Abstract
In spite of the critical environmental impacts of mining and the associated geopolitical supply risk, the strategic importance of rare metal tungsten is escalated by rapid expansions in industrialization, particularly in the ongoing low-carbon/energy era, which requires technologies that allow an economic, social, and ecologically friendly tungsten recovery from primary and secondary resources. The current recycling practices of tungsten carbide (WC)-based scraps have been accepted as economically and partially environmentally beneficial and can promote tungsten closed-loop recycling; however, low functional recycling rates and significant metal losses at varied stages hinder the economic recovery of metals. The current review presents the global situation of tungsten and WC flow with a focus on various sustainable methods to recycle spent tungsten and related metals. A detailed discussion of establishing a highly resilient circular economy with sustainable development goals is highlighted by juxtaposing the philosophy of the circular economy, integrated sustainability, and the metal life cycle approach. The article also discusses Industry 5.0 trends, such as sustainable digitalization and twin transition, to overcome the barriers associated with achieving efficient circular recycling. It is shown that cross-disciplinary methodologies, the integration of diverse technologies (digital/green), and the incorporation of state-of-the-art recycling techniques open up the future potential in the recycling sector.
1. Introduction
Since its inception in 1923, cemented carbides (based on tungsten carbide hard metals) have conquered a wide application range in mining, construction (tools for machining), automotive, cutting, drilling (tunneling), and similar applications, stimulating many fields of production technology [1,2,3]. Cemented carbides are metal matrix composites encompassing a hard carbide phase (mostly WC, 50% to 90% by weight) distributed/embedded/cemented as an aggregate into a soft metallic binder phase (usually Co, Fe, Ni; 0% to 35% by weight), exploiting the widely recognized powder metallurgical [4,5,6,7,8,9] and additive manufacturing (3D printing) [3] techniques. Frequently, the alloying of other metals (or their carbides), such as Ti, Ta, Nb, etc., to achieve the desired properties is also conducted [10,11,12,13,14]. The final composites are noted for their impeccable combination of properties, such as excellent hardness, toughness, refractoriness, and mechanical strength, which immensely benefit applications (up to 600 °C) mainly concerning extreme wear loss (mainly abrasion and impact) [11,15,16,17,18]. In addition, cemented carbides have proven their dominance over superhard materials (such as diamond, c-BN, or Al2O3-based) in several domains of the tooling industry due to their perfect balance of toughness and hardness (the low toughness of superhard materials leads to extreme brittle fracturing) [14,19,20]. Apart from the tooling and machining industry, the stability and impassiveness of ‘gray–white metal’ tungsten in air, acids, or bases has led to its increasing use in many other fields of chemistry (mordant, analytical reagent, catalyst, water treatment agent), radiation shielding, etc. [21]. Other uses of tungsten include rocket nozzles (aerospace), turbine blades, electrodes, heating elements, filaments in bulbs, car batteries, etc. Nevertheless, a remarkable amount of tungsten (nearly 50–55%) accounts for cemented carbide tools (almost 40–95 wt.% tungsten content) production [22,23,24]. All these factors have gained tungsten a reputation as a strategic commodity of high significance globally.
By 2022, the world’s hold (mine production, resource) of tungsten was 84,000 metric tons, which stretched mainly across China, Vietnam, Russia, Bolivia, Rwanda, Austria, Spain, Portugal, and some other countries in Africa [25]. Some mines that have closed in the past years in Australia, South Korea, and the USA are now considering re-opening. Altogether, China is the largest producer, exporter, and consumer of the world’s total (primary) tungsten (71,000 Mt, almost 84%) [26,27], although, in recent times, and especially in the European Union (EU), a significant growth (yearly) of tungsten production has been reported in Austria, Spain (doubled in 2021 by 500 Mt), and Portugal [28]. Tungsten’s importance in numerous applications for industries and its low substitutability (especially in hard metals and steel additives) gains it the highest ‘economic importance’. In addition, the ‘supply risk’ of tungsten is relatively high (higher than rare earth elements) since 50% of the EU tungsten is outsourced (Figure 1). Additionally, the high risks associated with tungsten supply chains can be expected anytime due to several factors, such as geopolitical reasons, export restrictions by producing countries, increased demand in local markets, etc. [29,30,31].
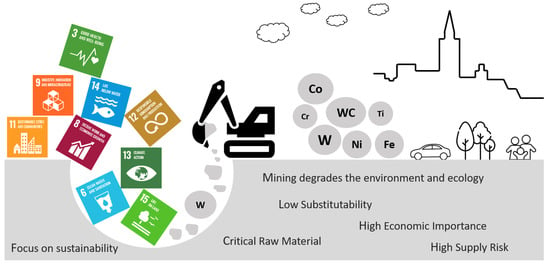
Figure 1.
A scheme portraying the reasons behind WC recycling [22,23,32,33,34,35,36].
Primary tungsten (generated by atmospheric weathering) is usually extracted from ore (mainly wolframite, ferberite, scheelite, or huebnerite) through a sequence of chemical processing stages. The extracting, refining, and processing of raw materials (or tungsten) results in huge energy consumption, substantial air and water pollution, high cost, etc. [31]. All of these factors lead to an increased carbon footprint and a reduced ecological and economic balance. With an astonishing boom in population, technology, and the demand for manufacturing, the global need for tungsten and its tools is growing more than ever; however, a major hindrance behind the long-term sustainability and use of ‘rare metal’ tungsten lies in its finite availability (non-renewable resource) [23]. On the other hand, the inclusion of tungsten and cobalt both in the EU list of critical raw materials (CRM) has posed a serious need to find an alternative for future usage [32]. At a time when the manufacturing sector continues to navigate the fourth industrial revolution, Industry 5.0, the next revolution, is at the doors with a strong emphasis on sustainability (recycling, energy efficiency) and making production (materials) respect the boundaries of our planet [33,34,35]; hence, it is high time for the world to understand the point of sustainable development through efficient metal use, metal reclamation (recycling), environmental protection, economic recovery and re-use of the world’s finite resources of tungsten [31,36,37,38,39,40,41,42,43]. In other words, it is necessary to achieve effective recycling methods for tungsten carbide scraps in order to recover tungsten (W) and the binder metal, such as nickel (Ni) or cobalt (Co). Scrap recycling to obtain tungsten is herein named secondary tungsten (generated by alteration rather than atmospheric weathering). Figure 1 shows a scheme depicting the reasons behind tungsten carbide recycling.
The current work presents a detailed review on various recycling methods for cemented tungsten carbide hard metal. Starting with an overview of tungsten carbide and its global usage, material flow, and processing (Section 1 and Section 2), the article describes its effective recycling/recovery methods (Section 3 and Section 4). A comparative assessment of different recycling methods has been made based on various factors, such as input/output feedstock, environment, energy, etc. Future improvements in regard to WC recycling have been elaborated in detail to efficiently manage the scraps generated. Thereafter, Section 5 discloses the methods to drive improvement in the recycling sector and create a highly sustainable economy by collating philosophies of circular economy, metal’s life cycle, and integrated sustainability. A detailed discussion on Industry 5.0 objectives, twin transitions, and the importance of digitalization in enhancing waste management is brought up in the context of WC recycling (Section 5). Figure 2 presents a schematic illustration of the areas addressed in the current review article.
Figure 2.
A scheme referring to the areas of discussion covered in the current work.
2. Processing and Usage of Tungsten
2.1. Resourcing and Treatment
As perceived, the world’s tungsten resources are geographically distributed but unequally spread across the planet. Figure 3 shows the yearly tungsten production/resource and the world’s reserves (in metric tons) as reported by United States Geological Survey (USGS) survey newsletters. The USA’s statistics were undeclared in terms of the total production.
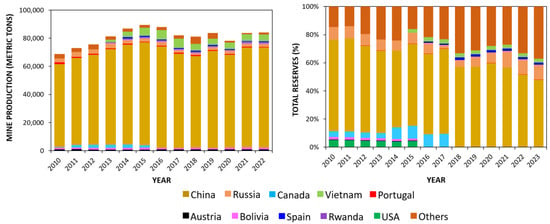
Figure 3.
Yearly tungsten mining production (in metric tons) and tungsten reserves across world (in percentage). Statistics from USGS newsletters [44].
The mining methods for primary tungsten vary as per the chemical and physical characteristics of the raw tungsten. Primary tungsten is obtained from natural resources and is generally mined underground (sub-surface) from its ore concentrates of wolframite and scheelite, mainly; however, the rare existence of an open pit or surface mining can also be seen. Tungsten mines are rather small and seldom produce more than 2000 tons of ore per day [45]. The Masan Resources Nui Phao mine situated in Vietnam is the world’s largest tungsten mine and generates more than 5000 tons of ore per day (30% of the world total supply of tungsten) [46]. Naturally occurring tungsten ores comprise less than 0.1–5% WO3 content [47]. On the other hand, the ore concentrates merchandised internationally require 65–75% WO3 content [45]. For this reason, the excavation of a massive ore deposit is necessary to obtain a minor quantity of tungsten. Moreover, it is necessary to separate a very large number of impurities in order to meet the international requirement for concentrate labeling. Recently, some major players in the market reported that new advanced techniques of tungsten ore processing (encompassing crushing, milling, and ore separation) could lead to an improvement in the tungsten yield by 7–12% and energy savings by 5% [48].
Currently, the methods to obtain tungsten involve an initial step of pre-treatment of scheelite and wolframite concentrates [49], which is usually performed by either ore roasting at ~650 °C, leaching in inorganic acid or alkali solution (alkaline pressure digestion), or both [31]. A common practice involves dissolving the concentrates in either soda or a concentrated NaOH solution [50] and then the acquired sodium tungstate solution is purified by precipitation and filtration using a solvent extraction or ion exchange technique prior to transformation into an ammonium tungstate solution ((NH4)2WO4). Furthermore, a high purity ammonium–paratungstate (APT) is achieved by crystallization at around 100 °C [47]. Upon oxidation of APT and a series of decomposition reactions, WO3 is obtained. Finally, a hydrogen reduction of WO3 results in a pure metallic accessible tungsten powder. Figure 4 shows a general flowchart for obtaining tungsten (tungsten carbide hard metal).
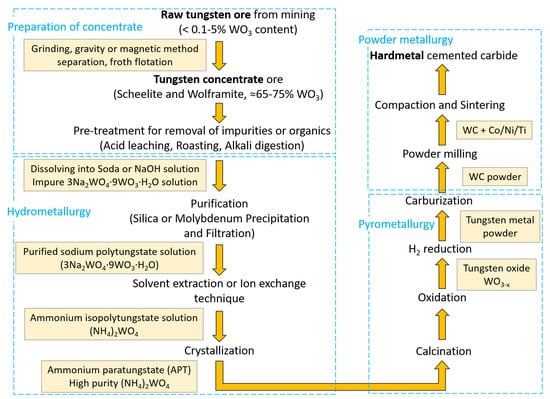
Figure 4.
A simplified flowchart showing various processes involved to obtain tungsten carbide hard metal from its raw mined ore [31,47,49,50,51,52].
2.2. Products and Scrap
Every product (material) is designed to serve a specific function (high value); however, upon its effective service for a certain period, it often submits to ineffective or poor functioning (low value), possibly due to its sub-parts deteriorating or completely breaking down. This could be caused by numerous factors (in the material), such as loss of strength, stability or an excessive wear, corrosion, deformation, fracture, etc. [3]. At the end, such unfit products are discarded and form scrap. Figure 5 shows the typical appearance of WC scraps collected after use.

Figure 5.
Hard (blue line) and soft (red line) tungsten and tungsten-containing materials scrap classification. Grinding sludge and lathe chip/scarf form soft scraps. Inserts, wire drawing dies, tunnel boring drill bits, and steel rolls form hard scraps. Courtesy of Indian Bureau of Mines [53].
Products can be divided into first-use and end-use products [54]. Cemented carbide rods or bars form the first-use products (also called half-finished products) since they require additional processing or value-addition steps to be ready for their end-use, while a cutting tool or cemented carbide inserts embedded into a base metal mantle to form a drill bit (for mining) form end-use products (also called the finished product). The scrap generated by first-use products are often termed as primary or new scrap (Figure 6, processing scrap), while the ones from end-use products are called secondary or old scrap (Figure 6, scrap from used part). For example, grinding sludge generated out of hard metal rod or bar machining processes (for drill bits, etc.) falls under primary or new scrap. Figure 6 classifies the types of scrap with their brief explanation. Figure 7 shows a typical product–scrap flowchart in terms of total tungsten demand (percentage usage) as per the current state.
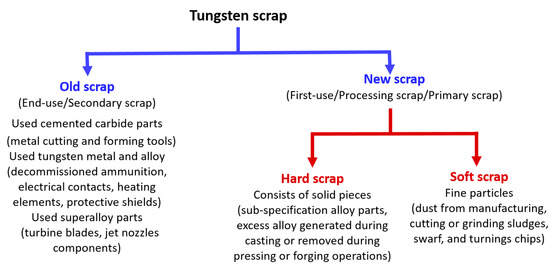
Figure 6.
Tungsten scrap classification.
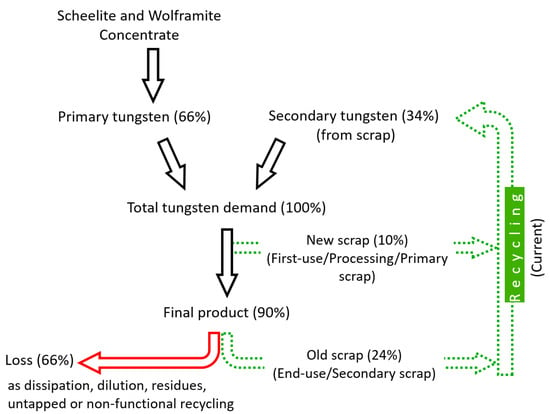
Figure 7.
A simplified tungsten flowchart representing a typical product-to-scrap life cycle. Statistics from International Tungsten Industry Association (ITIA) newsletter [55,56]. Losses are discussed in detail in Section 4.6.1.
2.3. Current and Future Usage of Tungsten and Its Carbide
A high tolerance for heat and pressure has made tungsten carbide a boon material for many decades. With its slow initial acceptance in technology, tungsten and its carbide have gained significant popularity from critical functional machineries (armor shields, deep drilling, dry cutting of difficult-to-machine materials, etc.) to our daily lives (jewelry, paints, dies, etc.). With its largest global contribution being machining and cutting tools (55–80%), this strategic material usage has expanded to fossil fuels combustion plants (tungsten-containing superalloy turbine blade) [57], electric vehicle batteries (for advanced fast-charge lithium-ion batteries) [58,59], medical applications (micro-needle sensors) [60,61], and in renewable energy generation (tungsten-based oxide in semiconductors, photo absorbers, hydrogen production, etc.) [62,63,64]. Figure 8 shows the most prevalent field and sub-field applications of tungsten carbides.
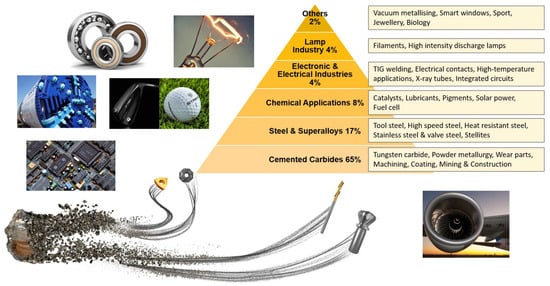
Figure 8.
Global usage of tungsten amongst industries [54,65,66,67].
Currently, the global energy demands have risen more than the supply can match. The future of a sustainable world needs robust and improved efficiency of conventional technologies to cut back on the energy losses in several areas. Regardless of the solution the world may see, tungsten-based materials will undoubtfully play a significant role in meeting these challenges. Additionally, the expansion of the production of super-alloys and other high-tech materials will require the use of functional materials and advanced tools with excellent properties, which will keep tungsten carbide tools in the market and in high demand [68,69]. In addition, recent advances have shown the enormous potential of tungsten-based materials to enhance the energy efficiency in the industries of deep drilling (10 km and more), fossil or renewable power generation (higher process temperatures), power transmission and distribution (through smart chip technology and ICs and reducing losses in solar, wind, geothermal, and biological applications, as well as thermonuclear reactors (W-based structure), etc. [67,70,71,72,73]. At the same time, it is crucial to stick to the idea of ‘do more and use less’.
3. Recycling Tungsten and Sustainable Development: Scrap as a Commodity
Processing tungsten directly from mines is undeniably a significant source of greenhouse gas emissions. Leal-Ayala et al. reported that primary tungsten production requires close to 10,000 kWh per ton of tungsten, in comparison to the secondary tungsten production (recycling) route i.e., <6000 kWh per ton of tungsten [26]. Recycling requires considerably less energy than the initial process of refining raw minerals into metals, meaning lower emissions (more than 40% reduced energy consumption [26]). A report by Sandvik Mining and Rock Solutions says that making tools from recycled carbide requires 70 percent less energy and emits 64 percent less CO2 [37].
Recycling in general terms can be defined as the practice of collecting and treating waste or materials to be scrapped and remanufacturing them into new products. It reduces the need for extracting, refining, and processing raw materials, all of which are a significant contributor to air and water pollution (additionally, spent or discarded products go to landfills, incineration, etc.). The re-use of material largely benefits the economy (saves energy, money) and community (embeds a practice of conserving resources, creating jobs). From the point of view of tungsten being a ‘rare material’, re-use or recycling ensures its sustainable supply to meet future demands. It is worthy to mention that recycling tungsten carbide (and most metals in general) directly contributes to achieving SDGs 3, 8, 9, 11, 12, 13, 14, and 15 in several ways through harmonizing three core elements (or systems) of sustainability, i.e., economic growth, social inclusion, and environmental protection [74,75,76,77]. Figure 9 shows a typical scheme to elaborate the relationship between recycling and sustainable development. Additionally, the figure shows the key factors of integrated sustainability, adapted from UN Global Compact Report 2017 [78].
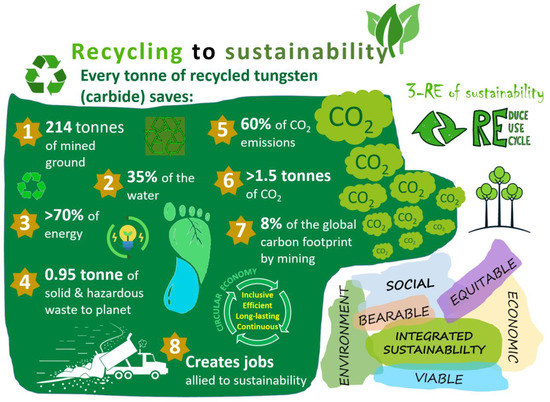
Figure 9.
A scheme showing the connection between tungsten recycling and its contribution to sustainable development [26,37,74,75,76,77]. Key factors of integrated sustainability adapted from UN Global Compact Report [78].
Why Is the Market for Tungsten (Carbide) Scrap Growing?
It is worth mentioning that 15 tons of mined ground is required to produce the same quantity of tungsten as when recycling just 70 kg of its carbide. As per the recycling industry’s statistics, recycling 1.2 kg of scrap or waste returns 1 kg of tungsten carbide (more than an 80% recovery rate), as can be seen in Figure 10 [79].
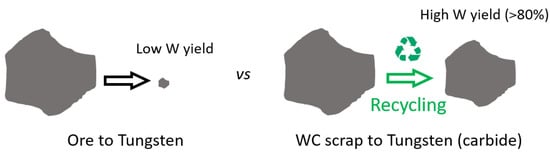
Figure 10.
A scheme showing a comparison between conventional technique of ore processing and recycling scrap to obtain tungsten. Recycled scrap yields a higher amount of tungsten [79].
The European Commission’s report in 2018 stated that the contribution of tungsten to the EU’s demand of CRMs was a remarkable 42% (end-of-life recycling), the second most recycled product in the EU’s CRMs list (vanadium being first) [80], whereas, in 2018, ITIA stated the global recycling input rate (input of secondary tungsten, i.e., old + new scrap/total input) of tungsten to be 35% [55]. The end-of-life recycling rate (amount of old scrap forming secondary tungsten) was reported to be 30% [55]. In regards to tungsten carbide products, the present-day total global recycling rate is 46% [55]; however, for hard metal tools and heavy metal parts, the recycling rates reported are 50–75% [81].
In regard to ‘strategic and rare metal’ tungsten, the global market in 2017 was 105,000 Mt. In 2022, it was evaluated at 119,000 Mt, and is predicted to reach a revised size of 170,000 Mt by 2030, representing the cumulative effect of an average annual growth (CAGR) of ≈4.5% [28,80]. More tungsten products will finally lead to increased scrap recycling and, thus, to a larger need to process scraps.
Several factors are responsible for the growing tungsten (carbide) recycling market [52]. Figure 11 demonstrates a scheme illustrating the growing market of tungsten (recycling) and its influencing factors.
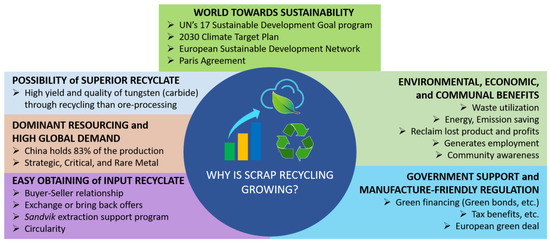
Figure 11.
A scheme illustrating the factors accelerating the market growth of tungsten (carbide) scrap and its recycling [26,28,37,51,52,76,78,79,80,81,82,83,84,85,86,87].
4. Recycling Techniques
Currently, there are a number of technologies for the processing or regeneration of WC. Some ‘direct methods’ introduce specific tungsten scrap for efficient and fast processing, while others use chemicals and energy to process them [54]. With the development of technology, methods are being refined and optimized to further improve the recycling efficiency (the amount of tungsten in scrap consumed divided by the amount of tungsten in scrap generated), energy consumption (low), volume of scrap recycling (high yield), etc., leading to a more sustainable economy [87].
It is easiest to collect, transport, and recycle the new scrap (generated out of the initial production). Almost no tungsten loss is seen during new scrap recycling. Dealing with the scraps generated from discarded end-products poses challenges due to the uncertainty of the raw material contained in it, the part complexity, etc. In addition, depending on the type of old scrap, different logistics (supply chain), sorting, or dismantling methods must be chosen, leading to a dedicated industrial line. The correct choice of recycling method depends on the type of scrap input and the desired recycled product. Figure 12 lists the most common methods for recycling tungsten (and its carbides). Some other methods are documented elsewhere [88,89,90,91].
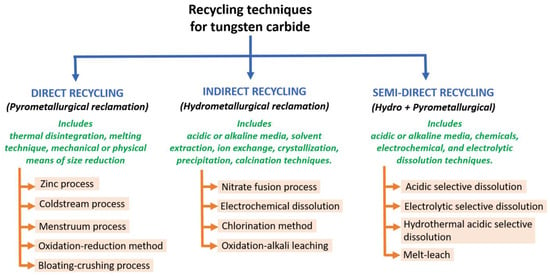
Figure 12.
Classification of different types of tungsten (cemented carbide) recycling methods [22,25,47,51,54,85,89,90,91,92,93,94,95,96,97,98].
4.1. Direct Recycling Techniques (Pyrometallurgy)
In direct recycling, as-obtained scrap (recyclate) is directly converted to powder form, utilizing physical, chemical (low-intensity), or combinational processing techniques. To this end, direct methods limit themselves to the processing of close to high-purity tungsten scraps (60–99%), mostly comprising a similar composition, foreign substance contamination, grain size, dimension (for sorting), and cleaning process as the final product [95]. In spite of this, direct recycling is widely acknowledged for its minimum energy consumption, minimum waste generation, high conversion efficiency, high-quality products, and low processing costs. Figure 12 shows the most used direct recycling methods [96].
4.1.1. Zinc Process
Among all the direct methods, the zinc process is the most widely acclaimed recycling process for tungsten materials and cemented carbides. Gaining attention in 2005 due to an increased tungsten price, the zinc process is the most cost-efficient and environmentally friendly hard metal recycling (recovery) method [95]. Here, the recyclate or the cemented carbide scrap is cleaned and sorted, then is loaded together with zinc into graphite boats in a special furnace at 900–1050 °C in an Ar/N2 atmosphere. During the procedure, the molten zinc reacts with the binder phase (cobalt) of the hard metal. There is volume expansion that is supplementary with this reaction and, as a consequence, the hard metal swells to a ‘porous cake’, as shown in Figure 13 [54]. After the reaction has ended, the vacuum distillation of Zn is accomplished at 1000–1050 °C and a pressure range of 6–13 Pa, deserting the dry powdery material that can be easily disintegrated or fine-milled [47,98]. The distilled Zn is recovered so that it can be used again. The fine-milled reclaimed powder serves as an input for the secondary hard metal product. A major advantage of the zinc process lies in its high tungsten (carbide) yield (almost 95%) [26] and lack of chemical modification of the raw material. In addition, no change in the hard metal powder grain size is seen after reclamation. The reclaimed powder comprises a binder metal, such as Co or, in the case of cutting tools, cubic carbides TaC, TiC, and NbC are retained. Other advantages of zinc process include close to no emissions, as the process is a furnace process, and lower CO2 emissions as compared to other methods.
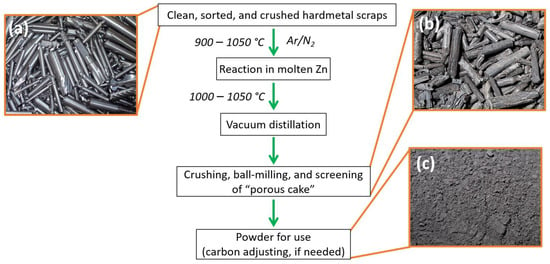
Figure 13.
Flowchart showing recycling of WC hard metal scraps using the zinc process. (a) Clean and sorted hard metal; (b) ‘porous cake’; and (c) crushed powder ready for use.
4.1.2. Cold Stream Process
The cold stream process is a high-velocity impact procedure involving the recyclate material (scrap) being incorporated in an accelerated gas stream passing through a Venturi system and, finally, being projected against a stationary target, resulting in shattering, disintegrating, or fragmenting of the scraps into small particles [96,98,99]. The shattered particles (<6 mesh) are then collected from the impact chamber by suction. The rapidly expanding gas exiting the Venturi nozzle (usually a supersonic nozzle) results in a strong cooling effect through adiabatic expansion (greater than the heat caused by pulverization). The process advantages include simplicity, low operating cost, low operating temperature (avoids WC oxidation and property degradation), high purity retention, and particle size control. Nevertheless, a major concern regarding the cold stream process is its inability to separate tungsten carbide from the binding material [85]. Figure 14 shows a typical flowchart to recover cemented carbide powder from scrap using a cold stream process.
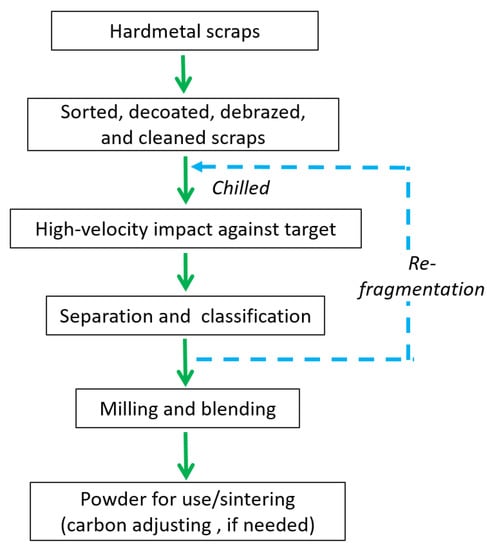
Figure 14.
Flowchart showing recycling of WC hard metal scraps using the cold stream process.
4.1.3. Oxidation-Reduction Method
In the oxidation-reduction method of recycling, heavy tungsten carbide scrap (WC-Co/Ni/Fe) is reclaimed by exposing the carbide to oxidation in air at about 800 °C (evading overheating), to create a mixture of tungsten oxide and metal tungstate. Afterward, the oxide–tungstate blend is subsequently milled, screened, and reduced in a hydrogen atmosphere at 900 °C to 1000 °C. Lastly, the blend is mixed with carbon through a process known as carburizing to form a tungsten carbide powder mixture [22,90]. After consolidation, the obtained tungsten carbide powder mix results in sintered tungsten carbide again. In order to gain pure W and a Co/Ni/Fe metallic element, oxidation is generally followed by either electrochemical or hydrometallurgical processes [90,100,101], described under the semi-direct method of recycling. Figure 15 shows a typical flowchart to recover cemented carbide powder from scrap using an oxidation-reduction method.
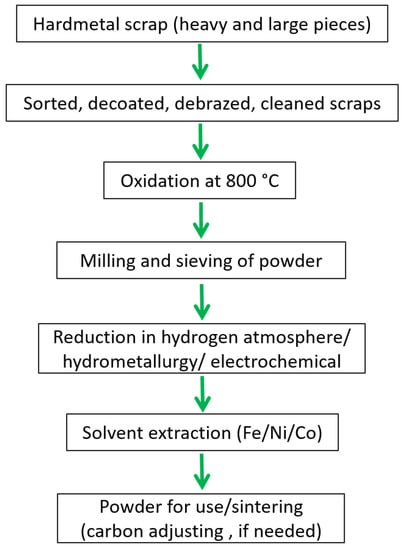
Figure 15.
Flowchart showing recycling of WC hard metal scraps using the oxidation-reduction method.
4.1.4. Menstruum Process
The menstruum or melt-bath process concerns the direct recycling of large and heavy hard metal scraps (W-Fe-Ni, W-Fe-Cu-Ni, and Fe-W) unlike zinc-melt, cold stream, or oxidation-reduction routes. It involves dissolving hard metal scraps in Fe–C or Co–C melts at twice the temperature (1550–1600 °C) of the zinc-melt process using an induction furnace [89]. Then, the hard metal reacts with the carbon of the melt to generate WC, which is often found at the bottom of the melt due to its higher density. Furthermore, upon draining the excess liquid in the melt, WC mixed with other Fe or Co compounds is collected for further purification utilizing HCl solution leaching or zinc-melt to obtain pure WC powder [85,102], as shown in Figure 16.
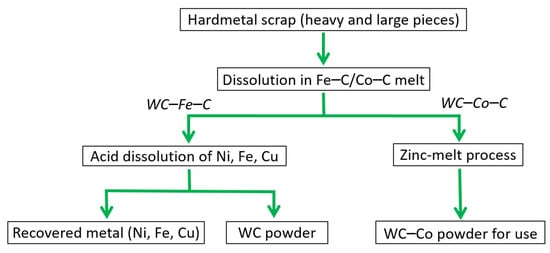
Figure 16.
Flowchart showing recycling of WC hard metal scraps using the Fe/Co-melt bath or menstruum process.
4.1.5. Bloating–Crushing Method
In the bloating–crushing method, the tungsten carbide scrap is initially thermally bloated and then crushed. This method of recycling benefits from ‘the embrittling effect’ caused by heating the cemented carbide (to about 1800 °C), followed by rapid quenching [96]. The embrittlement is the result of WC grain growth, the development of brittle phases, and thermally induced stresses. Lastly, the embrittled product is easily crushed into powders and re-used for the consolidation of tungsten carbide bulk.
4.2. Indirect or Chemical Recycling Techniques (Hydrometallurgy)
Certain scrap materials containing tungsten carbide do not meet the stringent purity requirements for direct processes. Consequently, alternative approaches have been developed to treat these materials. Indirect recycling methods involve chemically transforming the constituent metals into intermediate products, which are subsequently processed to obtain pure metals [103]. This method can be applied to a wide range of tungsten-containing materials, wherein tungsten is converted into an intermediate product, such as ‘virgin’ ammonium paratungstate (APT). Additionally, the recycling of WC-Co grinding sludge, a blend of abrasives, lubricants, and hard metal chips, is frequently conducted through indirect chemical processes. This is necessary because the sludge contains oil, coolants, and various impurities or pollutants.
4.2.1. Nitrite Fusion Method
In a pioneering study by Bhosale et al. [97], a method known as nitrite fusion was utilized to unlock the recycling potential of tungsten carbide scrap. The nitrite fusion method unfolds as tungsten carbide scrap undergoes a fusion process with nitrate salts, typically alkali metal nitrates, in an elevated temperature environment. These nitrate salts act as agents of oxidation, facilitating the transformation of tungsten carbide into soluble tungstate compounds that readily dissolve in water. Through subsequent stages of leaching and precipitation, the tungsten is meticulously separated and recovered from the solution, giving rise to the creation of pure tungsten compounds for further processing or reuse. The process employs nitrate salts, such as sodium nitrate (NaNO3), or combinations of NaNO3 and sodium bicarbonate (Na2CO3) to unleash a metamorphosis wherein tungsten transmutes into water-soluble sodium tungstate (Na2WO4), as shown in Figure 17. The nitrate fusion process embodies notable merits, including adaptability in terms of the types of scrap materials and the amount of scrap processed, remarkable efficiency, and exceedingly high recovery rates. Substances that are challenging to oxidize, such as tungsten heavy metal hard scrap or tungsten copper hard scrap, can be effectively treated using this approach. Nevertheless, it is vital to acknowledge the challenges posed by this method, such as the exothermic reactions involved, raise safety concerns, and the generation of copious amounts of toxic nitrous oxide fumes presents an immense pollution predicament that demands effective mitigation strategies [97].
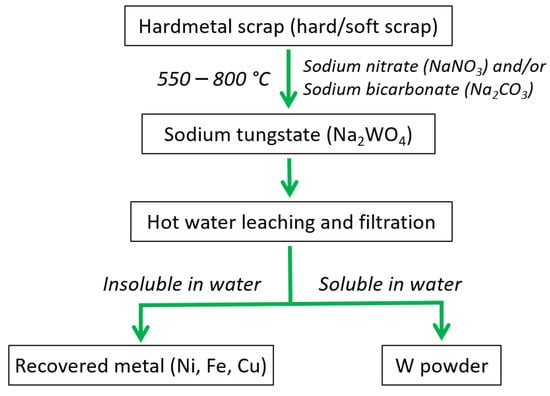
Figure 17.
Flowchart showing recycling of WC hard metal scraps using nitrite fusion method.
4.2.2. Electrochemical Process
Electrometallurgical methods (including electrochemical, electrodissolution, anodic dissolution, anodic-leaching, electro-leaching, and electrowinning) have gained a reputation in WC recycling due to a single-step process that consumes relatively less energy, contingent upon the choice of electrolytes utilized [104]; however, among all of them, the electrochemical technique stands out in terms of delivering exceptionally pure results, surpassing the efficacy of alternative approaches [22]. It involves the use of an electrochemical cell, where the tungsten carbide scrap is immersed in an electrolyte solution and subjected to an electric current, as shown in Figure 18. This process promotes the dissolution of tungsten carbide into the electrolyte, allowing for the recovery of tungsten ions. These ions can then be further processed to obtain pure tungsten or can be used in the production of new tungsten-based materials. As illustrated in Figure 18, when exposed to an alkaline environment, WC undergoes anodic decomposition, resulting in the formation of a sodium tungstate solution. Meanwhile, cobalt (Co) is deposited on the cathode. The hard metal tungsten carbide, with its high melting point, remains largely undissolved and is retained in the form of anode slimes or a skeleton at the anode. The dissolved ammonium tungsten in the electrolyte can be recovered and, subsequently, various intermediate forms can be utilized to obtain pure W. The electrochemical recycling of tungsten carbide offers several advantages, including high efficiency, scalability, and the ability to recover valuable tungsten resources while minimizing waste and environmental impact [22]. In [105], it was shown that the electrochemical method can draw out nickel binder dissolution and the conversion of tungsten carbide into an intermediate substance. The findings indicate the successful electrochemical conversion of tungsten to WO3 and the complete dissolution of nickel, resulting in the production of high-purity APT and nickel sulphate (NiSO4) as valuable commercial products, as shown in Figure 18. The attractiveness of this process lies in the recovery of both tungsten and the binder metal.
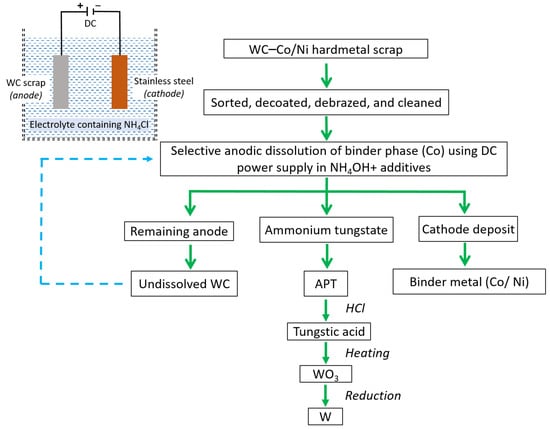
Figure 18.
Representation of the electrochemical cell and flowchart showing recycling of WC hard metal scraps using electrochemical method [22,104,105].
4.2.3. Chlorination Process
In the chlorination process, as shown in Figure 19, the WC-Co/Ni scrap undergoes a primary chlorination step (carried out in a gaseous Cl2 atmosphere at elevated temperatures, typically 800–1000 °C) to generate metal chlorides, such as WCl5 and WCl6 (oxychlorides, such as WOCl and WO2Cl2, are also generated). Furthermore, rapidly cooling (quenching) the metal chlorides in water results in the creation of hydrated crystals, which generate internal tension and lead to the fragmentation of the material. This is followed by a mechanical reduction process to yield WC-Co/Ni powder or to obtain intermediate products, such as APT, ammonium tungstate, or tungstic acid, using alkali/acid leaching. The drawback of this method is that it requires intricate equipment and several processing steps to obtain metal powders or other forms suitable for practical use (from metal chlorides) [47,106].
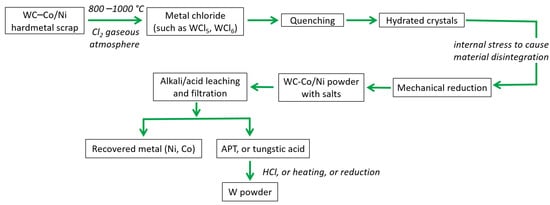
Figure 19.
Flowchart showing recycling of hard metal scraps using chlorination process [106].
4.3. Semi-Direct Recycling Techniques (Pyro + Hydrometallurgy)
The semi-direct recycling approach for cemented carbides offers the potential for selectively chemically dissolving the binder metal (typically cobalt) while preserving the integrity of the hard phase(s), i.e., WC. The dissolution process selectively targets the binder metal(s) while leaving the WC phase unaffected. Following this process, the remaining brittle tungsten skeleton can be crushed and reused in the production of new items [56,107,108]. Furthermore, cobalt can be reclaimed through the various methods described above (alkali/acid leaching). This approach is widely acclaimed for binary or ternary-phase tungsten carbide alloys’ recycling [47]. Few researchers have conveyed the development and optimization of the semi-direct approach (using selective acid leaching, etc.) resulting in minimal WC oxidation in the product (a concern in direct and indirect recycling). Kücher et al. [107] reported a study on regulating the oxidation reduction potential in order to selectively leach the binder metal (such as Co) from a hard metal substrate in a 2.0 mol/L HCl solution.
Although the semi-direct approach presents a significant opportunity for cost-effective recycling of hard metals [109], it plays a relatively small role when compared to indirect recycling and the zinc process reclamation method [110], which account for the majority of the global annual total reclaimed hard metals. Table 1 shows a relative assessment of the necessities, advantages, and shortcomings associated with direct, indirect, and semi-direct recycling of cemented carbide.

Table 1.
A comparative assessment of requirements, advantages, and disadvantages associated with direct, indirect, and semi-direct recycling of WC scraps [47,85,95,101,102,111].
4.4. Progress in Bio-Derived Sustainable Organic Acids for Leaching Practice in WC Hard Metal Recycling
As described above, WC-containing scraps are often put to acid leaching in inorganic acid solutions, such as hydrochloric acid (HCl) or nitric acid (HNO3), which enables the selective dissolution of the binder metal while leaving the WC phase largely unaffected, making it suitable for subsequent reuse (indirect and semi-direct approaches) [93,112]. The acid reacts with the binder metal, typically Co, resulting in the formation of soluble metal salts, while the WC particles remain largely unaffected; however, this widely used practice concerning the use of inorganic acid is surrounded by serious dangers to humans and the environment. Mishandling of inorganic acids may result in corrosion of equipment and infrastructure, harm to the environment and ecosystem through careless disposal, higher energy consumption through prolonged reaction times at elevated temperatures, etc. In this regard, the use of bio-derived organic acids is a solution [113]. Their easy availability, renewable nature, affordable cost, and safety features show promising results for metal leaching purposes. Additionally, their solubility in water (as well as in other green solvents, such as ethanol) is a bonus. The most widely reported organic acids used in leaching treatment of WC hard and soft scraps are acetic acid [57,95], succinic acid [114], lactic acid [114], itaconic acid [114], lactobionic and maltobionic acids, formic acid [113,114], malic acid [113,115], citric acid [104,112,114], tartaric acid [113], and maleic acid [114,116].
4.5. Effect of WC Hard Metal Recycling on Final Products
4.5.1. Total Resource Recovery
Total resource recovery in tungsten carbide recycling is an essential aspect of sustainable resource management and the promotion of a circular economy. The tungsten present in cemented carbide scrap is not the sole valuable metal of significance. The presence of other elements, such as Co, Ta, Ti, Cr, Ni, and Mo also holds significance. While the direct method of zinc recycling is widely utilized for recycling WC scrap from end-use and first-use, its primary emphasis is on the retrieval of W powder. Other elements, apart from W, are not the major objective of this method; however, in light of alarm regarding resource conservation, achieving total resource recovery, sustainability has become extremely important in Industry 5.0. At present, the global attitude to recycling stresses the recovery of all resources from scrap materials, leaving no waste behind. Understanding this peak level of recycling compels a re-evaluation of the existing recycling procedures, with the aim of retrieving all the elements from cemented carbide scrap. Nonetheless, attaining total resource recovery encounters challenges, such as intricate material composition, contamination, and economic viability. Advanced separation technologies, stakeholder engagement, research and development, and supportive policies are key solutions to overcome these challenges and improve total resource recovery in tungsten carbide recycling.
4.5.2. Recycling and Its Related Costs
Despite the energy-saving benefits, the cost of tungsten recycling may not always be lower compared to purchasing ore concentrate. The cost of tungsten scrap can, at times, be higher than that of tungsten concentrate, depending on market conditions (for example, USD 15,000 per ton of carbide scrap vs. USD 14,000 per ton of Chinese concentrate [26]); however, the rewards of recycling extend beyond energy and cost savings. They also encompass material efficiency, supply security, and reduced environmental impacts. Tungsten mining and extraction routes result in substantial losses (10–40%), whereas recycling means achieving high yields (>95%) [117]. While recycling alone cannot fully replace the primary production due to increasing demand [42,118], it can contribute to securing an efficient secondary supply of tungsten. The main costs related to scrap re-entry come mainly from modification/cleaning/sorting or the reagent utilized on scrap of the WC materials before the recycling process, mainly indirect (chemical) recycling methods (Figure 12); therefore, prioritizing a reduced number of conversion steps is crucial to optimize the effectiveness of the recycling process.
4.5.3. Market of Re-Produced/Recycled Products
Recycling of tungsten carbide has experienced a consistent growth in recent years. Recycling companies, such as Sandvik, have implemented internal programs to collect and purchase used carbide tools and products, aiming to transform them back into secondary products for further use [37]. Among the recycling processes, the zinc recycling method has gained significant industrial recognition. One of the key rewards of the zinc recycling process is the production of powders suitable for manufacturing products with properties that meet marketable standards [95]. Presently, commercial hard metal products combine approximately 30 to 60% recycled powder blended with virgin powder to create the final products. The major players of tungsten tools and product recycling include Sandvik AB, Kennametal, Ceratizit Group, Tejing Tungsten, and IMC Group [38,81].
4.5.4. Environmental Concerns
Recycling facilities consistently encounter difficulties concerning renewable energy, the disposal of waste liquids, and the release of harmful gases that contribute to pollution. Owing to widespread utilization in various sectors, it is crucial to address the potential workplace dangers associated with tungsten carbide and its constituent metals. Inhaling WC particles poses health risks, including fibrosis and carcinogenic affects [119,120,121,122]. Additionally, the risks associated with the environment (soil) are also noted to be serious [123]. With recent recycling approaches, simply retrieving the metal for a specific purpose is not sufficient. An ideal recycling facility should achieve the highest level of operation with respect to conversion expenses, energy expenditures, purity of the WC-Co scrap, and environmental benefits [93,124,125]. Consequently, obtaining high-grade products from the scrap with the fewest conversion steps remains a challenging endeavor. As a result, recycling methods that exhibit the least environmental impact are anticipated to exhibit superior efficiency and sustainability.
Table 2 shows a comparative assessment of different recycling techniques concerning WC scraps.

Table 2.
Comparative assessment of direct, indirect, and semi-direct recycling of WC scraps [47,52,85,92,104,111,126].
4.6. Future of WC Recycling
Recycling plays a crucial role in both cost-effectiveness and environmental conservation by reusing scrap wastes, thereby reducing the need for consuming natural resources [51]. It is reported that recovering tungsten and cobalt from waste tungsten carbides can account for 20% to 30% of the overall supply, resulting in a significant reduction in raw material costs of around 15% to 50% [85]. According to the ITIA, it is projected that 35–40% of tungsten will be recycled (end-of-life recycling rate or functional recycling rate) in the future due to its high demand, highlighting the vital contribution of recycling industries to the growth of the WC market. Thanks to increasing focus in WC scrap recycling and its technology, the impact on the environment appears to decrease [88]; however, to benefit fully from recycling, several other factors need to be addressed, focusing on achieving ‘integrated sustainability’.
The significance of recycling has been extensively discussed, yet little attention has been given to the concept of ‘recycling quality’, which is mainly found in the scientific literature and EU legislation and has not been practically implemented [126]. In the context of sustainability, the focus is on achieving ‘high-quality’ or ‘functional recycling’. In relation to WC recycling, which has been discussed throughout this manuscript, it can be defined as functional recycling (i.e., secondary production). Functional recycling involves the separation and sorting of metal (tungsten or binder metal) from the discarded products to obtain recyclates that can be reintroduced into the raw material manufacturing process (e.g., hard metal) with minor adjustments, such as the addition of specific elements (e.g., carbon or a binder metal), to produce the desired grade of WC. In essence, functional recycling is closely associated with high-quality recycling, distinguishing it from low-quality or non-functional recycling [127].
4.6.1. The Untapped Losses or Non-Functional Recycling: A Challenge to Efficient Scrap Reuse
Losses or residues occur when WC, its constituent metals (binders), or both are not fully recovered through any of the recycling streams stated above. Losses also result from in-use dissipation (wear of WC cutting tools, etc.), improper recovery of molten slags developed during melting metallurgy, supply chain fault, informal recycling (non-government controlled and inappropriate technologies results in poor recycling efficiency), unstandardized practice, poor recycling knowledge, etc. These losses or residues generate an open metal life cycle and may end up in open environmental emissions (landfills, water resources, etc.) [128,129,130]. Surprisingly, these losses are not reflected in the reported recycling rate and often go unbothered or untapped. All this results in low quality recycling and lowering the metal life cycle (for example, W ~6 years, Co ~4 years, Ni ~59 years) [129]. In 2018, ITIA reported the tungsten (carbide) losses (not entering the recycling channel) to be around 67,000 Mt [54,55].
Non-functional recycling refers to the share of recycling where the waste or discard is collected and merged into a larger material stream as a foreign element or impurity. While this practice prevents environmental dispersion, it results in the loss of the metal’s functionality since it becomes almost impossible to recover the metal from the larger material stream. Although non-functional recycling is classified as a form of recycling, it contributes to an open metal life cycle, as discussed earlier [129,130]. It should be understood that in order to achieve an efficient sustainable system, it is required to create a closed-loop metal flow and, moreover, a higher rate of functional recycling, which can only be achieved once these untapped losses (and non-functional recycling) are brought down. Figure 20 shows a typical metal life cycle flow (which is also valid for tungsten and hard metals) in view of scrap use and loss of residues.
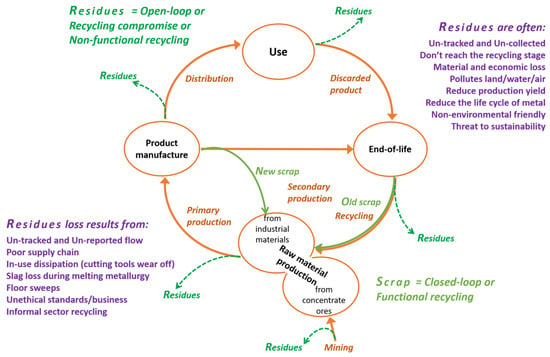
Figure 20.
A typical life cycle of a metal (hard metal), involving production, product manufacture, use, and end-of-life. The loss of residues at each period and the reuse of scraps are specified. Residue losses include emissions to the environment and non-functionally recycled wastes ending up in other material flows and flows to waste disposal facilities (landfills, slags, dilution) [54,55,128,129,130,131,132].
5. Ways to Improve the Recycling Efficiency and Sustainability of WC Hard Metal
5.1. Circular Economy
The availability of tungsten is crucial for technologically advanced economies, prompting the EU and other international entities to launch various initiatives aimed at ensuring secure and uninterrupted access to these resources. In this regard, circular economy is the solution [39,40]. Circular economies take raw materials used to make goods and loops them back into the economy instead of throwing them away (Figure 21b) [31,133,134]. It must be understood that the consumption/use phase in circular economy, where the product or material achieves its highest value, is the most important phase. The longer the product is in the consumption phase, for example, is serviced, repaired, and upgraded, the less the need for new manufacturing. In a perfectly efficient circular economy, the consumption phase gets longer and longer with novel material/product design offering the highest life cycle value (Figure 21c illustrates futuristic or new generation circular economy) [32].
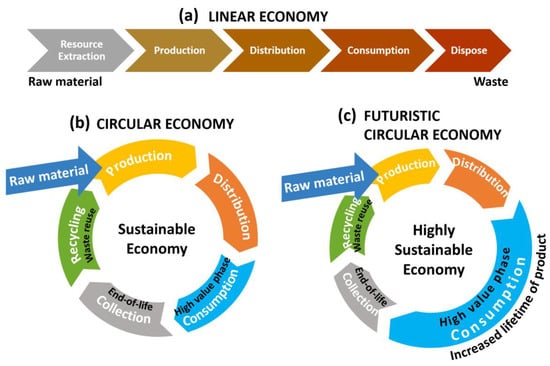
Figure 21.
(a) Linear economy vs. (b) current generation circular economy vs. (c) futuristic circular economy. Linear economy solely focuses on profitability, while circular approaches profitability through sustainability. Futuristic circular economy goals are aimed at extending the high-value consumption/use phase of the product [31,81,133,134,135].
An enhanced circularity of CRMs coupled with efficient extraction and minimal processing are approaches towards sustainability [82,136]. The aim is to adopt a holistic and highly flexible approach to recycling, encompassing not only tungsten but also other valuable elements, such as tantalum (Ta), cobalt (Co), and nickel (Ni). By broadening the focus beyond tungsten, the goal is to maximize the recovery and utilization of incorporated valuable elements throughout the recycling process [81,84,137]. The state of the art in tungsten circularity within industries encompasses various practices aimed at promoting resource efficiency, recycling, and its economy; however, the current industry is extremely dynamic and continually evolving as new technologies, policies, and practices emerge. There are currently several issues that need to be addressed in order to maximize the benefits of a closed loop economy in the tungsten carbide industry.
Challenges in Circularity of Tungsten Carbide
Collection and sorting of tungsten carbide scrap: The collection and sorting of tungsten carbide scrap at present is the biggest hurdle within the recycling process (collection phase, Figure 21) [26]. The scrap is often mixed with diverse substances, rendering the task of distinguishing and segregating them arduous and time-intensive. This problem is especially pronounced in sectors where tungsten carbide scrap is produced in limited volumes and dispersed across various sites, as observed in the machining industry [131].
Contamination and quality control: The contamination of tungsten carbide scrap is another significant concern in the recycling process (collection phase) [109,126]. Even minor quantities of contaminants, such as oil or alternative metals, can diminish the quality of the recycled tungsten carbide and have adverse effects on its functionality. To guarantee compliance with the specified standards, it is imperative to establish quality assurance protocols for the recycled tungsten carbide [8,70].
Processing technologies: The conversion of tungsten carbide scrap into reusable material (production phase, Figure 21) presents a notable obstacle owing to its exceptional hardness and resistance to wear (resistance against mechanical crushing) [15,92,98,99]. Conventional recycling methods, such as melting and casting, may not yield satisfactory outcomes for tungsten carbide (due to the low binder content) [11,15,138]. Advanced processing technologies, such as powder metallurgy and sintering, are indispensable for achieving efficient recycling of tungsten carbide; however, the utilization of these techniques demands specialized equipment and expertise, which can be both expensive and challenging to acquire [47].
Economic and market challenges: Recycled tungsten carbide typically fetches a lower price compared to newly manufactured tungsten carbide, diminishing its appeal to both recyclers and consumers (distribution phase, Figure 21) [47,56,87]. Moreover, the demand for recycled tungsten carbide can vary in response to the supply and demand dynamics of the industries reliant on its utilization.
Policy and regulatory barriers: Restrictions pertaining to the disposal of hazardous waste might impose constraints on the transportation and storage of tungsten carbide scrap, thereby impeding recycling efforts (distribution phase). Similarly, policies associated with product guarantees and legal responsibilities may deter the utilization of recycled tungsten carbide in specific applications [30,127,131].
To overcome these obstacles, several approaches can be implemented. Firstly, the establishment of effective collection and sorting systems for tungsten carbide scrap can mitigate contamination issues and ensure the production of high-quality recycled material [27,139,140]. Secondly, the exploration of novel processing technologies can provide alternative and more efficient avenues for tungsten carbide recycling [38,108,140,141]. Thirdly, the implementation of incentives and policies that incentivize the utilization of recycled tungsten carbide across diverse applications can foster a resilient market for recycled materials and encourage their widespread adoption [30,39,74,81,82,84,131]. Lastly, fostering collaboration among industry stakeholders and facilitating knowledge exchange can address common challenges and facilitate the integration of circular economy principles within the tungsten carbide sector [27,77,81,142].
5.2. Achieving Integrated Sustainability
Sustainable development is an urgent global priority that necessitates the adoption of resource-efficient and environmental friendly practices [8,76,143]. Recycling is a significant parameter towards a sustainable future, but it is not the only parameter driving towards complete ‘integrated sustainability’ [144,145,146]. While recycling plays a crucial role in waste reduction and resource conservation, achieving integrated sustainability requires a multifaceted approach that addresses various aspects of environment, social, and economic well-being. It must be understood that to achieve or boost circular economy practices or a complete material (use) closed loop, cumulative sustainability from government, society, and the stakeholders (businessman) needs to be satisfied [32,75,135,142]. In practical terms, integrated sustainability involves integrating sustainable practices throughout the entire life cycle of a product, project, or organization (procurement, distribution, and utilization) [147,148]. It refers to the incorporation of sustainable practices and principles into various aspects of a system or organization to create long-term balance and a resilient approach for development that meets the needs of the present without compromising the ability of future generations to meet their own needs [144,149]. Figure 22 represents an ‘integrated sustainability’ tree identifying the most necessitated aspects (and their sub-aspects) of environment, society, institutions, government, and economy (business), requiring strong interlinking or integration in order to advance to complete sustainability.
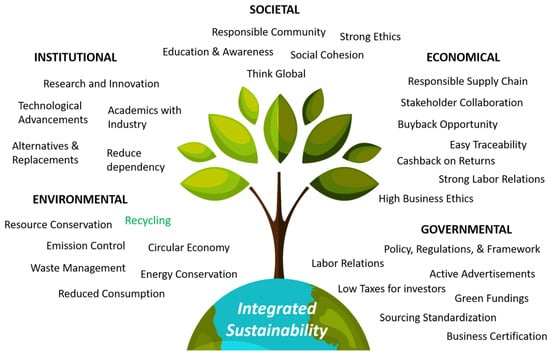
Figure 22.
Integrated sustainability tree—the most necessitated aspects and sub-aspects to be interlinked, with recycling being one of the key branches of this massive tree [74,75,76,77,95,128,129,131,135,145,146,147,148,149,150,151,152,153].
In regards to tungsten (carbide) recycling, achieving integrated sustainability involves adopting sustainable practices throughout the entire life cycle of tungsten, from mining and extraction to recycling and reuse [30,147].
Integrated sustainability starts with responsible tungsten mining practices. This includes implementing environmentally sound mining techniques, minimizing ecosystem disruption, and ensuring the safety and well-being of workers [27,76,146]. Sustainable mining practices also involve proper land reclamation and rehabilitation after extraction. It encourages the use of advanced technologies for efficient tungsten extraction and sorting processes [82,154]. These technologies help minimize energy consumption, water usage, and emissions during the extraction stage. They also improve the recovery rates of tungsten from ore, reducing the need for further extraction. On the other hand, a strong emphasis on the supply chain transparency in tungsten recycling should be made, which involves tracing the origin of tungsten-containing recyclate and ensuring their compliance with ethical and environmental standards [31,38,84,150]. Transparent supply chains help prevent the use of conflict minerals and promote responsible sourcing practices. Education, research, and such institutional help is of immense importance in fostering knowledge sharing, innovation, and the development of best practices in tungsten recycling (such as ‘Design for X’ or ‘Design for Excellence’) [77,82,155,156,157,158]. This includes optimizing energy consumption during the recycling, refining, and manufacturing stages. The development of energy-efficient technologies, such as advanced smelting and refining processes, can be investigated to minimize energy requirements and the associated emissions [31,64,110].
The importance of financial support and mechanisms, such as tax benefits to green industries, green bonds, green fundings, green loans, and support for start-ups, in achieving integrated sustainability should not be undermined [159,160]. Green bonds/funds/loans are financial instruments specifically designed to fund environmentally friendly projects and start-ups, including recycling initiatives. By issuing green bonds, recycling companies or projects can raise capital from investors who are interested in supporting sustainable initiatives. The proceeds from these bonds can be used to finance the expansion of recycling facilities, upgrade equipment, implement innovative recycling technologies, or develop new recycling infrastructure [159]. In all of these financial mechanisms, it is important to ensure transparency and accountability. Proper reporting and verification processes should be in place to ensure that the funds raised or invested are being utilized for the intended green purposes and that the recycling projects are delivering the expected environmental and social benefits. Last but not least, strong law and order through policy and framework should exist in order to monitor sustainable integrity among the branches [145,146].
5.3. Sustainable Digitalization for Futuristic ‘Smart and Green’ Industry 6.0: Strengthened by Twin Transition and Industry 5.0
The world is in the era of digitalization, when much of our daily business is heavily dependent on innovative digital and computer technologies. Due to their fast data sharing and processing, these modern technologies have found applications in socio-economic, environmental, sustainable, and climate research applications to augment the efficiency of a given system in study [161,162,163]. Digitalization uses a set of tools for the integration of digital technologies into focused tasks [164]. The tedious effort of digitizing a vast amount of data collected over years or decades, utilizing the techniques of real-time analysis, machine learning, and artificial intelligence, has resulted in profitably understanding/tracking/improving a cycle, phenomena, etc., in several fields, including the engineering, tribology, or waste management of engineered components [165,166,167,168,169,170,171] which can be achieved in relatively quicker time (Figure 23).

Figure 23.
The potential of digitalization (robotics, artificial intelligence, IoT, cloud computing, and data analytics) to enhance waste/scrap management and recycling. Information adapted from European Scientific Advisory Board on Climate Change [172].
With the quick pace of industrial revolutions and advancements into Industry 5.0, the focus is set on co-leading the digital and green transitions [33]. The twin transition, as shown in Figure 24, refers to the simultaneous and interconnected processes of transitioning towards both digitalization and sustainability by putting research and innovation to work to create a sustainable, human-centric, and resilient ‘Smart Industry of the future’ [173]. Furthermore, it acknowledges the potential of digitalization to enhance resource efficiency, enable renewable energy integration, optimize supply chains, and promote circular economy principles. It also recognizes the importance of sustainability considerations in digitalization efforts to ensure that technological advancements contribute to environmental and social well-being [161]. This twin transition is often termed as ‘sustainable digitalization’ across researchers and policymakers [174,175,176].
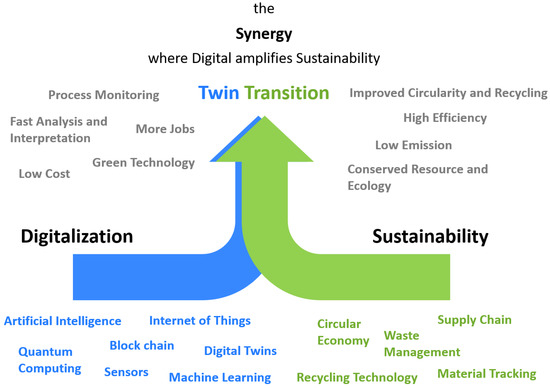
Figure 24.
Twin transition toward a sustainable digitalized future [161,162,174,175,177,178].
In the context of tungsten recycling, for example, sensors and Internet of Things devices can be used to monitor and collect data on various aspects of the recycling process, such as material composition, quality, and quantity. These data can then be analyzed using data analytics and artificial intelligence to identify opportunities for process optimization, waste reduction, and energy efficiency (smart grids). Blockchain technology, for instance, can be utilized to securely track and record the origin, processing, and certification of recycled metals [163]. This enhances trust and accountability in the supply chain, ensuring responsible sourcing and compliance with environmental standards. Sustainability involves adopting sustainable practices throughout the recycling value chain through implementing environmentally friendly and energy-efficient technologies in the recycling process, minimizing waste and emissions, and promoting responsible sourcing and material recovery. It also entails integrating circular economy principles by prioritizing material reuse, remanufacturing, and recycling to minimize the mining of raw tungsten resource [31,175]. Essentially, the ability to make well-informed sustainable decisions [145] utilizing digital technologies is the soul of twin transition. Figure 25 shows the digitalization aspects required in order to create a sustainable and perfectly efficient recycling approach.
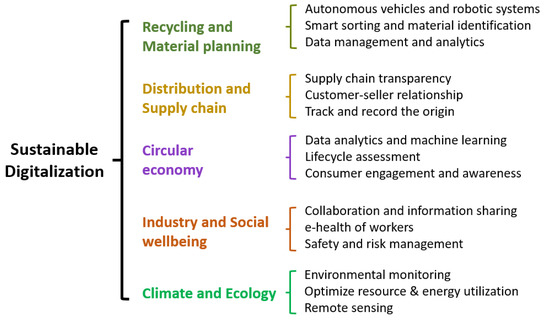
Figure 25.
Summary of the digitalization aspects related to a sustainable recycling approach [26,32,150,161,162,163,174,178].
Moving forward from the ‘technology-driven’ Industry 4.0, it is now widely accepted that serious steps must be taken towards saving environment in the ‘value-driven’ Industry 5.0 [33,171]; however, the impression of Industry 5.0 perfecting and extending the attributes and promises of Industry 4.0 advocates that they are to be considered hand-in-hand, i.e., of ‘technology-driven’ Industry 4.0 and ‘value-driven’ Industry 5.0 [33]. This points us to an era of ‘Techno-Social revolution’ while being ‘human-centric’ [179,180,181]. Poor recycling methods may violate the principle of human-centered design [182]. Disorganized recycling is one of the major contributions to air, water, and land pollution and, broadly, to all environmental concerns [141]. It must be understood that recycling is only one part of product/material life cycle. The other large parts (i.e., business, technology, supply chain, etc.) join hands to make recycling happen. The greater idea is to stabilize the climate in the long term while delivering ‘human-centric’ design/methods/systems at every step [158,183,184]. Thanks to the digitalization efforts in recycling, the tracking of material flow, environment effect, etc., is more efficient than it has ever been (Figure 26).
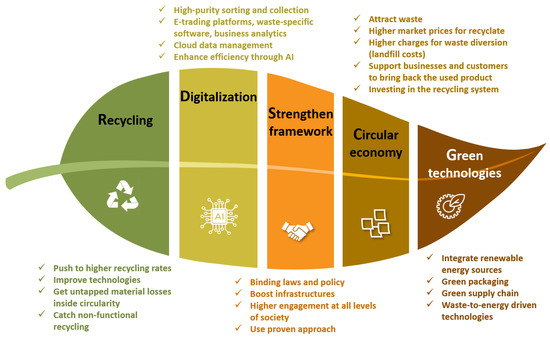
Figure 26.
The sustainability leaf of Industry 5.0 signifying the need for integration in areas of recycling, technologies, policy structure, circularity, and green technologies [38,74,76,81,82,127,134,137,146,158,161,162,163,164,174,175,178,183,184].
By aligning the principles of Industry 5.0, integrated sustainability, and twin transition, recycling industries can drive positive change. They can create more sustainable recycling and resilient systems that combine human ingenuity, technological advancements in recycling, and sustainable practices to address environmental challenges, increase recycling efficiency, promote social equity, and drive economic prosperity [74,147,174,179]. It is predicted that if low carbon energy technologies align themselves with information and communications technologies and become part of a recycling group, there is an increased prospect that the next industrial revolution will be ‘smart and green’ [177,185]. Figure 27 shows the objective-transition from Industry 4.0 to Industry 5.0, to futuristic Industry 6.0. The ‘smart and green’ Industry 6.0 is expected to drive the future by intelligent data collection and sorting (cloud computing) [174], proactive decision making [141], omni-channel interactions [186], hyperconnected business environments [187], and smart renewable energy sources integration [76].
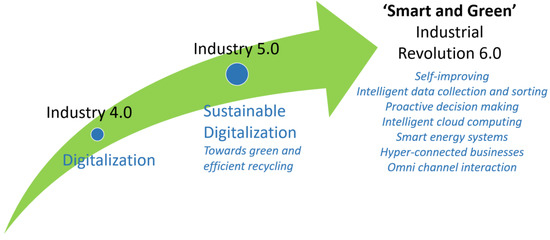
Figure 27.
The transformation/transition focus of Industry 4.0 (digitalization) to Industry 5.0 (sustainable digitalization) to futuristic Industry 6.0 (smart and green revolution) [141,142,146,148,173,181,184,186,187].
6. Summary and Outlook
With the transition towards sustainability driven by the emergence of ‘techno-social’ Industry 5.0, the focus on sustainable recycling has become more crucial than ever. The current work comprehensively reviews various recycling methods of WC hard metal scraps, since they remain an increasingly important raw material source for the global tungsten industry. The diverse chemical and physical composition of WC scrap requires the adoption of various recycling techniques to ensure the recovery of substantial quantities of WC with exceptional purity. Several direct (mechanical), indirect (chemical), and semi-direct (mechano-chemical) methods have been reported for recycling different grades of WC hard metal scraps. Direct methods enable the recovery of recyclates with the same composition as the input product, while indirect or semi-direct methods involve chemical modifications to extract pure tungsten or intermediate products, such as binder metals. The concept of an ‘improved’ circular economy by exploiting methodologies of ‘Design for X’ has been presented to create a highly sustainable closed-loop scrap circularity. In addition to recycling, various economic, societal, and governmental factors contributing to achieving integrated sustainability have been identified to meaningfully improve the recycling sector. The importance of digitalization in WC recycling and the utilization of Industry 5.0 tools to enhance recycling rates and create closed material/product life cycles has been conclusively shown. The review has contributed insight into the potential expectations for the future Industry 6.0.
Author Contributions
Conceptualization, R.K. and M.A.; methodology, R.K., A.K. and M.A.; investigation, R.K., A.K. and D.G.; writing—original draft preparation, R.K.; writing—review and editing, R.K., M.A., I.H. and P.K.; resources and funding, M.A., D.G., I.H. and P.K.; supervision, M.A. and I.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from the Estonian Research Council grants (PRG643, MNHA22057), EIT Raw Materials (‘RENEW’ VHE22005), and M-ERA.Net project (‘DuplexCER’ MNHA22040).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Some or all of the data, and models that support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schröter, K. Sintered Hardmetal Alloy and Procedure for Its Fabrication. German Reichspatentamt Patent No. 420689, 30 March 1923. [Google Scholar]
- Spriggs, G.E. A history of fine grained hardmetal. Int. J. Refract. Met. Hard Mater. 1995, 13, 241–255. [Google Scholar] [CrossRef]
- Kumar, R.; Antonov, M.; Beste, U.; Goljandin, D. Assessment of 3D printed steels and composites intended for wear applications in abrasive, dry or slurry erosive conditions. Int. J. Refract. Met. Hard Mater. 2020, 86, 105126. [Google Scholar] [CrossRef]
- Walbrühl, M.; Linder, D.; Ågren, J.; Borgenstam, A. Diffusion modeling in cemented carbides: Solubility assessment for Co, Fe and Ni binder systems. Int. J. Refract. Met. Hard Mater. 2017, 68, 41–48. [Google Scholar] [CrossRef]
- Guo, Z.; Xiong, J.; Yang, M.; Jiang, C. WC–TiC–Ni cemented carbide with enhanced properties. J. Alloys Compd. 2008, 465, 157–162. [Google Scholar] [CrossRef]
- Upadhyaya, G.S. Materials science of cemented carbides—An overview. Mater. Des. 2001, 22, 483–489. [Google Scholar] [CrossRef]
- Exner, H.E. Physical and chemical nature of cemented carbides. Int. Met. Rev. 2013, 24, 149–170. [Google Scholar] [CrossRef]
- Rizzo, A.; Goel, S.; Luisa Grilli, M.; Iglesias, R.; Jaworska, L.; Lapkovskis, V.; Novak, P.; Postolnyi, B.O.; Valerini, D. The Critical Raw Materials in Cutting Tools for Machining Applications: A Review. Materials 2020, 13, 1377. [Google Scholar] [CrossRef]
- Kumar, R.; Hussainova, I.; Rahmani, R.; Antonov, M. Solid lubrication at high-temperatures—A review. Matarials 2022, 15, 1695. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.G.P.; Schubert, W.D.; Lux, B. The role of the binder phase in the WC-Co sintering. Mater. Res. 2001, 4, 59–62. [Google Scholar] [CrossRef]
- García, J.; Ciprés, V.C.; Blomqvist, A.; Kaplan, B. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mater. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Kumar, R.; Antonov, M.; Liu, L.; Hussainova, I. Sliding wear performance of in-situ spark plasma sintered Ti-TiBw composite at temperatures up to 900 °C. Wear 2021, 476, 203663. [Google Scholar] [CrossRef]
- Kumar, R.; Aydinyan, S.; Ivanov, R.; Liu, L.; Antonov, M.; Hussainova, I. High-Temperature Wear Performance of hBN-Added Ni-W Composites Produced from Combustion-Synthesized Powders. Materials 2022, 15, 1252. [Google Scholar] [CrossRef] [PubMed]
- Antonov, M.; Zahavi, A.; Kumar, R.; Tamre, M.; Klimczyk, P. Performance of Al2O3 cBN materials and the perspective of using hyperspectral imaging during cutting tests. Proc. Est. Acad. Sci. 2021, 70, 524–532. [Google Scholar] [CrossRef]
- Kübarsepp, J.; Juhani, K.; Tarraste, M. Abrasion and Erosion Resistance of Cermets: A Review. Materials 2021, 15, 69. [Google Scholar] [CrossRef]
- Antonov, M.; Veinthal, R.; Yung, D.L.; Katušin, D.; Hussainova, I. Mapping of impact-abrasive wear performance of WC–Co cemented carbides. Wear 2015, 332–333, 971–978. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, L.; Antonov, M.; Ivanov, R.; Hussainova, I. Hot Sliding Wear of 88 wt.% TiB–Ti Composite from SHS Produced Powders. Materials 2021, 14, 1242. [Google Scholar] [CrossRef]
- Torres, H.; Slawik, S.; Gachot, C.; Prakash, B.; Ripoll, M.R. Microstructural design of self-lubricating laser claddings for use in high temperature sliding applications. Surf. Coat. Technol. 2018, 337, 24–34. [Google Scholar] [CrossRef]
- Kumar, R.; Antonov, M.; Klimczyk, P.; Mikli, V.; Gomon, D. Effect of cBN content and additives on sliding and surface fatigue wear of spark plasma sintered Al2O3-cBN composites. Wear 2022, 494–495, 204250. [Google Scholar] [CrossRef]
- Rahmani, R.; Karimi, J.; Kamboj, N.; Kumar, R.; Brojan, M.; Tchórz, A.; Skrabalak, G.; Lopes, S.I. Fabrication of localized diamond-filled copper structures via selective laser melting and spark plasma sintering. Diam. Relat. Mater. 2023, 136, 109916. [Google Scholar] [CrossRef]
- Tungsten Oxides & Acid|International Tungsten Industry Association (ITIA). Available online: https://www.itia.info/tungsten-oxides-acid.html (accessed on 12 March 2023).
- Katiyar, P.K.; Randhawa, N.S. A comprehensive review on recycling methods for cemented tungsten carbide scraps highlighting the electrochemical techniques. Int. J. Refract. Met. Hard Mater. 2020, 90, 105251. [Google Scholar] [CrossRef]
- Monet, P. The World Is Running Low on Tungsten; Why You Should Care? 2012. Available online: https://www.forbes.com/sites/ciocentral/2012/03/14/the-world-is-running-low-on-tungsten-why-you-should-care/?sh=6de2a3c1361f (accessed on 6 February 2023).
- Lunn, D.D.; Lunn, R.M. Cemented Carbides—A Success Story. ITIA Newsl. Int. Tungsten Ind. Assoc. 2010. Available online: https://www.itia.info/assets/files/Newsletter_2010_06.pdf (accessed on 7 February 2023).
- Dvořáček, J.; Sousedíková, R.; Vrátný, T.; Jureková, Z. Global tungsten demand and supply forecast. Arch. Min. Sci. 2017, 62, 3–12. [Google Scholar] [CrossRef]
- Leal-Ayala, D.R.; Allwood, J.M.; Petavratzi, E.; Brown, T.J.; Gunn, G. Mapping the global flow of tungsten to identify key material efficiency and supply security opportunities. Resour. Conserv. Recycl. 2015, 103, 19–28. [Google Scholar] [CrossRef]
- Tang, L.; Wang, P.; Graedel, T.E.; Pauliuk, S.; Xiang, K.; Ren, Y.; Chen, W.Q. Refining the understanding of China’s tungsten dominance with dynamic material cycle analysis. Resour. Conserv. Recycl. 2020, 158, 104829. [Google Scholar] [CrossRef]
- Mateus, A.; Lopes, C.; Martins, L.; Gonçalves, M.A. Current and foreseen tungsten production in portugal, and the need of safeguarding the access to relevant known resources. Resources 2021, 10, 64. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Filho, W.L.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Domaracka, L.; Matuskova, S.; Tausova, M.; Senova, A.; Kowal, B. Efficient Use of Critical Raw Materials for Optimal Resource Management in EU Countries. Sustainability 2022, 14, 6554. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Vogel-Heuser, B.; Wang, L. Industry 4.0 and Industry 5.0—Inception, conception and perception. J. Manuf. Syst. 2021, 61, 530–535. [Google Scholar] [CrossRef]
- Peng, H.; Forsberg, K. Progress on the Recovery of Critical Raw Materials. JOM 2022, 74, 1932–1933. [Google Scholar] [CrossRef]
- Kulu, P.; Tarbe, R.; Käerdi, H.; Goljandin, D. Abrasivity and grindability study of mineral ores. Wear 2009, 267, 1832–1837. [Google Scholar] [CrossRef]
- Sandvik Coromant recycling helps US veterans. Met. Powder Rep. 2014, 69, 11. [CrossRef]
- Sandvik Mining and Rock Technology. Sandvik Introduces Industry’s First ‘Opt-Out’ Carbide Recycling. Available online: https://www.rocktechnology.sandvik/en/news-and-media/news-archive/2023/03/sandvik-introduces-industrys-first-opt-out-carbide-recycling/ (accessed on 23 March 2023).
- Schlosser, M.; Abiad, V. Sandvik Coromant Recycling Concept. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare earth elements: Industrial applications and economic dependency of Europe. Proc. Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- CRM Alliance. Recycling Critical Raw Materials. Available online: https://www.crmalliance.eu/recycling-crms (accessed on 10 February 2023).
- Graedel, T.E.; Gunn, G.; Espinoza, L.T. Metal resources, use and criticality. In Critical Metals Handbook; Wiley: Hoboken, NJ, USA, 2013; pp. 1–19. [Google Scholar] [CrossRef]
- Tonge, P.; Roy, A.; Patel, P.; Beall, C.J.; Stoyanov, P. Tribological Evaluation of Lead-Free MoS2-Based Solid Film Lubricants as Environmentally Friendly Replacements for Aerospace Applications. Lubricants 2022, 10, 7. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Tungsten Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/tungsten-statistics-and-information (accessed on 20 March 2023).
- International Tungsten Industry Association (ITIA). Tungsten Mining & Beneficiation. Available online: https://www.itia.info/mining-beneficiation.html (accessed on 14 March 2023).
- Mining Digital. Masan Resources Nui Phao Mine: The World’s Largest Tungsten Mine. Available online: https://miningdigital.com/smart-mining/masan-resources-nui-phao-mine-worlds-largest-tungsten-mine (accessed on 14 March 2023).
- Shemi, A.; Magumise, A.; Ndlovu, S.; Sacks, N. Recycling of tungsten carbide scrap metal: A review of recycling methods and future prospects. Miner. Eng. 2018, 122, 195–205. [Google Scholar] [CrossRef]
- Increasing Yield on Tungsten and Tantalum Ore Production by Means of Advanced and Flexible Control on Crushing, Milling and Separation Process. OptimOre H2020 EU Project. 2014. Available online: https://cordis.europa.eu/project/id/642201 (accessed on 21 March 2023).
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- International Tungsten Industry Association (ITIA). Tungsten Processing. Available online: https://www.itia.info/tungsten-processing.html (accessed on 14 March 2023).
- Furberg, A.; Arvidsson, R.; Molander, S. Environmental life cycle assessment of cemented carbide (WC-Co) production. J. Clean. Prod. 2019, 209, 1126–1138. [Google Scholar] [CrossRef]
- Polini, R.; Marcucci, A.; Nunziante, P.; De Filippis, P.; Marcheselli, G. Toward Greener Synthesis of WC Powders for Cemented Tungsten Carbides Manufacturing. ACS Sustain. Chem. Eng. 2023, 16, 29. [Google Scholar] [CrossRef]
- Bhavan, I. Indian Minerals Yearbook 2019, 58th ed.; Tungsten (Final Release); Government of India Ministry of Mines Indian Bureau of Mines: Odisha, India, 2021. [Google Scholar]
- Zeiler, B.; Bartl, A.; Schubert, W.D. Recycling of tungsten: Current share, economic limitations, technologies and future potential. Int. J. Refract. Met. Hard Mater. 2021, 98, 105546. [Google Scholar] [CrossRef]
- Zeiler, B.; Schubert, W.-D.; Bartl, A.; Wien, T.U. Recycling of Tungsten: Current Share, Economic Limitations and Future Potential. Tungsten ITIA. 2018. Available online: https://www.itia.info (accessed on 17 March 2023).
- Edtmaier, C.; Schiesser, R.; Meissl, C.; Schubert, W.D.; Bock, A.; Schön, A.; Zeiler, B. Selective removal of the cobalt binder in WC/Co based hardmetal scraps by acetic acid leaching. Hydrometallurgy 2005, 76, 63–71. [Google Scholar] [CrossRef]
- Viswanathan, R.; Bakker, W. Materials for ultrasupercritical coal power plants—Turbine materials: Part II. J. Mater. Eng. Perform. 2001, 10, 96–101. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Fu, W.; Hu, Y.X.; Vaughan, J.; Wang, L.Z. The role of tungsten-related elements for improving the electrochemical performances of cathode materials in lithium ion batteries. Tungsten 2021, 3, 245–259. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Z.; Han, K.; Zhang, L.; Ding, X.; Wang, X.; Mai, L. Nano-Sized Niobium Tungsten Oxide Anode for Advanced Fast-Charge Lithium-Ion Batteries. Small 2022, 18, 2107365. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.K.; Miyakoda, K.; Saito, A.; Yasoda, Y.; Kajiwara, K.; Kimura, M.; Tsuchiya, K. Microneedle pH Sensor: Direct, Label-Free, Real-Time Detection of Cerebrospinal Fluid and Bladder pH. ACS Appl. Mater. Interfaces 2017, 9, 21651–21659. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, T.; Suzuki, M.; Aoyagi, S. Development of Minimally Invasive Microneedle Made of Tungsten- Sharpening Through Electrochemical Etching and Hole Processing for Drawing up Liquid Using Excimer Laser. J. Robot. Mechatron. 2013, 25, 755–761. [Google Scholar] [CrossRef]
- Janáky, C.; Rajeshwar, K.; De Tacconi, N.R.; Chanmanee, W.; Huda, M.N. Tungsten-based oxide semiconductors for solar hydrogen generation. Catal. Today 2013, 199, 53–64. [Google Scholar] [CrossRef]
- Garcia-Esparza, A.T.; Cha, D.; Ou, Y.; Kubota, J.; Domen, K.; Takanabe, K. Tungsten Carbide Nanoparticles as Efficient Cocatalysts for Photocatalytic Overall Water Splitting. ChemSusChem 2013, 6, 168–181. [Google Scholar] [CrossRef]
- Mim, R.S.; Aldeen, E.S.; Alhebshi, A.; Tahir, M. Recent advancements in strategies to improve performance of tungsten-based semiconductors for photocatalytic hydrogen production: A review. J. Phys. D Appl. Phys. 2021, 54, 503001. [Google Scholar] [CrossRef]
- Christian, J.; Gaur, R.P.S.; Wolfe, T.; Trasorras, J.R.L. Tungsten Chemicals and their Applications. ITIA Newsletter. June 2011. Available online: https://www.itia.info/assets/files/newsletters/Newsletter_2011_06.pdf (accessed on 15 March 2023).
- International Tungsten Industry Association (ITIA). Tungsten Applications. Available online: https://www.itia.info/applications.html (accessed on 15 March 2023).
- Wang, Y.; Liu, T.; Li, H.; Liu, B.; Yang, L. Tungsten-based photocatalysts with UV–Vis–NIR photocatalytic capacity: Progress and opportunity. Tungsten 2019, 1, 247–257. [Google Scholar] [CrossRef]
- Akgün, M.; Demir, H. Optimization of Cutting Parameters Affecting Surface Roughness in Turning of Inconel 625 Superalloy by Cryogenically Treated Tungsten Carbide Inserts. SN Appl. Sci. 2021, 3, 277. [Google Scholar] [CrossRef]
- Khan, M.A.; Gupta, K. A study on machinability of nickel based superalloy using micro-textured tungsten carbide cutting tools. Mater. Res. Express 2020, 7, 016537. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, H.; Hu, Q.; Zheng, S.; Li, L.; Xu, J.; Pang, H. Tungsten-Based Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1707500. [Google Scholar] [CrossRef]
- Fernandes, C.; Taurino, I. Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants. Sensors 2022, 22, 3062. [Google Scholar] [CrossRef] [PubMed]
- AbuAlRoos, N.J.; Azman, M.N.; Amin, N.A.B.; Zainon, R. Tungsten-based material as promising new lead-free gamma radiation shielding material in nuclear medicine. Phys. Medica 2020, 78, 48–57. [Google Scholar] [CrossRef]
- Sanyal, G.; Vaidyanathan, A.; Rout, C.S.; Chakraborty, B. Recent developments in two-dimensional layered tungsten dichalcogenides based materials for gas sensing applications. Mater. Today Commun. 2021, 28, 102717. [Google Scholar] [CrossRef]
- Hatayama, H. The metals industry and the Sustainable Development Goals: The relationship explored based on SDG reporting. Resour. Conserv. Recycl. 2022, 178, 106081. [Google Scholar] [CrossRef]
- Barbier, E.B.; Burgess, J.C. The SDGs and the Systems Approach to Sustainability. In Economics of the SDGs; Springer: Berlin/Heidelberg, Germany, 2021; pp. 15–37. [Google Scholar] [CrossRef]
- Hodgkinson, J.H.; Smith, M.H. Climate change and sustainability as drivers for the next mining and metals boom: The need for climate-smart mining and recycling. Resour. Policy 2021, 74, 101205. [Google Scholar] [CrossRef]
- Fuso Nerini, F.; Sovacool, B.; Hughes, N.; Cozzi, L.; Cosgrave, E.; Howells, M.; Tavoni, M.; Tomei, J.; Zerriffi, H.; Milligan, B. Connecting climate action with other Sustainable Development Goals. Nat. Sustain. 2019, 2, 674–680. [Google Scholar] [CrossRef]
- United Nations. United Nations Global Compact Progress Report: Business Solutions to Sustainable Development; United Nations: San Francisco, CA, USA, 2017. [Google Scholar]
- Hyperion Materials & Technologies. Hyperion Carbide Recycling. Available online: https://www.hyperionmt.com/globalassets/nosearchfiles/product-pdfs/recycling/Hyperion_Carbide_Recycling (accessed on 21 March 2023).
- Bobba, S.; Claudiu, P.; Huygens, D.; Dias, P.A.; Gawlik, B.; Tzimas, E. Report on Critical Raw Materials and the Circular Economy; European Union: Maastricht, The Netherlands, 2018; Available online: https://op.europa.eu/en/publication-detail/-/publication/d1be1b43-e18f-11e8-b690-01aa75ed71a1 (accessed on 23 March 2023).
- Hool, A.; Schrijvers, D.; van Nielen, S.; Clifton, A.; Ganzeboom, S.; Hagelueken, C.; Harada, Y.; Kim, H.; Ku, A.Y.; Meese-Marktscheffel, J.; et al. How companies improve critical raw material circularity: 5 use cases: Findings from the International Round Table on Materials Criticality. Miner. Econ. 2022, 35, 325–335. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D. Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Fetting, C. The European Green Deal; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Cimprich, A.; Young, S.B.; Schrijvers, D.; Ku, A.Y.; Hagelüken, C.; Christmann, P.; Eggert, R.; Habib, K.; Hirohata, A.; Hurd, A.J.; et al. The role of industrial actors in the circular economy for critical raw materials: A framework with case studies across a range of industries. Miner. Econ. 2022, 36, 301–319. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Lee, J.C.; Bae, M.; Kumar, V. Reclamation of tungsten from carbide scraps and spent materials. J. Mater. Sci. 2019, 54, 83–107. [Google Scholar] [CrossRef]
- Kumar, R.; Malaval, B.; Antonov, M.; Zhao, G. Performance of polyimide and PTFE based composites under sliding, erosive and high stress abrasive conditions. Tribol. Int. 2020, 147, 106282. [Google Scholar] [CrossRef]
- Kumar, R.; Antonov, M.; Holovenko, Y.; Surzenkov, A. Erosive Wear Resistance of Nature-inspired Flexible Materials. Tribol. Lett. 2020, 68, 51. [Google Scholar] [CrossRef]
- Shedd, K.B. Tungsten Recycling in the United States in 2000; U.S. Geological Survey Circular; United States Geological Survey: Reston, VA, USA, 2000. Available online: http://pubs.usgs.gov/circ/circ1196-R/ (accessed on 21 March 2023).
- Venkateswaran, S.; Schubert, W.D.; Lux, B.; Ostermann, M.; Kieffer, B. W-scrap recycling by the melt bath technique. Int. J. Refract. Met. Hard Mater. 1996, 14, 263–270. [Google Scholar] [CrossRef]
- Joost, R.; Pirso, J.; Viljus, M.; Letunovitš, S.; Juhani, K. Recycling of WC-Co hardmetals by oxidation and carbothermal reduction in combination with reactive sintering. Est. J. Eng. 2012, 18, 127–139. [Google Scholar] [CrossRef]
- Pirso, J.; Viljus, M.; Letunovits, S.; Juhani, K. Reactive carburizing sintering—A novel production method for high quality chromium carbide–nickel cermets. Int. J. Refract. Met. Hard Mater. 2006, 24, 263–270. [Google Scholar] [CrossRef]
- Pacini, A.; Lupi, F.; Rossi, A.; Seggiani, M.; Lanzetta, M. Direct Recycling of WC-Co Grinding Chip. Materials 2023, 16, 1347. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Srivastava, R.R.; Kim, M.S.; Nam, D.D.; Lee, J.C.; Huynh, H.T. Efficient recycling of WC-Co hardmetal sludge by oxidation followed by alkali and sulfuric acid treatments. Met. Mater. Int. 2016, 22, 897–906. [Google Scholar] [CrossRef]
- Freemantle, C.S.; Sacks, N. Recycling of cemented tungsten carbide mining tool scrap. J. S. Afr. Inst. Min. Metall. 2015, 115, 1207–1213. [Google Scholar] [CrossRef]
- Brookes, K.J.A. Hardmetals recycling and the environment. Met. Powder Rep. 2014, 69, 24–30. [Google Scholar] [CrossRef]
- Erik, L.; Schubert, W.-D. Tungsten: Properties, Chemistry, Technology of the Elements, Alloys, and Chemical Compounds; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Bhosale, S.N.; Mookherjee, S.; Pardeshi, R.M. Current Practices in Tungsten Extraction and Recovery. High Temp. Mater. Process. 1990, 9, 147–162. [Google Scholar] [CrossRef]
- Goljandin, D.; Kulu, P.; Käerdi, H.; Bruwier, A. Disintegrator as Device for Milling of Mineral Ores. Mater. Sci. 2005, 11, 398–402. [Google Scholar]
- Peetsalu, P.; Goljandin, D.; Kulu, P.; Mikli, V.; Käerdi, H. Micropowders producted by disintegrator milling. Powder Metall. Prog. 2003, 3, 99–110. [Google Scholar]
- Lee, G.-G.; Ha, H. Effects of Mechanical Milling on the Carbothermal Reduction of Oxide of WC/Co Hardmetal Scrap. Met. Mater. Int 2016, 22, 260–266. [Google Scholar] [CrossRef]
- Jung, W.G. Recovery of tungsten carbide from hard material sludge by oxidation and carbothermal reduction process. J. Ind. Eng. Chem. 2014, 20, 2384–2388. [Google Scholar] [CrossRef]
- Gu, W.H.; Jeong, Y.S.; Kim, K.; Kim, J.C.; Son, S.H.; Kim, S. Thermal oxidation behavior of WC–Co hard metal machining tool tip scraps. J. Mater. Process. Technol. 2012, 212, 1250–1256. [Google Scholar] [CrossRef]
- Lin, J.; Lin, J.; Lee, S. Process for Recovering Tungsten Carbide from Cemented Tungsten Carbide Scraps by Selective Electrolysis. U.S. Patent 384,016, 24 January 1995. Available online: https://patents.google.com/patent/US5384016A/en (accessed on 8 June 2023).
- Katiyar, P.K.; Randhawa, N.S.; Hait, J.; Jana, R.K.; Singh, K.K.; Mankhand, T.R. Anodic Dissolution Behaviour of Tungsten Carbide Scraps in Ammoniacal Media. Adv. Mater. Res. 2014, 828, 11–20. [Google Scholar] [CrossRef]
- Kuntyi, O.I.; Ivashkin, V.R.; Yavorskii, V.T.; Zozulya, G.I. Electrochemical processing of WC-Ni pseudoalloys in sulfuric acid solutions to ammonium paratungstate and nickel(II) sulfate. Russ. J. Appl. Chem. 2007, 80, 1856–1859. [Google Scholar] [CrossRef]
- Jönsson, K.A.; Nordstjernan, R. Process for Chlorination of Material Containing Hard Metal. U.S. Patent 384,016, 2 February 1971. Available online: https://patents.google.com/patent/US3560199A/en (accessed on 8 June 2023).
- Kücher, G.; Luidold, S.; Czettl, C.; Storf, C. Successful control of the reaction mechanism for semi-direct recycling of hard metals. Int. J. Refract. Met. Hard Mater. 2020, 86, 105131. [Google Scholar] [CrossRef]
- Kücher, G.; Luidold, S.; Czettl, C.; Storf, C. Lixiviation kinetics of cobalt from cemented carbides. Int. J. Refract. Met. Hard Mater. 2018, 70, 239–245. [Google Scholar] [CrossRef]
- Freemantle, C.S.; Sacks, N.; Topic, M.; Pineda-Vargas, C.A. Impurity characterization of zinc-recycled WC-6 wt.% Co cemented carbides. Int. J. Refract. Met. Hard Mater. 2014, 44, 94–102. [Google Scholar] [CrossRef]
- Schwab, B.; Schneider, W.-D. Zinc Recycling via the Imperial Smelting Technology—Latest Developments and Possibilities. In Recycling of Metals and Engineercd Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 193–200. [Google Scholar] [CrossRef]
- Acharyulu, S.L.N.; Rao, P.R. An integrated approach to the optimum utilization of national tungsten resources: Technology gaps. Bull. Mater. Sci. 1996, 19, 179–199. [Google Scholar] [CrossRef]
- Lin, J.C.; Lin, J.Y.; Jou, S.P. Selective dissolution of the cobalt binder from scraps of cemented tungsten carbide in acids containing additives. Hydrometallurgy 1996, 43, 47–61. [Google Scholar] [CrossRef]
- Cera, M.; Trudu, S.; Amadou, A.O.; Asunis, F.; Farru, G.; De Gaudenzi, G.P.; De Gioannis, G.; Serpe, A. Trends and perspectives in the use of organic acids for critical metal recycling from hard-metal scraps. Int. J. Refract. Met. Hard Mater. 2023, 114, 106249. [Google Scholar] [CrossRef]
- Oumarou Amadou, A.; Cera, M.; Trudu, S.; Piredda, M.; Cara, S.; De Gaudenzi, G.P.; Matharu, A.S.; Marchiò, L.; Tegoni, M.; Muntoni, A.; et al. A comparison among bio-derived acids as selective eco-friendly leaching agents for cobalt: The case study of hard metals waste enhancement. Front. Environ. Chem. 2023, 4, 1216245. [Google Scholar] [CrossRef]
- Seo, B.; Kim, S. Cobalt extraction from tungsten carbide-cobalt (WC-Co) hard metal scraps using malic acid. Int. J. Miner. Process. 2016, 151, 1–7. [Google Scholar] [CrossRef]
- Amadou, A.O.; De Gaudenzi, G.P.; Marcheselli, G.; Cara, S.; Piredda, M.; Spiga, D.; Matharu, A.S.; De Gioannis, G.; Serpe, A. A new facile solvometallurgical leaching method for the selective Co dissolution & recovery from hard metals waste. Int. J. Refract. Met. Hard Mater. 2021, 98, 105534. [Google Scholar] [CrossRef]
- Newman, H.R. The Mineral Industry of Austria; United States Geological Survey: Reston, VA, USA, 2006. [Google Scholar]
- Glöser, S.; Espinoza, L.T.; Gandenberger, C.; Faulstich, M. Raw material criticality in the context of classical risk assessment. Resour. Policy 2015, 44, 35–46. [Google Scholar] [CrossRef]
- Stefaniak, A.B. Persistence of tungsten oxide particle/fiber mixtures in artificial human lung fluids. Part. Fibre Toxicol. 2010, 7, 38. [Google Scholar] [CrossRef]
- Leyssens, B.L.; Vinck, C.; Van Der Straeten, F.W. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Tvermoes, B.E.; Unice, K.M.; Finley, B.L.; Kerger, B.D. A review of the health hazards posed by cobalt. Crit. Rev. Toxicol. 2013, 43, 316–362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Torres, H.; Aydinyan, S.; Antonov, M.; Varga, M.; Ripoll, M.R.; Hussainova, I. Microstructure and high temperature tribological behaviour of self-lubricating Ti-TiBx composite doped with Ni[sbnd]Bi. Surf. Coat. Technol. 2022, 447, 128827. [Google Scholar] [CrossRef]
- Datta, S.; Vero, S.E.; Hettiarachchi, G.M.; Johannesson, K. Tungsten Contamination of Soils and Sediments: Current State of Science. Curr. Pollut. Rep. 2017, 3, 55–64. [Google Scholar] [CrossRef]
- Premchand. Processing of low grade tungsten ore concentrates by hydrometallurgical route with particular reference to India. Bull. Mater. Sci. 1996, 19, 295. [CrossRef]
- Srivastava, R.R.; Kim, M.S.; Lee, J.C.; Jha, M.K.; Kim, B.S. Resource recycling of superalloys and hydrometallurgical challenges. J. Mater. Sci. 2014, 49, 4671. [Google Scholar] [CrossRef]
- Kücher, G.; Luidold, S.; Czettl, C.; Storf, C. First evaluation of semidirect recycling methods for the reclamation of cemented carbides based on a literature survey. Matéria 2018, 23, e12018. [Google Scholar] [CrossRef]
- Tonini, D.; Albizzati, P.F.; Caro, D.; De Meester, S.; Garbarino, E.; Blengini, G.A. Quality of recycling: Urgent and undefined. Waste Manag. 2022, 146, 11–19. [Google Scholar] [CrossRef]
- King, A.H. Our elemental footprint. Nat. Mater. 2019, 18, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Charpentier Poncelet, A.; Helbig, C.; Loubet, P.; Beylot, A.; Muller, S.; Villeneuve, J.; Laratte, B.; Thorenz, A.; Tuma, A.; Sonnemann, G. Losses and lifetimes of metals in the economy. Nat. Sustain. 2022, 5, 717–726. [Google Scholar] [CrossRef]
- Graedel, T.E.; Allwood, J.; Birat, J.P.; Buchert, M.; Hagelüken, C.; Reck, B.K.; Sibley, S.F.; Sonnemann, G. What Do We Know about Metal Recycling Rates? J. Ind. Ecol. 2011, 15, 355–366. [Google Scholar] [CrossRef]
- Atherton, J. Declaration by the Metals Industry on Recycling Principles. Int. J. Life Cycle Assess. 2007, 12, 59–60. [Google Scholar] [CrossRef]
- CMeskers, E.M. Coated Magnesium: Designed for Sustainability? Ph.D. Thesis, Technische Universiteit Delft, Delft, The Netherlands, 2008. [Google Scholar]
- Weed, B. ESG and the Circular Economy. Interplace. 8 October 2022. Available online: https://interplace.io/p/esg-and-the-circular-economy#details (accessed on 22 March 2023).
- Khajuria, A.; Atienza, V.A.; Chavanich, S.; Henning, W.; Islam, I.; Kral, U.; Liu, M.; Liu, X.; Murthy, I.K.; Oyedotun, T.D.T.; et al. Accelerating circular economy solutions to achieve the 2030 agenda for sustainable development goals. Circ. Econ. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Corvellec, H.; Stowell, A.F.; Johansson, N. Critiques of the circular economy. J. Ind. Ecol. 2022, 26, 421–432. [Google Scholar] [CrossRef]
- Achzet, B.; Helbig, C. How to evaluate raw material supply risk—An overview. Resour. Policy 2013, 38, 435–447. [Google Scholar] [CrossRef]
- Tercero Espinoza, L.; Schrijvers, D.; Chen, W.Q.; Dewulf, J.; Eggert, R.; Goddin, J.; Habib, K.; Hagelüken, C.; Hurd, A.J.; Kleijn, R.; et al. Greater circularity leads to lower criticality, and other links between criticality and the circular economy. Resour. Conserv. Recycl. 2020, 159, 104718. [Google Scholar] [CrossRef]
- Kainer, U.K. Metal Matrix Composites: Custom-Made Materials for Automotive and Aerospace Engineering. In Metal Matrix Composites: Custom-made Materials for Automotive and Aerospace Engineering; Wiley: Hoboken, NJ, USA, 2006; pp. 1–314. [Google Scholar] [CrossRef]
- Lo, S.C.; Cheng, T.M.; Hu, C.C.; Lai, C.H. Separation of tungsten and cobalt from cemented tungsten carbide by rapid breakdown anodization. Sep. Purif. Technol. 2023, 310, 123140. [Google Scholar] [CrossRef]
- Luidold, S. Recycling of Technologic Metals: Fundamentals and Technologies. In Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–238. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, P.; Yang, J.; Liu, X. Designing a smart incentive-based recycling system for household recyclable waste. Waste Manag. 2021, 123, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, P.; Ciotti, G.; Cottes, M.; Meneghetti, A. Integrating industrial waste heat recovery into sustainable smart energy systems. Energy 2019, 175, 941–951. [Google Scholar] [CrossRef]
- Kumar, R. Development of Solid Lubricated Composites for High-Temperature Tribological Applications. Ph.D. Thesis, Tallinn University of Technology: Tallinn, Estonia, 2022. [Google Scholar]
- Ihlen, Ø.; Roper, J. Corporate Reports on Sustainability and Sustainable Development: ‘We Have Arrived’. Sustain. Dev. 2014, 22, 42–51. [Google Scholar] [CrossRef]
- Tokos, H.; Pintarič, Z.N.; Krajnc, D. An integrated sustainability performance assessment and benchmarking of breweries. Clean Technol. Environ. Policy 2012, 14, 173–193. [Google Scholar] [CrossRef]
- Giljum, S.; Behrens, A.; Hinterberger, F.; Lutz, C.; Meyer, B. Modelling scenarios towards a sustainable use of natural resources in Europe. Environ. Sci. Policy 2008, 11, 204–216. [Google Scholar] [CrossRef]
- Remmen, A. Life Cycle Management: A Business Guide to Sustainability; United Nations Environment Programme: Nairobi, Kenya, 2007. [Google Scholar]
- Warren, J.P.; Rhodes, E.; Carter, R. A Total Product System Concept. Greener Manag. Int. 2014, 2001, 89–104. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Domingues, J.P.; Dima, A.M. Mapping the Sustainable Development Goals Relationships. Sustainability 2020, 12, 3359. [Google Scholar] [CrossRef]
- Koplin, J.; Seuring, S.; Mesterharm, M. Incorporating sustainability into supply management in the automotive industry—The case of the Volkswagen AG. J. Clean. Prod. 2007, 15, 1053–1062. [Google Scholar] [CrossRef]
- Barbier, E.B.; Burgess, J.C. The sustainable development goals and the systems approach to sustainability. Economics 2017, 11, 20170028. [Google Scholar] [CrossRef]
- United Nations Secretary-General; World Commission on Environment and Development. Report of the World Commission on Environment and Development: Note/By the Secretary-General; United Nations: San Francisco, CA, USA, 1987; Available online: https://digitallibrary.un.org/record/139811 (accessed on 19 May 2023).
- United Nations. The 17 GOALS|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 12 June 2023).
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Smith, J.M.; Rechenberg, C.; Cruey, L.; Magness, S.; Sandman, P. The impact of recycling education on the knowledge, attitudes, and behaviors of grade school children. Education 1997, 118, 262–267. [Google Scholar]
- Eastman, C. Design for X: Concurrent Engineering Imperatives; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kuo, T.C.; Huang, S.H.; Zhang, H.C. Design for manufacture and design for ‘X’: Concepts, applications, and perspectives. Comput. Ind. Eng. 2001, 41, 241–260. [Google Scholar] [CrossRef]
- Soomro, S.A.; Casakin, H.; Georgiev, G.V. Sustainable Design and Prototyping Using Digital Fabrication Tools for Education. Sustainability 2021, 13, 1196. [Google Scholar] [CrossRef]
- Barua, S.; Chiesa, M. Sustainable financing practices through green bonds: What affects the funding size? Bus. Strateg. Environ. 2019, 28, 1131–1147. [Google Scholar] [CrossRef]
- Gianfrate, G.; Peri, M. The green advantage: Exploring the convenience of issuing green bonds. J. Clean. Prod. 2019, 219, 127–135. [Google Scholar] [CrossRef]
- Mondejar, M.E.; Avtar, R.; Diaz, H.L.B.; Dubey, R.K.; Esteban, J.; Gómez-Morales, A.; Hallam, B.; Mbungu, N.T.; Okolo, C.C.; Prasad, K.A.; et al. Digitalization to achieve sustainable development goals: Steps towards a Smart Green Planet. Sci. Total Environ. 2021, 794, 148539. [Google Scholar] [CrossRef] [PubMed]
- Balogun, A.L.; Marks, D.; Sharma, R.; Shekhar, H.; Balmes, C.; Maheng, D.; Arshad, A.; Salehi, P. Assessing the Potentials of Digitalization as a Tool for Climate Change Adaptation and Sustainable Development in Urban Centres. Sustain. Cities Soc. 2020, 53, 101888. [Google Scholar] [CrossRef]
- Ceipek, R.; Hautz, J.; Petruzzelli, A.M.; De Massis, A.; Matzler, K. A motivation and ability perspective on engagement in emerging digital technologies: The case of Internet of Things solutions. Long Range Plann. 2021, 54, 101991. [Google Scholar] [CrossRef]
- FAppio, P.; Frattini, F.; Petruzzelli, A.M.; Neirotti, P. Digital Transformation and Innovation Management: A Synthesis of Existing Research and An Agenda for Future Studies; Wiley Online Library: Hoboken, NJ, USA, 2021; Volume 38, pp. 4–20. [Google Scholar] [CrossRef]
- Gupta, P.K.; Shree, V.; Hiremath, L.; Rajendran, S. The use of modern technology in smart waste management and recycling: Artificial intelligence and machine learning. Stud. Comput. Intell. 2019, 823, 173–188. [Google Scholar]
- Al, A.; Ahmed, A.; Asadullah, A. Artificial Intelligence and Machine Learning in Waste Management and Recycling. Eng. Int. 2020, 8, 43–52. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Marian, M.; Profito, F.J.; Aragon, N.; Shah, R. The Use of Artificial Intelligence in Tribology—A Perspective. Lubricants 2020, 9, 2. [Google Scholar] [CrossRef]
- Subrahmanyam, M.; Sujatha, C. Using neural networks for the diagnosis of localized defects in ball bearings. Tribol. Int. 1997, 30, 739–752. [Google Scholar] [CrossRef]
- Cavaleri, L.; Asteris, P.G.; Psyllaki, P.P.; Douvika, M.G.; Skentou, A.D.; Vaxevanidis, N.M. Prediction of surface treatment effects on the tribological performance of tool steels using artificial neural networks. Appl. Sci. 2019, 9, 2788. [Google Scholar] [CrossRef]
- Marian, M.; Tremmel, S. Current Trends and Applications of Machine Learning in Tribology—A Review. Lubricants 2021, 9, 86. [Google Scholar] [CrossRef]
- Ciulli, E. Tribology and Industry: From the Origins to 4.0. Front. Mech. Eng. 2019, 5, 55. [Google Scholar] [CrossRef]
- European Scientific Advisory Board on Climate Change. Available online: https://climate-advisory-board.europa.eu/ (accessed on 14 June 2023).
- European Union. Industry 5.0: Human-Centric, Sustainable and Resilient; Publication Office of European Union: Maastricht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Chen, X.; Kurdve, M.; Johansson, B.; Despeisse, M. Enabling the twin transitions: Digital technologies support environmental sustainability through lean principles. Sustain. Prod. Consum. 2023, 38, 13–27. [Google Scholar] [CrossRef]
- Ortega-Gras, J.J.; Bueno-Delgado, M.V.; Cañavate-Cruzado, G.; Garrido-Lova, J. Twin Transition through the Implementation of Industry 4.0 Technologies: Desk-Research Analysis and Practical Use Cases in Europe. Sustainability 2021, 13, 13601. [Google Scholar] [CrossRef]
- Guandalini, I. Sustainability through digital transformation: A systematic literature review for research guidance. J. Bus. Res. 2022, 148, 456–471. [Google Scholar] [CrossRef]
- Fouquet, R.; Hippe, R. Twin transitions of decarbonisation and digitalisation: A historical perspective on energy and information in European economies. Energy Res. Soc. Sci. 2022, 91, 102736. [Google Scholar] [CrossRef]
- Bruno, R.L.; Matusiak, M.; Osaulenko, K.; Radosevic, S. ‘Digitalisation’ and ‘Greening’ as Components of Technology Upgrading and Sustainable Economic Performance. Sustainability 2023, 15, 1838. [Google Scholar] [CrossRef]
- Chayko, M. Techno-social Life: The Internet, Digital Technology, and Social Connectedness. Sociol. Compass 2014, 8, 976–991. [Google Scholar] [CrossRef]
- Vespignani, A. Predicting the behavior of techno-social systems. Science 2009, 325, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Padovano, A.; Umbrello, S. Value-Oriented and Ethical Technology Engineering in Industry 5.0: A Human-Centric Perspective for the Design of the Factory of the Future. Appl. Sci. 2020, 10, 4182. [Google Scholar] [CrossRef]
- Wakkary, R. Things We Could Design: For More than Human-Centered Worlds; The MIT Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Adel, A. Future of industry 5.0 in society: Human-centric solutions, challenges and prospective research areas. J. Cloud Comput. 2022, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zheng, P.; Yin, Y.; Shih, A.; Wang, L. Toward human-centric smart manufacturing: A human-cyber-physical systems (HCPS) perspective. J. Manuf. Syst. 2022, 63, 471–490. [Google Scholar] [CrossRef]
- Fouquet, R. Make low-carbon energy an integral part of the knowledge economy. Nature 2017, 551, S141. [Google Scholar] [CrossRef]
- Yeğin, T.; Ikram, M. Developing a Sustainable Omnichannel Strategic Framework toward Circular Revolution: An Integrated Approach. Sustainability 2022, 14, 11578. [Google Scholar] [CrossRef]
- Bányai, T. Real-Time Decision Making in First Mile and Last Mile Logistics: How Smart Scheduling Affects Energy Efficiency of Hyperconnected Supply Chain Solutions. Energies 2018, 11, 1833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).