Scholar] [CrossRef]
- Seon, G.; Kim, H.S.; Cho, J.M.; Kim, M.; Park, W.K.; Chang, Y.K. Effect of post-treatment process of microalgal hydrolysate on bioethanol production. Sci. Rep. 2020, 10, 16698. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.L.; Chen, W.H.; Sheen, H.K.; Chang, J.S.; Lin, C.S.; Ong, H.C.; Show, P.L.; Ling, T.C. Bioethanol production from acid pretreated microalgal hydrolysate using microwave-assisted heating wet torrefaction. Fuel 2020, 279, 118435. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Zaini, I.N.; Irhamna, A.R.; Sasongko, D.; Aziz, M. Novel configuration of supercritical water gasification and chemical looping for highly-efficient hydrogen production from microalgae. Renew. Sust. Energy Rev. 2019, 112, 369–381. [Google Scholar] [CrossRef]
- Saka, C.; Kaya, M.; Bekiroğullari, M. Chlorella vulgaris microalgae strain modified with zinc chloride as a new support material for hydrogen production from NaBH4 methanolysis using CuB, NiB, and FeB metal catalysts. Int. J. Hydrogen Energy 2020, 45, 1959–1968. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hakobyan, L.; Trchounian, A. Characterization of light-dependent hydrogen production by new green microalga Parachlorella kessleri in various conditions. Photochem. Photobiol. B Biol. 2017, 175, 207–210. [Google Scholar] [CrossRef]
- Vargas, S.R.; dos Santos, P.V.; Zaiat, M.; do Carmo Calijuri, M. Optimization of biomass and hydrogen production by Anabaena sp. (UTEX 1448) in nitrogen-deprived cultures. Biomass Bioenergy 2018, 111, 70–76. [Google Scholar] [CrossRef]
- Çakmak, E.K.; Ugurlu, A. Enhanced biogas production of red microalgae via enzymatic pretreatment and preliminary economic assessment. Algal Res. 2020, 50, 101979. [Google Scholar] [CrossRef]
- Llamas, M.; Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Tuning microbial community in non-conventional two-stage anaerobic bioprocess for microalgae biomass valorization into targeted bioproducts. Bioresour. Technol. 2021, 337, 125387. [Google Scholar] [CrossRef]
- Hosseini, A.; Jazini, M.; Mahdieh, M.; Karimi, K. Efficient superantioxidant and biofuel production from microalga Haematococcus pluvialis via a biorefinery approach. Bioresour. Technol. 2020, 306, 123100. [Google Scholar] [CrossRef]
- Kumari, P.; Varma, A.K.; Shankar, R.; Thakur, L.S.; Mondal, P. Phycoremediation of wastewater by Chlorella pyrenoidosa and utilization of its biomass for biogas production. J. Environ. Chem. Eng. 2021, 9, 104974. [Google Scholar] [CrossRef]
- Assemany, P.; de Paula Marques, I.; Calijuri, M.L.; Reis, A. Complementarity of substrates in anaerobic digestion of wastewater grown algal biomass. Waste Biomass Valorization 2020, 11, 5759–5770. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Alvarado-Morales, M.; Kovalovszki, A.; Corbière, M.; Angelidaki, I. Energy recovery from wastewater microalgae through anaerobic digestion process: Methane potential, continuous reactor operation and modelling aspects. Biochem. Eng. J. 2018, 139, 1–7. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Abunasser, N.; Garcia, M.E.D.; Chen, M.; Ng, K.S.; Salley, S.O. Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl. Energy 2011, 88, 3324–3330. [Google Scholar] [CrossRef]
- Elbaroty, G.S. Optimization cultivation of Chlamydomonas reinhardtii in a tubular photobioreactor (2000 Liter) for biomass and green bioenergy (biodiesel) production. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1439–1457. [Google Scholar]
- Ramaraj, R.; Kawaree, R.; Unpaprom, Y. Direct transesterification of microalga Botryococcus braunii biomass for biodiesel production. Emergent Life Sci. Res. 2016, 2, 1–7. [Google Scholar]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Shirazi, Y.; Viamajala, S.; Varanasi, S. In situ and ex situ catalytic pyrolysis of microalgae and integration with pyrolytic fractionation. Front. Chem. 2020, 8, 786. [Google Scholar] [CrossRef]
- Garcia Alba, L.; Torri, C.; Samorì, C.; Van Der Spek, J.; Fabbri, D.; Kersten, S.R.; Brilman, D.W. Hydrothermal treatment (HTT) of microalgae: Evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Z.; Feng, L. Effects of reaction parameter on catalytic hydrothermal liquefaction of microalgae into hydrocarbon rich bio-oil. J. Energy Inst. 2021, 94, 22–28. [Google Scholar] [CrossRef]
- Priharto, N.; Ronsse, F.; Prins, W.; Carleer, R.; Heeres, H.J. Experimental studies on a two-step fast pyrolysis-catalytic hydrotreatment process for hydrocarbons from microalgae (Nannochloropsis gaditana and Scenedesmus almeriensis). Fuel Process. Technol. 2020, 206, 106466. [Google Scholar] [CrossRef]
- Wang, S.; Hu, S.; Shang, H.; Barati, B.; Gong, X.; Hu, X.; Abomohra, A.E.F. Study on the co-operative effect of kitchen wastewater for harvest and enhanced pyrolysis of microalgae. Bioresour. Technol. 2020, 317, 123983. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zou, W.; Peng, B.; Guo, C.; Zou, X. Lipid Droplets from Plants and Microalgae: Characteristics, Extractions, and Applications. Biology 2023, 12, 594. [Google Scholar] [CrossRef]
- Xue, J.; Balamurugan, S.; Li, T.; Cai, J.X.; Chen, T.T.; Wang, X.; Yang, W.D.; Li, H.Y. Biotechnological approaches to enhance biofuel producing potential of microalgae. Fuel 2021, 302, 121169. [Google Scholar] [CrossRef]
- Soós, V.; Shetty, P.; Maróti, G.; Incze, N.; Badics, E.; Bálint, P.; Ördög, V.; Balázs, E. Biomolecule composition and draft genome of a novel, high-lipid producing Scenedesmaceae microalga. Algal Res. 2021, 54, 102181. [Google Scholar] [CrossRef]
- Lu, H.; Cheng, J.; Zhu, Y.; Li, K.; Tian, J.; Zhou, J. Responses of Arthrospira ZJU9000 to high bicarbonate concentration (HCO3−: 171.2 mM): How do biomass productivity and lipid content simultaneously increase? Algal Res. 2019, 41, 101531. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, J.; Fa, Y.; Liu, X.; Lindblad, P. Enhancing microalgal lipid accumulation for biofuel production. Front. Microbiol. 2022, 13, 1024441. [Google Scholar] [CrossRef]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Yamada-Oshima, Y.; Asami, K.; Kanamori, T.; Yuasa, H.; Shimojima, M.; Ohta, H. Recycling of the major thylakoid lipid MGDG and its role in lipid homeostasis in Chlamydomonas reinhardtii. Plant Physiol. 2021, 187, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Lucker, B.F.; Zienkiewicz, K.; Miller, T.E.; Zienkiewicz, A.; Sears, B.B.; Kramer, D.M.; Benning, C. Galactoglycerolipid lipase PGD1 is involved in thylakoid membrane remodeling in response to adverse environmental conditions in Chlamydomonas. Plant Cell 2018, 30, 447–465. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, K.M.; McNichol, J.; McGinn, P.J.; O’Leary, S.J.; Melanson, J.E. Triacylglycerol profiling of microalgae strains for biofuel feedstock by liquid chromatography–high-resolution mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.L.B.; Laurens, L.M. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Lv, S.; Zhang, J.; Ni, H.; Wang, X.; Zhu, Y.; Chen, L. Study on the coupling relationship of low temperature fluidity and oxidation stability of biodiesel. Appl. Sci. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Bouchnak, I.; Coulon, D.; Salis, V.; D’Andréa, S.; Bréhélin, C. Lipid droplets are versatile organelles involved in plant development and plant response to environmental changes. Front. Plant Sci. 2023, 14, 1193905. [Google Scholar] [CrossRef]
- Lee, C.S.; Oh, H.S.; Oh, H.M.; Kim, H.S.; Ahn, C.Y. Two-phase photoperiodic cultivation of algal–bacterial consortia for high biomass production and efficient nutrient removal from municipal wastewater. Bioresour. Technol. 2016, 200, 867–875. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Wen, H.; Xue, Y.; Qu, Y.; Li, Y. Effects of multi-temperature regimes on cultivation of microalgae in municipal wastewater to simultaneously remove nutrients and produce biomass. Appl. Microbiol. Biotechnol. 2019, 103, 8255–8265. [Google Scholar] [CrossRef]
- Taleb, A.; Pruvost, J.; Legrand, J.; Marec, H.; Le-Gouic, B.; Mirabella, B.; Legeret, B.; Bouvet, S.; Peltier, G.; Li-Beisson, Y. Development and validation of a screening procedure of microalgae for biodiesel production: Application to the genus of marine microalgae Nannochloropsis. Bioresour. Technol. 2015, 177, 224–232. [Google Scholar] [CrossRef]
- Kim, B.H.; Ramanan, R.; Kang, Z.; Cho, D.H.; Oh, H.M.; Kim, H.S. Chlorella sorokiniana HS1, a novel freshwater green algal strain, grows and hyperaccumulates lipid droplets in seawater salinity. Biomass Bioenergy 2016, 85, 300–305. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, P.; Dasgupta, S.; Archana, G.; Bagchi, D. Gradient Strategy for Mixotrophic Cultivation of Chlamydomonas reinhardtii: Small Steps, a Large Impact on Biofuel Potential and Lipid Droplet Morphology. Bioenergy Res. 2023, 16, 163–176. [Google Scholar] [CrossRef]
- Garg, A.; Jain, S. Process parameter optimization of biodiesel production from algal oil by response surface methodology and artificial neural networks. Fuel 2020, 277, 118254. [Google Scholar] [CrossRef]
- Kasani, A.A.; Esmaeili, A.; Golzary, A. Software tools for microalgae biorefineries: Cultivation, separation, conversion process integration, modeling, and optimization. Algal Res. 2022, 61, 102597. [Google Scholar] [CrossRef]
- Galanopoulos, C.; Kenkel, P.; Zondervan, E. Superstructure optimization of an integrated algae biorefinery. Comput. Chem. Eng. 2019, 130, 106530. [Google Scholar] [CrossRef]
- Geraili, A.; Romagnoli, J.A. A multiobjective optimization framework for design of integrated biorefineries under uncertainty. AIChE J. 2015, 61, 3208–3222. [Google Scholar] [CrossRef]
- Nan, Y.; Liu, J.; Lin, R.; Tavlarides, L.L. Production of biodiesel from microalgae oil (Chlorella protothecoides) by non-catalytic transesterification in supercritical methanol and ethanol: Process optimization. J. Supercrit. Fluids 2015, 97, 174–182. [Google Scholar] [CrossRef]
- Banerjee, S.; Ray, A.; Das, D. Optimization of Chlamydomonas reinhardtii cultivation with simultaneous CO2 sequestration and biofuels production in a biorefinery framework. Sci. Total Environ. 2021, 762, 143080. [Google Scholar] [CrossRef]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef]
- Das, D.; Khanna, N.; Dasgupta, C.N. Biohydrogen Production: Fundamentals and Technology Advances; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]


| Cultivation Mode | Carbon Source | Energy Supply | Light Availability | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Autotrophic | Inorganic carbon | Light | Obligatory | Low cost, low energy consumption, high pigments production. | Low growth rate and biomass, specific photobioreactor required. |
| Heterotrophic | Organic carbon | Organic | No requirement | High biomass productivity and lipid accumulation due to high growth rate, process of scaling up is simplified, organic substrates can be used to alter biomass composition. | Higher cost, easy to be contaminated by other microorganisms, only a few microalgal species that can grow in a heterotrophic environment, inability to synthesize metabolites triggered by light. |
| Mixotrophic | Inorganic and organic carbon | Light and organic carbon | No obligatory | Increased growth rate, biomass, density and lipid accumulation, extended phase of exponential growth, stopping the photoinhibition effect and reducing biomass loss due to respiration during the dark hours, switch between photoautotroph and heterotroph regimens at any time. | High cost, contamination problems, limited microalgae species will grow. |
| Algal turf scrubber | Organic and inorganic carbon | Light and organic carbon | Obligatory | Improved nutrient status, pollutant removal, high biomass productivity rate, easy harvesting and low maintaince, decreased the overall production cost | Requirement of sufficient space and infrastructure |
| Technique with Image | Principle | Advantages | Disadvantages |
|---|---|---|---|
Flocculation | Aggregation of cells is achieved by enlarging their size through the addition of a flocculant, which can be in the form of chemicals (such as ferric chloride, ferric sulfate, and ammonium sulfate) or microbes (bacteria). | Fast and easy technique, used for large scale, less cell damage, applied to a wide variety of species, less energy requirements. | Chemicals may be expensive, high pH required, separating the coagulant from harvested biomass is difficult, limited culture medium recycling, increased microbial contamination. |
Filtration | Large cells (size > 70 µm) can be filtered under pressure or suction whereas smaller cells (size < 30 µm require ultrafilters to be harvested. | High recovery efficiency, cost effective, no chemical required, low energy consumption, low shear stress. | Slow hence requires pressure or vacuum, not effective for small algae, membrane fouling/clogging and replacement increases operational and maintenance costs. |
Flotation | Trapping algal cells by bubbling air. | Well-suited for large-scale applications, economically efficient with minimal space demands, short operation time. | Depends on bubble distribution into the suspension, needs surfactants. |
Centrifugation | Sedimentation based on the velocity, cell size and density. | Fast and effective technique, high recovery efficiency (>90), applicable to all microalgae. | Expensive technique with high energy requirement, high operation and maintenance costs, risk of cell destruction. |
Precipitation | Certain algae undergo self-precipitation, they settle at the bottom when circulation is halted. | No energy or chemicals are needed, it occurs naturally. | Species-specific, time periods vary depending on the species, not every species is self-precipitated. |
| Parameters | CO2 Sequestration | Direct Air Capture (DAC) | Carbon Capture and Storage (CCS) |
|---|---|---|---|
| Source of CO2 capture | This method involves capturing carbon dioxide from various sources, including industrial processes, power plants, and natural systems like forests and oceans (algae). It encompasses a wide range of approaches, both natural (e.g., photosynthesis in algae) and engineered (e.g., mineralization). | DAC is designed to capture carbon dioxide directly from the ambient air. It targets atmospheric CO2 and is not dependent on specific point sources. DAC is ideal for offsetting emissions from sectors that are challenging to decarbonize, such as transportation. | CCS focuses on capturing CO2 emissions from specific industrial point sources, such as power plants and industrial facilities. It is primarily aimed at mitigating emissions from large, stationary sources. |
| Capture efficiency | The capture efficiency of CO2 sequestration methods can vary widely depending on the specific approach. For example, biological methods like algae cultivation can be highly efficient, while geological methods like enhanced oil recovery may capture a significant but variable portion of emissions. | DAC technologies are designed to be highly efficient at capturing CO2 from the atmosphere. They are engineered to achieve capture rates close to 100% of the CO2 passing through the system. | CCS technologies typically capture a substantial portion of CO2 emissions from point sources, but capture rates can vary depending on the technology and the type of industrial process. |
| Scalability | The scalability of CO2 sequestration methods can vary. Some approaches, like algae cultivation, may require extensive culture medium, while others, like mineralization, can be implemented on a smaller scale. | DAC systems can be scaled up or down relatively easily, making them adaptable to different emission scenarios. They can be distributed across diverse locations, depending on the need. | CCS facilities are typically large and centralized, making them less adaptable to different locations and requiring significant infrastructure investment. |
| Environmental impact | The environmental impact of CO2 sequestration methods can vary widely. Biological approaches may have positive environmental co-benefits, while some geological methods may pose risks related to underground storage. | DAC systems have a relatively low environmental footprint, but the energy requirements for DAC can impact the overall environmental assessment. | CCS can have environmental implications related to underground storage, potential leakage, and the energy required for capture and compression. |
| Energy requirements | Energy requirements vary significantly depending on the method, with some processes requiring minimal energy and others being more energy-intensive. | Generally energy-intensive due to the need for air circulation and chemical processes. | Energy is required for capturing, compressing, and transporting CO2 to storage sites, making it energy-intensive as well. |
| Carbon neutrality | May have a carbon-neutral or even carbon-negative impact. | Has the potential to achieve carbon neutrality but requires a sustainable source of energy to power the capture process. | Typically does not achieve carbon neutrality as it focuses on preventing emissions rather than removing carbon from the atmosphere. |
| Economic feasibility | Economic feasibility varies widely depending on the specific method, scale, and potential revenue streams from sequestered carbon. | Considered more costly compared to some other methods due to its energy-intensive nature but may become more economically viable with advancements in technology. | Economic feasibility depends on factors such as government policies, incentives, and infrastructure costs. It has been applied in various industries. |
| Technological maturity | Maturity varies by method, with some approaches well-established and others still in development. | Relatively newer technology with ongoing research and development efforts. | More mature technology with decades of operational experience in certain industries, such as enhanced oil recovery. |
| Primary application | Applied across various sectors, including algal cultivation, and industrial emissions reduction. | Often used to offset emissions from sectors that are challenging to decarbonize, such as transportation. | Primarily used in heavy industry and power generation to reduce emissions from stationary sources. |
| Parametric Study | Algae Species | Biofuels | Production | Ref. |
|---|---|---|---|---|
| Effect of reactive oxygen, nitrogen species, time and depletion of cations | Chlorella sp. | Bioethanol | 0.43 g/g sugars | [211] |
| Effect of acid concentration, temperature and time | C. vulgaris | Bioethanol | 0.07 g/g microalgae | [212] |
| Effect of culture duration and fermentation time | Nannochloropsis oculata and Tetraselmis suecica | Bioethanol | 7.26% | [213] |
| Effect of moisture contents of wet algae, temperature, pressure and hydrogenation on process integration | Acutodesmus obliquus | Biohydrogen | 13,944 kg/h | [214] |
| Catalytic activity and effect of ZnCl2, CuB loading, NaBH4 concentration and temperature | C. Vulgaris | Biohydrogen | 17,833 mL/min g catalyst | [215] |
| Organic carbon sources and lighting regime | Parachlorella kessleri | Biohydrogen | 2.2 mmol/L | [216] |
| Effect of temperature, pH, light and glucose supplementation | Anabaena sp. | Biohydrogen | 57.6% | [217] |
| Temperature 55 °C | Porphyridium cruentum | Biogass | 179 mL CH4/g VS | [218] |
| Temperature 35 °C | C. vulgaris | Biogass | 168.9 mL CH4/g COD | [219] |
| Temperature 37 °C | Haematococcus pluvialis | Biogass | 91.9 mL CH4/g VS | [220] |
| Temperature 37 °C | Chlorella pyrenoidosa | Biogass | 147 mL CH4/g VS | [221] |
| Temperature 37 °C | Scenedesmus obliquus | Biogass | 0.16 m3 CH4/kg VS | [222] |
| Temperature 53 °C | Nannochloropsis limnetica | Biogass | 0.41 m3 CH4/kg VS | [223] |
| Temperature 35–55 °C | Chlorococum sp. | Biodiesel | 0.010–0.015 g FAME/g DW biomass | [224] |
| Light (180 µmol photons m−2 s−1 ) on a 14:10 (light: dark) photoperiod with salinity (NaCl 18 g/L) | Chaetoceros gracilis | Biodiesel | 0.36 g FAME/g DW biomass | [225] |
| Light (180 µmol photons m−2 s−1 ) on a 14:10 (light: dark) photoperiod with salinity (NaCl 18 g/L) | Tetraselmis suecica | Biodiesel | 0.18 g FAME/g DW biomass | [225] |
| Light (180 µmol photons m−2 s−1 ) on a 14:10 (light: dark) photoperiod with salinity (NaCl 18 g/L) | Chlorella sorokiniana | Biodiesel | 0.18 g FAME/g DW biomass | [225] |
| Red light intensity 350 µE m−2 s−1 | Dunaliella tertiolecta | Biodiesel | 0.22 g FAME/g DW biomass | [226] |
| Temperature 20 °C | Nannochloropsis oculata | Biodiesel | 0.07–0.24 g lipids/g DW biomass | [108] |
| Temperature 25 °C | C. vulgaris | Biodiesel | 0.06–0.14 g lipids/g DW biomass | [108] |
| Nutrients limitations (0.1 mM Sulfur, 7.0 mM Nitrogen and 0.43 mM phosphorus) | Chlamydomonas reinhardtii | Biodiesel | 60% | [227] |
| Light intensity (51 µmol photons m−2 s−1) with pH 7.8 | Botryococcus braunii | Biodiesel | 85.7% | [228] |
| CO2 (390 ppm) | C. vulgaris | Bio-oil | 46.60% | [229] |
| Increased alkanity | Chlorella sorokiniana | Bio-oil | 62% | [230] |
| Hydrothermal treatment | Desmodesmus sp. | Bio-oil | 49.40% | [231] |
| Effect of different metals (Co, Ni, and Fe) | Spirulina | Bio-oil | 43.60% | [232] |
| Catalytic hydrotreatment | Nannochloropsis gaditana | Bio-oil | 24.60% | [233] |
| Kitchen wastewater | Scenedesmus obliquus | Bio-oil | 55.59% | [234] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.P.; Yadav, P.; Kumar, I.; Solanki, M.K.; Roychowdhury, R.; Kumar, A.; Gupta, R.K. Advancement of Abiotic Stresses for Microalgal Lipid Production and Its Bioprospecting into Sustainable Biofuels. Sustainability 2023, 15, 13678. https://doi.org/10.3390/su151813678
Singh RP, Yadav P, Kumar I, Solanki MK, Roychowdhury R, Kumar A, Gupta RK. Advancement of Abiotic Stresses for Microalgal Lipid Production and Its Bioprospecting into Sustainable Biofuels. Sustainability. 2023; 15(18):13678. https://doi.org/10.3390/su151813678
Chicago/Turabian StyleSingh, Rahul Prasad, Priya Yadav, Indrajeet Kumar, Manoj Kumar Solanki, Rajib Roychowdhury, Ajay Kumar, and Rajan Kumar Gupta. 2023. "Advancement of Abiotic Stresses for Microalgal Lipid Production and Its Bioprospecting into Sustainable Biofuels" Sustainability 15, no. 18: 13678. https://doi.org/10.3390/su151813678
APA StyleSingh, R. P., Yadav, P., Kumar, I., Solanki, M. K., Roychowdhury, R., Kumar, A., & Gupta, R. K. (2023). Advancement of Abiotic Stresses for Microalgal Lipid Production and Its Bioprospecting into Sustainable Biofuels. Sustainability, 15(18), 13678. https://doi.org/10.3390/su151813678












