Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation for Yeast Isolates
2.2. Screening for Lipid Accumulation by Oleaginous Yeast
2.3. Enhancement of Lipid Accumulation by Oleaginous Yeast
2.4. Biodiesel Production from Microbial Lipids Chemically
2.5. Determination of the Quality of Biodiesel
2.6. Biosorption Isotherms of Congo Red Using De-Oiled Biomass Wastes
2.6.1. Preparation of De-Oiled Yeast Biomasses as a Biosorbent
2.6.2. Effect of Contact Time on the Biosorption Efficiency of Congo Red by Dried De-Oiled Yeast Biomasses
2.6.3. Effect of pH on Biosorption Efficiency of Congo Red by De-Oiled Yeast Biomasses
2.7. Biosorption Isotherms of Congo Red by De-Oiled Yeast Biomasses
2.8. Congo Red Biosorption Evolution
2.9. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Deoiled Yeast Biomass
3. Results
3.1. Isolation and Screening for Lipid Accumulation by Isolated Yeast Isolates
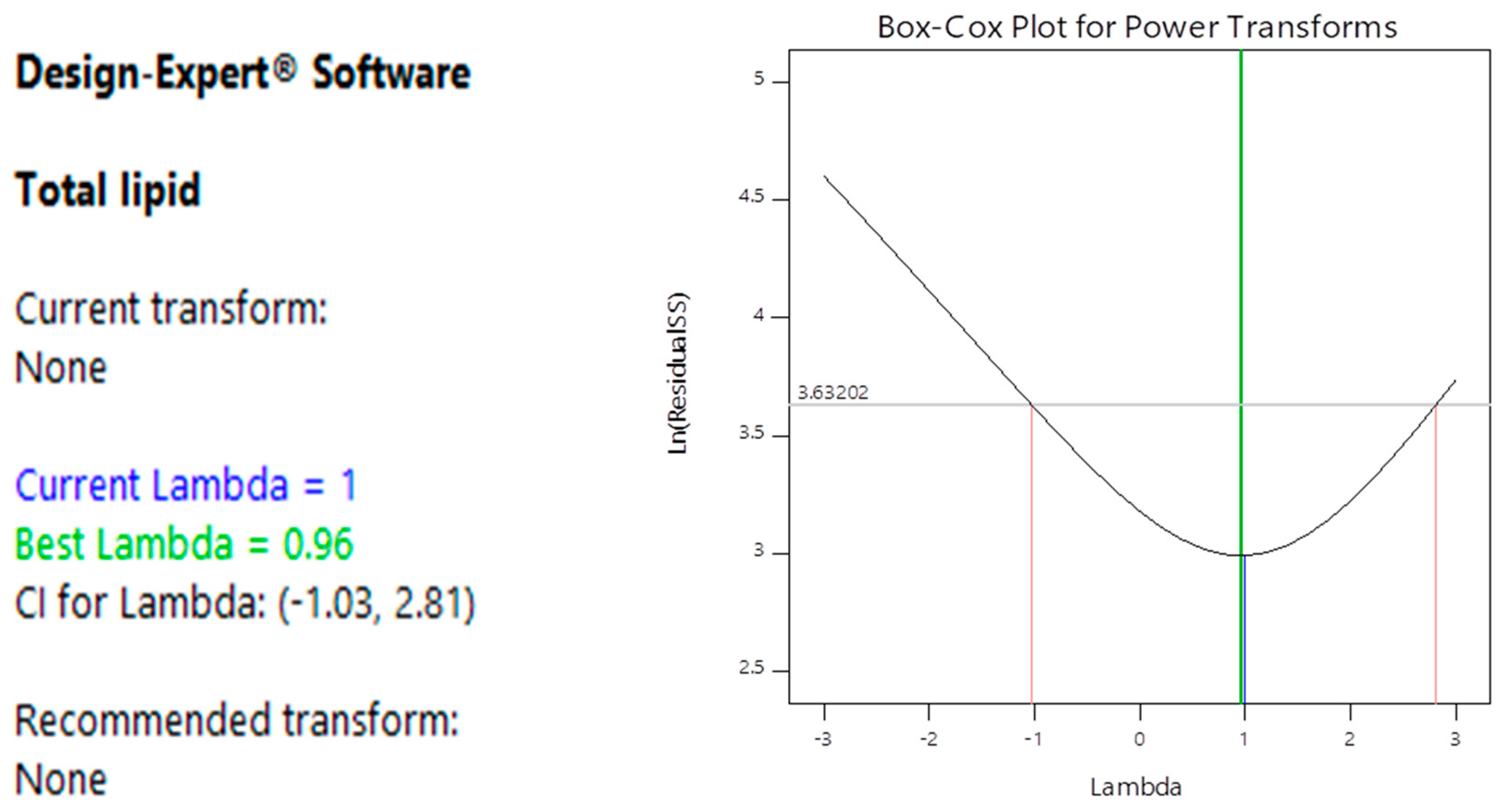
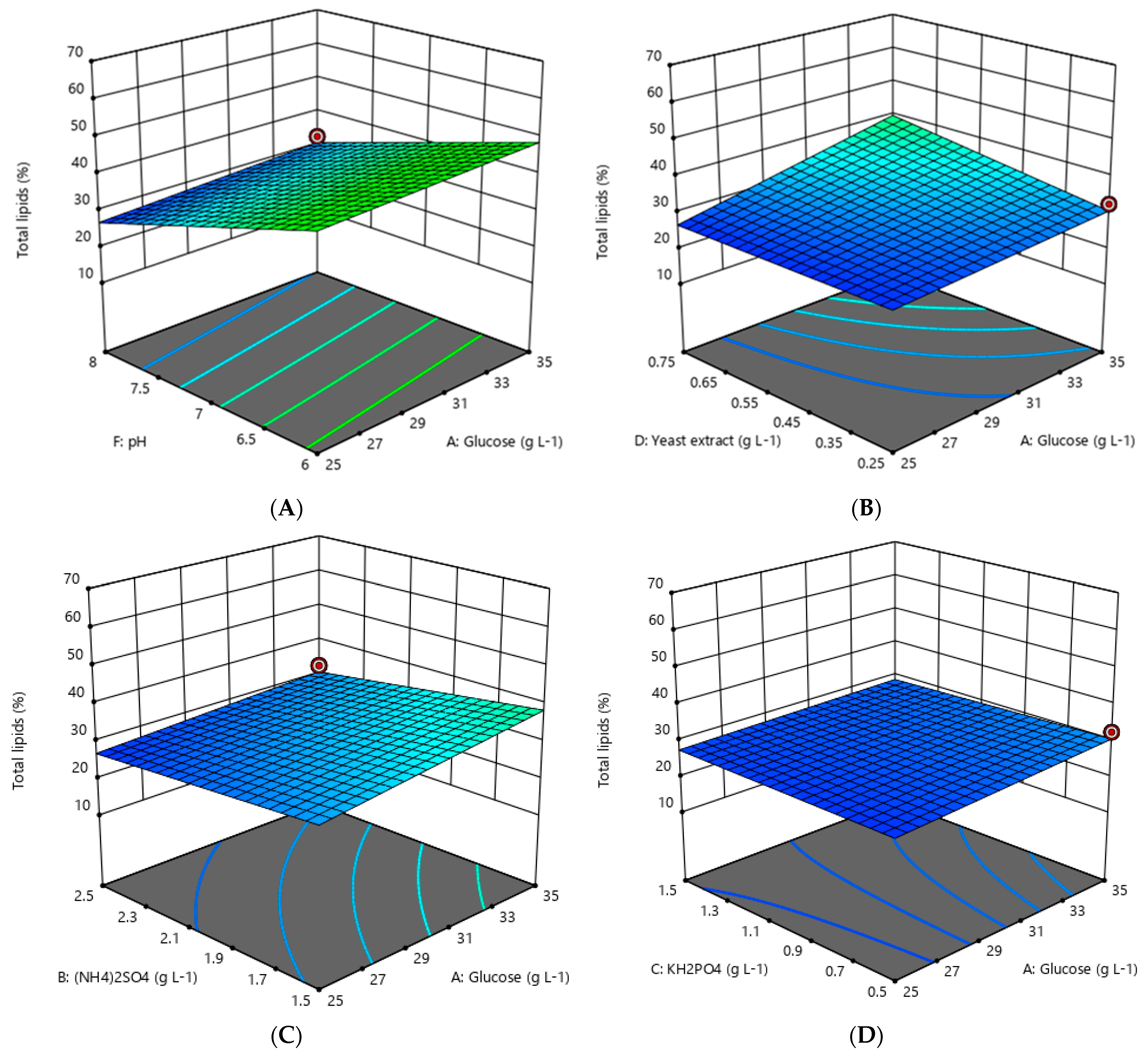
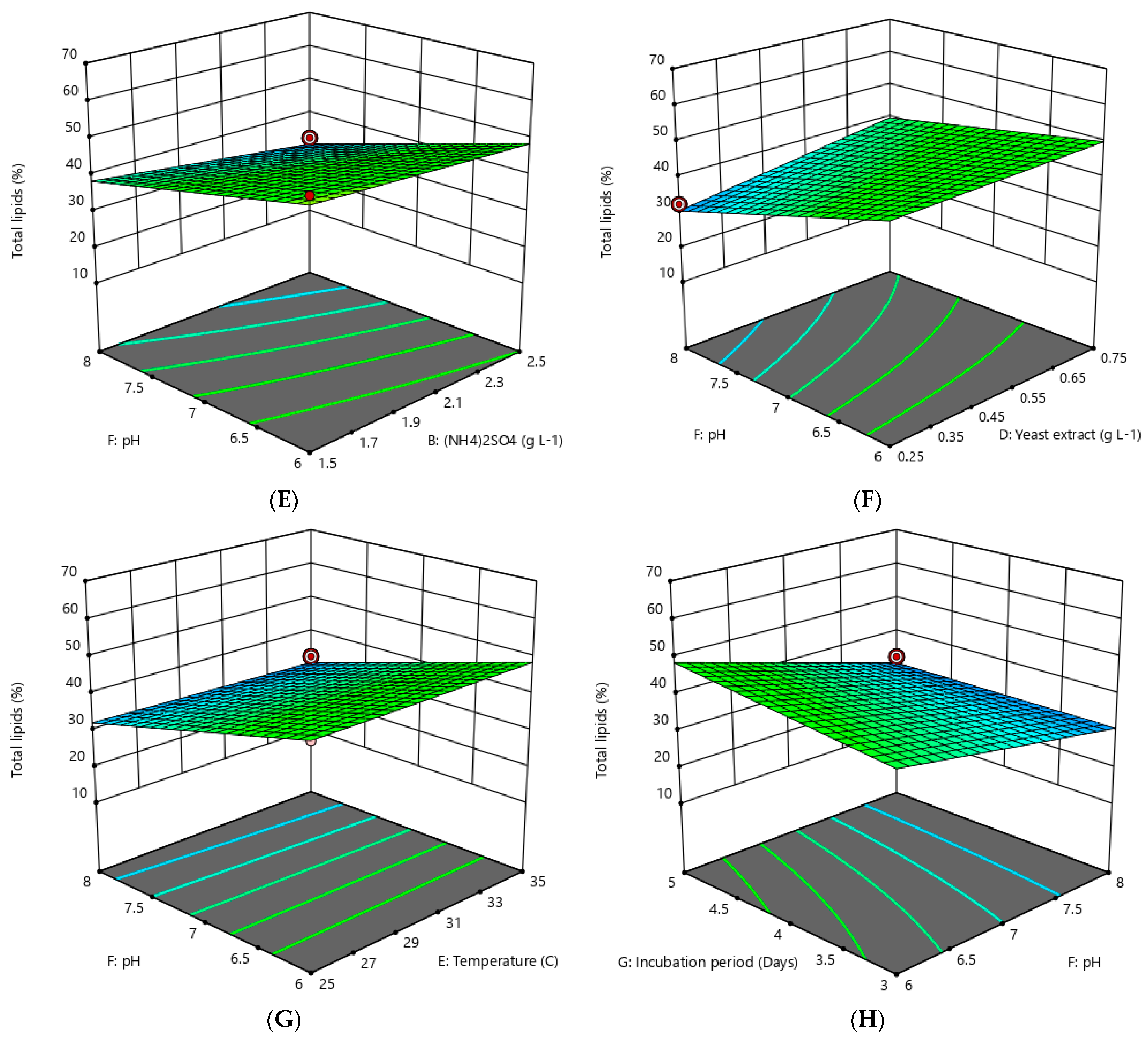
3.2. Optimization of Lipid Accumulation by Oleaginous Yeast
3.3. Biodiesel Synthesis from Extracted Lipids of Oleaginous Yeast Biomasses
3.4. Quality of the Produced Biodiesel
3.5. Biosorption of Congo Red
3.5.1. Impact of pH on Removal of Congo Red
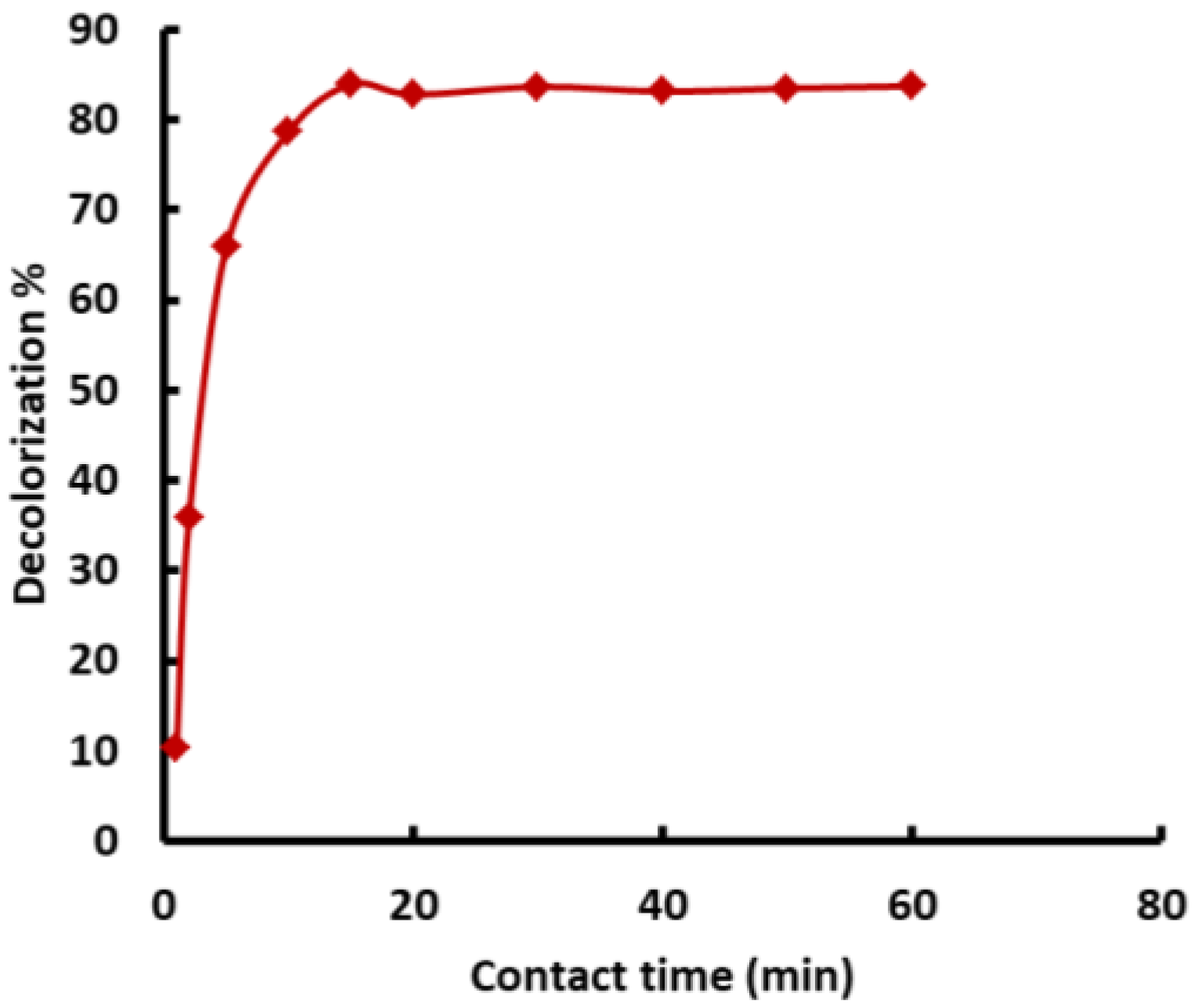
3.5.2. Impact of Contact Time
3.6. Biosorption Isotherm
- Ceq = the Congo red equilibrium concentration (mg L−1),
- qeq = equilibrium adsorption amount (mg g−1).
- qmax = maximum biosorption capability of the dye per gram of de-oiled biomass (mg g−1).
- b = Langmuir constant (L mg−1) represents the de-oiled yeast biomass/Congo red affinity (L mg−1). The values (b) and qmax can be determined from the values of the intercept and slope of Ceq/qe versus the Ceq plot.
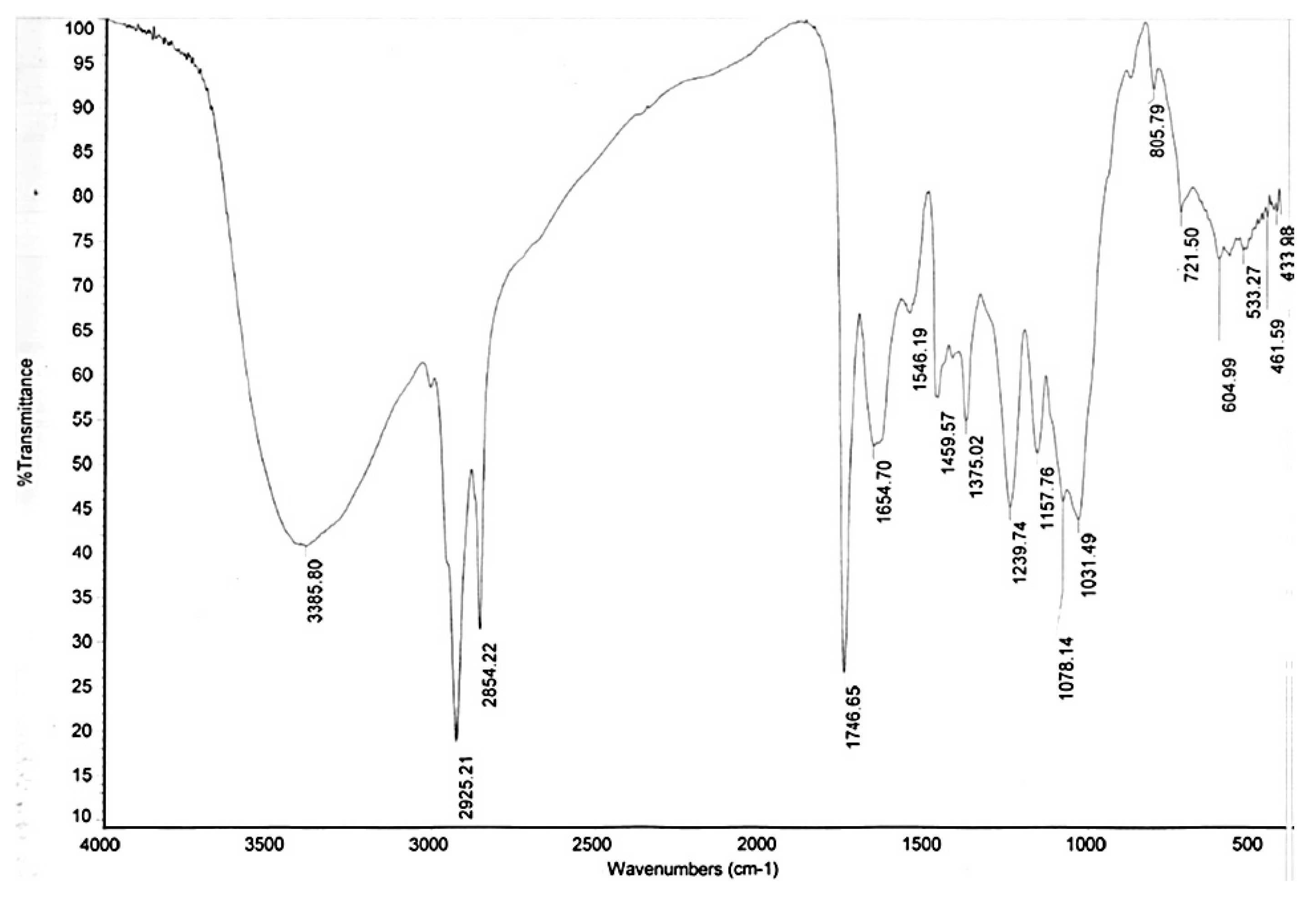
3.7. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of De-Oiled Biomass Wastes
4. Discussion
4.1. Optimization of Lipid Accumulation by Oleaginous Yeast
4.2. Biosorption of Congo Red by De-Oiled Yeast Biomasses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CaCl2 2H2O | Calcium chloride dihydrate |
| CCD | Central composite design |
| CR | Congo red |
| FAMEs | fatty acid methyl esters |
| FTIR | Fourier-transform infrared spectroscopy |
| GC/MS | Gas chromatography/mass spectrum |
| KBr | Potassium bromide |
| KH2PO4 | Potassium dihydrogen phosphate |
| MgSO4 7H2O | Magnesium sulfate heptahydrate |
| NaCl | Sodium chloride |
| (NH4)2SO4 | Ammonium sulfate |
| PBD | Plackett–Burman design |
| YMA | Yeast malt extract agar medium |
References
- Holechek, J.L.; Geli, H.M.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Chuba, V.; Lavrinenko, A.; Chuba, V.; Tsyvenkova, N. Justification of fuel mixture composition of petroleum based diesel fuel and diesel biofuel based on plant oil. In Engineering for Rural Development. Proceedings of the International Scientific Conference (Latvia); Latvia University of Life Sciences and Technologies: Jelgava, Latvia, 2021. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bagy, M.M.K.; Morsy, F.M.; Hassan, E.A. Enhancement of biodiesel, hydrogen and methane generation from molasses by Cunninghamella echinulata and anaerobic bacteria through sequential three-stage fermentation. Energy 2014, 78, 543–554. [Google Scholar] [CrossRef]
- Najjar, A.A.; Hassan, E.A.; Zabermawi, N.M.; Almasaudi, S.B.; Moulay, M.; Harakeh, S.; Abd El-Aal, M. Efficacy of the Immobilized Kocuria flava Lipase on Fe3O4/Cellulose Nanocomposite for Biodiesel Production from Cooking Oil Wastes. Catalysts 2022, 12, 977. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ibrahim, H.A.; Barakat, K.M.; Shaltout, N.A.; Sayed, W.M.E.; Abou-Shanab, R.A.; Sadowsky, M.J. Potential of Marine Biota and Bio-waste Materials as Feedstock for Biofuel Production. In Waste Management; CRC Press: Boca Raton, FL, USA, 2022; pp. 123–139. [Google Scholar]
- Bagy, M.M.K.; Abd-Alla, M.H.; Morsy, F.M.; Hassan, E.A. Two stage biodiesel and hydrogen production from molasses by oleaginous fungi and Clostridium acetobutylicum ATCC 824. Int. J. Hydrogen Energy 2014, 39, 3185–3197. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Karishma, S. Bio-derived catalysts for production of biodiesel: A review on feedstock, oil extraction methodologies, reactors and lifecycle assessment of biodiesel. Fuel 2022, 316, 123379. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bagy, M.M.K.; Morsy, F.M.; Hassan, E.A. Improvement of fungal lipids esterification process by bacterial lipase for biodiesel synthesis. Fuel 2015, 160, 196–204. [Google Scholar] [CrossRef]
- Kumbhar, M.B.; Lokhande, P.E.; Chavan, U.S.; Salunkhe, V.G.A. Global Scenario of Sustainable Technologies and Progress in a Biodiesel Production. In Biodiesel Technology and Applications; Inamuddin Ahamed, M.I., Boddula, R., Rezakazemi, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 215–240. [Google Scholar] [CrossRef]
- Azizian, H.; Kramer, J.K. A rapid method for the quantification of fatty acids in fats and oils with emphasis on trans fatty acids using Fourier transform near infrared spectroscopy (FT-NIR). Lipid 2005, 40, 855–867. [Google Scholar] [CrossRef]
- Balat, M. Potential alternatives to edible oils for biodiesel production—A review of current work. Energy Conver. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Rincón, L.; Jaramillo, J.; Cardona, C. Comparison of feedstocks and technologies for biodiesel production: An environmental and technoeconomic evaluation. Ren. Energy 2014, 69, 479–487. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, J.H.; Chien, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Conv. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Wang, F.M.; Juan, H.Y.; Su, C.H. Biodiesel production by direct transesterification of wet spent coffee grounds using switchable solvent as a catalyst and solvent. Biores. Technol. 2020, 296, 122334. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Musmar, S.E.A.; Kabir, F.; Batool, I.; Rasheed, M.A.; Jamil, F.; Khan, S.U.; Tlili, I. Biodiesel production from Melia azedarach and Ricinus communis oil by transesterification process. Catalysts 2020, 10, 427. [Google Scholar] [CrossRef]
- Chopra, J.; Rangarajan, V.; Sen, R. Recent developments in oleaginous yeast feedstock based biorefinery for production and life cycle assessment of biofuels and value-added products. Sustain. Energy Technol. Assess. 2022, 53, 102621. [Google Scholar] [CrossRef]
- Bao, W.; Li, Z.; Wang, X.; Gao, R.; Zhou, X.; Cheng, S.; Men, Y.; Zheng, L. Approaches to improve the lipid synthesis of oleaginous yeast Yarrowia lipolytica: A review. Ren. Sustain. Energy Rev. 2021, 149, 111386. [Google Scholar] [CrossRef]
- Uğuz, G.; Atabani, A.E.; Mohammed, M.N.; Shobana, S.; Uğuz, S.; Kumar, G.; Ala’a, H. Fuel stability of biodiesel from waste cooking oil: A comparative evaluation with various antioxidants using FT-IR and DSC techniques. Biocat. Agric. Biotechnol. 2019, 21, 101283. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Grossart, H.P.; Hassan, E.A.; Masigol, H.; Arias-Andres, M.; Rojas-Jimenez, K. Inland Water Fungi in the Anthropocene: Current and Future Perspectives. In The Encyclopedia of Inland Waters, 2nd ed.; Kendra Cheruvelil; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Llorca, M.; Farré, M.; Eljarrat, E.; Díaz-Cruz, S.; Rodríguez-Mozaz, S.; Wunderlin, D.; Barcelo, D. Review of emerging contaminants in aquatic biota from Latin America: 2002–2016. Environ. Toxicol. Chem. 2017, 36, 1716–1727. [Google Scholar] [CrossRef]
- Rodríguez-Castillo, G.; Molina-Rodríguez, M.; Cambronero-Heinrichs, J.C.; Quirós-Fournier, J.P.; Lizano-Fallas, V.; Jiménez-Rojas, C.; Masís-Mora, M.; Castro-Gutiérrez, V.; Mata-Araya, I.; Rodríguez-Rodríguez, C.E. Simultaneous removal of neonicotinoid insecticides by a microbial degrading consortium: Detoxification at reactor scale. Chemosphere 2019, 235, 1097–1106. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2015) 1756) Text with EEA Relevance. 2015. Available online: https://leap.unep.org/countries/eu/national-legislation/commission-implementing-decision-eu-2015495-establishing-watch (accessed on 4 June 2023).
- Sun, B.; Li, Q.; Zheng, M.; Su, G.; Lin, S.; Wu, M.; Li, C.; Wang, Q.; Tao, Y.; Dai, L.; et al. Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials: A review. Environ. Poll. 2020, 265, 114908. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Vo, D.V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Beh, C.H.; Mizuno, M.; Isobe, T.; Shiroishi, M.; Kanda, T.; Amano, Y. Screening and investigation of dye decolorization activities of basidiomycetes. J. Biosci. Bioeng. 2008, 105, 69–72. [Google Scholar] [CrossRef][Green Version]
- Greluk, M.; Hubicki, Z. Efficient removal of Acid Orange 7 dye from water using the strongly basic anion exchange resin Amberlite IRA-95. Desalination 2011, 278, 219. [Google Scholar] [CrossRef]
- Tišma, M.; Zelić, B.; Vasić-Rački, Đ. White-rot fungi in phenols, dyes and other xenobiotics treatment—A brief review. Croatian J. Food Sci. Technol. 2010, 2, 34–47. [Google Scholar]
- Kurade, M.B.; Waghmode, T.R.; Jadhav, M.U.; Jeon, B.H.; Govindwar, S.P. Bacterial–yeast consortium as an effective biocatalyst for biodegradation of sulphonated azo dye Reactive Red 198. RSC Adv. 2015, 5, 23046–23056. [Google Scholar] [CrossRef]
- Chung, K.T.; Chen, S.C.; Wong, T.Y.; Li, Y.S.; Wei, C.I.; Chou, M.-W. Mutagenic studies of benxidine and its analogues: Structure activity relationships. Toxicol. Sci. 2000, 56, 351–356. [Google Scholar] [CrossRef][Green Version]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Jamil, S.N.; Abdullah, L.C.; Choong, T.S.; Lau, K.L.; Abdullah, M. Simultaneous adsorption of cationic dyes from binary solutions by thiourea-modified poly(acrylonitrile-co-acrylic acid): Detailed isotherm and kinetic studies. Materials 2019, 12, 2903. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Liu, X.; Jiang, X.; Zhang, Z.; Zhang, T.; Zhang, L. The investigation of synergistic and competitive interaction between dye Congo red and methyl blue onmagnetic MnFe2O4. Chem. Eng. J. 2014, 246, 88–96. [Google Scholar] [CrossRef]
- Folch, J.; Ascoli, I.; Lees, M.; Meath, J.A.; LeBaron, F.N. Preparation of lipide extracts from brain tissue. J. Biol. Chem. 1951, 191, 833–841. [Google Scholar] [CrossRef]
- Anschau, A.; Caruso, C.S.; Kuhn, R.C.; Franco, T.T. Validation of the sulfo-phospho-vanillin (SPV) method for the determination of lipid content in oleaginous microorganisms”. Braz. J. Chem. Eng. 2017, 34, 19–27. [Google Scholar] [CrossRef]
- Banno, I. Studies on the sexuality of Rhodotorula. J. General Appl. Microbiol. 1967, 13, 167–196. [Google Scholar] [CrossRef]
- Sampaio, J.P. Rhodosporidium banno (1967). In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1523–1539. [Google Scholar]
- Vicente, G.; Bautista, L.F.; Gutiérrez, F.J.; Rodríguez, R.; Martínez, V.; Rodríguez-Frómeta, R.A.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Direct transformation of fungal biomass from submerged cultures into biodiesel. Energy Fuels 2010, 24, 3173–3178. [Google Scholar] [CrossRef]
- Najjar, A.; Hassan, E.A.; Zabermawi, N.; Saber, S.H.; Bajrai, L.H.; Almuhayawi, M.S.; Abujamel, T.S.; Almasaudi, S.B.; Azhar, L.E.; Moulay, M.; et al. Optimizing the catalytic activities of methanol and thermotolerant Kocuria flava lipases for biodiesel production from cooking oil wastes. Sci. Rep. 2021, 11, 13659. [Google Scholar] [CrossRef]
- Singh, G.; Sinha, S.; Kumar, K.K.; Gaur, N.A.; Bandyopadhyay, K.K.; Paul, D. High density cultivation of oleaginous yeast isolates in ‘mandi’waste for enhanced lipid production using sugarcane molasses as feed. Fuel 2020, 276, 118073. [Google Scholar] [CrossRef]
- Xue, S.J.; Li, X.C.; Huang, X.; Liu, J.; Li, Y.; Zhang, X.T.; Zhang, J.Y. Diversity investigation of cultivable yeasts associated with honeycombs and identification of a novel Rhodotorula toruloides strain with the robust concomitant production of lipid and carotenoid. Bioresour. Technol. 2023, 370, 128573. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Sajish, S.; Singh, S.; Nain, L. Yeasts for Single Cell Oil Production from Non-conventional Bioresources. In Microbial Biotechnology for Renewable and Sustainable Energy; Springer Nature: Singapore, 2022; pp. 337–364. [Google Scholar]
- Zhao, Y.; Song, B.; Li, J.; Zhang, J. Rhodotorula toruloides: An ideal microbial cell factory to produce oleochemicals, carotenoids, and other products. World J. Microbiol. Biotechnol. 2022, 38, 13. [Google Scholar] [CrossRef]
- Patel, A.; Sartaj, K.; Pruthi, P.A.; Pruthi, V.; Matsakas, L. Utilization of Clarified Butter Sediment Waste as a Feedstock for Cost-Effective Production of Biodiesel. Foods 2019, 8, 234. [Google Scholar] [CrossRef]
- Papanikolaou, S. Oleaginous Yeasts: Biochemical Events Related with Lipid Synthesis and Potential Biotechnological Applications. Ferment. Technol. 2012, 1, 1000–1103. [Google Scholar] [CrossRef]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganism 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Yellapu, S.K.; Bharti Kaur, R.; Kumar, L.R.; Tiwari, B.; Zhang, X.; Tyagi, R.D. Recent developments of downstream processing for microbial lipids and conversion to biodiesel. Bioresour. Technol. 2018, 256, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Morales-Palomo, S.; González-Fernández, C. Microbial lipids from organic wastes: Outlook and challenges. Bioresour Technol. 2021, 323, 124612. [Google Scholar] [CrossRef]
- Guo, M.; Cheng, S.; Chen, G.; Chen, J. Improvement of lipid production in oleaginous yeast Rhodosporidium toruloides by ultraviolet mutagenesis. Eng. Life Sci. 2019, 19, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Alankar, S.S.L.; Sajesh, N.; Rastogi, S.; Sakhuja, S.; Rajeswari, G.; Kumar, V.; Chandel, A.K.; Jacob, S. Bioprocessing of fermentable sugars derived from water hyacinth into microbial lipids and single cell proteins by oleaginous yeast Rhodosporidium toruloides NCIM 3547. Biomass Convers. Biorefinery 2021, 1–15. [Google Scholar] [CrossRef]
- Osman, M.E.; Abdel-Razik, A.B.; Zaki, K.I.; Mamdouh, N.; El-Sayed, H. Isolation, molecular identification of lipid-producing Rhodotorula diobovata: Optimization of lipid accumulation for biodiesel production. J. Genet. Eng. Biotechnol. 2022, 20, 32. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, X.; Hua, Y.; Feng, B.; Zhao, Z.K. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur. J. Lipid Sci. Technol. 2008, 110, 405–412. [Google Scholar] [CrossRef]
- Awad, D.; Bohnen, F.; Mehlmer, N.; Brueck, T. Multi-factorial-guided media optimization for enhanced biomass and lipid formation by the oleaginous yeast Cutaneotrichosporon oleaginosus. Front. Bioeng. Biotechnol. 2019, 7, 54. [Google Scholar] [CrossRef]
- Karim, A.; Islam, M.A.; Mishra, P.; Muzahid, A.J.M.; Yousuf, A.; Khan, M.; Faizal, C.K.M. Yeast and bacteria co-culture-based lipid production through bioremediation of palm oil mill effluent: A statistical optimization. Biomass Convers. Biorefinery 2021, 13, 2947–2958. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Subramaniam, R.; Dufreche, S.; Zappi, M.; Bajpai, R. Microbial lipids from renewable resources: Production and characterization. J. Ind. Microb. Biotechnol. 2010, 37, 1271–1287. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuel 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.J. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, K.; Sundararaju, P.; Srinivasan, N.; Uthandi, S. Bioconversion of sago processing wastewater into biodiesel: Optimization of lipid production by an oleaginous yeast, Candida tropicalis ASY2 and its transesterification process using response surface methodology. Microb. Cell Factories 2021, 20, 167. [Google Scholar] [CrossRef]
- Xavier, M.C.A.; Coradini, A.L.V.; Deckmann, A.C.; Franco, T.T. Lipid production from hemicellulose hydrolysate and acetic acid by Lipomyces starkeyi and the ability of yeast to metabolize inhibitors. Biochem. Eng. J. 2017, 118, 11–19. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mohan, S.V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate. Bioresour. Technol. 2018, 254, 284–289. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; He, Q.; Zhao, M.; Gong, Z. Microbial Lipid Production from High Concentration of Volatile Fatty Acids via Trichosporon cutaneum for Biodiesel Preparation. Appl. Biochem. Biotechnol. 2022, 194, 2968–2979. [Google Scholar] [CrossRef]
- Saran, S.; Mathur, A.; Dalal, J.; Saxena, R.K. Process optimization for cultivation and oil accumulation in an oleaginous yeast Rhodosporidium toruloides A29. Fuel 2017, 188, 324–331. [Google Scholar] [CrossRef]
- Raut, G.; Jagtap, S.; Kumar, V.R.; RaviKumar, A. Enhancing lipid content of oleaginous Yarrowia lipolytica biomass grown on waste cooking oil and its conversion to biodiesel by statistical optimization. Biomass Convers Biorefinery 2022, 1–18. [Google Scholar] [CrossRef]
- Bandhu, S.; Dasgupta, D.; Akhter, J.; Kanaujia, P.; Suman, S.K.; Agrawal, D.; Kaul, S.; Adhikari, D.K.; Ghosh, D. Statistical Design and Optimization of Single Cell Oil Production from Sugarcane Bagasse Hydrolysate by an Oleaginous Yeast Rhodotorula sp. IIP-33 Using Response Surface Methodology; SpringerPlus, Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2014; Volume 3, pp. 1–11. [Google Scholar]
- Berikten, D.; Hosgun, E.Z.; Bozan, B.; Kivanc, M. Improving lipid production capacity of new natural oleaginous yeast: Pichia cactophila firstly. Biomass Convers. Biorefinery 2021, 12, 1311–1321. [Google Scholar] [CrossRef]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitman, J.; Lee, S.C. Sporangiospore Size Dimorphism Is Linked to Virulence of Mucor circinelloides. PLoS Path. 2011, 7, 1553–7366. [Google Scholar] [CrossRef] [PubMed]
- Sayeda, A.A.; Mohsen, S.A.; Osama, H.; Azhar, A.H.; Saher, S.M. Biodiesel production from Egyptian isolate Fusarium oxysporum NRC2017. Bull. Nation. Res. Centre 2019, 43, 210. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, K.S.; Lee, C.F.; Hsu, C.L.; Huang, C.W.; Jang, H.D. Microbial lipid production by oleaginous yeast Cryptococcus sp. in the batch cultures using corncob hydrolysate as carbon source. Biomass Bioenergy 2015, 72, 95–103. [Google Scholar] [CrossRef]
- Naveira-Pazos, C.; Veiga, M.C.; Kennes, C. Accumulation of lipids by the oleaginous yeast Yarrowia lipolytica grown on carboxylic acids simulating syngas and carbon dioxide fermentation. Biores. Technol. 2022, 360, 127649. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Islam, M.A.; Yousuf, A.; Khan, M.M.R.; Faizal, C.K.M. Microbial lipid accumulation through bioremediation of palm oil mill wastewater by Bacillus cereus. ACS Sustain. Chem. Eng. 2019, 7, 14500–14508. [Google Scholar] [CrossRef]
- Alvarez, H.M.; Hernández, M.A.; Lanfranconi, M.P.; Silva, R.A.; Villalba, M.S. Rhodococcus as biofactories for microbial oil production. Molecules 2021, 26, 4871. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel production from lignocellulosic biomass using oleaginous microbes: Prospects for integrated biofuel production. Front. Microbiol. 2021, 12, 658284. [Google Scholar] [CrossRef]
- Shah, A.M.; Mohamed, H.; Zhang, Z.; Song, Y. Isolation, characterization and fatty acid analysis of Gilbertella persicaria DSR1: A potential new source of high value single-cell oil. Biomass Bioenergy 2021, 151, 106156. [Google Scholar] [CrossRef]
- Fazili, A.B.A.; Shah, A.M.; Zan, X.; Naz, T.; Nosheen, S.; Nazir, Y.; Ullah, S.; Zhang, H.; Song, Y. Mucor circinelloides: A model organism for oleaginous fungi and its potential applications in bioactive lipid production. Microb. Cell Factories 2022, 21, 29. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Paul, T.; Sinharoy, A.; Baskaran, D.; Pakshirajan, K.; Pugazhenthi, G.; Lens, P.N. Bio-oil production from oleaginous microorganisms using hydrothermal liquefaction: A biorefinery approach. Crit. Rev. Environ. Sci. Technol. 2022, 52, 356–394. [Google Scholar] [CrossRef]
- Shaigani, P.; Awad, D.; Redai, V.; Fuchs, M.; Haack, M.; Mehlmer, N.; Brueck, T. Oleaginous yeasts-substrate preference and lipid productivity: A view on the performance of microbial lipid producers. Microb. Cell Factories 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Buosi, G.M.; da Silva, E.T.; Spacino, K.; Silva, L.R.C.; Ferreira, B.A.D.; Borsato, D. Oxidative stability of biodiesel from soybean oil: Comparison between synthetic and natural antioxidants. Fuel 2016, 181, 759–764. [Google Scholar] [CrossRef]
- Bouras, H.D.; Yeddou, A.R.; Bouras, N.; Hellel, D.; Holtz, M.D.; Sabaou, N.; Chergui, A.; Nadjemi, B. Biosorption of Congo red dye by Aspergillus carbonarius M333 and Penicillium glabrum Pg1: Kinetics, equilibrium and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2017, 80, 915–923. [Google Scholar] [CrossRef]
- Singh, G.; Dwivedi, S.K. Mechanistic, adsorption kinetics and confirmatory study of Congo red dye removal by native fungus Aspergillus niger. Biomass Convers. Biorefinery 2022, 1–19. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, T. Biosorption of methylene blue from wastewater by an extraction residue of Salvia miltiorrhiza Bge. Bioresour. Technol. 2016, 219, 330–337. [Google Scholar] [CrossRef]
- Dada, E.O.; Ojo, I.A.; Alade, A.O.; Afolabi, T.J.; Jimoh, M.O.; Dauda, M.O. Biosorption of bromo-based dyes from wastewater using low-cost adsorbents: A review. J. Sci. Res. Rep. 2020, 26, 34–56. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Xu, J.; Lu, X.; Wang, C.; Xu, H.; Yuan, H.; Zhang, J. Brewer’s grains with different pretreatments used as bio-adsorbents for the removal of Congo red dye from aqueous solution. BioResources 2020, 15, 6928–6940. [Google Scholar] [CrossRef]
- El Haddad, M.; Slimani, R.; Mamouni, R.; Laamari, M.R.; Rafqah, S.; Lazar, S. Evaluation of potential capability of calcined bones on the biosorption removal efficiency of safranin ascationic dye from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2013, 44, 13–18. [Google Scholar] [CrossRef]
- Vairavel, P.; Murty, V.R. Decolorization of Congo red dye in a continuously operated rotating biological contactor reactor. Desalin. Water Treat 2020, 196, 299–314. [Google Scholar]
- Ghoniem, A.A.; Moussa, Z.; Alenzi, A.M.; Alotaibi, A.S.; Fakhry, H.; El-Khateeb, A.Y.; Saber, W.I.; Elsayed, A. Pseudomonas alcaliphila NEWG-2 as biosorbent agent for methylene blue dye: Optimization, equilibrium isotherms, and kinetic processes. Sci. Rep. 2023, 13, 3678. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, M.; Bera, D.; Adhikari, S. Biosorption of an azo dye Reactive Blue 4 from aqueous solution using dead and CMC immobilized biomass of Rhizopus oryzae (MTCC 262). Bioremediat. J. 2021, 25, 326–346. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- George, G.; Saravanakumar, M.P. Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn–Al LDH: Application on crystal violet and malachite green dye adsorption—Isotherm, kinetics and Box-Behnken design. Environ. Sci. Pollut. Res. 2018, 25, 30236–30254. [Google Scholar] [CrossRef]
- Kumar, R.; Laskar, M.A.; Hewaidy, I.F.; Barakat, M.A. Modified adsorbents for removal of heavy metals from aqueous environment: A review. Earth Syst. Environ. 2019, 3, 83–93. [Google Scholar] [CrossRef]
- Le, H.Q.; Sekiguchi, Y.; Ardiyanta, D.; Shimoyama, Y. CO2—Activated Adsorption: A New Approach to Dye Removal by Chitosan Hydrogel. ACS Omega 2018, 3, 14103–14110. [Google Scholar] [CrossRef]





| Factor Code | Tested Factor | Unit | Level | |
|---|---|---|---|---|
| Low (−1) | High (+1) | |||
| A | Glucose | g L−1 | 25 | 35 |
| B | (NH4)2SO4 | g L−1 | 1.50 | 2.50 |
| C | KH2PO4 | g L−1 | 0.50 | 1.50 |
| D | Yeast extract | g L−1 | 0.25 | 0.75 |
| E | Temperature | °C | 25 | 35 |
| F | pH | 6 | 8 | |
| G | Incubation period | Days | 3 | 5 |
| Test | Total Lipids % | Yeast Dry Biomass (g L−1) | |
|---|---|---|---|
| Yeast Isolates | |||
| Y1110 | 29.14 ± 2.16 | 6.82 ± 0.52 | |
| Y1118 | 35.02 ± 1.96 | 4.73 ± 1.14 | |
| Y1119 | 14.91 ± 0.96 | 5.71 ± 0.83 | |
| Y1120 | 18.16 ± 1.14 | 9.06 ± 0.72 | |
| Y1124 | 47.83 ± 1.37 | 7.80 ± 1.50 | |
| Y1133 | 30.42 ± 2.05 | 5.62 ± 0.94 | |
| Y1136 | 21.07 ± 1.46 | 8.04 ± 0.92 | |
| Y1201 | 16.72 ± 1.53 | 7.94 ± 1.05 | |
| Y1204 | 26.55 ± 0.88 | 4.95 ± 0.47 | |
| Y1205 | 19.03 ± 0.70 | 8.13 ± 0.68 | |
| Y1206 | 41.43 ± 1.85 | 5.92 ± 1.21 | |
| Y1208 | 39.85 ± 1.09 | 7.05 ± 0.90 | |
| Y1210 | 13.28 ± 0.64 | 4.22 ± 1.83 | |
| Parameter | Result |
|---|---|
| Phenotypic Characteristics | |
| Colony color | Pink colored |
| Shape | Globose to subglobose cells |
| Budding | Present |
| Pseudohyphae | May be present |
| Biochemical reactions | |
| Urease test | + |
| Nitrate reductase | − |
| Indole production | − |
| Soluble starch | − |
| Glucose | + |
| Xylose | + |
| Sucrose | + |
| Galactose | + |
| Lactose | − |
| Maltose | + |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1857.31 | 5 | 371.46 | 111.84 | <0.0001 | Significant |
| A-glucose | 1349.70 | 1 | 1349.70 | 406.38 | <0.0001 | Significant |
| B-(NH4)2SO4 | 11.56 | 1 | 11.56 | 3.48 | 0.1113 | not significant |
| E-Temperature | 58.09 | 1 | 58.09 | 17.49 | 0.0058 | Significant |
| AB (glucose:(NH4)2SO4) | 120.15 | 1 | 120.15 | 36.18 | 0.0010 | Significant |
| BE ((NH4)2SO4: Temperature) | 31.33 | 1 | 31.33 | 9.43 | 0.0219 | Significant |
| Residual | 19.93 | 6 | 3.32 | |||
| Cor Total | 1877.24 | 11 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1655.89 | 18 | 91.99 | 1.9700 | 0.0471 | Significant |
| A—Glucose | 569.90 | 1 | 569.90 | 12.2100 | 0.0015 | Significant |
| B—(NH4)2SO4 | 106.35 | 1 | 106.35 | 2.2800 | 0.1413 | not significant |
| C—KH2PO4 | 11.94 | 1 | 11.94 | 0.2557 | 0.6167 | not significant |
| D—Yeast extract | 2.73 | 1 | 2.73 | 0.0584 | 0.8106 | not significant |
| E—Temperature | 1.59 | 1 | 1.59 | 0.0340 | 0.8550 | not significant |
| F—pH | 385.06 | 1 | 385.06 | 8.2500 | 0.0073 | Significant |
| G—Incubation period | 42.21 | 1 | 42.21 | 0.9043 | 0.3490 | not significant |
| AB (Glucose: (NH4)2SO4) | 20.97 | 1 | 20.97 | 0.4492 | 0.5077 | not significant |
| AC (Glucose: KH2PO4) | 9.89 | 1 | 9.89 | 0.2118 | 0.6486 | not significant |
| AD (Glucose: yeast extract) | 133.58 | 1 | 133.58 | 2.8600 | 0.1007 | not significant |
| AE (Glucose: temperature) | 7.32 | 1 | 7.32 | 0.1568 | 0.6948 | not significant |
| AF (Glucose: pH) | 1.48 | 1 | 1.48 | 0.0317 | 0.8597 | not significant |
| AG (Glucose: incubation period) | 4.67 | 1 | 4.67 | 0.1001 | 0.7538 | not significant |
| BF ((NH4)2SO4: pH) | 28.28 | 1 | 28.28 | 0.6058 | 0.4423 | not significant |
| CF (KH2PO4: pH) | 3.10 | 1 | 3.10 | 0.0665 | 0.7983 | not significant |
| DF (yeast extract: pH) | 98.99 | 1 | 98.99 | 2.1200 | 0.1554 | not significant |
| EF (temperature: pH) | 6.31 | 1 | 6.31 | 0.1351 | 0.7157 | not significant |
| FG (pH: incubation period) | 81.07 | 1 | 81.07 | 1.7400 | 0.1972 | not significant |
| Residual | 1446.94 | 31 | 46.68 | |||
| Lack of fit | 1237.77 | 26 | 47.61 | 1.1400 | 0.4909 | not significant |
| Pure error | 209.17 | 5 | 41.83 | |||
| Cor Total | 3102.83 | 49 |
| Character | Value | Standard | |
|---|---|---|---|
| US Biodiesel Standard ASTM D6751 | EU Biodiesel Standard EN 14214 | ||
| Density (g·cm−3) | 0.89 | NS | 0.86–0.90 |
| Viscosity (40 °C; mm2 s−1) | 3.99 | 1.9–6.0 | 3.5–5.0 |
| SN | 151.19 | NS | NS |
| IV | 34.34 | NS | 120 max |
| HHV (MJ kg −1) | 42.72 | NS | NS |
| CN | 63.86 | 47–65 | 51 min |
| Concentration of linolenic acid (C18:2) (%) | 0.36 | NS | 12 max |
| FAME with ≥4 double bonds (%) | ND | NS | 1 max |
| Yeast Isolates | Maximum Lipid Content % | References |
|---|---|---|
| Yarrowia lipolytica | 26.02 | [61] |
| Rhodotorula toruloides | 43.80 | [62] |
| Candida tropicalis ASY2 | 45.96 | [63] |
| Lipomyces starkeyi | 28.40 | [64] |
| Cryptococcus curvatus MTCC 2698 | 28.30 | [65] |
| Trichosporon cutaneum | 49.10 | [66] |
| Rhodosporidium toruloides A29 | 53.51 | [67] |
| Yarrowia lipolytica NCIM 3589 | 64.50 | [68] |
| Rhodotorula toruloides Y1124 | 64.80 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuhayawi, M.S.; Hassan, E.A.; Almasaudi, S.; Zabermawi, N.; Azhar, E.I.; Najjar, A.; Alkuwaity, K.; Abujamel, T.S.; Alamri, T.; Harakeh, S. Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal. Sustainability 2023, 15, 13412. https://doi.org/10.3390/su151813412
Almuhayawi MS, Hassan EA, Almasaudi S, Zabermawi N, Azhar EI, Najjar A, Alkuwaity K, Abujamel TS, Alamri T, Harakeh S. Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal. Sustainability. 2023; 15(18):13412. https://doi.org/10.3390/su151813412
Chicago/Turabian StyleAlmuhayawi, Mohammed S., Elhagag A. Hassan, Saad Almasaudi, Nidal Zabermawi, Esam I. Azhar, Azhar Najjar, Khalil Alkuwaity, Turki S. Abujamel, Turki Alamri, and Steve Harakeh. 2023. "Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal" Sustainability 15, no. 18: 13412. https://doi.org/10.3390/su151813412
APA StyleAlmuhayawi, M. S., Hassan, E. A., Almasaudi, S., Zabermawi, N., Azhar, E. I., Najjar, A., Alkuwaity, K., Abujamel, T. S., Alamri, T., & Harakeh, S. (2023). Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal. Sustainability, 15(18), 13412. https://doi.org/10.3390/su151813412







