Abstract
This work provides an overview of the importance of recycling PET waste to reduce the environmental impact of plastic waste, conserve natural resources and energy, and create jobs in the recycling industry. Many countries have implemented regulations and initiatives to promote the recycling of PET waste and reduce plastic pollution, such as extended producer responsibility (EPR) systems, bans on certain single-use plastics, and deposit–return systems for plastic bottles. The article further underscores the versatility of recycled PET, as it can be transformed into various products such as fibers, sheets, film, and strapping. These recycled materials find applications in numerous sectors including clothing, carpets, upholstery, and industrial fibers. Recognizing the importance of collaboration among governments, industries, and individuals, we emphasize the need for sustainable PET waste management practices and the promotion of recycled materials. The article also provides information on India’s experiences with PET waste management and regulations in other countries. It is important to note that the global production and consumption of PET have increased significantly in recent years, with the packaging industry being the largest consumer of PET. This has resulted in a significant increase in the generation of PET waste, which poses a significant environmental and health hazard if not managed properly. PET waste can end up in landfills, where it can take hundreds of years to decompose, or it can end up in the oceans, where it can harm marine life and the environment. Therefore, the proper management and recycling of PET waste are essential to mitigate these negative impacts. In terms of India’s experiences with PET waste management, several initiatives have been implemented to promote the recycling of PET waste. For example, the government has launched the Swachh Bharat Abhiyan campaign, which aims to promote cleanliness and sanitation in the country to promote waste segregation and recycling.
1. Introduction
Polyethylene terephthalate (PET) is a widely used thermoplastic with excellent properties, making it a popular choice for various packaging and single-use plastic products. Its exceptional tensile strength, processability, transparency, thermal stability, barrier properties, toughness-to-weight ratio, and chemical resistance have contributed to its widespread use [1]. However, the significant consumption of PET has led to a substantial amount of PET waste, especially in the form of single-use packaging. This has raised concerns about the environmental impact of plastic waste, such as marine pollution and landfill accumulation.
To address these concerns, there is a growing global movement to reduce single-use plastics and increase PET recycling efforts. Various strategies have been proposed, including promoting reusable products, exploring sustainable packaging options like bioplastics, implementing deposit–return systems for plastic bottles and other packaging, and investing in recycling programs and infrastructure. These efforts aim to minimize plastic consumption, increase recycling rates, and encourage the adoption of sustainable materials in the packaging industry.
Sustainable packaging solutions have gained momentum in recent years due to consumer awareness and environmental considerations. PET has emerged as a leading candidate in the pursuit of eco-friendly packaging materials due to its recyclability and potential for a circular economy. The ability to collect, process, and transform used PET products into new packaging materials offers a significant opportunity to reduce reliance on virgin resources and minimize waste. PET’s transparency also plays a crucial role, allowing vibrant colors and product visibility, enhancing brand recognition, and engaging consumers. Furthermore, PET’s exceptional barrier properties protect sensitive contents, extending product freshness and shelf life.
As consumer lifestyles evolve, PET packaging continues to meet the demands for convenience and on-the-go products. Its lightweight nature reduces material waste and provides portability and ease of use for single-serve applications.
This paper aims to discuss effective handling and management techniques for PET packaging waste, focusing on best practices for the collection, sorting, and recycling processes. The objective is to optimize resource utilization and minimize environmental impacts throughout the lifecycle of PET packaging [2,3,4].
By promoting responsible waste management and environmental stewardship, we seek to inspire individuals, businesses, and policymakers to embrace sustainable packaging solutions. Through education and advocacy, we aim to drive positive change and foster collective efforts in reducing the environmental impact of PET packaging.
Overall, this paper addresses the pressing need for sustainable PET packaging practices, emphasizing the importance of proper waste management and the adoption of environmentally friendly alternatives. By raising awareness and providing guidance, we strive to contribute to a greener and more sustainable future in the packaging industry.
On the other hand, because of its growing consumption and non-biodegradability [5], its waste has increased over the years and poses a severe environmental hazard when discarded after use. PET waste contributes 12% by volume of the world’s solid waste, with its volume increasing at an alarming rate [6,7]. PET waste discarding began to pose severe economic and environmental issues with the upsurge in its amount. Economic, environmental, and energy concerns emphasize the large-scale recycling of PET. PET recycling does not only serve as a partial solution to the substantial PET waste problem, but also subsidizes the energy conservation and raw products of the petrochemical industry [8]. The production and consumption of plastic/packaging/PET materials are directly associated with the generation of plastic/packaging/PET waste.
The primary aim of this review article is to explore strategies for reducing and eliminating plastic, packaging, and PET waste through various environmental recycling methods. The objective is to lessen the burden on landfills and mitigate the environmental impact of these materials.
In summary, this paper focuses on several objectives related to PET packaging. Firstly, it highlights the importance of PET as a widely used thermoplastic with excellent properties. Secondly, it discusses effective waste management techniques to address the environmental concerns stemming from PET waste, especially single-use packaging. Thirdly, the paper addresses the challenges and opportunities related to PET packaging, exploring innovative solutions that support a more circular economy and reduce reliance on traditional PET materials. Furthermore, the paper aims to promote responsible practices in the packaging industry, encouraging the adoption of sustainable alternatives and recycling efforts. By achieving these objectives, the ultimate goal is to contribute to a more sustainable and environmentally conscious approach to PET packaging
2. Production and Consumption of PET
2.1. PET Production
The global polyethylene terephthalate (PET) market is expected to witness significant growth in the coming years. According to market forecasts, the market is estimated to surpass USD 72.88 billion by the end of 2030 in terms of revenue, exhibiting a compound annual growth rate (CAGR) of 6.7% during the forecast period from 2022 to 2030 [9]. PET, which is produced in various forms such as films, bottles, fibers, and resin, is widely recognized as an economically viable option in the packaging industry, particularly for beverages. PET bottles and PET fibers continue to dominate the market, accounting for 30.3% and 63.5% of the total global PET production, respectively, while polyester film and resin account for 6.2 shown in Figure 1 [10,11,12]. The market’s steady growth can be attributed to factors such as increased demand for sustainable packaging solutions, growth in the beverage industry, and the versatile applications of PET in various sectors. As the market progresses, it will be crucial for industry stakeholders to monitor emerging trends and invest in innovative technologies to meet the growing demand for PET products. The size of the global polyethylene terephthalate (PET) resin market was estimated at USD 80.9 million in 2021, and is anticipated to increase from USD 85.11 million in 2022 to USD 127.67 million by 2030, expanding at a CAGR of 5.2% during the forecast period (2023–2030) [13].
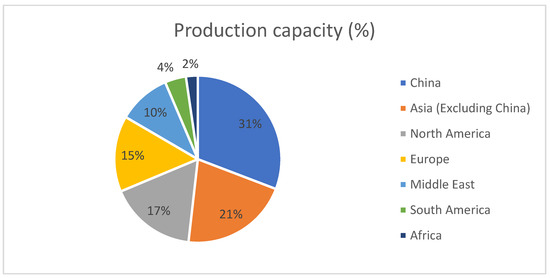
Figure 1.
Worldwide PET resin production capacity [11].
2.2. PET Consumption Market
The Middle East is currently experiencing rapid growth in various industries, making it the fastest-growing market globally, with a Compound Annual Growth Rate (CAGR) of 7.15% in terms of value. This growth is primarily driven by two key factors: the increasing manufacturing output of electrical and electronics products, and the rising demand for food and beverage packaging. Saudi Arabia and the United Arab Emirates are projected to remain the largest consumers in the region, with consumption volumes reaching around 47% and 41%, respectively, by 2029. These countries have established themselves as major players in the Middle East market, driven by their economic development and industrial expansion. The growing manufacturing output of electrical and electronics products in the Middle East is contributing to the demand for PET resin. This can be attributed to the region’s focus on diversifying its economy and reducing dependence on oil revenue. The production of consumer electronics, such as smartphones, tablets, and other electronic devices, has been increasing steadily, resulting in a higher demand for PET resin in electrical encapsulation and device manufacturing. The rising demand for food and beverage packaging is fueling the growth of the PET resin market in the Middle East. The region’s expanding population, along with changing lifestyles and an increasing preference for convenience-sized products, has led to a surge in demand for PET-based packaging materials. PET offers excellent properties for packaging applications, including its lightweight nature, transparency, and ability to preserve the quality and freshness of packaged products. Overall, the Middle East market’s robust growth in the electrical and electronics industry, as well as the food and beverage packaging sector, is driving the increasing consumption of PET resin. Saudi Arabia and the United Arab Emirates are expected to remain key players in the region, driving the market forward with their significant consumption volumes as shown in Figure 2 [14].
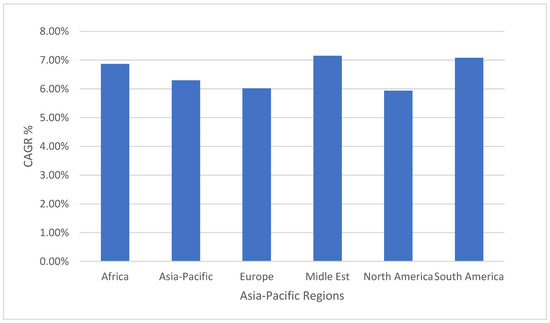
Figure 2.
Polyethelene terephthalate (PET) market, CAGR, %, by region, 2023–2029 [14].
PET (polyethylene terephthalate) has a wide range of applications in various industries, including packaging, electrical, and electronics. It is commonly used for packaging foods and beverages, particularly in convenience-sized soft drinks, water bottles, and other packaging forms. Additionally, PET is utilized in electrical encapsulation, electrical devices, solenoids, and smart meters. The packaging sector accounts for a significant portion of the overall PET resin market’s revenue, with approximately 96% in 2022. The global packaging industry is the largest end-user industry for PET resin. Factors such as a rising population, increasing income levels, and changing lifestyles contribute to the growth of the global plastic packaging industry. This, in turn, drives the demand for PET resin, especially in end-user segments such as FMCG (fast-moving consumer goods), food and beverages, pharmaceuticals, and others. The production of plastic packaging is expected to increase from 140 million tons in 2023 to around 180 million tons in 2029, reflecting the rising demand in the packaging industry. The electrical and electronics industry is the fastest-growing industry in terms of revenue, projected to register a Compound Annual Growth Rate (CAGR) of 7.88% during the forecast period of 2023–2029. This growth is primarily driven by the expanding consumer electronics market, which is expected to reach a value of USD 1103 billion in 2023. The factors contributing to this growth include the demand for lightweight products, the growth of e-commerce, a focus on sustainability, and higher adoption rates among major consumer product manufacturers. Furthermore, the revenue from electrical and electronics production is anticipated to achieve a CAGR of 6.71% during the same forecast period. Consequently, the demand for PET resin in the global market is expected to witness significant growth in the near future [15].
3. Scenario of PET and Plastic Waste and Its Recycling
3.1. Global Scenario
Rapid urbanization and increased demand for PET materials, such as plastic packaging, have led to a significant increase in the production and consumption of these materials. This, in turn, has contributed to a rise in the amount of municipal solid waste (MSW) generation. Single-use plastic packaging, which is designed for immediate disposal, is a major contributor to this problem. According to Figure 3 [16], almost 97% of plastic wrapping is thrown away after a single use. India aims to entirely remove single-use plastic by 2022. It is estimated that in 2015, around 158 million tons of plastic material were discarded globally after a single use. This includes plastic packaging, disposable plastic products, and other single-use items. This amount is projected to increase in the coming years due to population growth, urbanization, and increasing consumption. It is important to note that this number is an estimation and might have some variation. Peru has banned the use of single-use plastics in its 76 protected cultural and natural areas, including popular tourist destinations like Machu Picchu, Manu, and Huascarán, as well as in national museums. This move aims to reduce the amount of solid waste generated in these areas, as tourists are a major source of plastic waste, particularly single-use wrapping and PET bottles. At Machu Picchu alone, invitees generate an average of 14 tons of solid waste per day. Plastic packaging accounted for around 50% of the plastic waste generated worldwide in 2015.
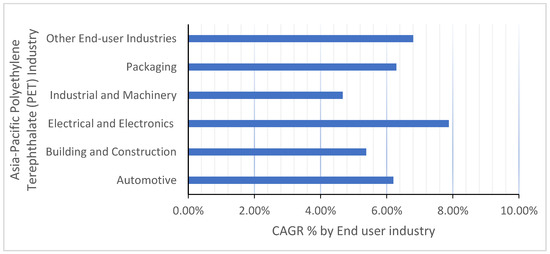
Figure 3.
Polyethelene terephthalate (PET) market, CAGR, %, by end user industry, 2023–2029 [16].
In March 2019, the European Union voted to ban the top 10 single-use plastic items found on European beaches by 2021, so as to reduce plastic pollution on beaches and in the ocean. The EU also set a target of 90% of plastic bottles to be recycled by 2025 [17]. Member states were directed to work out the details of the ban before the 2021 deadline. Plastic packaging is a major contributor to coastal litter, accounting for 61% of litter found on worldwide coastlines, at around 300 million tons [18]. In 2015, plastic packaging waste accounted for 47% of the plastic waste generated worldwide. Half of this plastic packaging waste came from Asia, with China being the largest generator of plastic packaging waste worldwide shown in Figure 4. The United States was the largest generator of plastic packaging waste per capita, followed by Japan and the European Union. It is important to note that this information is based on a study from 2015, and the numbers might have changed since then. The Figure 5 depicts the plastic packaging waste generation in 2014, represented in million metric tons (MMt), and the corresponding per capita amount in kilograms (kg) [19].
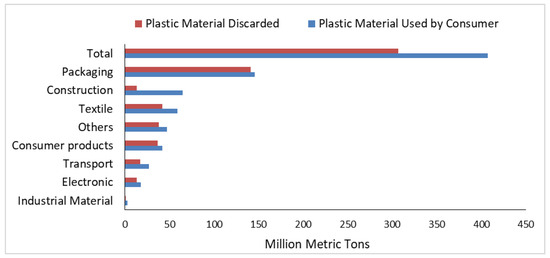
Figure 4.
The global plastic material discarded after single use in the year 2015 [19].
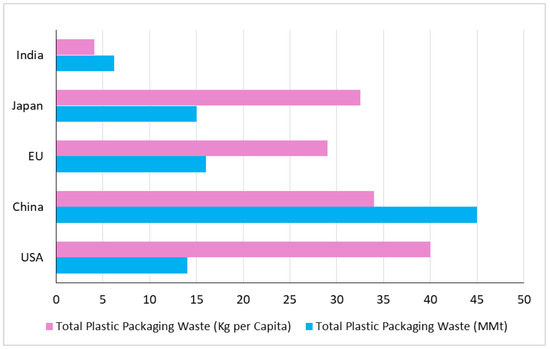
Figure 5.
Plastic packaging waste generation in 2014 (MMt) [19].
Plastic products, including those of PET, are often dumped after use and generally form part of MSW. MSW is often a rich source of numerous kinds of recyclable materials such as plastic, paper, glass, and metal. This practice is still in vogue across continents such as the EU, Australia, the USA, India, etc. The general composition of MSW generated in the USA in 2013 is presented in Table 1 [20], which indicates that plastic waste, including PET waste, comprises about 13% of the MSW. Data from a report published by the EPA [21] show that plastic containers and packaging discarding are the primary sources of PET waste in MSW in the USA. Figure 6 shows the MSW generated from some prominent countries [22].

Table 1.
Composition of MSW generated in the USA in 2013 [20].
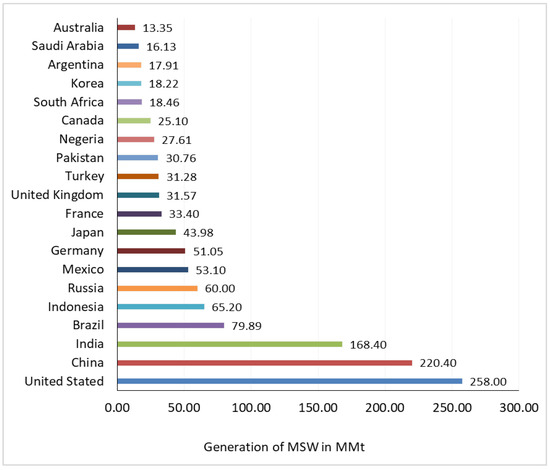
Figure 6.
MSW generation in some prominent countries in 2017 [22].
3.2. Indian Scenario
The same as in the USA, PET also forms a significant fraction of the plastic waste in MSW in India [22]. Table 2 shows the average plastic waste generated in some of the metropolitan cities in India [23,24].

Table 2.
Plastic waste generated (tons per day) in four metropolitan cities in India [24].
A survey conducted in 60 major cities of India in 2012 [25] revealed that the daily generation of plastic waste in the country was about 15.3 kilotons, or 5.6 million tons, per year. While 60% of the plastic waste was collected and recycled, the remaining 40% went uncollected. This information is from a survey conducted in 2012, and the numbers might have varied since then. Normally, the cities with higher populations and higher GDP per capita create more waste (including plastic). Here, we look at Delhi and Mumbai, with almost the same population, but almost double the amount of waste was created in Delhi. The populations of Chennai and Kolkata are almost one-third that of Mumbai, but they created the same quantity of plastic waste because of the poor management of waste recycling. Several states in India have implemented bans on single-use plastics and have seen positive results. For example, in Maharashtra, a state in India, there was a reported 40% decrease in plastic waste in the seven months following the implementation of the ban. This indicates that the ban has been effective in reducing plastic waste in the area. However, it is important to note that this information is based on a specific case, and the results may vary in other regions or with different types of bans [26].
3.3. Worldwide Recycling Efforts
According to new research, after China’s “Green Fence” action plan banned plastic waste imports in 2018, a significant portion of plastic waste exported from the US for recycling was shipped to other countries in Southeast Asia, such as Thailand, Malaysia, and Vietnam. In the first six months of 2018, around half of the plastic waste exported from the US for recycling was sent to these three countries. This indicates that the ban in China caused a shift in the destinations of plastic waste exported from the US. However, it is important to note that this information is based on a specific period, and the current situation may be different [27]. The previous year, the US exported more than 70% of its plastic waste to China and Hong Kong.
The recycling of PET waste is being adopted on a large scale in many countries across the globe to protect the environment and maintain sustainability [28]. Asia’s largest PET bottle recycling factory, INCOM Resources Recovery, with an annual recycling capacity of 50 kt, is located in Beijing, China. The used PET plastic bottles are converted into clean PET plastic material for making new bottles through mechanical recycling. The recycling rate of PET bottles in China is reported to be almost 90% [29].
Japan is also involved in recycling waste plastics, where around 77% of plastic waste was recycled in 2010, up from 39% in 1996 and 73% in 2006 [30]. In 2010, Japan recycled 72% of PET bottles, compared with 48% in the EU and 39% in the UK. Further, in 2013, 0.47 million tons of post-use PET bottles were broken down through mechanical recycling. The recycled material was used for sheeting, textiles, industrial materials, and household products such as egg boxes. Further, enormous amounts of rPET are transported to Hong Kong, China, and some other parts of Asia, where it is used for making toys and games.
In collaboration with Alkem Nigeria Limited, a fiber processing manufacturer, Coca-Cola USA started a buyback and recycling scheme for used PET bottles in Nigeria. The joint venture-initiated Nigeria’s first bottles-to-fiber recycling plant in 2005, and from 2005 to 2012 the total volume of bottles recycled increased from 135 tons to 6200 tons. This initiative not only helped to reduce plastic waste but also created jobs for an estimated 1500 people. In 2015, the Coca-Cola Company updated its PET bottling plant and created a 100% bio-PET version, aiming to switch entirely to bio-based plastics by 2020 [31].
More and more PET recycling plants are being set up in several countries due to the increase in PET waste. According to a study by the National Association for PET Container Resources (NAPCOR), an initiative was taken up in January 2013 to add four more PET recycling plants in the United States, with an additional capacity of 200 million pounds [32]. Similarly, a state-of-the-art PET recycling plant was built in England in 2012 with an operational capacity of over 50% of the UK’s PET waste, and it has been reported to have sorted over 250 million plastic bottles since opening [33]. The plastic/PET waste, co-mingled with MSW, is first sorted by type (metals and plastics), and then further sorted by plastic properties, color, and quality, before subjecting them all to reprocessing.
3.4. Recycling Efforts in India
Recycling PET waste started in India in 1994, but the industry grew slowly. The mostly mechanical recycling process is being adopted to produce rPET fibers. As of 2014, about 25 industries were involved in PET waste recycling. Currently, M/s Ganesh Ecosphere (Shri Shyam Sunder Sharmma founder of the company) is reported to be the largest recycler of PET waste, with an annual capacity of 57,600 tons. M/s Crystic Resins India Pvt. Ltd., Reliance Industries, M/s Dhansari, and M/s JBT are the other major industries involved in this field. Bisleri, one of India’s largest packaged mineral water industries, has also started recycling PET bottles [34]. The initiative involves the collection of PET bottles, crushing them into fine flakes, and then using the same for the manufacture of fibers. The rPET fibers are being widely used for the production of carpets, t-shirts, handbags, window blinds, and other useful products. As already mentioned in Section 5.1 second para reverse vending machines are also being installed in different cities to collect and process used PET bottles.
Sustainable packaging is a growing concern for plastic manufacturers and fast-moving consumer goods (FMCG) companies. Companies like Unilever, one of the world’s largest FMCG companies, are taking steps to address this issue. Unilever announced plans to make all its packaging “reusable, recyclable or compostable” by 2025. This goal is a part of Unilever’s efforts to reduce its environmental footprint and promote sustainable packaging. Such steps taken by companies are expected to drive the development and adoption of more sustainable packaging solutions in the future.
Western India is the hub of the plastic recycling machinery market, its share being about 46%, followed by North India at 28%, South India at 22%, and East India at only 4%. Gujarat in Western India hosts a significant cluster of plastic recycling machinery manufacturers/suppliers as mentioned in Table 3 [35].

Table 3.
Summary of where in India has introduced regulations on plastic/packaging products state-wise [35].
4. Unsustainable Disposal Methods of PET/Plastic Waste in Practice
Large quantities of MSW (containing PET and plastic waste) are still being disposed of through unsustainable methods, causing severe environmental damage. These methods are discussed below.
4.1. Disposal in Landfill
Plastic waste dumping in landfills, along with MSW, is a widely adopted practice, despite knowing that it leads to the wastage of valuable land resources, and with the awareness that is not considered a sustainable waste management practice. Besides occupying a vast area of land, it also generates odors and aerosols, causes visual disruption, and releases harmful chemicals and gasses through escape as leachates from landfills. Although the relative proportion of land occupied by plastic/PET waste is low in comparison to that of MSW, the damage caused to the environment by the same is enormous due to its non-biodegradable characteristics. Plastics/PET decompose very slowly and inhibit the breakdown of useful biodegradable materials from the soil. Despite this, the continued practice of dumping in landfills may be due to a lack of sufficient funds, and technical know-how, required to implement sustainable methods.
The average proportion of MSW dumped in landfills in the European Union is about 38%. Some of the leading European countries have stopped landfilling, or only a small proportion (up to 19%) is dumped into landfills, while the rest is diverted for recycling/compositing, or for waste-to-energy generation [40]. Australia also continues to depend on landfills to dispose of municipal solid wastes. Tata Energy Research Institute (TERI), India, estimated that about 1400 sq. Km of land area (equivalent to the city of Delhi, India) would be required by 2047 for dumping municipal waste in India [41].
Th landfill deposition of MSW is more prevalent in the USA, where about 68% of MSW is dumped in landfills. This is attributed to opposition by environmental groups to adopting waste-to-energy processes. However, a large number of landfills (560 out of 1900) are implementing technologies to capture landfill gas (methane) and produce electricity, which is recognized as a renewable energy source. Although it is a welcome step, only about a third of the potent greenhouse gas is converted into electricity in reality.
4.2. Burning in an Open Environment
Burning PET waste mixed with plastic waste in the open environment is also a hazardous process. It is considered an inappropriate way of managing plastic waste, because of the emission of toxic gasses produced from the decomposition of plastic/PET molecular chains and additives. During the process, halogens in the plastics fraction form volatile metal halides, which, through catalytic effects, aid in the formation of dioxins and furans [42]. The adverse environmental impacts due to the incineration of plastic waste in the open atmosphere include the generation of airborne particulates and greenhouse gas emissions. Therefore, the open burning of PET and plastic waste is not practiced in many European countries, and all the waste is utilized in the recycling processes. The government of India also enforced a ban on the open burning of plastic waste [43]. The open burning of plastic and other solid waste is also prohibited in Sri Lanka, and the same is considered a punishable offense there [44].
4.3. Littering
Littering is another inappropriate way of disposing of plastic/PET waste. Such waste, which fails to reach landfills or incinerators, is identified as the cause of manifold ecological problems, such as the choking of water bodies, an impediment to the growth of flora and fauna, etc. The dumping of plastic waste into water bodies such as rivers or oceans contributes to the deaths of millions of seabirds, thousands of marine mammals, and countless fish every year [45]. The city of Mumbai, India experienced tremendous monsoon flooding in the year 2005, resulting in around 1000 deaths [46], and the same was blamed on plastic bags that choked the gutters and drains, preventing rainwater from draining through underground systems. A similar tragedy occurred in 1988 and 1998 in Bangladesh, which led to the banning of plastic bag usage in 2002 [47]. Sri Lanka and other countries adopted a ban for similar reasons. Mauretania imposed a ban on plastic bags as cattle were getting sick from eating plastic bags. Indonesia, the world’s second-biggest plastics polluter after China, has pledged to reduce plastic waste emission into the ocean by 75% by 2025. According to BBC News, South Africa and Cameroon have recently joined other countries to declare a tax on the use of thin bags to prevent the contamination of Africa’s fields and cities.
5. Environment-Friendly (Sustainable) Methods of PET and Plastic Waste Management
Worldwide efforts are in progress to discourage practicing the above unsustainable disposal methods and develop environment-friendly (sustainable) methods to manage PET and plastic waste, which also results in the generation of some useful products.
Thermo-plastic wastes such as HDPE, PP, etc., are being used in bitumen road construction [48]. It was reported that about 1 ton of plastic waste in molten form is mixed with 5 tons of bitumen to construct 1 km of road. The usage of PET waste as a polymeric admixture to bitumen was attempted at a temperature of 200–220 °C. The same resulted in an increase in Marshall Stability by up to 76% at a dosage of 5.3% PET waste. An increase in the density and flow value of the mix was also reported [49]. Crushed PET waste and vehicle tire waste were used as fine aggregates in road construction [50,51].
Recycling PET and plastic waste is attracting worldwide attention as a sustainable option to manage the same. However, to implement these methods, it is necessary first to segregate PET and plastic waste from MSW (for waste-to-energy conversion), and segregate PET waste from plastic/PET waste (for material recycling).
5.1. Collection of PET and Plastic Waste from Source
To promote the collection of plastic waste before dumping it into MSW, people worldwide are encouraged to segregate their waste at the source as recyclable and non-recyclable waste, and deposit the same in separate bins placed at the roadside. Placing colored bins (such as blue, green, and yellow) at convenient locations in which such waste can be deposited is being widely adopted in many countries, such as the UK, Singapore, Germany, etc. Such a system is also practiced in many cities in India, such as Chennai, Mumbai, Bengaluru, Delhi, and Kolkata. The colored bins are also being made available to individuals on a yearly rent basis for the deposition of plastic waste, and these are collected once a week. An automated underground pipe system has been installed in some of the cities of the world, through which the waste deposited in colored bins in residential areas, business premises, town centers, hospitals, and airports is transported with the help of air (at a velocity of about 70 km/h) to a centralized processing plant. One such system is in operation in the city of Gandhinagar, Gujarat, India. However, the old practice of manual door-to-door waste collection on pneumatic vehicles is still in vogue in many cities of India.
Reverse vending machines, which accept used beverage cans and PET bottles and give cash in return, are in use in countries like the USA, Germany, Australia, China, etc. Sometimes, monetary incentives (in terms of mobile recharging, travel tickets, movie tickets, parking tickets, etc.) are being offered to encourage people to place used plastic bottles in collection bins. In India, the first reverse vending machine, which can accept and crush up to 500 bottles in a day, was set up at Church Gate Railway Station in Mumbai, India, on June 2015. Users will get an instant “reward” when used containers are fed into the machine, thus motivating them towards repeated use. When the device accepts the bottles, three options will appear on the screen; rewards, mobile recharge, and a discount from an outlet with which the machine provider has ties for concessions. The user can select any of the options, and a printout for the same will be issued. Bisleri, one of the largest bottled water manufacturing companies in India, and Inorbit Mall jointly started a PET bottle collection center in Inorbit Mall, Vashi, and Mumbai, India in February 2012. It is India’s first ever plastic (PET) bottle collection and recycling machine (with no incentive).
5.2. Separation of Plastic Waste from MSW
5.2.1. Electrostatic Separator
This method is frequently used to separate directing and non-conducting constituents in MSW, and is usually applied to isolated aluminum or copper from plastics or paper [52]. Furthermore, a technique that uses eddy currents is also employed to separate plastic particles from metal and plastic mixture. These procedures are appropriate only to separate good conductors, such as metallic particles, from dielectrics, such as plastics. However, this method is unsuitable for separating dielectric particles such as mixed plastic combinations.
5.2.2. Triboelectric Separation
Triboelectric separation is a type of electrostatic separation that utilizes frictional charging. Its use for the separation of plastics is a relatively new approach. In this technique, electric charges are imparted to the particles of two polymers in a mixture, and then they are separated by passing through an external electric field [53]. The deposition of positive or negative electric charges on a polymer depends on its ability to hold or lose electrons. A material with a higher affinity for electrons gains electrons and gets negatively charged, whereas a material with a low affinity loses electrons and gets positively charged [54]. An air cyclone is employed as a charging device to produce a higher frictional speed, and a triboelectric cyclone separator has been developed, which has been successfully tested for separating plastics in the laboratory.
5.2.3. Air Separator
This method is common in recycling facilities to separate different materials from a waste stream, such as plastic bottles from aluminum cans. The method is based on the principle of density, where materials with different densities are separated by controlling the airflow generated by a fan. The process starts with the waste stream being fed into the separator. The measured airflow produced by the fan splits the waste into light and heavy fractions, with the light fraction being extracted upwards and the heavy fraction being separated into a second conveyor [55,56,57]. The less-heavy fraction then reaches a rotary valve, where the rotor reduces the airspeed and the fraction is discharged onto another conveyor. This separation method is efficient and cost-effective, as it allows for separating different materials in a single step, making it easier to recycle the different materials. However, it is important to note that the separation process’s effectiveness depends on the waste stream’s specific characteristics, such as the size and density of the materials.
5.3. Separation of PET Waste from Plastic Waste
The waste plastic consequently collected directly (as described in Section 4.1) or separated from MSW (as described in Section 5.2) is additionally separated from discrete PET in the rest of the plastic waste. There are various methods for separating PVC and PET from plastic waste, as they often look similar and are problematic to differentiate. One method is the use of spectroscopy methods, which employ different types of spectroscopy, such as FTIR and XRF, to identify different types of plastics/polymers. Another method is the use of dry separation techniques, which utilize different physical properties such as the density, size, and shape of the materials to separate them. Air separation, eddy current separation, and electrostatic separation are examples of dry separation techniques. Wet separation techniques are also employed for separating PVC and PET from plastic waste. The liquid medium is used to dissolve or soften certain plastics, making separating them easier. The disadvantage of wet separation techniques is that they require a post-sorting process for re-use or discharge, expensive reagents, and the drying/dewatering of the plastics extracted from the process [58]. It is important to note that the separation process’s effectiveness depends on the waste stream’s specific characteristics, such as the size and density of the materials, and the type of separation technique used (Table 4).

Table 4.
PET waste separation from plastic waste.
6. Methods of Management of Plastic Waste
6.1. Waste-to-Energy Conversion
6.1.1. Pyrolysis
Thermal depolymerization or pyrolysis is a process in which plastic/PET waste is heated to high temperatures (typically between 400 and 500 °C) in the absence of oxygen (in an oxygen-free environment) to break down the polymer chains and convert the plastic into smaller molecules. These smaller molecules can then be converted into various products such as gases (syngas), liquids (pyrolytic oil), and solids (char).
The syngas produced from this process can be used as a fuel source in boilers or engines to generate electricity. The pyrolytic oil can be used as a fuel for heating or feedstock for producing chemicals and new plastics. The solid char can also be used as a fuel or feedstock to produce activated carbon [78,79].
The main advantage of this process is that it allows for the recovery of energy from plastic waste and the production of new raw materials, which can be used to make new plastics, thus reducing the dependence on fossil fuels and the amount of plastic waste in the environment. However, this process is still in the research phase, and commercial applications are limited. The process is also expensive, and there is a need for more research to improve its efficiency and reduce the cost of the process [80,81]. It is considered green technology, as it does not contaminate water and the environment. Plasma Pyrolysis Technology (PPT) is a type of closed environment burning technique primarily used to burn medical waste. Still, it can also be used to burn plastic waste at high temperatures, such as 850 °C. A plasma-based pyrolysis system has been designed and put into use in the state of Gujarat, India for INR 1.2 million (Bhasin, 2009) [82].
A project entitled “Plastic Waste Disposal using Plasma Pyrolysis Technology” was funded by the Central Pollution Control Board (CPCB) and undertaken by the Facilitation Centre for Industrial Plasma Technology (FCIPT), Gandhi Nagar (Gujarat). The Institute for Plasma Research received funding to investigate the technology of plasma pyrolysis with economic support from the Department of Science and Technology (DST), and the New Delhi and Technology Information, Forecasting & Assessment Council (TIFAC). The study was supplemented by applying different kinds of waste plastic such as multilayer, metalized packaging, thin carry bags, etc. Throughout the experimentation, pollutant emissions such as dioxins, furans, particular matter (PM), oxides of nitrogen, and carbon monoxide were observed (CPCB, 2016) [83]. In this method, the high maximum temperature was reached using a plasma torch in an oxygen-starved environment, which destroys plastic in an eco-friendly and efficient manner.
M/s Envac have installed waste treatment plants in many countries across the globe based on the pyrolysis process to produce biogas, liquid fuel, and bio-fertilizer. One such system has been in operation in the city of Gandhinagar, Gujarat, India, since April 2015. The controlled burning of waste to produce (electrical) energy is also being practiced worldwide. India’s first commercial waste-to-energy plant with a handling capacity of 1300 tons of MSW per day and a production capacity of 16 MW energy has been in operation in Delhi, India, since 2010 (Westinghouse Plasma Corporation, 2014) [84].
6.1.2. Gasification
Gasification is the controlled burning of PET and plastic trash at temperatures between 600 and 800 °C in the presence of air or occasionally purified oxygen, with the latter approach being more expensive. Syngas, a combination of gases created through this process, is the initial result, and can be utilized as a fuel alternative to natural gas or as a source of raw materials for the manufacture of petrochemicals. Anke-Brems et al. (2013) have discussed the many technologies that are involved in the process, and how frequently a fluidized bed gasifier is employed for the purpose [85,86]. It should be noted that PET waste was also included in the plastic waste used in the operations above. Two plants built by M/s Westinghouse Plasma Gasification, each with a 1.6 MW capacity and able to consume roughly 72 tons of garbage per day [87], are located in the Indian state of Maharashtra.
6.2. Material Recycling
6.2.1. PET Recycling Techniques
The first report on recycling PET bottle waste came from the USA in 1977 [88], and since then, numerous investigations on PET recycling techniques have been conducted [89,90,91]. Used PET is being recovered at a steadily higher rate. With a rate of about 19.5%, PET leads the list of materials recycled from all solid plastic trash [21].
Mechanical Recycling
Mechanical recycling, also known as material recycling, primarily involves the washing of PET waste, followed by its crushing and grinding to reduce its size into flakes, re-extrusion into fibers, and the processing of the same for the production of new PET goods. There are two mechanical recycling methods that are currently in vogue for the production of fibers from PET waste:
- A more common process for turning flakes into fibers is direct extrusion. Molten PET is extruded into a mold during the extrusion molding process, where it cools and solidifies to take the shape of the mold. For the production of huge products, PET extrusion molding is used. PET can be easily produced as an extruded material for bottles and jars;
- The melt-extrusion process performed at 280 °C, where PET flakes are extruded into pellets or granules and then melt-extruded into fibers for extrusion molding (Bottle to Bottle PET recycling). It is worth noting that PET can be easily produced in the form of extruded material for bottles and jars, and blowing manufacturing can also be used for jars. This suggests that the same manufacturing process can be used for both bottles and jars made from PET. Technologies and plants are also available for the bottle-to-bottle process, in which PET bottle flakes are directly converted and then blown to make a bottle in an integrated system.
Chemical Recycling
Chemical recycling involves converting the PET chain by chemical processes into oligomers and other compounds. This process can be divided into two main categories: depolymerization and functionalization. Depolymerization consists in breaking down the PET chain into its monomers, which can then be used to produce new PET. Functionalization, on the other hand, involves chemically modifying the PET chain to produce new chemicals with different properties and applications [92,93,94,95,96]. One example of depolymerization is the process of methanolysis, in which PET is dissolved in methanol and heated to produce a mixture of terephthalic acid and ethylene glycol, which can be recycled to produce new PET. Another example is the process of glycolysis, which involves using a glycolysis agent to break down the PET chain into its monomers.
Chemical recycling can help reduce plastic waste’s environmental impact, as it allows for the recovery and reuse of plastic materials, rather than relying on the traditional mechanical recycling process. However, it is an expensive process and requires specialized equipment and skilled personnel. It also poses a risk of pollution and the generation of harmful by-products.
In comparison to previous review papers on the management of polyethylene terephthalate (PET) plastic waste, our study offers several distinct contributions. While existing reviews have provided valuable insights into PET waste management practices, they often focus on general waste management techniques without a specific emphasis on PET waste, or lack a comprehensive analysis of regulatory frameworks and applications of recycled PET waste. In contrast, our study specifically concentrates on mechanical and chemical recycling methods for PET waste, exploring their advantages, limitations, and potential applications. Additionally, we delve into the rules and regulations implemented in different countries, shedding light on the varying approaches to PET waste management. Moreover, we examine the wide range of applications for recycled PET waste across industries such as textiles, packaging, and construction. These unique aspects differentiate our work from previous reviews and contribute to a more comprehensive understanding of PET waste management strategies [97].
Glycolysis is a chemical recycling process that involves the breaking down of PET into its constituent monomers, which are then used to produce new PET. This process typically involves heating the PET with a catalyst and a small amount of glycol, such as ethylene glycol or diethylene glycol. The glycol acts as a solvent and a reactant, helping to break down the PET and facilitate the formation of new monomers. The resulting monomers can then be purified and used to produce new PET. This method is found to be more environmentally friendly than traditional mechanical recycling methods (Table 5), as it allows for a greater degree of control over the final product and reduces the need for virgin raw materials. However, it is also more complex and costly, requiring specialized equipment and expertise [98].

Table 5.
Chemical recycling techniques of PET waste.
In glycolysis, PET waste is chemically reacted with a diol, such as ethylene glycol, at high temperatures. The reaction breaks down the PET polymer into its constituent monomers, ethylene glycol, and terephthalic acid. These monomers can then be purified and used as raw materials to produce new PET or other chemicals. The glycolysis of PET was first described in a patent in 1965 [115], and it involves the transesterification/glycolysis of a PET molecule with glycol and conversion of the same into a BHET molecule in the presence of transesterification catalysts. In this process, PET degradation is carried out using EG [116], DEG [117], propylene glycol, and di-propylene glycol [118] (Table 6). However, like glycolysis, a partial depolymerisation reaction resulting in an intermediate product, i.e., BHET and oligomers, can be used directly to produce new PET. It requires mild reaction conditions, such as atmospheric pressure and a temperature range of 180–250 °C. Therefore, attempts have been made for enhancing the rate of glycolysis and BHET monomer yield by use of efficient catalysts and the optimization of the reaction conditions, and these exertions have resulted in enhancements in BHET monomer yield and reaction time from 65% over 8 h to around 90%, and a reduced reaction time of about 30 min [118,119,120]. The most intensively studied method for increasing the glycolysis rate is the use of transesterification catalysts. Metal-based catalysts stimulate glycolysis reaction mechanisms [121,122].

Table 6.
Glycolysis de-polymerization of PET by numerous reactions (Raheem et al. 2019) [6].
6.3. Methods of Management of PET Waste
The management of PET waste involves various methods and strategies to minimize its environmental impact and promote sustainable practices. Here are some common methods of PET waste management:
- Recycling—Recycling is a key method for managing PET waste. PET bottles and other PET products can be collected, sorted, and processed through mechanical or chemical recycling methods to produce new PET products or other useful materials. Recycling helps to conserve resources, reduce energy consumption, and divert PET waste from landfills;
- Waste separation and collection—Effective waste separation and collection systems are essential for proper PET waste management. Establishing recycling programs, providing separate bins or containers for PET waste, and educating the public about the importance of recycling are crucial steps. Communities, businesses, and governments can work together to implement efficient waste collection systems;
- Extended producer responsibility (EPR)—EPR is an approach whereby producers or manufacturers take responsibility for the entire lifecycle of their products, including proper disposal and recycling. By implementing EPR programs, manufacturers of PET products can design their packaging to be more recyclable, support recycling infrastructure, and take part in the collection and recycling of PET waste;
- Waste-to-energy conversion—In cases where PET waste cannot be effectively recycled, waste-to-energy conversion methods can be considered. Technologies such as incineration or gasification can convert PET waste into energy sources like heat or electricity. However, it is important to ensure that these processes are conducted in an environmentally sound manner, and meet appropriate emissions standards;
- Education and awareness—Educating the public about the importance of proper PET waste management is crucial. Promoting awareness campaigns, providing information on recycling practices, and encouraging responsible consumer behavior can help in reducing PET waste generation and increasing recycling rates;
- Reducing single-use plastics—Addressing the root cause of PET waste involves reducing the consumption of single-use plastics. Encouraging the use of reusable alternatives, promoting sustainable packaging solutions, and supporting initiatives to phase out or limit single-use plastics can significantly reduce the amount of PET waste generated;
- Research and innovation—Continuous research and innovation are important for finding new and improved methods of PET waste management. This includes developing advanced recycling technologies, exploring new uses for recycled PET materials, and finding ways to optimize waste management processes.
Implementing a combination of these methods, along with policy support and collaboration between various stakeholders, can contribute to the effective management of PET waste and the transition to a more sustainable circular economy.
7. PET Recycling Techniques
PET waste can be recycled using both mechanical and chemical recycling techniques. Mechanical recycling involves collecting and sorting PET waste, followed by shredding, washing, melting, and reprocessing the waste into new plastic pellets or fibers. This method is widely used and helps to produce various PET-based products. On the other hand, chemical recycling utilizes different processes such as glycolysis, hydrolysis, methanolysis, or solvolytic depolymerization to break down PET waste into its monomer building blocks, or other valuable chemicals. Chemical recycling offers the potential to recover high-quality monomers for PET production and address challenges associated with complex PET products (Table 7). Continued research and development efforts are being made to optimize both mechanical and chemical recycling techniques for PET waste recycling.

Table 7.
Different glycolysis methods for PET recycling.
7.1. Mechanical Recycling
Mechanical recycling is the most common and widely employed method for recycling PET waste. It involves several steps:
- Collection—PET waste, such as used PET bottles, is collected through recycling programs, waste management systems, or dedicated collection points. The proper collection and segregation of PET waste are crucial for effective recycling;
- Sorting and cleaning—Collected PET waste undergoes sorting to separate it from other types of plastic and non-recyclable materials. The sorted PET waste is then thoroughly cleaned to remove contaminants like labels, caps, and residual liquids;
- Shredding and granulating—The cleaned PET waste is shredded into smaller pieces or flakes. The flakes are then further processed into granules or pellets. Shredding and granulation increase the surface area of the PET material, making it easier to handle during subsequent processing;
- Melting and purification—The PET flakes or granules are melted down to a liquid state in high-temperature extruders. During this process, any remaining impurities, such as dyes or additives, are filtered or removed. The purified molten PET is then cooled and solidified;
- Reprocessing—The solidified PET material is usually cut into small pellets or chips, which can be used as feedstock in various manufacturing processes. These processes can include injection molding, blow molding, or extrusion to produce a wide range of PET-based products, such as fibers, films, sheets, bottles, containers, and packaging materials.
7.2. Chemical Recycling
The following Table 7 represents various methods of glycolysis used for the treatment of polyethylene terephthalate (PET) plastic waste. This table highlights different approaches used in glycolysis, such as kinetics of PET waste glycolytic degradation, the microwave glycolysis depolymerization of PET, etc., and Table 8 compares them based on key parameters including reaction conditions, catalysts used, reaction time, yield of monomers and temperature. The table provides a comprehensive overview of the different methods of glycolysis, enabling a comparative analysis of their effectiveness and feasibility for PET plastic waste management.
Relative Advantages of Glycolysis over Methanolysis and Hydrolysis
Glycolysis of PET is an area of widespread research because of the advantages of this process over methanolysis and hydrolysis, which include its suppleness, simplicity, low capital costs [153], eco-friendliness, and high yield. It also has a lower reaction time, and the process can be simply adapted to the conventional plants used for PET production. It stands out as the best PET recycling process over the other methods for the reason that it is carried out in an extensive series of temperatures from 180 °C to 240 °C [118], and achieves the uppermost proficiency and eminence of the product when a catalyst is used [154,155]. Another additional benefit of glycolysis is that the BHET can be mixed with fresh BHET, and the combination can be used for other (DMT-based or TPA-based) PET production lines [105,115].
On the other hand, hydrolysis is comparatively easy compared to glycolysis and methanolsis. Among the three depolymerizing agents used in these three processes, i.e., water (hydrolysis), methanol (methanolysis), and ethylene glycol (glycolysis), water is the weakest nucleophile [8]. The other disadvantage of hydrolysis is the use of higher temperatures (200–250 °C) and pressures (1.4–2 MPa), besides a longer duration needed for depolymerization. Commercially, hydrolysis is not widely used to produce food-grade recycled PET because of the cost associated with the purification of the TPA produced during the process [140].
The main disadvantage of the methanolysis method is the high cost associated with the separation and refining of the mixture of the reaction products (glycols, alcohols, and phthalate derivatives). Furthermore, the water formed during the process causes a toxic effect on the catalyst, also causing the formation of various azeotropes. Also, with the existing inclination to use TPA, instead of DMT, as the raw material for the production of PET, the DMT produced by methanolysis must to be converted into TPA, which significantly adds to the cost of the methanolysis process [156].
Mechanical vs. Chemical Recycling.
The relative advantages and disadvantages of mechanical and chemical recycling are presented in Table 8.

Table 8.
Advantages and disadvantages of recycling methods.
Table 8.
Advantages and disadvantages of recycling methods.
| Mechanical Recycling | |
|---|---|
| Advantages | Disadvantages |
| Recycling PET by melt reprocessing is relatively simple, requires lower investments, utilizes established equipment [157,158], is flexible in relation to feedstock volume, and has little adverse environmental effect. |
|
| Chemical Recycling | |
| Advantages | Disadvantages |
|
|
8. Applications of Recycled PET (rPET) Products
Recycled PET goods have found numerous uses and are being employed in place of virgin PET. Table 9 indicates broad areas of applications of rPET [161], and as may be seen, large quantities of rPET are used to produce fibers.

Table 9.
Application of rPET flakes.
Table 10 presents some details of the application areas where products obtained from PET recycling are being used worldwide.

Table 10.
Applications of products obtained from PET recycling.
9. Governmental Regulations for the Management of PET and Plastic/Waste
Keeping in view the growing volumes of plastic waste, concerted efforts were initiated long ago to manage plastic waste in many countries by formulating legal guidelines. As PET waste is a part of plastic waste, no separate guidelines for PET waste have been made. The Bureau of Indian Standards (BIS) issued an Indian Standard IS: 14534 in 2016 recommending different ways of plastic waste recycling, and suggested appropriate types of end-products that can be developed from plastic waste recycling (IS:14534, 2016) [186]. The Waste Framework Directive, revised in 2008, streamlined waste legislation, integrating rules on issues such as the management of dangerous waste and waste oils. The salient details of codal provisions for the management of MSW (plastic waste) set out in some countries are summarized in Table 11.

Table 11.
Codes and regulations on MSW/plastic/PET waste in some of the countries.
The discussion of this paper delves into the findings and insights generated by exploring the current techniques and approaches used for the management of polyethylene terephthalate (PET) plastic waste. The research question, “What are the current techniques and approaches used for the management of PET plastic waste, with a specific focus on mechanical and chemical recycling methods, rules and regulations in different countries, and applications of recycled PET waste?” guided our investigation and analysis.
Our examination of mechanical recycling methods revealed the significance of sorting, cleaning, and reprocessing techniques in recovering PET waste for reuse. We identified the advantages of mechanical recycling, such as its potential to reduce environmental impact, conserve resources, and contribute to a circular economy. However, challenges such as contamination, degradation, and limited recycling capacity were also noted, indicating the need for continuous improvement and innovation in this area.
The exploration of chemical recycling methods, including glycolysis, pyrolysis, and depolymerization, highlighted their potential for use in converting PET waste into valuable raw materials or feedstocks. These methods offer opportunities for closed-loop recycling and the production of high-quality materials, contributing to a more sustainable PET waste management system. Nonetheless, technological advancements, scalability, and cost-effectiveness remain areas for further research and development.
The discussion also assessed the rules and regulations implemented in different countries, underscoring the importance of policy frameworks in shaping PET waste management practices. Variations in regulations across nations were observed, reflecting diverse approaches to addressing PET waste challenges. Sharing best practices, harmonizing standards, and promoting international collaboration are essential for effective global PET waste management.
Furthermore, the applications of recycled PET waste in various fields were explored, highlighting the potential for utilizing these materials in sectors such as textile manufacturing, packaging, and construction. The adoption of recycled PET in these industries can contribute to resource conservation, waste reduction, and the development of sustainable products.
Overall, this discussion emphasizes the importance of an integrated approach to PET waste management, combining mechanical and chemical recycling methods, while considering the regulatory landscape and exploring diverse applications. It underscores the need for continued research, innovation, and collaboration among stakeholders to achieve a more sustainable and efficient PET waste management system on a global scale.
10. Conclusions
This article presents an overview of the current status of plastic production and consumption, and the share of PET in the same. The focus is on plastic waste disposal and management challenges, particularly for single-use PET items. The article highlights the various regulations in place in different countries for the disposal of Municipal Solid Waste (MSW) and plastic waste, and the methods of waste management that are sustainable and unsustainable. The article also reviews the modern methods of waste disposal that are being adopted worldwide and in India, such as mechanical and chemical recycling. Mechanical recycling, which includes washing, grinding, and pelletizing, is in extensive use due to its simplicity, established technical know-how, and lower investment. Although less popular, chemical recycling yields numerous chemical products that can be used in various applications. Thermal–mechanical recycling methods are also employed to generate electrical energy and fuel gas from plastic waste. The article emphasizes the importance of promoting sustainable development by making stringent regulations for dealing with PET and plastic waste. It also underlines the need for industries that produce this waste to take responsibility for its recycling and reuse, as it can be a profitable business. The end products of recycled PET are used in a wide variety of fields, such as cement concrete, the textile industry, the automobile industry, etc. The installation of new PET recycling plants or the augmentation of the capacity of existing plants is taking place all over the world, and PET recycling is becoming a big industry by itself.
This study has explored and analyzed the current techniques and approaches used for the management of polyethylene terephthalate (PET) plastic waste. We have gained valuable insights into PET waste management practices. The examination of mechanical and chemical recycling methods, along with the discussion of rules and regulations in different countries, and the applications of recycled PET waste, provides a comprehensive understanding of the subject matter. This study emphasizes the need for integrated waste management strategies and sustainable practices to address the growing environmental concerns associated with PET plastic waste. The findings presented in this paper contribute to the existing knowledge in the field, and provide recommendations by which policymakers and waste management stakeholders can develop effective and sustainable PET waste management solutions.
In conclusion, the management and recycling of single-use PET and plastic waste is a complex issue that requires cooperation from governments, industry, and individuals. Through continued research and development, increased collection and sorting efforts, the promotion of the use of recycled materials, and the development of more efficient and cost-effective recycling methods, it is possible to improve the recycling rate and reduce the environmental impact of plastic waste. PET (polyethylene terephthalate) is a widely used packaging material due to its non-reactivity, durability, and shatterproof properties. However, this has led to an increase in PET waste generation, particularly in the form of single-use items. Recycling PET waste into useful products is considered an eco-friendly and sustainable method of waste management.
The current scenario of recycling techniques and management for single-use PET and plastic waste is a mixed one, with challenges such as contamination and a lack of proper infrastructure. However, there have been several efforts to improve the recycling of single-use PET and plastic waste, including the development of new recycling technologies, increasing collection and sorting, promoting the use of recycled materials, and improving awareness and education. Additionally, more efficient and cost-effective methods, such as chemical recycling, bioplastics, and proper recycling infrastructure, are needed to effectively manage and recycle single-use PET and plastic waste. Therefore, cooperation between governments, industry, and individuals is crucial to ensure the sustainable management of PET waste and to promote the use of recycled materials.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to the Directors of CSIR—Central Institute of Mining and Fuel Research (CSIR-CIMFR) and CRRI—Central Road Research Institute (CSIR-CRRI) for their support and permission to submit this paper. They would also like to thank S. K. Sudip Maity (Coordinator of AcSIR-CIMFR) for his constant inspiration and guidance. The authors acknowledge and appreciate the financial support provided by the University Grants Commission (UGC) in New Delhi, India, which allowed them to carry out this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BRICS—Brazil, Russia, India, China and South Africa; BHET—Bis-2,hydroxy-ethylene terephthalate; CAGR—Compound Annual Growth Rate; CPCB—Central Pollution Control Board; DMT—dimethyl terephthalate; DEG—diethylene glycol; DSEWPC—Department of Sustainability, Environment, Water, Population and Communities; EG—ethylene glycol; EPA—Environmental Protection Agency; FMCG—fast-moving consumer goods; GBI—Global Business Intelligence; HDPE—high-density polyethylene; kt—kilotons; PS/EPS—polystyrene/expanded polystyrene; MMT—million metric tons; MT—million tons; PET—poly(ethylene terephthalate); NAPCOR—National Association for PET Container Resources; PP—polypropylene; PVC—poly(vinyl chloride); rPET—recycled PET; RCRA—Resource Conservation and Recovery Act; TERI—The Energy Research Institute; TPA—terephthalic acid; TPD—tons per day.
References
- Carraher, C.E. Polymer Chemistry, 5th ed.; McGraw-Hill Book Company, Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Welle, F. Twenty years of PET bottle to bottle recycling-An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Helms, B.A.; Russell, T.P. Reaction: Polymer chemistries enabling cradle-tocradle life cycles for plastics. Chem 2016, 1, 816–818. [Google Scholar] [CrossRef]
- Demirel, B.; Yaraş, A.; Elçiçek, H. Crystallization behavior of PET materials. BalıkesirÜniversitesi Fen Bilim. 2016, 13, 26–35. [Google Scholar]
- Leng, Z.; Padhan, R.K.; Sreeram, A. Production of a sustainable paving material through chemical recycling of waste PET into crumb rubber modified asphalt. J. Clean. Prod. 2018, 180, 682–688. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Irwan, J.; Asyraf, R.; Othman, N.; Koh, H.; Annas, M.M.K.; Faisal, S. The mechanical properties of PET fiber reinforced concrete from recycled bottle wastes. Adv. Mater. Res. 2013, 795, 347–351. [Google Scholar]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.H.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egyptian J. Petrol. 2016, 25, 53–64. [Google Scholar] [CrossRef]
- Coherent Market Insight: Polyethylene-Terephthalate (PET) Market Analysis. Burlingame CA, United States. Available online: https://www.coherentmarketinsights.com/market-insight/polyethylene-terephthalate-market-279 (accessed on 10 July 2023).
- Thompson, R.; Swan, S.; Moore, C.; Vom Saal, F.S. Our plastic age. Phil. Trans. R. Soc. B 2009, 364, 1973–1974. [Google Scholar] [CrossRef]
- Cischem. Com Co., Ltd. Publication. The World Market Analysis of PET Value Chain; Cischem. Com Co., Ltd. Publication: New Delhi, India, 2010. [Google Scholar]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- The Size of the Global Polyethylene Terephthalate (PET) Resin Market Was Estimated at USD 80.9 Million in 2021 and Is Anticipated to Increase from USD 85.11 Million in 2022 to USD 127.67 Million by 2030, Expanding at a CAGR of 5.2% during the Forecast Period (2023–2030). Available online: https://www.skyquestt.com/report/polyethylene-terephthalate-pet-resin-market (accessed on 28 February 2023).
- Polyethylene Terephthalate (PET) Market Size & Share Analysis-Growth Trends & Forecasts Up To 2029. Available online: https://www.mordorintelligence.com/industry-reports/polyethylene-terephtalate-market (accessed on 10 July 2023).
- Market Research Report. Available online: https://www.fortunebusinessinsights.com/industry-reports/polyethylene-terephthalate-pet-market-101743 (accessed on 30 April 2023).
- Mordor Intelligence: Asia-Pacific Polyethylene Terephthalate (Pet) Market Trends. Available online: https://www.mordorintelligence.com/industry-reports/asia-pacific-polyethylene-teraphtalate-pet-market/market-trends (accessed on 10 July 2023).
- Lohr, A.; Savelli, H.; Beunen, R.; Kalz, M.; Ragas, A.; Belleghem, F.V. Solutions for global marine litter pollution. Curr. Opin. Environ. Sustain. 2017, 28, 90–99. [Google Scholar] [CrossRef]
- Phan, A. How We Can Turn Plastic Waste into Green Energy. Available online: https://theconversation.com/how-we-can-turn-plastic-waste-into-green-energy-104072 (accessed on 1 December 2018).
- Schlanger, Z. The World Will Finally Have to Confront Its Massive Plastic Problem Now That China Won’t Handle It. Recycling. Available online: https://getpocket.com/explore/item/the-world-will-finally-have-to-confront-its-massive-plastic-problem-now-that-china-won-t-handle-it (accessed on 20 June 2018).
- EPA. Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2012; Environmental Protection Agency, Office of Solid Waste and Emergency Response: Washington, WA, USA, 2014.
- EPA. Advancing Sustainable Materials Management: Facts and Fig. 2013; USEPA: Washington, DC, USA, 2015.
- Wang, T. Generation of Municipal Solid Waste Worldwide in 2017, by Select Country (in Million Metric Tons). Available online: https://www.statista.com/statistics/916749/global-generation-of-municipal-solid-waste-by-country (accessed on 25 September 2018).
- Seetharaman, G.; Bureau, E.T. India Wants to Double Consumption of Cheap Material in 5 Yrs, What About Its Plastic Waste. The Economic Times, 25 June 2017. [Google Scholar]
- Ramadevi, K.; Manju, R. Experimental investigation on the properties of concrete with plastic PET (Bottle) fibres as fine aggregates. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 42–46. [Google Scholar]
- CPCB. Material on Plastic Waste Management, June 2012; CPCB: New Delhi, India, 2012. [Google Scholar]
- Sampathkumar, Y. Plastic Bans Spread in India. Winners and Losers Aren’t Who You’d Expect. Available online: https://www.researchgate.net/publication/340244918_Single-use_Plastic_Ban_and_its_Public_Health_Impacts_A_Narrative_Review (accessed on 8 February 2019).
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef] [PubMed]
- Carey, J. On the brink of a recycling revolution? Proc. Natl. Acad. Sci. USA 2017, 114, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Deng, Y.; Li, S.; Yu, K.; Liu, Y.; Shang, M.; Wang, J.; Shu, J.; Sun, Z.; Chen, M.; et al. Waste Electrical and Electronic Equipment Reutilization in China. Sustainability 2021, 13, 11433. [Google Scholar] [CrossRef]
- PWMI. Introduction of Plastic Recycling in Japan; Plastic Waste Management Institute: Tokyo, Japan, 2013. [Google Scholar]
- The Coca-Cola Company. Coca-Cola Produces world’s First PET Bottle Made Entirely from Plants. Available online: https://www.businesswire.com/news/home/20150603005726/en/Coca-Cola-Produces-World%E2%80%99s-First-PET-Bottle-Made-Entirely-from-Plants (accessed on 4 November 2016).
- Parsons, H. PET Supply Chain Must Innovate to Boost Recycling, Meet Demand: NAPCOR. Available online: https://www.beveragedaily.com/Article/2015/02/12/PET-packaging-recycling-NAPCOR-bale-yield-utilization (accessed on 12 February 2015).
- Lamb, G. Engineering Polymers: Recycling and Post-Consumer Waste. Strategic Business Insights. Available online: https://www.strategicbusinessinsights.com//about/featured/2013/2013-06-post-consumer-waste.shtml (accessed on 30 June 2013).
- Afaqs. Available online: http://www.afaqs.com/news/company_briefs/?id=53021_Bisleri+installs+PET+bottles+recycling+machine+at+Inorbit+Mall (accessed on 14 February 2012).
- VDMA. Market Research Report on Plastic Waste Management Practices &Current Status of Plastic Recycling in India. Available online: https://www.scribd.com/document/375442539/2016-07-27-Market-Research-Report-on-Plastic-Waste-Study (accessed on 31 December 2017).
- Naik, Y. India just Banned All Forms of Disposable Plastic in Its Capital; Mumbai Mirror: Mumbai, India, 2017. [Google Scholar]
- The Himachal Pradesh Non-Biodegradable Garbage (Control) Act. 1995. Available online: https://www.indiacode.nic.in/bitstream/123456789/5300/1/the_himachal_pradesh_non-biodegradable_garbage.pdf (accessed on 10 July 2023).
- The Punjab Plastic Carry Bags (Manufacture, Usage & Disposal) Control (Amendment) Act, 2016. (Punjab Act. No. 21 of 2016). Available online: https://www.indianemployees.com/acts-rules/details/punjab-plastic-carry-bags-manufacture-usage-and-disposal-control-amendment-act-2016 (accessed on 10 July 2023).
- NDTV (India). Haryana Bans Use of Plastic Carry Bags; NDTV: Haryana, India, 2010. [Google Scholar]
- Plastics Europe. Plastics–The Facts 2015 An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2015-Plastics-the-facts.pdf (accessed on 16 December 2015).
- Annepu, R.J. Sustainable Solid Waste Management in India. Master’s Thesis, Columbia University, New York, NY, USA, 2012. [Google Scholar]
- Antrekowitsch, H.; Potesser, M.; Spruzina, W.; Prior, F. Metallurgical recycling of electronic scrap. In Proceedings of the 135th The Minerals, Metals and Materials Society (TMS) Annual Meeting and Exhibition, San Antonio, TX, USA, 12–16 March 2006. [Google Scholar]
- Plastic Waste (Management and Handling) Rules. 2011. Available online: https://thc.nic.in/Central%20Governmental%20Rules/Plastic%20Waste%20Management%20Rules,%202016.pdf (accessed on 18 March 2016).
- The National Environmental Act No. 47 of 1980. Available online: https://leap.unep.org/countries/lk/national-legislation/national-environmental-act-1980-no-47-1980 (accessed on 1 January 1980).
- Gjerde, K.M. Ecosystems and Biodiversity in Deep Waters and High Seas; UNEP Regional Seas Reports and Studies No. 178; UNEP/IUCN: Geneva, Switzerland, 2006; Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/13602/rsrs178.pdf (accessed on 10 July 2023).
- Ellis, S.; Kantner, S.; Saab, A.; Watson, M. Plastic Grocery Bags: The Ecological Footprint. VIPIRG, Victoria, 1–19. Available online: https://www.yumpu.com/en/document/view/35448984/plastic-grocery-bags-the-ecological-footprint-vipirg (accessed on 22 December 2005).
- Coyle, K. Environmental Literacy Council: Paper or Plastic? Available online: http://www.enviroliteracy.org/article.php/1268.html/pdf (accessed on 20 November 2005).
- Johnson, K.A.; Victor, N.B.; Trinity, A.T. Use of waste plastic materials for road construction in GHANA. Case Stud. Constr. Mater. 2017, 6, 1–7. [Google Scholar]
- Prasad, K.V.R.; Mahendra, S.P.; Kumar, N.S.; Rakesh, S.G.; Vijay, V.; Likith, T.; Yogesh, B.P. Study on utilization of waste plastic in bituminous mixes for road construction. In Proceedings of the International Conference on Futuristic Innovations and Developments in Civil Engineering (ICFiDCe), Sivakasi, Tamil Nadu, India, 18 April 2013; pp. 198–203. [Google Scholar]
- Kumar, K.N.; Rajakumara, H.N. Study of using waste rubber tyres in construction of bituminous road. Int. J. Sci. Eng. Res. 2016, 7, 23–27. [Google Scholar]
- Pedro, D.; Brito, d.J.; Veiga, R. Mortars Made with Fine Granulate from Shredded Tires. J. Mater. Civ. Eng. 2013, 25, 519–529. [Google Scholar] [CrossRef]
- Zhang, S.; Forssberg, E.; Arvidson, B.; Moss, W. Separation mechanism and criteria of a rotating drum eddy-current separator operation. Resour. Conserv. Recycl. 1999, 25, 215–232. [Google Scholar] [CrossRef]
- Lee, J.K.; Shin, J.H. Triboelectrostatic Separation of PVC Materials from Mixed Plastics for Waste Plastic Recycling. Korean J. Chem. Eng. 2002, 19, 267–272. [Google Scholar] [CrossRef]
- Dodbiba, G.; Shibayama, A.; Miyazaki, T.; Fujita, T. Electrostatic separation of the shredded plastic mixtures using a tribo-cyclone. Phys. Sep. Sci. Eng. 2001, 11, 63–92. [Google Scholar] [CrossRef]
- Arai, S.; Ito, S.; Oi, E.; Yotsumoto, H.; Kikuchi, E.; Sakamoto, H. Study of the dry separation of plastics using a column-type air separator. In Proceedings of the Annual Meeting of MIMIJ, Japan; 1995; p. 110. [Google Scholar]
- Ito, S.; Hasuda, T.; Arai, S. Plastics separation by pneumatic separator using differentiated acceleration-deceleration zone. In Proceedings of the 5th International Symposium on East Asian Recycling Technology, Tsukuba, Japan, 15–17 June 1999; Volume 273–276, pp. 15–17. [Google Scholar]
- Nakajima, J.; Nakazawa, H.; Sato, H.; Kudo, Y. Study on air separation of PVC and PET. J. Mini. Mater. Proc. Inst. Jpn. 2001, 117, 123–126. [Google Scholar]
- Dodbiba, G.; Fujita, T. Progress in Separating Plastic Materials for Recycling. Phys. Sep. Sci. Eng. 2004, 13, 165–182. [Google Scholar] [CrossRef]
- Williams, P.C. Implementation of Near-Infrared Technology. In Near-Infrared Technology in the Agricultural and Food Industries, 2nd ed.; Williams, P.C., Norris, K., Eds.; American Association of Cereal Chemists: Paul, MN, USA, 2001. [Google Scholar]
- Lang, G. New on-line process analyzers expand NIR capabilities. Instrum. Control. Syst. 1991, 64, 99–102. [Google Scholar]
- Pickuth, A. Advanced in bridging the gap between laboratory and process on- and in-line NIR spec-trometry of liquids by the combined technologies of AOTF (acousto-optic tunable filter) remote sensing probes with fiber optics, sampling point multiplexing, and uses of chemometric software packages. In Near Infrared Spectrometry; Horwood: Chichester, UK, 1992; pp. 153–158. [Google Scholar]
- Fuller, M.P.; Meyers, M.E. Online FT-MIR/NIR process analysis. Microchem. Acta 1988, 1, 31–34. [Google Scholar] [CrossRef]
- Pascoe, R.; O’Connell, B. Development of a method for separation of PVC and PET using flame treatment and flotation. Miner. Eng. 2003, 16, 1205–1212. [Google Scholar] [CrossRef]
- Kassouf, A.; Maalouly, J.; Rutledge, D.N.; Chebib, H.; Ducruet, V. Rapid discrimination of plastic packaging materials using MIR spectroscopy coupled with independent components analysis (ICA). Waste Manag. 2014, 34, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.M.; Masoumi, H.; Mirian, S.S.; Tabrizchi, M. Sorting of polypropylene resins by color in MSW using visible reflectance spectroscopy. Waste Manag. 2010, 29, 2209–2213. [Google Scholar] [CrossRef]
- Sarranti, S.; Gargiulo, A.; Bonifazi, G. The utilization of hyperspectral imaging for impurities glass contaminants detection. Waste Manag. Res. 2010, 24, 48–59. [Google Scholar]
- Florestan, J.; Lachambre, A.; Mermilliod, N.; Boulou, J.C.; Marfisi, C. Recycling of plastics: Automatic identification of polymers by spectroscopic methods. Resour. Conser. Recycl. 1994, 10, 67–741. [Google Scholar] [CrossRef]
- Tamon, T.; Fujii, S.; Inada, K.; Motomura, K.; Nishihara, I.; Fujita, T. A proposal of plastic sensing by infrared absorption using laser diode. In Proceedings of the 16th SICE (The Society of Instrument and Control Engineering) Symposium on Sensing Forum, Japan, 1999; pp. 147–152. Available online: https://dokumen.tips/documents/identification-of-plastics-by-infrared-absorption-using-ingaasp-laser-diode.html?page (accessed on 10 July 2023).
- Inada, K.; Matsuda, R.; Fujiwara, C.; Nomura, M.; Tamon, T.; Nishihara, I.; Takao, T.; Fujita, T. Identification of plastics by infrared absorption using InGaAsp laser diode. Resour. Conser. Recycl. 2001, 33, 131–146. [Google Scholar] [CrossRef]
- Marques, G.A.; Tenório, J.A.S. Use of froth flotation to separate PVC/PET mixtures. Waste Manag. 2000, 20, 265–269. [Google Scholar] [CrossRef]
- Pascoe, R.D. Sorting of plastics using physical separation techniques. Recycling and reuse of waste materials. In Proceedings of the International Symposium. 2003; pp. 173–188. Available online: https://www.icevirtuallibrary.com/doi/abs/10.1680/rarowm.32521.0017 (accessed on 10 July 2023).
- Saisinchai, S. Separation of PVC from PET/PVC mixtures using flotation by calcium lignosulfonate depressant. Eng. J. 2013, 18, 45–54. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E.; Pugh, R.J. Selective flotation separation of plastics by particle control. Resour. Conser. Recycl. 2001, 33, 37–50. [Google Scholar] [CrossRef]
- Pongstabodee, S.; Kunachitpimol, N.; Damronglerd, S. Combination of three-stage sink-float method and selective flotation technique for separation of mixed post-consumer plastic waste. Waste Manag. 2008, 28, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Dodbiba, G.; Haruki, N.; Shibayama, A.; Miyazaki, T.; Fujita, T. Combination of sink–float separation and flotation technique for purification of shredded PET bottle from PE or PP flakes. Int. J. Miner. Process. 2002, 65, 11–29. [Google Scholar] [CrossRef]
- Kangal, M.O. Selective flotation technique for separation of PET and HDPE used in drinking water bottles. Miner. Process. Extr. Metall. Rev. 2010, 31, 214–223. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.Q.; Fu, J.G.; Gu, G.H. Flotability and flotation separation of polymer materials modulated by wetting agents. Waste Manag. 2014, 34, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Conver. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Lopez-Fonseca, R.; Duque-Ingunza, I.; De Rivas, B.; Flores-Giraldo, L.; Guti_errez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, E.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, K.C. Plasma Arc Gasification for Waste Management. Available online: https://www.yumpu.com/en/document/view/7381120/plasma-arc-gasification-for-waste-management-electronics-for-you (accessed on 16 February 2009).
- CPCB. Study on Plastic Waste Disposal through “Plasma Pyrolysis Technology”; CPCB: New Delhi, India, 2016. [Google Scholar]
- Westinghouse Plasma Corporation: Westinghouse Plasma Gasification, Hazardous Waste Management. Available online: https://d3pcsg2wjq9izr.cloudfront.net/files/41035/download/449710/ANRG_Energy-Evolved_Brochure.pdf (accessed on 15 July 2014).
- Brems, A.; Baeyens, J.; Vandecasteele, C.; Dewil, R. Polymeric Cracking of Waste Polyethylene Terephthalate to Chemicals and Energy. J. Air Waste Manag. Assoc. 2011, 61, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of plastic waste as waste-to-energy or waste-to-syngas recovery route. Nat. Sci. 2013, 5, 695–704. [Google Scholar] [CrossRef]
- Matsunami, J.; Yoshida, S.; Yokota, O.; Nezuka, M.; Tsuji, M.; Tamaura, Y. Gasification of Waste Tyre and Plastic (PET) by Solar Thermochemical Process for Solar Energy Utilization. Sol. Energy 1999, 65, 21–23. [Google Scholar] [CrossRef]
- Miller, C. Polyethylene Terephthalate. Waste Age. 2002, 33, 102–106. [Google Scholar]
- Helwani, Z.; Othman, M.; Aziz, N.; Kim, J.; Fernando, W. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Patterson, J. Continuous Depolymerization of Poly (Ethylene Terephthalate) via Reactive Extrusion. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2007. [Google Scholar]
- Yoshioko, T.; Motoki, T.; Okuwaki, A. Kinetics of hydrolysis of poly (ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 2001, 40, 75–79. [Google Scholar] [CrossRef]
- Carta, D.; Cao, G.; D’Angeli, C. Chemical recycling of poly (ethylene terephthalate) (PET) by hydrolysis and glycolysis. Environ. Sci. Pollut. Res. 2003, 10, 390–394. [Google Scholar] [CrossRef]
- Goje, A.S.; Mishra, S. Chemical kinetics, simulation, and thermodynamics of glycolytic depolymerization of poly (ethylene terephthalate) waste with catalyst optimization for recycling of value added monomeric products. Macromol. Mater. Eng. 2003, 288, 326–336. [Google Scholar] [CrossRef]
- Yamaye, M.; Hashime, T.; Yamamoto, K. Chemical recycling of poly (ethylene terephthalate). 2. Preparation of terephthalohydroxamic acid and terephthalohydrazide. Ind. Eng. Chem. Res. 2002, 41, 3993–3998. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, C.Y.; Lo, Y.; Mao, C.; Liao, W.T. Studies of glycolysis of poly (ethylene terephthalate) recycled from postconsumer soft-drink bottles. II. Factorial experimental design. J. Appl. Polym. Sci. 2001, 80, 956–962. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, C.Y.; Lo, Y.W.; Mao, C.F.; Liao, W.T. Studies of glycolysis of poly (ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. Influences of glycolysis conditions. J. Appl. Polym. Sci. 2001, 80, 943–948. [Google Scholar] [CrossRef]
- Thachnatharen, N.; Shahabuddin, S.; Sridewi, N. The Waste Management of Polyethylene Terephthalate (PET). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1127, 012002. [Google Scholar] [CrossRef]
- Raheem, A.B.; Hassan, A.; Noor, Z.Z.; Samsudin, S.; Abd-Hamid, M.; Bello, A.; Oladokun, O.; Sabeen, A.H.; Shamiri, A. Process simulation of bis (2-hydroxyethyl) terephthalate and its recovery using two stage evaporation systems. Chem. Eng. Trans. 2018, 63, 655–660. [Google Scholar]
- Ikladious, N.E. Recycling of poly (terephthalate): Identification of glycolysis product. J. Elastomers Plast. 2000, 32, 140–151. [Google Scholar] [CrossRef]
- Pardal, F.; Tersac, G. Kinetics of poly (ethylene terephthalate) glycolysis by diethylene glycol. Part II: Effect of temperature, catalyst and polymer morphology. Polym. Degrad. Stab. 2007, 92, 611–616. [Google Scholar] [CrossRef]
- Pardal, F.; Tersac, G. Reactivity of polyesters in glycolysis reactions: Unexpected effect of the chemical structure of the polyester glycolic unit. Polym. Degrad. Stab. 2006, 91, 2809–2812. [Google Scholar] [CrossRef]
- Subramian, P.M. Plastics recycling and waste management in the US. Resour. Conser. Recycl. 2000, 28, 253–263. [Google Scholar] [CrossRef]
- Cakić, S.; Ristić, I.; M-Cincović, M.; Nikolić, N.; Ilić, O.; Stojiljković, D.; B-Simendić, J. Glycolyzed products from PET waste and their application in synthesis of polyurethane dispersions. Prog. Org. Coat. 2012, 74, 115–124. [Google Scholar] [CrossRef]
- Karayannidis, G.; Nikolaidis, A.; Sideridou, I.; Bikiaris, D.; Achilias, D. Chemical recycling of PET by glycolysis: Polymerization and characterization of the dimethacrylatedglycolysate. Macromol. Mater. Eng. 2006, 291, 1338–1347. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical Recycling of Poly (ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146. [Google Scholar] [CrossRef]
- Grause, G.; Kaminsky, W.; Fahrbach, G. Hydrolysis of poly (ethylene terephthalate) in a zfluidized bed reactor. Polym. Degrad. Stab. 2004, 55, 571–575. [Google Scholar] [CrossRef]
- Rosmaninho, M.G.; Jardim, E.; Moura, F.C.C.; Ferreira, G.L.; Thom, V.; Yoshida, M.I.; Araujo, M.H.; Lago., R.M. Surface hydrolysis of postconsumer polyethylene terephthalate to produce adsorbents for cationic contaminants. J. Appl. Polym. Sci. 2006, 102, 5284–5291. [Google Scholar] [CrossRef]
- Sirek, M.; Jirousek, J. The Method of Chemical Recycling of Polyethylene Terephthalate. Waste. Patent WO/2001/068581, 20 September 2001. [Google Scholar]
- Goto, M.; Koyamoto, H.; Kodama, A.; Hirose, T.; Nagaoka, S. Depolymerization of polyethylene terephthalate insupercritical methanol. J. Phys. Condens. Matter 2002, 14, 11427–11430. [Google Scholar] [CrossRef]
- Spychaj, T.; Fabryey, E.; Spychaj, S.; Kacperski, M. Aminolysis and aminoglycolysis of waste poly (ethylene terephthalate). J. Mater. Cycles. Waste Manag. 2001, 3, 24–31. [Google Scholar]
- Myren TH, T.; Stinson, T.A.; Mast, Z.J.; Huntzinger, C.G.; Luca, O.R. Chemical and Electrochemical Recycling of End-Use Poly(ethylene terephthalate) (PET) Plastics in Batch, Microwave and Electrochemical Reactors. Molecules 2020, 25, 2742. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.R.; Harad, A.M. Aminolysis of polyethylene terephthalate waste. Polym. Degrad. Stab. 2006, 91, 1850–1854. [Google Scholar] [CrossRef]
- Hoang, C.N.; Dang, Y.H. Aminolysis of poly (ethylene terephthalate) waste with ethylenediamine and characterization of α, ω-diamine products. Polym. Degrad. Stab. 2003, 98, 697–708. [Google Scholar] [CrossRef]
- Jain, A.; Soni, R.K. Spectroscopic investigation of end products obtained by ammonolysis of poly (ethylene terephthalate) waste in the presence of zinc acetate as a catalyst. J. Polym. Res. 2007, 14, 475–481. [Google Scholar] [CrossRef]
- McDowell, J.T.; Klusio, N.C. Process for Reclaiming Linear Terephthalate Polyester. U.S. Patent 3222299, 7 December 1965. [Google Scholar]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical recycling of PET wastes with different catalysts. Int. J. Polym. Sci. 2015, 2015, 124524. [Google Scholar] [CrossRef]
- El-Mejjatti, A.; Harit1, T.; Riahi, A.; Khiari, R.; Bouabdallah, I.; Malek, F. Chemical recycling of poly (ethylene terephthalate). Application to the synthesis of multiblock copolyesters. Express Polym. Lett. 2014, 8, 544–553. [Google Scholar] [CrossRef]
- Bartolome, L.; Imran, M.; Cho, B.G.; Al-Masry, W.A.; Kim, D.H. Recent Developments in the Chemical Recycling of PET, In Material Recycling–Trends and Perspectives; Achilias, D., Ed.; INTECH Open Access: Rijeka, Croatia, 2012; pp. 65–84. [Google Scholar]
- Imran, M.; Kim, D.H.; Al-Masry, W.A.; Mahmood, A.; Hassan, A.; Haider, S.; Ramay, S.M. Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly(ethylene terephthalate) via glycolysis. Polym. Degrad. Stab. 2013, 98, 904–915. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value-added textiles. Fash. Text. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M. Glycolysis of polyethylene terephthalate waste. J. Appl. Polym. Sci. 2005, 97, 513–517. [Google Scholar] [CrossRef]
- Pingale, N.; Palekar, V.; Shukla, S. Glycolysis of postconsumer polyethylene terephthalate waste. J. Appl. Polym. Sci. 2010, 115, 249–254. [Google Scholar] [CrossRef]
- Shukla, S.; Kulkarni, K. Depolymerization of Poly (ethylene terephthalate) waste. J. Appl. Polym. Sci. 2002, 85, 1765–1770. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R.; Gitsov, I. A novel catalyst for the glycolysis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 1148–1152. [Google Scholar] [CrossRef]
- Xi, G.; Lu, M.; Sun, C. Study on depolymerization of waste poly (ethylene terephthalate) into monomer of bis (2-hydroxyethyl terephthalate). Polym. Degrad. Stab. 2005, 87, 117–120. [Google Scholar] [CrossRef]
- Shukla, S.; Palekar, V.; Pingale, N. Zeolite Catalyzed Glycolysis of Poly (ethylene terephthalate) Bottle Waste. J. Appl. Polym. Sci. 2008, 110, 501–516. [Google Scholar] [CrossRef]
- Lopez-Fonseca, R.; Duque-Ingunza, I.; De Rivas, B.; Arnaiz, S.; Gutierrez-Ortiz, J.I. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym. Degrad. Stab. 2010, 95, 1022–1028. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Li, Z.; Lu, X.; Zhang, S. Investigation of solid catalysts for glycolysis of polyethylene terephthalate. Chem. Eng. J. 2012, 185–186, 168–177. [Google Scholar] [CrossRef]
- Bartolome, L.; Imran, M.; Lee, K.G.; Sangalang, A.; Ahn, J.K. Superparamagnetic g-Fe2O3 nanoparticles as an easily recoverable catalyst for the chemical recycling of PET. Green Chem. 2014, 16, 279–286. [Google Scholar] [CrossRef]
- Aguado, A.; Martinez, L.; Becerra, L.; Arieta-Araunabena, M.; Arnaiz, S.; Asueta, A.; Robertson, I. Chemical zdepolymerization of PET complex waste: Hydrolysis vs. glycolysis. J. Mater. Cycles Waste Manag. 2014, 16, 201–210. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, Y.; Lu, X.; Zhang, S. First-row transition metal-containing ionic liquids as highly active catalysts for the glycolysis of poly (ethylene terephthalate) (PET). ACS Sustain. Chem. Eng. 2015, 3, 340–348. [Google Scholar] [CrossRef]
- Eshaq, G.; Elmetwally, A.E. (MgeZn)eAl layered double hydroxide as a regenerable catalyst for the catalytic glycolysis of polyethylene terephthalate. J. Mol. Liq. 2016, 214, 1–6. [Google Scholar] [CrossRef]
- Alzuhairi, M.; Khalil, B.; Hadi, R. Nano ZnO Catalyst for Chemical Recycling of Polyethylene terephthalate (PET). Eng. Technol. J. 2017, 35, 831–837. [Google Scholar] [CrossRef]
- Lima, G.R.; Monteiro, W.F.; Ligabue, R.; Santana, R.M.C. Titanate Nanotubes as New Nanostructured Catalyst for Depolymerization of PET by Glycolysis Reaction. Mater. Res. 2017, 20, 588–595. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eissa, A.-M.M.F.; Moustafa, M.E.; Eshaq, G.; Rabie, A.R.M.; Elmetwally, A.E. Glycolysis of poly (ethylene terephthalate) catalyzed by the Lewis base ionic liquid [bmim][OAc]. Ind. Eng. Chem. Res. 2014, 53, 18443–18451. [Google Scholar] [CrossRef]
- Viana, M.E.; Riul, A.; Carvalho, G.M.; Rubira, A.F.; Muniz, E.C. Chemical recycling of PET by catalyzed glycolysis: Kinetics of the heterogeneous reaction. Chem. Eng. J. 2011, 173, 210–219. [Google Scholar] [CrossRef]
- Chaudhary, S.; Surekha, P.; Kumar, D.; Rajagopal, C.; Roy, P.K. Microwav assisted glycolysis of poly (ethylene terepthalate) for preparation of polyester polyols. J. Appl. Polym. Sci. 2013, 129, 2779–2788. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Z.; Zhang, X.; Zhang, S.; Zhang, Y. Glycolysis of poly (ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. 2009, 45, 1535–1544. [Google Scholar] [CrossRef]
- Pticek Sirocic, A.; Fijacko, A.; Hrnjak-Murgic, Z. Chemical recycling of postconsumer poly (ethylene-terephthalate) bottlesedepolymerization study. Chem. Biochem. Eng. Q 2013, 27, 65–71. [Google Scholar]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Duque-Ingunza, I.; Lopez-Fonseca, R.; De Rivas, B.; Guti_errez-Ortiz, J. Synthesis of unsaturated polyester resin from glycolysed postconsumer PET wastes. J. Mater. Cycles Waste Manag. 2013, 15, 256–263. [Google Scholar] [CrossRef]
- Imran, M.; Kim, B.K.; Han, M.; Cho, B.G. Sub-and supercritical glycolysis of polyethylene terephthalate (PET) into the monomer bis (2-hydroxyethyl) terephthalate (BHET). Polym. Degrad. Stab. 2010, 95, 1686–1693. [Google Scholar] [CrossRef]
- Sangalang, A.; Bartolome, L. Generalized kinetic analysis of heterogeneous PET glycolysis: Nucleation-controlled depolymerization. Polym. Degrad. Stab. 2015, 115, 45–53. [Google Scholar] [CrossRef]
- Asakuma, Y.; Yamamura, Y.; Nakagawa, K.; Maeda, K.; Fukui, K. Mechanism of depolymerization reaction of polyethylene terephthalate: Experimental and theoretical studies. J. Polym. Environ. 2010, 19, 209–216. [Google Scholar] [CrossRef]
- Chen, F.; Wang, G.; Shi, C.; Zhang, Y.; Zhang, L.; Li, W.; Yang, F. Kinetics of glycolysis of poly (ethylene terephthalate) under microwave irradiation. J. Appl. Polym. Sci. 2013, 127, 2809–2815. [Google Scholar] [CrossRef]
- Yasir, A.H.; Khalaf, A.S.; Khalaf, M.N. Preparation and characterization of oligomer from recycled PET and evaluated as a corrosion inhibitor for C-steel material in 0.1 M HCl. Open J. Org. Polym. Mater. 2017, 7, 1–15. [Google Scholar] [CrossRef][Green Version]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Elmetwally, A.E. Ionic liquid coordinated ferrous acetate complex immobilized on bentonite as a novel separable catalyst for PET glycolysis. Ind. Eng. Chem. Res. 2015, 54, 12474–12481. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent developments in the chemical recycling of postconsumer poly (ethylene terephthalate) waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Pingale, N.; Shukla, S. Microwave assisted ecofriendly recycling of poly (ethylene terephthalate) bottle waste. Eur. Polym. J. 2008, 44, 4151–4156. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Geng, Y.; Zhou, Q.; Lu, X.; Zhang, S. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly (ethylene terephthalate) (PET). Green Chem. 2015, 17, 2473–2479. [Google Scholar] [CrossRef]
- Chen, F.; Wang, G.; Li, W.; Yang, F. Glycolysis of poly (ethylene terephthalate) over Mg-Al mixed oxides catalysts derived from hydrotalcites. Ind. Eng. Chem. Res. 2013, 52, 565–571. [Google Scholar] [CrossRef]
- Horn, H.W.; Jones, G.O.; Wei, D.S.; Fukushima, K.; Lecuyer, J.M.; Coady, D.J.; Hedrick, J.L.; Rice, J.E. Mechanisms of organo catalytic amidation and trans-esterification of aromatic esters as a model for the depolymerization of poly (ethylene) terephthalate. J. Phys. Chem. 2012, 116, 12389–12398. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, A.; Bhave, V.; Kathalewar, M.; Mare, S.; Raut, P. New polyester polyol derived from recycled poly (ethylene terephthalate) for coating application. Arch. Appl. Sci. Res. 2012, 4, 85–93. [Google Scholar]
- Abdelaal, M.Y.; Sobahi, T.R.; Makki, M.S.I. Chemical Degradation of Poly (Ethylene Terephthalate). Int. J. Polym. Mater. 2008, 57, 73–80. [Google Scholar] [CrossRef]
- Abdelaal, M.Y.; Sobahi, T.R.; Makki, M.S.I. Chemical transformation of PET waste through glycolysis. Constr. Build. Mater. 2011, 25, 3267–3271. [Google Scholar] [CrossRef]
- Paszun, D.; Spychaj, T. Chemical recycling of poly (ethylene terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar] [CrossRef]
- Badia, J.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly (ethylene terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.; Mishra, D. Thermolysis of waste plastics to liquid fuel: A suitable method for plastic waste management and manufacture of value added productsda world prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Dulio, V.; Po, R.; Borrelli, R.; Guarini, A.; Santini, C. Characterization of low-molecular-weight oligomers in recycled poly(ethylene terephthalate). Die Angew. Makromol. Chem. Macromol. Mater. Eng. 1995, 225, 109–122. [Google Scholar]
- Pawlaka, A.; Plutaa, M.; Morawieca, J.; Galeskia, A.; Pracella, M. Characterization of scrap poly (ethylene terephthalate). Eur. Polym. J. 2000, 36, 1875–1884. [Google Scholar] [CrossRef]
- National Association of PET Container Resources (NAPCOR). Report on Postconsumer PET Container Recycling Activity in 2015; NAPCOR: Middleton, WI, USA, 2015; Available online: https://plasticsrecycling.org/images/library/2015-APR-NAPCOR-rate-report.pdf (accessed on 10 July 2023).
- Karayannidis, G.P.; Achilias, D.S.; Sideridou, I.D.; Bikiaris, D.N. Alkyd resins derived from glycolized waste poly (ethylene terephthalate). Eur. Polym. J. 2004, 41, 201–210. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, D.; Son, C.; Lee, Y.; Kim, E.; Moon, I. Synthesis and applications of unsaturated polyester resins based on PET waste. Korean J. Chem. Eng. 2007, 24, 1076–1083. [Google Scholar] [CrossRef]
- Foti, D. Use of recycled waste pet bottles fibers for the reinforcement of concrete. Compos. Struct. 2013, 96, 396–404. [Google Scholar] [CrossRef]
- Sheikh, K.F.; Irwan, J.M.; Othman, N.; Wan, I.M.H. Pull-Out Strength of Polyethylene Terephthalate Bottle Fibre in Concrete Matrix. Malays. Constr. Res. J. 2017, 21, 75–85. [Google Scholar]
- Kim, J.J.; Park, C.; Lee, S.; Lee, S.; Won, J.C. Effect of the geometry of recycled PET fiber reinforcement on shrinkage cracking of cement of cement-based composites. Compos. Part B 2008, 39, 442–450. [Google Scholar] [CrossRef]
- Pelisser, F.; Montedo, O.R.K.; Gleize, P.J.P.; Roman, H.R. Mechanical Properties of Recycled PET Fibers in Concrete. Mater. Res. 2012, 15, 679–686. [Google Scholar] [CrossRef]
- Ingrao, C.; Giudice, A.L.; Tricase, C.; Rana, R.; Siracusa, V. Recycled-PET fibrebased panels for building thermal insulation: Environmental impact and improvement potential assessment for a greener production. Sci. Total Environ. 2014, 493, 914–929. [Google Scholar] [CrossRef]
- Gurudatt, K.; De, P.; Rakshit, A.K.; Bardhan, M.K. Spinning Fibers from Poly (ethylene terephthalate) Bottle-Grade Waste. J. Appl. Polym. Sci. 2003, 90, 3536–3545. [Google Scholar] [CrossRef]
- Saikia, N.; Brito, J.D. Waste Polyethylene Terephthalate as an Aggregate in Concrete. Mater. Res. 2013, 16, 341–350. [Google Scholar] [CrossRef]
- Schut, J.H. Bottle-to-Bottle PET Recycling Uses Silicone Modifier. Plastis Technoogy. Available online: https://www.ptonline.com/articles/bottle-to-bottle-pet-recycling-uses-silicone-modifier (accessed on 31 January 2007).
- Kim, S.B.; Yi, N.H.; Kim, H.Y.; Kim, J.H.J.; Song, Y.C. Material and structural performance evaluation of recycled PET fiber reinforced concrete. Cem. Concr. Compos. 2010, 32, 232–240. [Google Scholar] [CrossRef]
- Frigione, M. Recycling of PET Bottles as Fine Aggregates in Concrete. Waste Manag. 2010, 110, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Saikia, N.; Brito, d.J. Mechanical properties and abrasion behaviour of concrete containing shredded PET bottle waste as a partial substitution of natural aggregate. Constr. Build. Mater. 2014, 52, 236–244. [Google Scholar] [CrossRef]
- Juki, M.I.; Awang, M.; Annas, M.M.K.; Boon, K.H.; Othman, N.; Kadir, A.A.; Roslan, M.A.; Khalid, F.S. Relationship between compressive, splitting tensile and flexural strength of concrete containing granulated waste Polyethylene Terephthalate (PET) bottles–as fine aggregate. Adv. Mater. Res. 2013, 795, 356–359. [Google Scholar]
- Irwani, J.M.; Annaszpb, M.M.K.; Aeslina, A.K.; Othrnan, N.; Koh, H.B.; Asyraf, R.M.; Faisal, S.K. Cracking Propagation of Reinforced Concrete using Polyethylene Terephtalate (PET) bottles as Fine Aggregate. Adv. Mater. Res. 2014, 91, 474–478. [Google Scholar]
- Azhdarpour, A.M.; Nikoudel, M.R.; Taheri, M. The effect of using polyethylene terephthalate particles on physical and strength-related properties of concrete; a laboratory evaluation. Constr. Build. Mater. 2016, 109, 55–62. [Google Scholar] [CrossRef]
- Vishnu, A.; Mohana, V.; Maanasi, S.; Ponmalar, V. Use of Polyethylene Terephthalate in Concrete-A Brief Review. Int. J. Civ. Eng. Technol. 2017, 8, 1171–1176. [Google Scholar]
- Adibfar, M.; Kaghazchi, T.; Asasian, N.; Soleimani, M. Conversion of poly (ethylene terephthalate) waste into activated carbon: Chemical activation and characterization. Chem. Eng. Technol. 2014, 37, 979–986. [Google Scholar] [CrossRef]
- Sarker, M.; Kabir, A.; Rashid, M.M.; Molla, M.; Din Mohammad, A.S.M. Waste Polyethylene Terephthalate (PETE-1) Conversion into Liquid Fuel. J. Fundam. Renew. Energ. Appl. 2011, 1, R101202. [Google Scholar] [CrossRef]
- Vitkauskienė, I.; Makuška, R. Glycolysis of industrial poly (ethylene terephthalate) waste directed to bis (hydroxyethylene) terephthalate and aromatic polyester polyols. CHEMIJA 2008, 19, 29–34. [Google Scholar]
- Purohit, J.; Chawada, G.; Choubisa, B.; Patel, M.; Dholakiya, B. Polyester Polyol Derived from Waste Poly (Ethylene Terephthalate) for Coating Application on Mild Steel. Chem. Sci. J. 2012, 76, 1–7. [Google Scholar]
- Ghaderian, A.; Haghighi, A.H.; Taromi, F.A.; Abdeen, Z.; Boroomand, A.; Taheri, S.M.R. Characterization of rigidpolyurethane foam prepared from recycling of PET waste. Period. Polytech. Chem. Eng. 2015, 59, 296–305. [Google Scholar] [CrossRef]
- Kacperski, M.; Spychaj, T. Rigid Polyurethane Foams with Poly (ethylene terephthalate)/ Triethanolamine Recycling Products. Polym. Adv. Technol. 1999, 10, 620–624. [Google Scholar] [CrossRef]
- Saravari, O.; Vessabutr, B.; Pimpan, V. Synthesis of Urethane Oils from Waste Poly (ethylene terephthalate) Bottles. J. Appl. Polym. Sci. 2003, 92, 3040–3045. [Google Scholar] [CrossRef]
- WML. SECTION 4–Waste Management Legislation Waste Management–Overview. In Handbook on the Implementation of EC Environmental Legislation; Europion Union: Brussels, Belgium, 2008. [Google Scholar]
- National Waste Policy. Implementation Report 2012 and 2013. 2019. Available online: https://www.dcceew.gov.au/sites/default/files/documents/national-waste-policy-implementation-2013.pdf (accessed on 1 April 2014).
- Waste Management in China, 2005. Waste Management in China: Issues and Recommendations. Available online: https://globalrec.org/wp-content/uploads/2014/03/China-Waste-Management-2005.pdf (accessed on 1 May 2005).
- The Waste (England and Wales) Regulations, 2011. 2011 No. 988, Environmental Protection, England and Wales. Available online: https://www.legislation.gov.uk/uksi/2011/988/contents/made (accessed on 28 March 2011).
- Solid Waste Management Rules. 2015. Available online: http://www.indiaenvironmentportal.org.in/content/411423/draft-solid-waste-management-rules-2015 (accessed on 8 April 2016).
- Waste Management and Public Cleansing Law. 1970. Available online: https://www.env.go.jp/en/recycle/basel_conv/files/Waste_Management_and_Public_Cleansing.pdf (accessed on 10 July 2023).
- Boyle, C.A. Solid waste management in New Zealand. Waste Manag. 2000, 20, 517–526. [Google Scholar] [CrossRef]
- Waste Minimization Act. 2008. Available online: https://environment.govt.nz/acts-and-regulations/acts/waste-minimisation-act-2008 (accessed on 31 December 2008).
- Byung, J.K.; Chai, S.G.; Seung, J.Y.; Yong, H.L.; John, T.B. Korean Waste Management Law and Waste Disposal Forms; USACERL Special Report N-91/19. 1991. Available online: https://apps.dtic.mil/sti/pdfs/ADA235501.pdf (accessed on 10 July 2023).
- RCRA: Resource Conservation and Recovery Act, P.L. 94–580, 90 Stat. 2795 42 U.S.C. 6901, October 21, 1976. RCRA Recycling Bins. Available online: https://libraryguides.law.pace.edu/RCRA (accessed on 10 November 2011).
- Code of Federal Regulations: Part 243-Criteria for Municipal Solid Waste Landfills. 26. 2012. Available online: https://www.govinfo.gov/content/pkg/CFR-2012-title40-vol26/xml/CFR-2012-title40-vol26-part258.xml (accessed on 10 July 2023).
- Code of Federal Regulations: Part 243-Guidelines for the Storage and Collection of Residential, Commercial, and Institutional Solid Waste. 26. 2012. Available online: https://www.govinfo.gov/content/pkg/CFR-2012-title40-vol26/xml/CFR-2012-title40-vol26-part243.xml. (accessed on 10 July 2023).
- Occupational safety and health: Environmental, Climate ad Sustainable Development laws; FIN-2011-L-112217. Available online: https://www.ilo.org/dyn/natlex/natlex4.detail?p_isn=112217&p_lang (accessed on 10 July 2023).
- Keuc, A. Waste Management in Slovenia. Available online: http://www.umanotera.org/ (accessed on 10 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).