Use of Alcaligenes faecalis to Reduce Coliforms and Enhance the Stabilization of Faecal Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria
- Gram-negative bacteria Escherichia coli K12 MG1655 and Pseudomonas aeruginosa 4.8.1;
- Gram-positive non-sporulating bacteria Micrococcus luteus NCIMB 13267 and Staphylococcus aureus 209P;
- Yeast (eukaryote) Yarrowia lipolytica 367-2.
2.2. Cultivation
2.3. Faecal Sludge
2.4. Determination of Microbial Antagonism
2.4.1. Perpendicular Strokes Method
2.4.2. Disk Diffusion Test
2.5. Conducting an Experiment on the Biodegradation of FS Using A. faecalis
2.6. Determination of Colony-Forming Units
2.7. Tests for Coliform/E. coli
2.8. Determination of Bactericidal (MBC) and Minimum Inhibitory (MIC) Concentrations
2.9. Analytical Methods
2.10. Statistical Methods
3. Results
3.1. Antagonistic Properties and Resistance of A. faecalis DOS7
3.1.1. Perpendicular Strokes Method
3.1.2. Disk Diffusion Test
3.1.3. Resistance of A. faecalis Bacteria to the Action of test Microorganisms, Antibiotics and Biocides
3.2. Efficiency of FS Degradation with A. faecalis
3.2.1. Dynamics of the Main Biochemical Parameters of FS Biodegradation
3.2.2. Dynamics of Biochemical Indicators Associated with the Bioconversion of Nitrogen and Bicarbonates
3.3. Changes in the Total Cell Number and Infectious Potential of FS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devaraj, R.; Raman, R.K.; Wankhade, K.; Narayan, D.; Ramasamy, N.; Malladi, T. Planning fecal sludge management systems: Challenges observed in a small town in southern India. J. Environ. Manag. 2021, 281, 111811. [Google Scholar] [CrossRef]
- Odey, E.A.; Li, Z.; Zhou, X.; Kalakodio, L. Fecal sludge management in developing urban centers: A review on the collection, treatment, and composting. Environ. Sci. Pollut. Res. 2017, 24, 23441–23452. [Google Scholar] [CrossRef]
- Englund, M.; Carbajal, J.P.; Ferré, A.; Bassan, M.; Hoai Vu, A.T.; Nguyen, V.A.; Strande, L. Modelling quantities and qualities (Q&Q) of faecal sludge in Hanoi, Vietnam and Kampala, Uganda for improved management solutions. J. Environ. Manag. 2020, 261, 110202. [Google Scholar] [CrossRef]
- Adjama, I.; Agyemang Derkyi, N.S.; Uba, F.; Akolgo, G.A.; Opuko, R. Anaerobic Co-Digestion of Human Feces with Rice Straw for Biogas Production: A Case Study in Sunyani. Model. Simul. Eng. 2022, 7, 2608045. [Google Scholar] [CrossRef]
- Xu, P.-P.; Sun, K.; Du, J.; Xie, G.; Xing, D.; Liu, B.-F. Biochar prepared from human feces (HFBC) as a promoter in biohydrogen production using simulated feces as substrate. Chem. Eng. J. 2023, 461, 142074. [Google Scholar] [CrossRef]
- Diener, S.; Semiyaga, S.; Niwagaba, C.B.; Muspratt, A.M.; Gning, J.B.; Mbéguéré, M.; Ennin, J.E.; Zurbrugg, C.; Strande, L. A value proposition: Resource recovery from faecal sludge—Can it be the driver for improved sanitation? Resour. Conserv. Recycl. 2014, 88, 32–38. [Google Scholar] [CrossRef]
- Krueger, B.C.; Fowler, G.D.; Templeton, M.R.; Moya, B. Resource recovery and biochar characteristics from full-scale faecal sludge treatment and co-treatment with agricultural waste. Water Res. 2020, 169, 115253. [Google Scholar] [CrossRef]
- Semiyaga, S.; Mackay, A.E.O.; Niwagaba, C.B.; Katukiza, A.Y.; Kansiime, F. Decentralized options for faecal sludge management in urban slum areas of Sub-Saharan Africa: A review of technologies, practices and end-uses. Resour. Conserv. Recycl. 2015, 104, 109–119. [Google Scholar] [CrossRef]
- Andreev, N.; Ronteltap, M.; Boincean, B.; Lens, P.N.L. Lactic acid fermentation of human excreta for agricultural application. J. Environ. Manag. 2018, 206, 890–900. [Google Scholar] [CrossRef]
- Gwara, S.; Wale, E.; Odindo, A.; Buckley, C. Why do We Know So Much and Yet So Little? A Scoping Review of Willingness to Pay for Human Excreta Derived Material in Agriculture. Sustainability 2020, 12, 6490. [Google Scholar] [CrossRef]
- Gwara, S.; Wale, E.; Odindo, A.; Buckley, C. Attitudes and Perceptions on the Agricultural Use of Human Excreta and Human Excreta Derived Materials: A Scoping Review. Agriculture 2021, 11, 153. [Google Scholar] [CrossRef]
- Akram, U.; Quttineh, N.-H.; Wennergren, U.; Tonderski, K.; Metson, G.S. Optimizing Nutrient Recycling From Excreta in Sweden and Pakistan: Higher Spatial Resolution Makes Transportation More Attractive. Front. Sustain. Food Syst. 2019, 3, 50. [Google Scholar] [CrossRef]
- Butte, G.; Niwagaba, C.; Nordin, A. Assessing the microbial risk of faecal sludge use in Ugandan agriculture by comparing field and theoretical model output. Water Res. 2021, 197, 117068. [Google Scholar] [CrossRef] [PubMed]
- Kjær, P.; Jensen, M.; Phuc, P.D.; Knudsen, L.G.; Dalsgaard, A.; Konradsen, F. Hygiene versus fertiliser: The use of human excreta in agriculture—A Vietnamese example. Int. J. Hyg. Environ. Health 2008, 211, 432–439. [Google Scholar] [CrossRef]
- Fuhrimann, S.; Winkler, M.S.; Kabatereine, N.B.; Tukahebwa, E.M.; Halage, A.A.; Rutebemberwa, E.; Medlicott, K.; Schindler, C.; Utzinger, J.; Cissé, G. Risk of Intestinal Parasitic Infections in People with Different Exposures to Wastewater and Fecal Sludge in Kampala, Uganda: A Cross-Sectional Study. PLoS Neglected Trop. Dis. 2016, 10, e0004469. [Google Scholar] [CrossRef]
- Kerry, A.H.; Warish, A.; Rauh, E.; Channah, R.; McLain, J.; Rebecca, L.M. Comparing microbial risks from multiple sustainable waste streams applied for agricultural use: Biosolids, manure, and diverted urine. Curr. Opin. Environ. Sci. Health 2020, 14, 37–50. [Google Scholar] [CrossRef]
- Musaazi, I.G.; McLoughlin, S.; Murphy, H.M.; Rose, J.B.; Hofstra, N.; Tumwebaze, I.K.; Verbyla, M.E. A systematic review and meta-analysis of pathogen reduction in onsite sanitation systems. Water Res. X 2023, 18, 100171. [Google Scholar] [CrossRef]
- Li, M.; Song, G.; Liu, R.; Huang, X.; Liu, H. Inactivation and risk control of pathogenic microorganisms in municipal sludge treatment: A review. Front. Environ. Sci. Eng. 2022, 16, 70. [Google Scholar] [CrossRef]
- Fidjeland, J.; Magri, M.E.; Håkan, J.; Albihn, A.; Vinnerås, B. The potential for self-sanitisation of faecal sludge by intrinsic ammonia. Water Res. 2013, 47, 6014–6023. [Google Scholar] [CrossRef]
- Strande, L.; Ronteltap, M.; Brdjanovic, D. Faecal Sludge Management: Systems Approach for Implementation and Operation; IWA Publishing: London, UK, 2014. [Google Scholar] [CrossRef]
- Oyoo, V.; Riungu, J.N.; Dey, P.; Kirimi, J.G.; Matheka, R.M. Process performance evaluation of faecal matter treatment via black soldier fly. J. Water Sanit. Hyg. Dev. 2023, 13, 441–452. [Google Scholar] [CrossRef]
- Wong, J.W.; Selvam, A. Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. J. Hazard. Mater. 2009, 169, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Manga, M.; Muoghalu, C.C.; Acheng, P.O. Inactivation of faecal pathogens during faecal sludge composting: A systematic review. Environ. Technol. Rev. 2023, 12, 150–174. [Google Scholar] [CrossRef]
- Odey, E.A.; Zifu, L.; Xiaoqin, Z.; Yichang, Y. Locally produced lactic acid bacteria for pathogen inactivation and odor control in fecal sludge. J. Clean. Prod. 2018, 184, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Masís-Meléndez, F.; Segura-Montero, F.; Quesada-González, A. Control of septage sanitization by limes and lactic acid fermentation. J. Environ. Manag. 2021, 287, 112203. [Google Scholar] [CrossRef] [PubMed]
- Andreev, N.; Ronteltap, M.; Boincean, B.; Lens, P.N. Treatment of source-separated human feces via lactic acid fermentation combined with thermophilic composting. Compost. Sci. Util. 2017, 25, 220–230. [Google Scholar] [CrossRef]
- Odey, E.A.; Li, Z.; Zhou, X.; Yan, Y. Optimization of lactic acid fermentation for pathogen inactivation in fecal sludge. Ecotoxicol. Environ. Saf. 2018, 157, 249–254. [Google Scholar] [CrossRef]
- Zewde, A.A.; Li, Z.; Zhou, X.; Xu, Y. A Novel Fermentation Method for the Target Production of Propionic Acid Dominant Volatile Fatty Acids from Food Waste for Pathogen Inactivation. Waste Biomass Valorization 2023. [Google Scholar] [CrossRef]
- Loiko, N.; Kanunnikov, O.; Serdyukov, D.; Axelrod, V.; Tereshkin, E.; Vishnyakova, A.; Litti, Y. Didecyldimethylammonium Chloride- and Polyhexamethylene Guanidine-Resistant Bacteria Isolated from Fecal Sludge and Their Potential Use in Biological Products for the Detoxification of Biocide-Contaminated Wastewater Prior to Conventional Biological Treatment. Biology 2022, 11, 1332. [Google Scholar] [CrossRef]
- Loiko, N.; Kanunnikov, O.; Gannesen, A.; Kovalenko, V.; Vishnyakova, A.; Axelrod, V.; Litti, Y. Brain Natriuretic Peptide (BNP) Affects Growth and Stress Tolerance of Representatives of the Human Microbiome, Micrococcus luteus C01 and Alcaligenes faecalis DOS7. Biology 2022, 11, 984. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Singh, H.; Verma, J.; Vibha, K.; Singh, R.; Jawed, A.; Tripathi, C.K.M. Isolation, Screening, and Identification of Novel Isolates of Actinomycetes from India for Antimicrobial Applications. Front. Microbiol. 2016, 7, 1921. [Google Scholar] [CrossRef]
- Moriarty, M.J.; Semmens, K.; Bissonnette, G.K.; Jaczynski, J. Inactivation with UV-radiation and internalization assessment of coliforms and Escherichia coli in aquaponically grown lettuce. LWT 2018, 89, 624–630. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; American Public Health Association (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Loiko, N.; Kanunnikov, O.; Tereshkina, K.; Pankratov, T.; Belova, S.; Botchkova, E.; Vishnyakova, A.; Litti, Y. Biocides with Controlled Degradation for Environmentally Friendly and Cost-Effective Fecal Sludge Management. Biology 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.; Deka, H.; Chowdhury, D. Anthracene removal potential of green synthesized titanium dioxide nanoparticles (TiO2-NPs) and Alcaligenes faecalis HP8 from contaminated soil. Chemosphere 2023, 321, 138102. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Wang, K.; Zhang, X.; Hu, G.; Chen, E.; Qiu, J.; Yuan, C.; Jian, H. Quinolinic acid catabolism is initiated by a novel four-component hydroxylase QuiA in Alcaligenes faecalis JQ191. Environ. Res. 2023, 216, 114421. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, R.; Namdeo, A.; Verma, J.S.; Geed, S.R. Isolation of indole biodegrading bacterial species from petroleum contaminated site of northeast India: Growth kinetics and crude oil biodegradation assessment. J. Water Process Eng. 2023, 52, 103544. [Google Scholar] [CrossRef]
- Pous, N.; Bañeras, L.; Corvini, P.F.-X.; Liu, S.-J.; Puig, S. Direct ammonium oxidation to nitrogen gas (Dirammox) in Alcaligenes strain HO-1: The electrode role. Environ. Sci. Ecotechnol. 2023, 15, 100253. [Google Scholar] [CrossRef]
- Huang, C. Extensively drug-resistant Alcaligenes faecalis infection. BMC Infect. Dis. 2020, 20, 833. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of Salt Tolerance and Interactive Impact of Azotobacter chroococcum and/or Alcaligenes faecalis Inoculation on Canola (Brassica napus L.) Plants Grown in Saline Soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef]
- Yang, J.X.; Zhao, B.; An, Q.; Huang, Y.S.; Guo, J.S. Bioaugmentation with A. faecalis strain NR for achieving simultaneous nitrogen and organic carbon removal in a biofilm reactor. Bioresour. Technol. 2018, 247, 871–880. [Google Scholar] [CrossRef]
- Mastan, A.; Rane, D.; Dastager, S.G.; Vivek Babu, C.S. Plant Probiotic Bacterial Endophyte, Alcaligenes faecalis, Modulates Plant Growth and Forskolin Biosynthesis in Coleus forskohlii. Probiotics Antimicrob. Proteins 2020, 12, 481–493. [Google Scholar] [CrossRef]


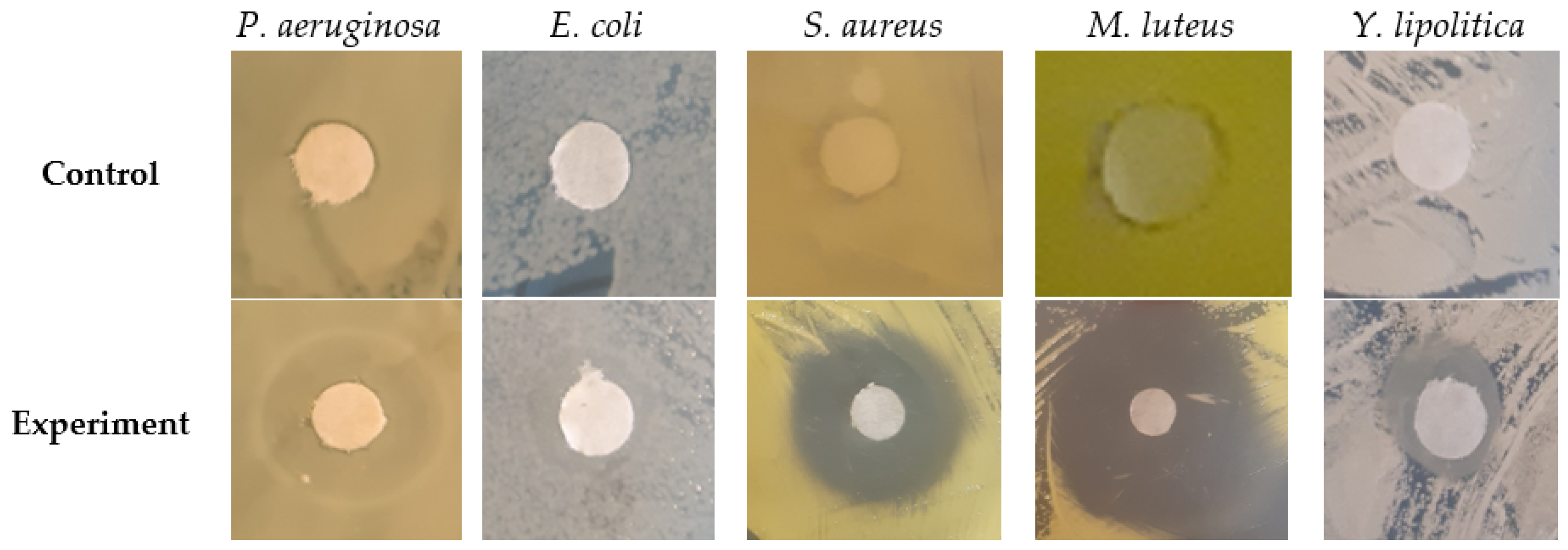

| Test Culture | Distance by Which the Growth of the Test Culture is Inhibited in the Perpendicular Strokes Experiment, mm | Diameter of Growth Inhibition Zone in the Disc Diffusion Experiment, mm |
|---|---|---|
| P. aeruginosa | 7 ± 1 ac | 15 ± 3 a,* |
| E. coli | 11 ± 2 abc | 11 ± 2 a |
| S. aureus | 18 ± 2 abc | 19 ± 2 a |
| M. luteus | 26 ± 3 b | 25 ± 3 a |
| Y. lipolitica | 5 ± 1 ac | 11 ± 1 a |
| No. | Antibiotic/Biocide | MIC, ppm | MBC, ppm |
|---|---|---|---|
| 1 | Ciprofloxacin | 16.3 | 19.6 |
| 2 | Tetracycline | 30.0 | 35.0 |
| 3 | Sodium dehydroacetate | 45,000 | >100,000 |
| 4 | Bronopol | 40 | 40 |
| 5 | 2,2-dibromo-3-nitrilopropionamide | 25 | 25 |
| 6 | Sharomix | 600 | 600 |
| 7 | Sodium percarbonate | 5000 | 6000 |
| 8 | Silver citrate | 15,000 | 25,000 |
| 9 | Didecyldimethylammonium chloride | 900 | 900 |
| 10 | Alkyldimethylbenzylammonium chloride | 600 | 600 |
| 11 | Polyhexamethylene guanidine | 500 | 500 |
| 12 | Chlorhexidine digluconate | 1000 | 2000 |
| Incubation Time, Day | TSS, mg/L | COD, mg/L | BOD5, mg/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tank1 | Tank2 | Tank3 | Tank1 | Tank2 | Tank3 | Tank1 | Tank2 | Tank3 | |
| 0 | 5362 ± 245 | 5641 ± 229 | 5477 ± 286 | 8600 ± 378 | 8712 ± 392 | 8464 ± 338 | 4050 ± 206 | 4242 ± 235 | 4117 ± 199 |
| 1 | 4715 ± 192 | 5038 ± 210 | 5020 ± 226 | 8760 ± 355 | 8980 ± 392 | 9640 ± 443 | 3734 ± 218 | 3786 ± 243 | 3667 ± 212 |
| 4 | 4248 ± 176 a | 2972 ± 119 b | 2475 ± 194 b | 8640 ± 461 a | 6460 ± 314 b | 6760 ± 284 ab | 3600 ± 302 | 3976 ± 251 | 3886 ± 282 |
| 6 | 4202 ± 213 a | 2442 ± 132 b | 1235 ± 107 c | 8320 ± 409 a | 6080 ± 206 b | 6240 ± 241 b | 3610 ± 225 | 3875 ± 227 | 3742 ± 229 |
| 11 | 3740 ± 160 a | 1998 ± 118 b | 1070 ± 69 c | 7234 ± 377 a | 4864 ± 288 b | 4622 ± 250 b | 3443 ± 237 a | 2794 ± 184 ab | 2061 ± 230 b |
| Incubation Time, Day | pH | Ntot, mg/L | N-NH4, mg/L | Bicarbonate Ion, mg/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tank1 | Tank2 | Tank3 | Tank1 | Tank2 | Tank3 | Tank1 | Tank2 | Tank3 | Tank1 | Tank2 | Tank3 | |
| 0 | 9.54 | 9.55 | 9.56 | 3846 ± 193 | 3869 ± 236 | 3715 ± 228 | 3218 ± 268 | 3254 ± 205 | 3234 ± 210 | 5000 ± 206 | 5035 ± 235 | 4916 ± 199 |
| 1 | 9.57 | 9.62 | 9.53 | 3716 ± 206 | 3609 ± 233 | 3571 ± 218 | 3424 ± 198 | 3513 ± 192 | 3531 ± 221 | 4734 ± 167 | 4368 ± 178 | 3941 ± 191 |
| 4 | 9.54 | 9.61 | 9.57 | 3123 ± 215 | 2957 ± 159 | 2876 ± 141 | 2857 ± 136 | 2851 ± 146 | 2790 ± 171 | 4606 ± 159 | 4180 ± 163 | 3862 ± 161 |
| 6 | 9.62 | 9.63 | 9.58 | 2939 ± 196 | 2834 ± 165 | 2766 ± 207 | 2674 ± 114 | 2760 ± 108 | 2667 ± 159 | 4553 ± 200 a | 3964 ± 185 ab | 3024 ± 156 b |
| 11 | 9.64 | 9.60 | 9.54 | 2818 ± 174 | 2790 ± 227 | 2692 ± 115 | 2541 ± 141 | 2697 ± 173 | 2613 ± 144 | 3769 ± 188 a | 3073 ± 187 ab | 2683 ± 142 b |
| Incubation Time, Day | Tank1 | Tank2 | Tank3 | |||
|---|---|---|---|---|---|---|
| Total Cell Titre | Coliform Percentage | Total Cell Titre | Coliform Percentage | Total Cell Titre | Coliform Percentage | |
| Starting point (40 min) | (2.9 ± 0.3) × 106 a | 30.5 | (4.3 ± 0.1) × 106 a | 29.4 | (8.0 ± 0.5) × 106 b | 28.6 |
| 1 | (4.0 ± 0.3) × 106 | 26.5 | (4.4 ± 0.4) × 106 | 8.6 | (4.1 ± 0.2) × 106 | 7.4 |
| 4 | (4.6 ± 0.3) × 106 a | 20.8 | (7.3 ± 0.5) × 106 b | 0 | (8.1 ± 0.4) × 106 b | 0 |
| 6 | (6.0 ± 0.5) × 105 a | 17.2 | (1.3 ± 0.1) × 107 b | 0 | (8.4 ± 0.7) × 106 c | 0 |
| 11 | (2.7 ± 0.3) × 104 a | 13.9 | (9.1 ± 0.8) × 106 b | 0 | (1.2 ± 0.1) × 107 b | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiko, N.; Kanunnikov, O.; Litti, Y. Use of Alcaligenes faecalis to Reduce Coliforms and Enhance the Stabilization of Faecal Sludge. Sustainability 2023, 15, 12580. https://doi.org/10.3390/su151612580
Loiko N, Kanunnikov O, Litti Y. Use of Alcaligenes faecalis to Reduce Coliforms and Enhance the Stabilization of Faecal Sludge. Sustainability. 2023; 15(16):12580. https://doi.org/10.3390/su151612580
Chicago/Turabian StyleLoiko, Nataliya, Oleg Kanunnikov, and Yuriy Litti. 2023. "Use of Alcaligenes faecalis to Reduce Coliforms and Enhance the Stabilization of Faecal Sludge" Sustainability 15, no. 16: 12580. https://doi.org/10.3390/su151612580
APA StyleLoiko, N., Kanunnikov, O., & Litti, Y. (2023). Use of Alcaligenes faecalis to Reduce Coliforms and Enhance the Stabilization of Faecal Sludge. Sustainability, 15(16), 12580. https://doi.org/10.3390/su151612580










