A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground
Abstract
:1. Introduction
2. Hazardous Ingredients of Spent Coffee Grounds
3. Properties and Potential Recycling Applications of Spent Coffee Grounds
4. Production Processes of PLA
4.1. Producing Lactic Acid from SCGs
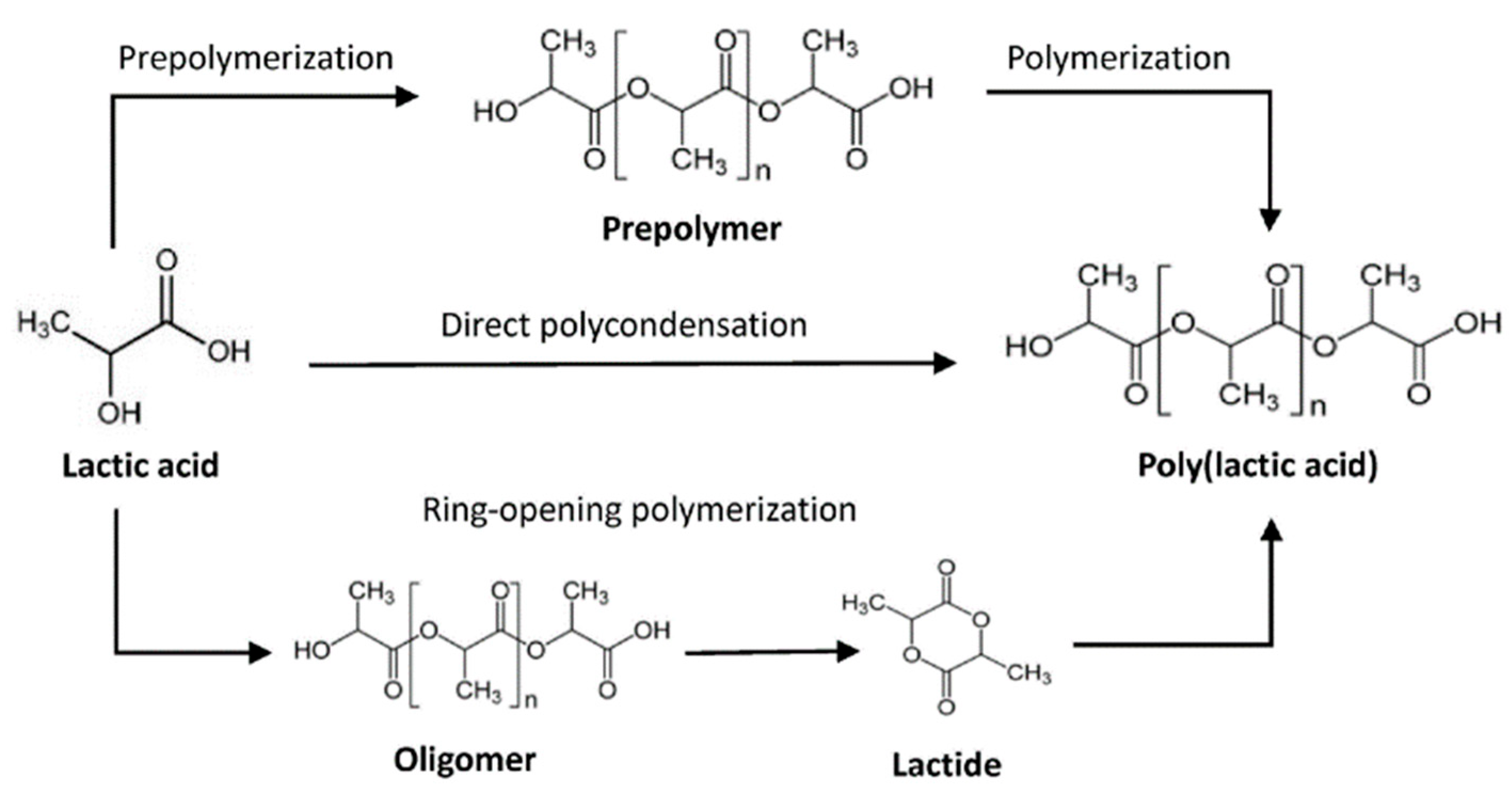
4.2. Producing PLA from Lactic Acid
5. The Feasibility Analysis
5.1. Possible Ways to Improve the Yield of PLA Production from SCGs
5.2. Cost Analysis of PLA Production from SCGs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization. ICO—World Coffee Production; International Coffee Organization: London, UK, 2021. [Google Scholar]
- International Coffee Organization. ICO—World Coffee Consumption; International Coffee Organization: London, UK, 2021. [Google Scholar]
- Coffee Farmers. Available online: https://www.fairtrade.org.uk/farmers-and-workers/coffee/ (accessed on 13 April 2023).
- Cerino-Córdova, F.J.; Dávila-Guzmán, N.E.; León, A.M.G.; Salazar-Rabago, J.J.; Soto-Regalado, E. Revalorization of Coffee Waste. In Coffee—Production and Research; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- British Coffee Association Coffee Consumption. Coffee Consumption. 2021. Available online: https://britishcoffeeassociation.org/coffee-consumption/ (accessed on 28 May 2023).
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of Different Rates of Spent Coffee Grounds (SCG) on Composting Process, Gaseous Emissions and Quality of End-Product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Blinová, L.; Sirotiak, M.; Pastierova, A.; Soldán, M. Review: Utilization of Waste from Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent Coffee Ground Characterization, Pelletization Test and Emissions Assessment in the Combustion Process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef]
- Chai, W.Y.; Krishnan, U.G.; Sabaratnam, V.; Tan, J.B.L. Assessment of Coffee Waste in Formulation of Substrate for Oyster Mushrooms Pleurotus Pulmonarius and Pleurotus Floridanus. Future Foods 2021, 4, 100075. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Active Biocatalyst for Biodiesel Production from Spent Coffee Ground. Bioresour. Technol. 2018, 266, 431–438. [Google Scholar] [CrossRef] [PubMed]
- De Bomfim, A.S.C.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Abut, S.; Kaya, M.; Eskicioglu, C.; Semaan, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; Mohanasundaram, R.; et al. Anaerobic Co-Digestion of Oil-Extracted Spent Coffee Grounds with Various Wastes: Experimental and Kinetic Modeling Studies. Bioresour. Technol. 2021, 322, 124470. [Google Scholar] [CrossRef] [PubMed]
- Kanniah, J.C. What Happens to Coffee Grounds after They’re Used? Perfect Daily Grind, 16 September 2020. [Google Scholar]
- Planet Ark Coffee Grounds. Available online: https://businessrecycling.com.au/pages/coffee-grounds.cfm? (accessed on 22 April 2022).
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Miłek, D.; Szewczyk, A.; Sławińska, I. Acute Toxicity of Experimental Fertilizers Made of Spent Coffee Grounds. Waste Biomass Valor. 2018, 9, 2157–2164. [Google Scholar] [CrossRef]
- Kueh, A.B.H. Spent Ground Coffee—Awaking the Sustainability Prospects. Environ. Toxicol. Manag. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Beyene, A.; Kebede, Y.; Addis, T.; Assefa, F.; Amsalu, A.; Mulat, W.; Kloos, H.; Triest, L. The Impact of Traditional Coffee Processing on River Water Quality in Ethiopia and the Urgency of Adopting Sound Environmental Practices. Environ. Monit. Assess. 2011, 184, 7053–7063. [Google Scholar] [CrossRef] [PubMed]
- Herpin, U.; Gloaguen, T.V.; da Fonseca, A.F.; Montes, C.R.; Mendonça, F.C.; Piveli, R.P.; Breulmann, G.; Forti, M.C.; Melfi, A.J. Chemical Effects on the Soil–Plant System in a Secondary Treated Wastewater Irrigated Coffee Plantation—A Pilot Field Study in Brazil. Agric. Water Manag. 2007, 89, 105–115. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent Coffee Grounds By-Products and Their Influence on Soil C–N Dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Lirio-Paredes, J.; Ogata-Gutiérrez, K.; Zúñiga-Dávila, D. Effects of Rhizobia Isolated from Coffee Fields in the High Jungle Peruvian Region, Tested on Phaseolus vulgaris L. Var. Red Kidney. Microorganisms 2022, 10, 823. [Google Scholar] [CrossRef]
- Blinová, L.; Sirotiak, M. Utilization of Spent Coffee Grounds for Removal of Hazardous Substances from Water: A Review. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2019, 27, 145–152. [Google Scholar] [CrossRef]
- Methane and Other Gases: A Range of Greenhouse Gases Contribute to Climate Change—Document—Gale OneFile: High School Edition. Available online: https://go-gale-com.ezproxy.lib.hkmu.edu.hk/ps/retrieve.do?tabID=T003&resultListType=RESULT_LIST&searchResultsType=SingleTab&retrievalId=f45e87da-bed2-4a15-8161-86c40dc4b3f4&hitCount=1&searchType=AdvancedSearchForm¤tPosition=1&docId=GALE%7CA172597067&docType=Article&sort=RELEVANCE&contentSegment=ZTSF-198501P&prodId=STOM&pageNum=1&contentSet=GALE%7CA172597067&searchId=R1&userGroupName=hkmu&inPS=true (accessed on 12 April 2023).
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A Comparative Life Cycle Assessment of Different Spent Coffee Ground Reuse Strategies and a Sensitivity Analysis for Verifying the Environmental Convenience Based on the Location of Sites. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Solomakou, N.; Tsafrakidou, P.; Goula, A.M. Valorization of SCG through Extraction of Phenolic Compounds and Synthesis of New Biosorbent. Sustainability 2022, 14, 9358. [Google Scholar] [CrossRef]
- Vítězová, M.; Dordevic Jancikova, S.; Dordević, D.; Vitez, T.; Elbl, J.; Hanišáková, N.; Jampilek, J.; Kushkevych, I. The Possibility of Using Spent Coffee Grounds to Improve Wastewater Treatment Due to Respiration Activity of Microorganisms. Appl. Sci. 2019, 9, 3155. [Google Scholar] [CrossRef]
- Mahmoud, E.; Atabani, A.E.; Badruddin, I.A. Valorization of Spent Coffee Grounds for Biogas Production: A Circular Bioeconomy Approach for a Biorefinery. Fuel 2022, 328, 125296. [Google Scholar] [CrossRef]
- Talalaj, I.A.; Biedka, P. Use of the Landfill Water Pollution Index (LWPI) for Groundwater Quality Assessment near the Landfill Sites. Environ. Sci. Pollut. Res. 2016, 23, 24601–24613. [Google Scholar] [CrossRef]
- Thenepalli, T.; Chilakala, R.; Ahn, J.-W. Environmental Effect of the Coffee Waste and Anti-Microbial Property of Oyster Shell Waste Treatment. J. Energy Eng. 2017, 26, 39–49. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Polyhydroxyalkanoates and Carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A Study on Chemical Constituents and Sugars Extraction from Spent Coffee Grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of Oil Extracted from Spent Coffee Grounds for Sustainable Production of Polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting Environmental Risks to Benefits by Using Spent Coffee Grounds (SCG) as a Valuable Resource. Environ. Sci. Pollut. Res. 2018, 25, 35776–35790. [Google Scholar] [CrossRef]
- UCL Business Founded by UCL Graduate Is Now World’s Largest Recycler of Coffee Grounds. Available online: https://www.ucl.ac.uk/enterprise/case-studies/2020/sep/business-founded-ucl-graduate-now-worlds-largest-recycler-coffee-grounds (accessed on 20 April 2023).
- Block, I.; Günter, C.; Duarte Rodrigues, A.; Paasch, S.; Hesemann, P.; Taubert, A. Carbon Adsorbents from Spent Coffee for Removal of Methylene Blue and Methyl Orange from Water. Materials 2021, 14, 3996. [Google Scholar] [CrossRef]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field Evaluation of Coffee Grounds Application for Crop Growth Enhancement, Weed Control, and Soil Improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Starbucks Japan Finds New Ways to Recycle Coffee Grounds. Available online: https://stories.starbucks.com/stories/2016/starbucks-japan-coffee-grounds/ (accessed on 20 April 2023).
- Katayama, A. Recycle Coffee Grounds with Zero Impact: Japanese Koji Turns Waste into Coffee Bars at Low Cost. Available online: https://www.forbes.com/sites/akikokatayama/2022/03/28/recycle-coffee-grounds-with-zero-impact-japanese-koji-turns-the-waste-into-delicious-coffee-bars-at-low-cost/ (accessed on 20 April 2023).
- Muñoz Velasco, P.; Mendívil, M.A.; Morales, M.P.; Muñoz, L. Eco-Fired Clay Bricks Made by Adding Spent Coffee Grounds: A Sustainable Way to Improve Buildings Insulation. Mater. Struct. 2016, 49, 641–650. [Google Scholar] [CrossRef]
- Chung, L.L.P.; Wong, Y.C.; Arulrajah, A. The Application of Spent Coffee Grounds and Tea Wastes as Additives in Alkali-Activated Bricks. Waste Biomass Valor. 2021, 12, 6273–6291. [Google Scholar] [CrossRef]
- Arulrajah, A.; Kua, T.-A.; Suksiripattanapong, C.; Horpibulsuk, S.; Shen, J.S. Compressive Strength and Microstructural Properties of Spent Coffee Grounds-Bagasse Ash Based Geopolymers with Slag Supplements. J. Clean. Prod. 2017, 162, 1491–1501. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Ihnen, A.C.; Petrock, A.M.; Chou, T.; Samuels, P.J.; Fuchs, B.E.; Lee, W.Y. Crystal Morphology Variation in Inkjet-Printed Organic Materials. Appl. Surf. Sci. 2011, 258, 827–833. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Cellulose Nanofibers from Coffee Grounds Could Be Used to Make Bioplastics. Available online: https://www.azonano.com/news.aspx?newsID=37223 (accessed on 12 April 2023).
- Battista, F.; Zuliani, L.; Rizzioli, F.; Fusco, S.; Bolzonella, D. Biodiesel, Biogas and Fermentable Sugars Production from Spent Coffee Grounds: A Cascade Biorefinery Approach. Bioresour. Technol. 2021, 342, 125952. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Silva, M.A.; Nebra, S.A.; Machado Silva, M.J.; Sanchez, C.G. The use of biomass residues in the brazilian soluble coffee industry. Biomass Bioenergy 1998, 14, 457–467. [Google Scholar] [CrossRef]
- Garcia, C.V.; Kim, Y.-T. Spent Coffee Grounds and Coffee Silverskin as Potential Materials for Packaging: A Review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Elida, F.S.; Azizah; Wiyono, H.T.; Muzakhar, K. Efficiency of Cellulase Production Using Coffee Pulp Waste under Solid State Fermentation by Aspergillus sp. VT12. AIP Conf. Proc. 2020, 2296, 020020. [Google Scholar]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Liu, Q.; Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. A Review: Research Progress in Modification of Poly(Lactic Acid) by Lignin and Cellulose. Polymers 2021, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.K.; Boyacioglu, S.; Kodal, M.; Gokce, O.; Ozkoc, G. Thermally Induced Shape Memory Behavior, Enzymatic Degradation and Biocompatibility of PLA/TPU Blends: “Effects of Compatibilization”. J. Mech. Behav. Biomed. Mater. 2017, 71, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Choochottiros, C.; Chin, I.-J. Potential Transparent PLA Impact Modifiers Based on PMMA Copolymers. Eur. Polym. J. 2013, 49, 957–966. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Competitiveness of Sustainable Bioplastic Feedstocks—A Monte Carlo Analysis for Polylactic Acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. ChemSusChem 2021, 14, 56–72. [Google Scholar] [CrossRef]
- EUBIO_Admin Bioplastic Market Data. European Bioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 28 May 2023).
- Pleissner, D.; Neu, A.-K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative Lactic Acid Production from Coffee Pulp Hydrolysate Using Bacillus Coagulans at Laboratory and Pilot Scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Fahim, I.; Hosny, M.; El-Badry, M.A. Optimization of Lactic Acid Production from Agro-Industrial Wastes Produced by Kosakonia Cowanii. Curr. Res. Green Sustain. Chem. 2022, 5, 100228. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces Cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef]
- Hudeckova, H.; Neureiter, M.; Obruca, S.; Frühauf, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Lactic Acid. Lett. Appl. Microbiol. 2018, 66, 306–312. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Cheng, Y.-S.; Huang, C.-H.; Huang, C.-W. Optimization of High Solids Dilute Acid Hydrolysis of Spent Coffee Ground at Mild Temperature for Enzymatic Saccharification and Microbial Oil Fermentation. Appl. Biochem. Biotechnol. 2016, 180, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. (Eds.) 2—Synthesis and Production of Poly(Lactic Acid). In Polylactic Acid; Plastics Design Library; William Andrew Publishing: Oxford, UK, 2013; pp. 71–107. ISBN 978-1-4377-4459-0. [Google Scholar]
- De Bomfim, A.S.C.; de Oliveira, D.M.; Voorwald, H.J.C.; de Carvalho Benini, K.C.C.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Daoud, W.A.; Fei, B.; Chen, L.; Kwan, T.H.; Ki Lin, C.S. Efficient ZnO Aqueous Nanoparticle Catalysed Lactide Synthesis for Poly(Lactic Acid) Fibre Production from Food Waste. J. Clean. Prod. 2017, 165, 157–167. [Google Scholar] [CrossRef]
- Breton Toral, A.; Trejo Estrada, S.R.; McDonald, A.G. Lactic Acid Production from Potato Peel Waste, Spent Coffee Grounds and Almond Shells with Undefined Mixed Cultures Isolated from Coffee Mucilage from Coatepec Mexico. Ferment. Technol. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef]
- Vitetta, L.; Coulson, S.; Thomsen, M.; Nguyen, T.; Hall, S. Probiotics, D-Lactic Acidosis, Oxidative Stress and Strain Specificity. Gut Microbes 2017, 8, 311–322. [Google Scholar] [CrossRef]
- Sungyeap Hong, C.L. An Overview of the Synthesis and Synthetic Mechanism of Poly(Lactic Acid). Mod. Chem. Appl. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Chiyanzy, I.; Brienzo, M.; García-Aparicio, M.; Agudelo, R.; Görgens, J. Spent Coffee Ground Mass Solubilisation by Steam Explosion and Enzymatic Hydrolysis. J. Chem. Technol. Biotechnol. 2015, 90, 449–458. [Google Scholar] [CrossRef]
- Kasai, N.; Konishi, A.; Iwai, K.; Maeda, G. Efficient Digestion and Structural Characteristics of Cell Walls of Coffee Beans. J. Agric. Food Chem. 2006, 54, 6336–6342. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.S.; Whalen-Pedersen, E.; Perkins, D.E.; Plumb, S.; Ceriali, S.; Wragg, A. Enzyme-Assisted Soluble Coffee Production. U.S. Patent 11/995,913, 18 December 2008. [Google Scholar]
- Gustavo, M.G.; Irwin, L.A. Method of Producing a Coffee Extract. CA Patent 2987164A1, 29 December 2016. [Google Scholar]
- Evangelista, S.R.; Silva, C.F.; Miguel, M.G.P.d.C.; Cordeiro, C.d.S.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of Coffee Beverage Quality by Using Selected Yeasts Strains during the Fermentation in Dry Process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Coffee and Yeasts: From Flavor to Biotechnology. Fermentation 2021, 7, 9. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Cenian, K.; Skiadas, I.; Gavala, H. Integration of Chlorogenic Acid Recovery and Bioethanol Production from Spent Coffee Grounds. Biochem. Eng. J. 2016, 116, 54–64. [Google Scholar] [CrossRef]
- Lee, J.W.; In, J.H.; Park, J.-B.; Shin, J.; Park, J.H.; Sung, B.H.; Sohn, J.-H.; Seo, J.-H.; Park, J.-B.; Kim, S.R.; et al. Co-Expression of Two Heterologous Lactate Dehydrogenases Genes in Kluyveromyces Marxianus for l-Lactic Acid Production. J. Biotechnol. 2017, 241, 81–86. [Google Scholar] [CrossRef]
- Manandhar, A. Shah Techno-Economic Analysis of Bio-Based Lactic Acid Production Utilizing Corn Grain as Feedstock. Processes 2020, 8, 199. [Google Scholar] [CrossRef]
- Sanaei, S.; Stuart, P.R. Systematic Assessment of Triticale-Based Biorefinery Strategies: Techno-Economic Analysis to Identify Investment Opportunities. Biofuels Bioprod. Biorefin. 2018, 12, S46–S59. [Google Scholar] [CrossRef]
- Chiarakorn, S.; Permpoonwiwat, C.K.; Nanthachatchavankul, P. Cost Benefit Analysis of Bioplastic Production in Thailand. Econ. Public Policy 2011, 3, 56–85. [Google Scholar]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-Economic Analysis of a Food Waste Valorisation Process for Lactic Acid, Lactide and Poly(Lactic Acid) Production. J. Clean. Prod. 2018, 181, 72–87. [Google Scholar] [CrossRef]
- International Coffee Organization—What’s New. Available online: https://www.ico.org/ (accessed on 13 April 2023).
- Lunt, J. The Business Case for Commercial Production of Bioplastics in Maine Sustainable Bioplastics Council of Maine; Maine Technology Institute: Brunswick, ME, USA, 2010. [Google Scholar]
| Possible Applications of Spent Coffee Grounds (SCG) | ||||
|---|---|---|---|---|
| Industry | Technologies/ Process/Uses | Examples | Main Limitations | Ref. |
| Renewable Energy | Extraction | Biodiesel |
| [8,32,36] |
| Extract + Straw | Bioenergy, pellets | |||
| Environmental Remediation | Absorption | Removal of heavy metal Deodorization |
| [15,35,37] |
| Pyrolysis | Biochar | |||
| Agriculture | Composting | Fertilizers, soil improver, plant cultivation |
| [32,35,38] |
| Substrate | Mushroom growth |
| ||
| Healthcare Industry | Extraction of Bioactive Compound | Oils to produce soaps; Phenolic substances |
| [32,39,40] |
| Food Industry | Food ingredient | |||
| Construction Industry | / | Bricks |
| [35,41,42,43] |
| Polymer production Industry | Hydrolysis Carbohydrates, Oil | Cellulose-type polymers (Bioplastic/Lactic acid) |
| [44,45,46,47,48,49] |
| Operating Condition | Micro-Organism | Highlight/Result | Ref. |
|---|---|---|---|
| 2.7% H2SO4 20 min (acid hydrolysis) + Enzymatic hydrolysis | Bacillus coagulans Lactobacillus rhamnousus |
| [64] |
| 1% H2SO4 30 min (acid hydrolysis) | S. cerevisiae |
| [63] |
| 5.3% H2SO4 118 min (acid hydrolysis) + ACCELLERASE 1500® | Lipomyces Stakeyi |
| [65] |
| 1.8% H2SO4 30 min (acid hydrolysis) + ACCELLERASE 1500® + downstream process {filtration, softening, electrodialysis, chromatography, distillation} | Bacillus coagulans |
| [61] |
| 5% HCl 20 min (acid hydrolysis) + downstream process {end product extraction, purification, precipitation, quantification of end product} | Kosakonia cowanii |
| [62] |
| Project | Feedstock | Min Cost per Ton (USD) | Max Cost per Ton (USD) |
|---|---|---|---|
| Manandhar and Shah (2020) [82] | Corn grains | 844 | 1251 |
| Sanaei and Stuart (2018) [83] | Triticale | 911 | 1496 |
| Wellenreuther et al. (2022) [58] | Corn grain and stover | 1004 | 1374 |
| Chiarakorn et al. (2011) [84] | Cassava roots | 2410 | 2620 |
| Kwan et al. (2018) [85] | Food waste powder | 1066 | 3558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mak, S.L.; Wu, M.Y.T.; Chak, W.Y.; Kwong, W.K.; Tang, W.F.; Li, C.H.; Lee, C.C.; Li, C.Y. A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground. Sustainability 2023, 15, 13498. https://doi.org/10.3390/su151813498
Mak SL, Wu MYT, Chak WY, Kwong WK, Tang WF, Li CH, Lee CC, Li CY. A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground. Sustainability. 2023; 15(18):13498. https://doi.org/10.3390/su151813498
Chicago/Turabian StyleMak, Shu Lun, Ming Yan Tanya Wu, Wai Ying Chak, Wang Kei Kwong, Wai Fan Tang, Chi Ho Li, Chi Chung Lee, and Chun Yin Li. 2023. "A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground" Sustainability 15, no. 18: 13498. https://doi.org/10.3390/su151813498
APA StyleMak, S. L., Wu, M. Y. T., Chak, W. Y., Kwong, W. K., Tang, W. F., Li, C. H., Lee, C. C., & Li, C. Y. (2023). A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground. Sustainability, 15(18), 13498. https://doi.org/10.3390/su151813498








