Abstract
Soil acidity is a major problem of agriculture in many parts of the world. Soil acidity causes multiple problems such as nutrient deficiency, elemental toxicity and adverse effects on biological characteristics of soil, resulting in decreased crop yields and productivity. Although a number of conventional strategies including liming and use of organic and inorganic fertilizers are suggested for managing soil acidity but cost-effective and sustainable amendments are not available to address this problem. Currently, there is increasing interest in using biochar, a form of biomass derived pyrogenic carbon, for managing acidity while improving soil health and fertility. However, biochar varies in properties due to the use of wide diversity of biomass, variable production conditions and, therefore, its application to different soils can result in positive, neutral and or negative effects requiring an in-depth understanding of biochar-acid soil interactions to achieve the best possible outcomes. Here, we present a comprehensive synthesis of the current literature on soil acidity management using biochar. Synthesis of literature showed that biochars, enriched with minerals (i.e., usually produced at higher temperatures), are the most effective at increasing soil pH, basic cation retention and promoting plant growth and yield. Moreover, the mechanism of soil acidity amelioration with biochar amendments varies biochar types, i.e., high temperature biochars with liming effects and low temperature biochars with proton consumption on their functional groups. We also provide the mechanistic interactions between biochar, plant and soils. Altogether, this comprehensive review will provide guidelines to agricultural practitioners on the selection of suitable biochar for the reclamation of soil acidity.
1. Introduction
Soil acidity is a major problem, which affects a significant portion of the earth’s surface and restricts agricultural production [1,2]. Acid soils cover more than 30% of the world’s ice-free land area, which is equivalent to 50% of arable land. In a variety of agro-ecosystems including agricultural systems, soil acidity is rising over time [1]. Many factors affect soil acidity such as fertilization, acid rainfall, leaching of nutrients, removal of agricultural residues and different agricultural practices [2]. Acidic soils have a number of negative effects on soil biology and plant performance, including base cation leaching, instability in the soil’s aggregate structure, an increase in metal toxicity, and a decrease in nutrient availability [3]. A high Al concentration in acid soils restricts root growth by inhibiting cell elongation and cell division. This ultimately results in a reduction in crop yield and productivity [4]. Given the prevalence of acid soil and the fact that it is becoming more intense, it is very important to develop sustainable measures for preventing future acidification as well as to remediate existing acid soils in order to maintain food security.
Numerous strategies including liming, integrated nutrient management, and addition of organic manure to acid soils have been proposed by researchers and agricultural professionals for ameliorating soil acidity and increasing agricultural productivity [5]. Liming is one of the most popular traditional soil acidity management techniques and possibly among the most widely adopted practices. When lime is applied, it increases soil pH, with positive impacts on crop productivity. The soil pH increases because lime provides basic cations such as Ca and Mg, thus Al toxicity is reduced [6]. Moreover, lime application can help to increase soil microbial activity with changes in soil bacterial and fungal colonization. Despite these benefits of lime application, it has some disadvantages. For instance, the application of lime in agricultural soil for a long period can cause re-acidification and may increase physical firmness, while it may cause leaching loss of mineral nutrients such as Mg2+ and NO3− [7].
As an alternative to the current practices, biochar, a form of pyrogenic carbon-rich materials, can be one of the promising amendments for increasing the crop yield and productivity in diverse soils, including acidic soils. Biochar, being resistant to microbial decomposition, is considered a natural and eco-friendly soil amendment since it can potentially reduce or buffer soil pH for a longer period [8]. Biochar consists of stable or fixed carbon, labile carbon and other volatile compounds, moisture, and ash components [9]. Ashes in biochar, such as alkaline oxides and carbonates, can contribute a significant amount of alkalinity; however, this is dependent on the feedstock and manufacturing process. Carbon molecules of biochar can remain stable for hundreds or thousands of years while they develop functional groups on its surfaces [8].
The carboxylic and phenolic functional groups in the biochar surface can buffer soil pH, while the intrinsic basic cations in the biochar can also help to minimize soil pH. The later effect may be shortlived, while the earlier effect will increase with the passage of time [7]. In addition, biochar can improve soil microbial functions, including symbiotic association with mycorrhiza that helps to acquire nutrients under acidic conditions [10,11].
Because of its large surface area and useful carboxylic and phenolic groups, biochar prevents the loss of minerals through leaching. As a result, it helps in improving nutrient uptake. However, biochar can also bind toxic elements such as Al and Fe, reducing stress on root growth [12].
Although there have been numerous studies on the use of biochar to increase soil fertility and productivity, there is still a knowledge gap on the mechanistic interactions of biochar with acid soils. However, we provide an in-depth synthesis on how different types of biochar can change soils with different pH. Altogether, this synthesis will provide a guideline for agricultural practitioners on choosing a suitable biochar for their soils of interest with the ultimate outcomes of enhancing crop yield and productivity.
2. Soil Acidity
The problem of acid soils is worldwide (Figure 1). Acid soils mostly occur in two main belts, (a) the northern belt, located in the cold, humid and temperate zone, including North America, South Asia, and Russia, and (b) the southern belt, located in the hot, humid, tropical regions, including South Africa, South America, Australia, and New Zealand [13]. Acid soils cover large proportions of land such as 16.7% of Africa, 6.1% of Australia and New Zealand, 9.9% of Europe, 26.4% of Asia, and 40.9% of America [10]. Acid soils cover a significant part of at least 48 developing countries located mainly in tropical areas, being more frequent in Oxisols and Ultisols in South America and in Oxisols in Africa [14]. However, the intensity of soil acidity is also diverse, with extremely acidic soils (pH < 3.5) located in Asia and South and North America (Figure 1).
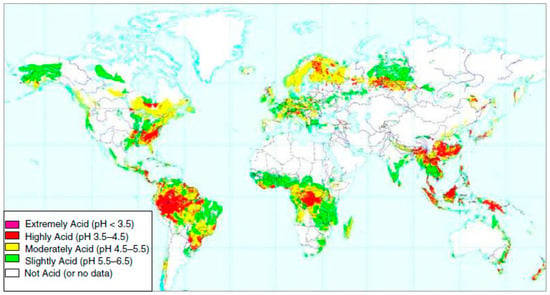
Figure 1.
World distribution of acid soils, adapted from [15].
2.1. Causes of Acidity Development in Soil
Soil acidification in agricultural lands especially results from the greater release of protons (H+) from the transformation reactions of carbon- (C), nitrogen- (N) and sulfur- (S) containing compounds. The changes in soil minerals are caused by different soil processes (e.g., redox potential and organo–mineral interactions) and soil–plant interactions such as the release of organic acids, protons (H+) and hydroxyl (–OH) ions as well as the uptake of nutrients by plants [16]. Moreover, soil acidity can increase through acid deposition from the atmosphere by acid rain. Excessive use of ammonia-based fertilizers in intensive crop cultivation practices can reduce soil pH. Organic matter decomposition or mineralization may contribute to soil acidity [14]. However, a net increase in proton concentrations with these multiple processes in soils results in soil acidification thoughsoils buffer the added acid or base depending on its properties (such as organic matter and clay content). Here, we summarized the most common pathways and management practices that contribute to soil acidity while detailed mechanisms were discussed in previous studies [16].
2.1.1. Leaching of Bases
One of the main causes of soil acidity is the leaching of base cations such as calcium, magnesium, potassium, and sodium from the soil profile [14]. Leaching is usually more prevalent in areas that receive high rainfall. For instance, acid soils in tropical countries (e.g., Cerrado soils in Brazil and highly weathered soils in South America and Asia) likely experience high leaching of base cations [14].
2.1.2. Fertilization
Excess fertilization, particularly nitrogen, contributes significantly to soil acidification [17]. Uptake of a cation by plants is balanced with the release of a proton (H+), while the conversion of NH4+-N to NO3−-N and the leaching of NO3− contribute twice the amount of H+ [18]. Since nitrogen is usually applied through ammonia-based fertilizers (e.g., mostly as urea), conversion and subsequent leaching could accelerate the acidification process. In fact, NO3− is not strongly adsorbed by the soil and can easily be leached from soils that are light in texture and receive high rainfall [19]. Moreover, the types of N fertilizer applied also have variable impacts on acidification (e.g., anhydrous NH3 acidifies more than urea). The rate of acidification is also higher when the rate of N-fertilizer application is higher [20]. Moreover, application of phosphorus fertilizer as superphosphates releases protons during mineralization, while elemental sulfur, applied through fungicides and fertilizers, also contributes to soil acidity [21].
2.1.3. Acid Rain and Weathering of Soil Minerals
Acid rain can significantly contribute to increased soil acidity since rain water often carries acids such as sulfuric acid (H2SO4) and nitric acid (HNO3) [22]. Essential nutrients from soil, such as calcium (Ca), magnesium (Mg), and potassium (K), can be washed away or leached by acid rain. Acidic soils can develop as a result of the natural weathering of rocks and minerals. Goethite (FeOOH), hematite (Fe2O3), magnetite (Fe3O4), amorphous Fe(OH)3, gibbsite (Al(OH)3), and boehmite (Al2O3·H2O) are some of the minerals that contribute to soil acidity [23].
2.1.4. Organic Matter Mineralization and Biogeochemical Cycling of Nutrients
The most important processes that generate protons (H+) and hydroxyl ions (OH−) are the mineralization of organic matter and the biogeochemical cycling of C, N, and S [24]. In C cycling, organic acids either developed from organic matter mineralization or were released by plant roots, contributing to soil acidity since they release protons during dissociation. Moreover, the dissolution of CO2 to carbonic acid also contributes to soil acidity [23]. The S cycle produces H+ ions as a result of the oxidation and mineralization of organic S. In the N cycle, transformation of nitrogen in soils (e.g., nitrification of ammonium) and nitrogen fixation generate more protons than they consume, resulting in an increase in soil acidity [21,25,26]
3. Soil Acidity Management
Depending on the type of soil, various approaches are practiced to manage soil acidity. Improved nutrient use efficiency, liming, use of organic materials, biochar, suitable crop rotations, and adoption of cultivars resistant to Al and Mn toxicity are important methods that are usually adopted to manage soil acidification [27]. The commonly used methods are liming and organic amendment, while biochar application has recently been recommended as one of the potential amendments.
3.1. Biochar as a Sustainble Amemndment for Soil Acidity Reclamation
3.1.1. Biochar and Its Properties
Biochar is a pyrogenic carbon-rich material that carries diverse properties. The pyrolysis temperature, residence period, chosen feedstock, and thermal conversion technology affect the properties of biochar [28]. Among the driving factors, pyrolysis temperature possibly has significant impacts on the characteristics of biochar (Figure 2). According to a study that synthesized the relationships between biochar production parameters and biochar properties, the CEC and volatile matter were negatively associated with the increasing pyrolysis temperature, suggesting that biochar produced at higher temperatures would have less CEC and volatile carbon. This decrease in CEC is linked to the formation of basic functional groups and a decrease in the quantity of acidic functional groups, particularly carboxylic functional groups at higher temperatures [29]. High-temperature (600–700 °C) biochars have well-organized aromatic carbon layers, and thus have low H:C and C:O ratios. However, the pH, specific surface area, ash and carbon content, and pore volume was positively associated with the increasing pyrolysis temperature [30]. Since biochar produced at higher temperatures carries a larger amount of ash, this leads to a higher biochar pH, and thus carries greater liming properties, while biochar produced at relatively lower temperatures carries higher carboxylic and phenolic functional groups that contribute to pH buffering during deprotonation [30]. The production of biochar is also influenced by factors other than pyrolysis temperature, including moisture content, lignin, and cellulose content in the biomass [31]. For example, biochar produced from solid waste and animal litter carries lower aromatic carbon and higher ash than biochar made from agricultural residue and wood biomass [30]. To generalize the biochar’s contribution to soil acidity amelioration, there are three biochar-mediated mechanisms. These are (a) its liming values, (b) its buffering capacity of soil pH and (c) its role in changing soil functions such as nutrient uptake and water uptake. These are discussed below.
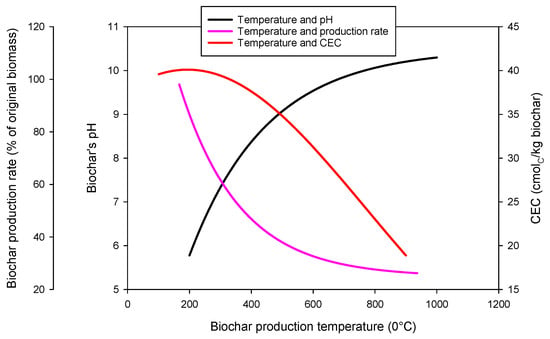
Figure 2.
Relationship between biochar production temperature and biochar properties [32].
Biochar Liming Potential
Biochar contains a variety of inorganic elements, including metal carbonate, sulfate, phosphate, silicate, and chloride. It has been demonstrated that the alkalinity of biochar is correlated with the amount of CaCO3, MgCO3, Mg(OH)2, and MgO [33]. In general, biochar made from waste biomass or grasses contains more minerals when it is produced at relatively high temperatures (>500 °C), and thus the liming value is relatively high (~12% CaCO3 equivalent) (Table 1). Based on the liming values, biochar can be classified into the following: Class 0 (<1% CaCO3− eq), Class 1 (1–10% CaCO3-eq), Class 2 (10–20% CaCO3-eq) and Class 3 (>20% CaCO3-eq) [34].

Table 1.
Biochar liming equivalence (%CaCO3-eq).
When biochar is applied to acidic soils the minerals, particularly, the basic cations (CaCO3, MgO, etc.) interact with soil-reactive species including the protons, and thus reduces soil acidity [37]. However, the contribution of biochar in changing soil pH through liming values may be short lived since the liming materials in the biochar might be exhausted after soil application [38,39].
Contribution of Biochar Functional Groups in Soil Acidity Reclamation
Biochar carries several functional groups, both acidic and basic, depending on the production conditions, feedstock, and modification treatments (Figure 3). These functional groups interact with soil-reactive species. For instance, the carboxylic, phenolic, oxonium and amine groups consume protons and hydroxyl groups depending on their state of deprotonation [40]. Potentiometric charge determination of biochar can provide detailed information about the pH-dependent change in the biochar. Since these functional groups are dissociated at different pH, the contribution of biochar in soil pH might be related to the soil pH. Geng et al. [41] investigated the role of biochar in reducing soil acidity and suggested that the carbonate, oxygen-containing functional groups, and silicates in biochar were the main explaining factors that modified acid soil properties. The authors further inferred that application of crop residue biochars may be a better option than traditional liming to ameliorate acidic soils. Changes in the biochar’s functional groups, either by natural process known as aging, or by artificial modification, e.g., oxidation or impregnation with minerals, could have similar consequences on soil acidity changes. For example, on aging, biochar could develop carboxylic or phenolic functional groups that could buffer soil pH by consuming protons and hydroxyl groups as observed in the Terra Preta soils that received charcoals [42].
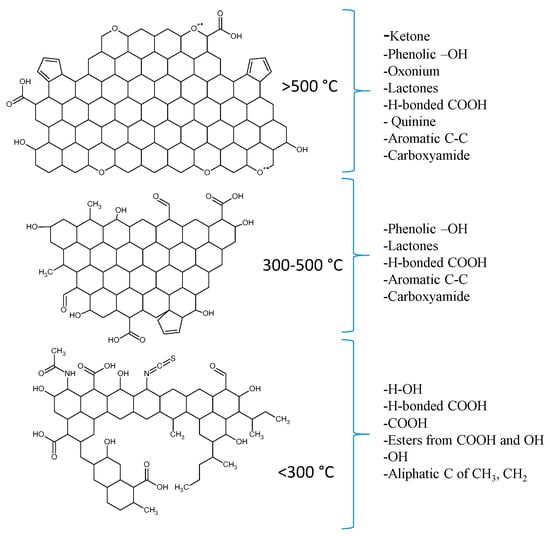
Figure 3.
Possible functional groups on the biochar surface at different pyrolysis temperatures.
Effects of Biochar on Soil pH
Application of biochar showed variable effects on soil pH when applied to soil with a different initial pH (Figure 4). Our meta-data synthesis showed that the change in soil pH was the highest (an average change of +4.78 units) in strongly acid soils (pH < 4) while the change was only +0.13 units for soil that had an original pH between 4 and 6. The change was slightly higher at +0.63 units when the soil pH was 6–8. When the pH was strongly alkaline, >8.0, biochar addition reduced the soil pH, suggesting its pH buffering capacity. It is important to note that similar changes in the pH of soils with a different initial pH (e.g., pH 4vs. pH 8) does not mean that the biochar’s contribution to soil acidity amelioration was equal since the pH is in the log scale.
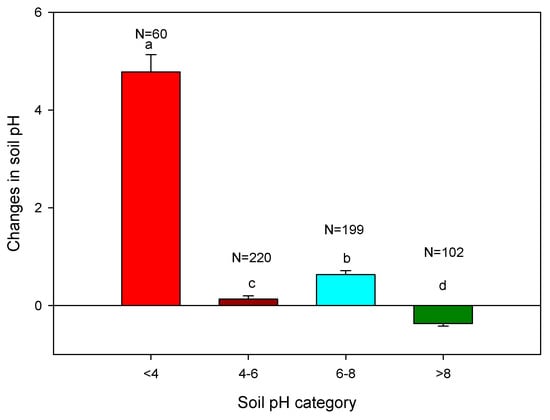
Figure 4.
Biochar application changes in soil pH compared with initial pH. The different letters above the bars indicate significant difference at 5% level of significant while N indicates number of observations.
Biochar-Mediated Changes in Soil Properties Reduce Soil Acidity
Interactions between biochar, soil, microbes, and plant roots may occur, leading to a number of changes in soil properties and functions. For instance, biochar application could change nutrient retention and uptake since it contributes to the reactive surfaces of soil (Table 2). A significant increase in soil available nitrogen has been reported. This increase is usually attributed to the retention of nutrients on the biochar surface. Particularly, the retention of nitrate and basic cations on the biochar surface may significantly reduce leaching, and thus can potentially contribute to reducing soil acidification [43]. However, biochar mediated an increase in biological nitrogen fixation (BNF) and could increase soil acidity. On the other hand, BNF studies reported an increase in soil pH, suggesting that the net impact is positive [44].
In acid soils, phosphorus fixation is one of the most dominant problems. Biochar application raises soil pH, which in turn raises soil phosphorus bioavailability [45]. Furthermore, organic compounds or macromolecules released by biochar may engage in competitive interactions with fixed phosphate on mineral surfaces. Humic acids produced from biochar are at least 10-fold more effective at brining the fixed phosphate from mineral surfaces to the soil available pool [46]. Increased bioavailability can increase plant P uptake. This enhanced phosphate uptake by plants releases more hydroxyl groups, contributing to ameliorating soil acidity [47].

Table 2.
Effect of biochar on nutrient bioavailability.
Table 2.
Effect of biochar on nutrient bioavailability.
| Biochar Types | Temperature (°C) | Soil Texture | Experimental Condition | Major Findings | References |
|---|---|---|---|---|---|
| Wheat straw biochar | 500 | Typical saline alluvial soil | Leaching experiment with urea, N fertilizer and biochar | Biochar application reduced NO3−, total N andNH4+ leaching. | [48] |
| Sycamore biochar | 500 | Sandy loam soil | Leaching experiment with manure, slurry, or fertilizer, each with or without 2% biochar | Mineral N leaching decreased | [49] |
| Peanut hull biochar | 600 | Sandy soil | Leaching experiment with 2% biochar | Reduce the amounts of NO−, NH4+, and PO4− | [50] |
| Pecan shell biochar | 300–600 | Norfolk foamy sand soil | Leaching experiment with 0.5%, 1%, 2% biochar | Extractable Ca, k, Mn, and P increased in an acid soil | [51] |
| Mixture of hickory and other woods | - | Midwestern agriculture soil | Experiment on incubating and leaching manure with additions of 0.5%, 1%, and 2% biochar | Water retention, CEC, pH, and nutrient content increase but no effect on saturated hydraulic conductivity | [52] |
| Forest slash biochar | 650 | Sand fraction | Biochar addition in compost | Leaching of dissolved organic C, N, and P enhanced | [53] |
In acid soils, biochar specifically binds toxic cations, e.g., Fe and Al. For example, many studies reported that the exchangeable Fe and Al concentration reduced significantly with biochar application [54]. The toxicity of Fe and Al may also be reduced since biochar increases soil properties that make Fe and Al less bioavailable. A reduction in the toxicity of Al and Fe can increase the performance of plants.
In acid soils, many studies reported that biochar increases the activities of soil microorganisms (Table 3). For instance, biochar has been shown to increase biological nitrogen fixation in many acid soils [55]. Moreover, similar increases in arbuscular mycorrhizal fungi and soil bacteria in acid soils have also been reported [56]. An increase in microbial activities helps in cycling of nutrients while arbuscular mycorrhizal fungi helps to acquire nutrients and water, resulting in the greater performance of plants in biochar-amended soils compared in controls [57].

Table 3.
Mechanisms by which microbial abundance is affected by biochar additions to soil, adapted from Lehmann et al. [58].
The impact of biochar on soil microbial populations and nutrient availability, however, can differ depending on the biochar feedstock, production conditions, application rate, and soil type. Furthermore, depending on the particular microbial species and their functional roles in nutrient cycling, the response of soil microorganisms to biochar amendments may vary [57]. Therefore, site-specific studies and monitoring are necessary to understand the interaction between biochar, soil microbes, and nutrient availability in acid soils.
Biochar Improves Crop Performance in Acidic Soils
On average, biochar enhanced crop performance, with the largest increase observed in acidic soils. Several meta-analyses showed that crop performance is the most pronounced in acidic soils (Figure 5). The average yield increase was 53% in acidic soils (pH < 6.0) [59]. Application of biochar with inorganic fertilizers was shown to have a more pronounced effect than biochar alone [57]. Nevertheless, the yield advantage depends on both soil and biochar properties.
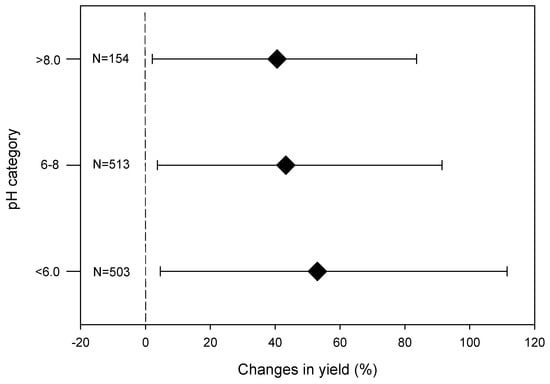
Figure 5.
Change in crop yield with biochar application in different soils (Mia et al. in preparation). The data were collected from published articles in 2010 and 2017. A meta-analysis was conducted following standard procedures.
In order to determine the effects of biochar on the grain yield (GY), the biological yield (BY), and the 1000 grain weight (TGW) of four important crops (wheat, maize, rice, and soy bean), a meta-analysis based on peer-reviewed articles was carried out. While other crops were not significantly impacted, biochar enhanced GY of maize and wheat by 28% and 13%, respectively, compared to the control. According to the analysis, biochar application rates between 1 and 10 t ha−1 significantly improved GY (65%), BY (38%) and TGW (23%).
Xu et al. [60] reviewed updated datasets from globally conducted 455, 131, and 95 independent experiments to identify the key factors influencing the responses of crop yield, soil organic carbon, and global warming potential to biochar (B) and biochar combined with chemical fertilizers (BF). The study’s goal was to investigate the variation in the effect of biochar application alone (B) and biochar combined with chemical fertilizers (BF) on crop yield, soil organic carbon (SOC), and global warming potential (GWP). Overall biochar and biochar combined with chemical fertilizer increased crop yield by 15.1% and 48.4%,respectively.
Biochar Effects on Soil Acidity Are Biochar Specific
It can be difficult for the farmer to decide the type of biochar to choose in order to obtain a particular advantage because of the heterogeneity in biochar properties and their paths of contributions (Table 4). Application of ash-rich or high-temperature biochar might can help in increasing soil pH. Except for its involvement in nutrient retention and microbial nutrient cycling, biochar can have a minor impact on managing soil acidity when the ash of biochar is exhausted [61]. In contrast to high-temperature biochar (ash rich), low-temperature biochar has a less direct effect on pH change since it only buffers pH through consumption of protons/hydroxyl ions during protonation and deprotonation [62]. Similar functional groups would exist for biochar on aging, and thus may play similar roles. With time, a large number of the functional groups could be incorporated in organic matter and soil minerals, reducing their potential effects on soil pH (Figure 4). The role of medium-temperature biochar would be in between these two categories.
Biochar–Acid Soil Interactions and Carbon Stabilization
Biochar, after soil application, interacts with soil minerals and organic matter (Figure 6). Particularly in acidic soils, where the relative abundance of Fe/Al-bearing minerals is high, these interactions can be quite intensive [63]. Diverse interactions of biochar with soil minerals include (a) ligand exchange/electrostatic interactions between negatively charged functional groups of biochar and positively charged mineral surfaces; (b) electrostatic interactions between negatively charged soil minerals, multivalent cations and negatively charged biochar; (c) ligand exchange/electrostaticinteractions between negatively charged soil minerals and positively charged biochar surfaces; (d) hydrophobic and π–π interactions between soil organic matter, biochar and soil minerals; (e) ligand/electrostatic interactions between soil organic matter and positively charged soil minerals; (f) electrostatic interactions between negatively charged functional groups of biochar, multivalent ions, and positively charged mineral surfaces. Apart from these chemical interactions, biochar can have physical interactions with soil minerals and soil organic matter while soil microorganisms (fungal hyphae and bacterial biomass) could enhance soil aggregation. Since biochar varies in properties (fresh vs. aged), these interactions can be quite variable, with more interactions with aged biochar and soil minerals. Nevertheless, the biochar and mineral interactions could increase soil carbon stabilization, and thus play a role in nutrient cycling [64].
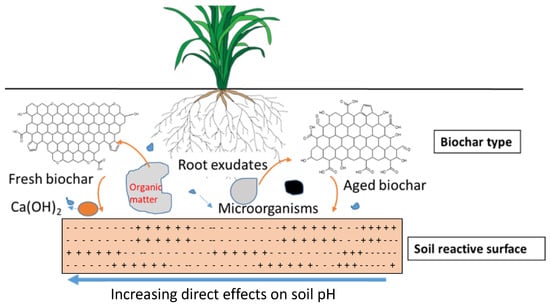
Figure 6.
Diagram showing the interactions of biochar with soil minerals (adapted from Nkoh et al. [65]).

Table 4.
Biochar effects on acid soil properties and performance.
Table 4.
Biochar effects on acid soil properties and performance.
| Biochar Types | Possible Effects on Soil pH | Possible Effects on Nutrient Retention and Uptake | Metal Ion Toxicity | Effect on Soil Microorganisms | Overall Effects | References |
|---|---|---|---|---|---|---|
| Low-temperature biochars (i.e., ≤450 °C) | pH buffering | Cation retention due to high CEC | A high toxic metal ion fixation on biochar’s functional groups | Less prominent effects since the surface area of biochar is low | Moderate effects are expected | [66,67,68] |
| Medium-temperature biochars (i.e., 400–600 °C) | Soil pH increase by ash and pH buffering | Both cation and anion retention, but in moderate capacities | A moderate toxic metal ion fixation on biochar’s functional groups | Moderate to high microbial activities | Moderate to significant effects are expected | [68,69,70] |
| High-temperature biochar (i.e., 600 °C or above) | A high change in soil pH and less pH buffering | High anion and moderate cation retention | A moderate toxic ion fixation | High microbial activity | A significant impact is expected | [67,71,72] |
| Oxidized or aged biochars | High pH buffering and moderate pH increase | A high cation retention and low anion retention | A toxic ion fixation | Low microbial activity | Moderate impacts | [73,74] |
Some Challenges of Biochar Application
Biochar properties are quite variable, with a number of factors including pyrolysis temperature, residence time, heating rate (fast or slow), oxygen limitation level, and feedstock properties (such as cellulose or lignin content, moisture status and particle size). When aiming to achieve a targeted benefit, these factors need to be optimized. However, production of function-specific (i.e., biochar with specific properties) industrial biochar is often challenging since suitable feedstock is not available or production is costly. In contrast, in many developing countries, appropriate biochar production technologies are not available. When modification of biochar by chemical oxidation or mineral impregnation is considered, many of the methods are expensive, laborious and time consuming. For example, chemical oxidation of biochar with hydrogen peroxide or another strong oxidizer (such as nitric acid) is suggested for creating surface functional groups, but these methods are often difficult to apply at large scales.
When farmers’ purchasing point of view is considered, it is often difficult for the farmers to identify biochar they need since there is no standard classification in labels of biochar packets. However, International Biochar Initiatives are striving to introduce biochar classification. Unambiguous guidelines would strengthen the application of biochar as a soil additive to preserve and improve soil health [75].
The type of biochar, the conditions of production, the characteristics of the soil, and the quantity of biochar used are potential issues that could restrict the use of biochar as an amendment [76]. It is vital to create standardized procedures and quality assurance controls. The cost of producing and using biochar at large scales can prevent its general acceptance. To make biochar more available to farmers and land managers, cost-effective production techniques and application methods must be created.
3.2. Comparison between Effects of Lime and Biochar
The roles of lime on soil pH and crop performance were recently reviewed by Enesi et al. [77]. The yield increase with liming was between 3% in sugar beet and 33% in pulses, while the change in soil pH was approximately one unit (i.e., increased to 5.55 from 4.78) [77]. However, depending on the rate of application, the initial soil pH, and type of crop, liming may have different effects on soil characteristics and crop performance (Figure 7). The articulated mechanisms for the claimed benefits are (a) to neutralize the released protons by the incorporation of liming materials, (b) to decrease the amount of Al and Mn and (c) to enhance the amount of nickel immobilization in the soil through co-precipitation and chemisorption.
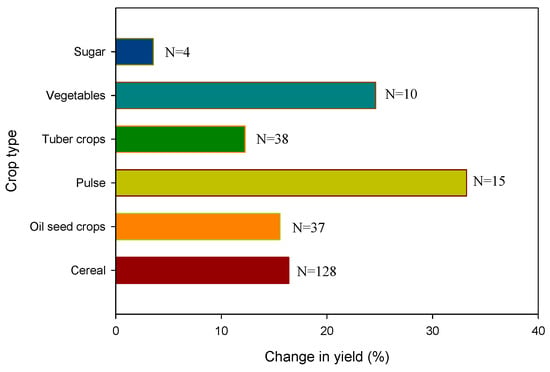
Figure 7.
Changes in crop yield (%) with liming [77].
When biochar is combined with lime, the rate of lime application can be reduced while an additive or synergistic affect can be achieved. For example, Mehnaz et al. [7] conducted a study using combined application of biochar and lime and observed that, compared to the control, rice husk biochar (RHB) combined with lime significantly buffered soil pH and increased nutrient availability (e.g., P by 137%), while reducing Al and Fe concentrations. These changes in soil properties significantly increased maize yield (by 77.59%) and nutrient uptake compared to the control.
The multi-season field trial demonstrated that the application of biochar, lime, ash, and washed biochar positively influenced maize growth and yield (Table 5). The amendments increased soil pH, reduced aluminum concentrations, and enhanced nutrient availability. Biochar exhibited superior performance compared to lime due to its additional nutrient contribution [78].

Table 5.
Effect of biochar and lime treatments on maize yield (t ha−1) over seven planting seasons [78].
4. Conclusions and Future Research Perspectives
Biochar has multiple potentialities including amelioration of soil acidity. Biochar, when applied to acid soils, can improve crop production in several ways: (a) through an increase in soil pH by adding liming materials such as CaCO3 and MgO; (b) through the consumption of protons by hydroxyl ions released from the dissociating phenolic functional groups; (c) by enhancing nutrient retention, bioavailability and uptake. However, the initial large effect of biochar’s liming material on soil acidity amelioration can be faded out with time since these materials would be consumed. Nevertheless, biochar-mediated ameliorating effects would remain active and may increase over time. Moreover, the combined application of biochar and lime or other nutrient elements could bring more pronounced effects than biochar application alone.
Despite these effects, there are considerable gaps in our understanding. Major areas of future research may involve
- (a)
- Long-term studies: Examining the changes in soil pH in field experiments in the longterm after application of artificially aged biochar.
- (b)
- Acid neutralization: Examining the role of liming vs. surface functionality:Biochar-mediated changes in soil acidity are brought about by the contribution of both liming and surface functionality. Therefore, it would be interesting to determine whether the liming material in biochar drives most of the effects.
- (c)
- Biochar–lime and/or nutrient interactions:Although several studies were conducted on biochar–lime interactions, our understanding is still limited about the interactions between biochar and lime and nutrients when applied in combination.
Author Contributions
Conceptualization, M.K.U. and S.M.; Statistical analysis, H.M.T., S.M. and A.A.S.; validation, M.K.U. and S.M.; writing—original draft preparation, H.M.T.; writing—review and editing, M.K.U., S.M., F.A., S.B.A.W., S.K., Z.A. and N.A.S.; visualization, H.M.T. and S.M.; supervision, M.K.U. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Putra Malaysia, Fundamental Research Grant Scheme (FRGS 1/2020/WAB04/Vote no 5540389), and D’ Khairan Farm SdnBhd (Vote no 6300349).
Acknowledgments
The authors are grateful to the Universiti Putra Malaysia, Selangor Darul Ehsan, Malaysia, for provision of the research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yadav, D.S.; Jaiswal, B.; Gautam, M.; Agrawal, M. Soil Acidification and Its Impact on Plants. In Plant Responses to Soil Pollution; Springer: Singapore, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Kalkhoran, S.S.; Pannell, D.J.; Thamo, T.; White, B.; Polyakov, M. Soil Acidity, Lime Application, Nitrogen Fertility, and Greenhouse Gas Emissions: Optimizing Their Joint Economic Management. Agric. Syst. 2019, 176, 102684. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil Acidification and the Importance of Liming Agricultural Soils with Particular Reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The Role of Arbuscular Mycorrhizas in Decreasing Aluminium Phytotoxicity in Acidic Soils: A Review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef]
- Thakuria, D.; Hazarika, S.; Krishnappa, R. Soil Acidity and Management Options. Indian J. Fertil. 2016, 12, 40–56. [Google Scholar]
- Behera, S.K.; Shukla, A.K. Spatial Distribution of Surface Soil Acidity, Electrical Conductivity, Soil Organic Carbon Content and Exchangeable Potassium, Calcium and Magnesium in Some Cropped Acid Soils of India. Land Degrad. Dev. 2015, 26, 71–79. [Google Scholar] [CrossRef]
- Mosharrof, M.; Uddin, M.K.; Sulaiman, M.F.; Mia, S.; Shamsuzzaman, S.M.; Haque, A.N.A. Combined Application of Biochar and Lime Increases Maize Yield and Accelerates Carbon Loss from an Acidic Soil. Agronomy 2021, 11, 1313. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar Properties and Eco-Friendly Applications for Climate Change Mitigation, Waste Management, and Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Steiner, C. Considerations in Biochar Characterization. Agric. Environ. Appl. Biochar Adv. Barriers 2016, 63, 87–100. [Google Scholar]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Mrunalini, K.; Behera, B.; Jayaraman, S.; Abhilash, P.C.; Dubey, P.K.; Swamy, G.N.; Prasad, J.V.N.S.; Rao, K.V.; Krishnan, P.; Pratibha, G. Nature-based Solutions in Soil Restoration for Improving Agricultural Productivity. Land Degrad. Dev. 2022, 33, 1269–1289. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar Amendment Improves Crop Production in Problem Soils: A Review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Kaur, C.; Selvakumar, G.; Ganeshamurthy, A.N. Acid Tolerant Microbial Inoculants: A Requisite for Successful Crop Production in Acidic Soils. In Phyto and Rhizo Remediation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 235–247. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Management of Soil Acidity of South American Soils for Sustainable Crop Production. Adv. Agron. 2014, 128, 221–275. [Google Scholar]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular Approaches Unravel the Mechanism of Acid Soil Tolerance in Plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil Acidification and the Liming Potential of Biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen Deposition Contributes to Soil Acidification in Tropical Ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.M.; Wiedemann, S.; Rowley, H.V.; Tucker, R.; Feitz, A.J.; Schulz, M. Assessing Agricultural Soil Acidification and Nutrient Management in Life Cycle Assessment. Int. J. Life Cycle Assess. 2011, 16, 431–441. [Google Scholar] [CrossRef]
- Zeng, M.; de Vries, W.; Bonten, L.T.C.; Zhu, Q.; Hao, T.; Liu, X.; Xu, M.; Shi, X.; Zhang, F.; Shen, J. Model-Based Analysis of the Long-Term Effects of Fertilization Management on Cropland Soil Acidification. Environ. Sci. Technol. 2017, 51, 3843–3851. [Google Scholar] [CrossRef]
- Bouman, O.T.; Curtin, D.; Campbell, C.A.; Biederbeck, V.O.; Ukrainetz, H. Soil Acidification from Long-Term Use of Anhydrous Ammonia and Urea. Soil Sci. Soc. Am. J. 1995, 59, 1488–1494. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hedley, M.J. Role of Carbon, Nitrogen, and Sulfur Cycles in Soil Acidification. In Handbook of Soil Acidity; Marcel Dekker: New York, NY, USA, 2003; pp. 29–56. [Google Scholar]
- Kumar, S. Acid Rain-the Major Cause of Pollution: Its Causes, Effects. Int. J. Appl. Chem. 2017, 13, 53–58. [Google Scholar]
- Zelazny, L.W.; Jackson, M.L.; Lim, C.H. Oxides, Hydroxides, and Aluminosilicates. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 101–150. [Google Scholar]
- Neina, D. The Role of Soil PH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Pettit, R.E. Organic Matter, Humus, Humate, Humic Acid, Fulvic Acid and Humin: Their Importance in Soil Fertility and Plant Health. CTI Res. 2004, 10, 1–7. [Google Scholar]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and Transformations of Micronutrients in Agricultural Soils as Influenced by Organic Matter Build-up: A Review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Golla, A.S. Soil Acidity and Its Management Options in Ethiopia: A Review. Int. J. Sci. Res. Manag. 2019, 7, 1429–1440. [Google Scholar]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar Role in the Sustainability of Agriculture and Environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface Chemical Functional Groups Modification of Porous Carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Wang, Y.; Herath, H.; Xi, Y.G.; Xiao, X.J. Effect of Crop Residue Biochar on Soil Acidity Amelioration in Strongly Acidic Tea Garden Soils. Soil Use Manag. 2014, 30, 119–128. [Google Scholar] [CrossRef]
- Camps-Arbestain, M.; Amonette, J.E.; Singh, B.; Wang, T.; Schmidt, H.P. A Biochar Classification System and Associated Test Methods. In Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; pp. 165–193. [Google Scholar]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar PH, Electrical Conductivity and Liming Potential. In Biochar: A Guide to Analytical Methods; Csiro Publishing: Melbourne, Australia, 2017; pp. 23–38. [Google Scholar]
- Hue, N. Soil Acidity: Development, Impacts, and Management. In Structure and Functions of Pedosphere; Springer: Berlin/Heidelberg, Germany, 2022; pp. 103–131. [Google Scholar]
- Sui, F.; Zuo, J.; Chen, D.; Li, L.; Pan, G.; Crowley, D.E. Biochar Effects on Uptake of Cadmium and Lead by Wheat in Relation to Annual Precipitation: A 3-Year Field Study. Environ. Sci. Pollut. Res. 2018, 25, 3368–3377. [Google Scholar] [CrossRef]
- Blok, C.; Van der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for Horticultural Rooting Media Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, D.; Nah, J.-W.; Jeon, Y.-J. Advances in Functionalizing Fucoidans and Alginates (Bio) Polymers by Structural Modifications: A Review. Chem. Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Geng, N.; Kang, X.; Yan, X.; Yin, N.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Biochar Mitigation of Soil Acidification and Carbon Sequestration Is Influenced by Materials and Temperature. Ecotoxicol. Environ. Saf. 2022, 232, 113241. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Hamdan, R.; Cooper, W.T. Physicochemical Changes in Pyrogenic Organic Matter (Biochar) after 15 Months of Field Aging. Solid Earth 2014, 5, 693–704. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How Does Biochar Influence Soil N Cycle? A Meta-Analysis. Plant Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological Nitrogen Fixation by Common Beans (Phaseolus vulgaris L.) Increases with Bio-Char Additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Huang, F.; Cai, Y.; Cai, K. Biochar Application under Low Phosphorus Input Promotes Soil Organic Phosphorus Mineralization by Shifting Bacterial PhoD Gene Community Composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef]
- Hiemstra, T.; Mia, S.; Duhaut, P.B.; Molleman, B. Natural and Pyrogenic Humic Acids at Goethite and Natural Oxide Surfaces Interacting with Phosphate. Environ. Sci. Technol. 2013, 47, 9182–9189. [Google Scholar] [CrossRef]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar Applied with Appropriate Rates Can Reduce N Leaching, Keep N Retention and Not Increase NH3 Volatilization in a Coastal Saline Soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Angst, T.E.; Patterson, C.J.; Reay, D.S.; Anderson, P.; Peshkur, T.A.; Sohi, S.P. Biochar Diminishes Nitrous Oxide and Nitrate Leaching from Diverse Nutrient Sources. J. Environ. Qual. 2013, 42, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of Biochar Amendment on Sorption and Leaching of Nitrate, Ammonium, and Phosphate in a Sandy Soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar Impact on Nutrient Leaching from a Midwestern Agricultural Soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Iqbal, H.; Garcia-Perez, M.; Flury, M. Effect of Biochar on Leaching of Organic Carbon, Nitrogen, and Phosphorus from Compost in Bioretention Systems. Sci. Total Environ. 2015, 521–522, 37–45. [Google Scholar] [CrossRef]
- Dai, Z.; Li, R.; Muhammad, N.; Brookes, P.C.; Wang, H.; Liu, X.; Xu, J. Principle Component and Hierarchical Cluster Analysis of Soil Properties following Biochar Incorporation. Soil Sci. Soc. Am. J. 2014, 78, 205–213. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Wu, D.; Sun, Y.; Zhang, H.; Gu, W.; Wang, Y.; Meng, J.; Chen, W. Biochar Can Improve Biological Nitrogen Fixation by Altering the Root Growth Strategy of Soybean in Albic Soil. Sci. Total Environ. 2021, 773, 144564. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Tian, L.; Li, S.; Li, X.; Shen, Y.; Tian, C. Long-Term Biochar Application Influences Soil Microbial Community and Its Potential Roles in Semiarid Farmland. Appl. Soil Ecol. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Ooi, G.T.; Razi, M.F.A.; Robinson, S.; Whitaker, J.; McNamara, N.P. Effects of Leucaena Biochar Addition on Crop Productivity in Degraded Tropical Soils. Biomass Bioenergy 2020, 142, 105710. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of Biochar Application on Crop Productivity, Soil Carbon Sequestration, and Global Warming Potential Controlled by Biochar C:N Ratio and Soil PH: A Global Meta-Analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar Production from Date Palm Waste: Charring Temperature Induced Changes in Composition and Surface Chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Weng, Z.; Van Zwieten, L.; Tavakkoli, E.; Rose, M.T.; Singh, B.P.; Joseph, S.; Macdonald, L.M.; Kimber, S.; Morris, S.; Rose, T.J.; et al. Microspectroscopic Visualization of How Biochar Lifts the Soil Organic Carbon Ceiling. Nat. Commun. 2022, 13, 5177. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Liang, A.; Li, Y.; Song, Q.; Li, X.; Li, D.; Hou, N. Insight into the Soil Aggregate-Mediated Restoration Mechanism of Degraded Black Soil via Biochar Addition: Emphasizing the Driving Role of Core Microbial Communities and Nutrient Cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Al Baquy, M.A.; Mia, S.; Shi, R.; Kamran, M.A.; Mehmood, K.; Xu, R. A Critical-Systematic Review of the Interactions of Biochar with Soils and the Observable Outcomes. Sustainability 2021, 13, 13726. [Google Scholar] [CrossRef]
- Kelly, C.N.; Peltz, C.D.; Stanton, M.; Rutherford, D.W.; Rostad, C.E. Biochar Application to Hardrock Mine Tailings: Soil Quality, Microbial Activity, and Toxic Element Sorption. Appl. Geochem. 2014, 43, 35–48. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Y.; Liu, Y.; Chen, J.; Wu, Q.; Ruan, Y.; Li, S.; Shi, J.; Zhao, L.; Sun, X.; et al. Pyrolysis Temperature of Biochar Affects Ecoenzymatic Stoichiometry and Microbial Nutrient-Use Efficiency in a Bamboo Forest Soil. Geoderma 2020, 363, 114162. [Google Scholar] [CrossRef]
- Liao, X.; Kang, H.; Haidar, G.; Wang, W.; Malghani, S. The Impact of Biochar on the Activities of Soil Nutrients Acquisition Enzymes Is Potentially Controlled by the Pyrolysis Temperature: A Meta-Analysis. Geoderma 2022, 411, 115692. [Google Scholar] [CrossRef]
- Azadi, N.; Raiesi, F. Biochar Alleviates Metal Toxicity and Improves Microbial Community Functions in a Soil Co-Contaminated with Cadmium and Lead. Biochar 2021, 3, 485–498. [Google Scholar] [CrossRef]
- Becerra-Agudelo, E.; López, J.E.; Betancur-García, H.; Carbal-Guerra, J.; Torres-Hernández, M.; Saldarriaga, J.F. Assessment of the Application of Two Amendments (Lime and Biochar) on the Acidification and Bioavailability of Ni in a Ni-Contaminated Agricultural Soils of Northern Colombia. Heliyon 2022, 8, e10221. [Google Scholar] [CrossRef]
- Frimpong Manso, E.; Nartey, E.K.; Adjadeh, T.A.; Darko, D.A.; Lawson, I.Y.D.; Amoatey, C.A. Use of Corn Cob and Rice Husk Biochar as Liming Materials in Acid Soils. West Afr. J. Appl. Ecol. 2019, 27, 32–50. [Google Scholar]
- Soudek, P.; Rodriguez Valseca, I.M.; Petrová; Song, J.; Vaněk, T. Characteristics of Different Types of Biochar and Effects on the Toxicity of Heavy Metals to Germinating Sorghum Seeds. J. Geochem. Explor. 2017, 182, 157–165. [Google Scholar] [CrossRef]
- Shamin, M. Biochar Aging Effects on Nitrogen and Phosphorus Dynamics in Grassland Plants and Soils: A Molecular Understanding of Biochar Properties and Mechanisms. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2018. [Google Scholar]
- Dao, M.T.; Nguyen, T.T.T.; Nguyen, X.D.; La, D.D.; Nguyen, D.D.; Chang, S.W.; Chung, W.J.; Nguyen, V.K. Toxic Metal Adsorption from Aqueous Solution by Activated Biochars Produced from Macadamia Nutshell Waste. Sustainability 2020, 12, 7909. [Google Scholar] [CrossRef]
- Loehrlein, M. Soil Health. In Sustainable Landscaping; CRC Press: Boca Raton, FL, USA, 2020; pp. 169–188. [Google Scholar] [CrossRef]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzieva, S.; et al. Sustainable Approach and Safe Use of Biochar and Its Possible Consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Enesi, R.O.; Dyck, M.; Chang, S.; Thilakarathna, M.S.; Fan, X.; Strelkov, S.; Gorim, L.Y. Liming Remediates Soil Acidity and Improves Crop Yield and Profitability—A Meta-Analysis. Front. Agron. 2023, 5, 1194896. [Google Scholar] [CrossRef]
- Hale, S.E.; Nurida, N.L.; Jubaedah; Mulder, J.; Sørmo, E.; Silvani, L.; Abiven, S.; Joseph, S.; Taherymoosavi, S.; Cornelissen, G. The Effect of Biochar, Lime and Ash on Maize Yield in a Long-Term Field Trial in a Ultisol in the Humid Tropics. Sci. Total Environ. 2020, 719, 137455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).