Recovery of Tellurium from Waste Anode Slime Containing High Copper and High Tellurium of Copper Refineries
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Method
2.2.1. Leaching
2.2.2. Roasting of Anode Slime
2.2.3. Electrowinning of Te
3. Results and Discussion
3.1. Alkali Leaching of Anode Slimes
3.1.1. Preliminary Leaching Test
3.1.2. Alkali Leaching under Oxidizing Conditions
3.1.3. Soda Ash Roasting-Alkali Leaching
3.1.4. Roasting without Soda Ash
3.1.5. Leaching in Recycled Spent Electrolyte
3.2. Electrowinning of Tellurium
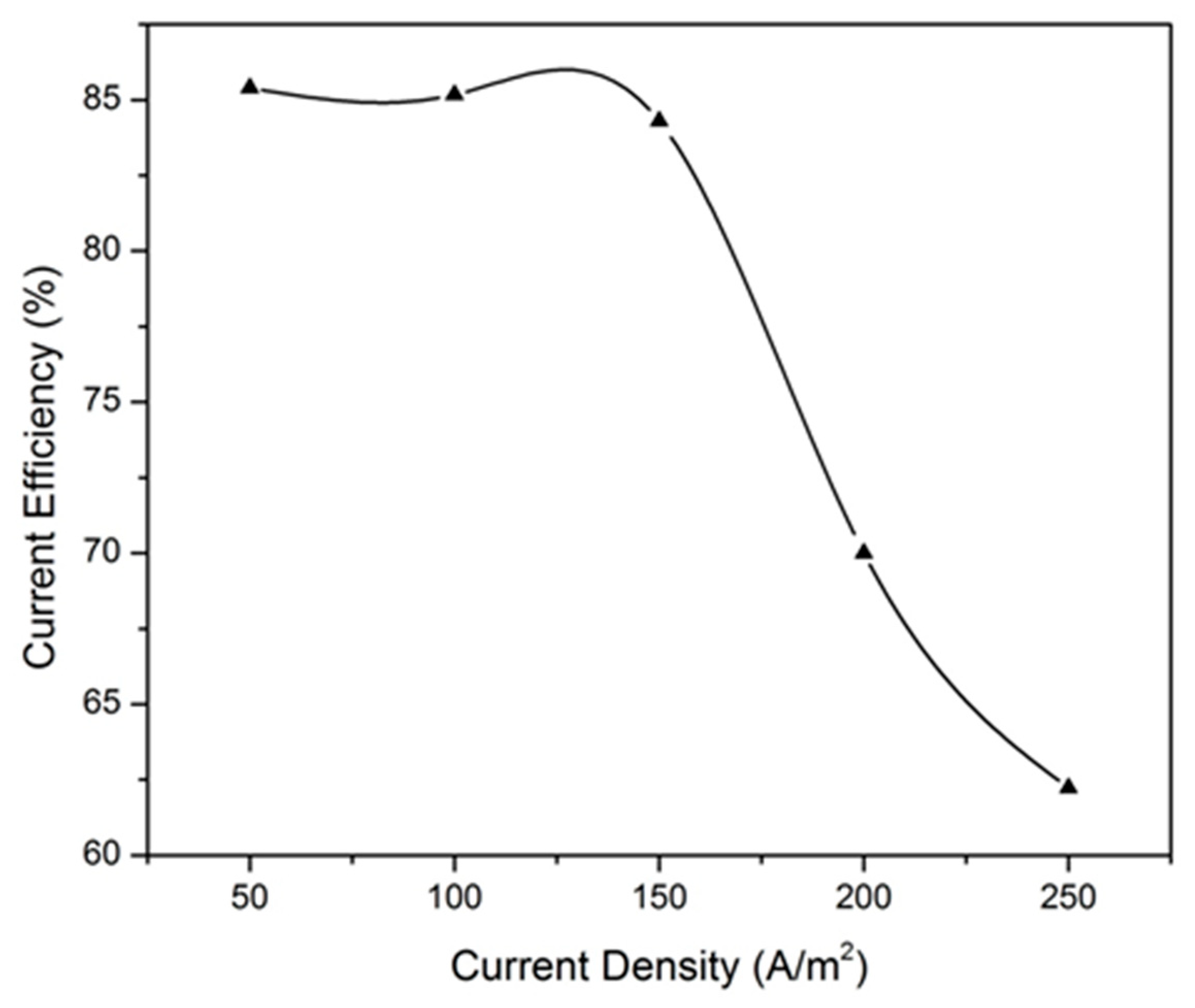
3.2.1. Effect of Current Density
Purity of Te Powder
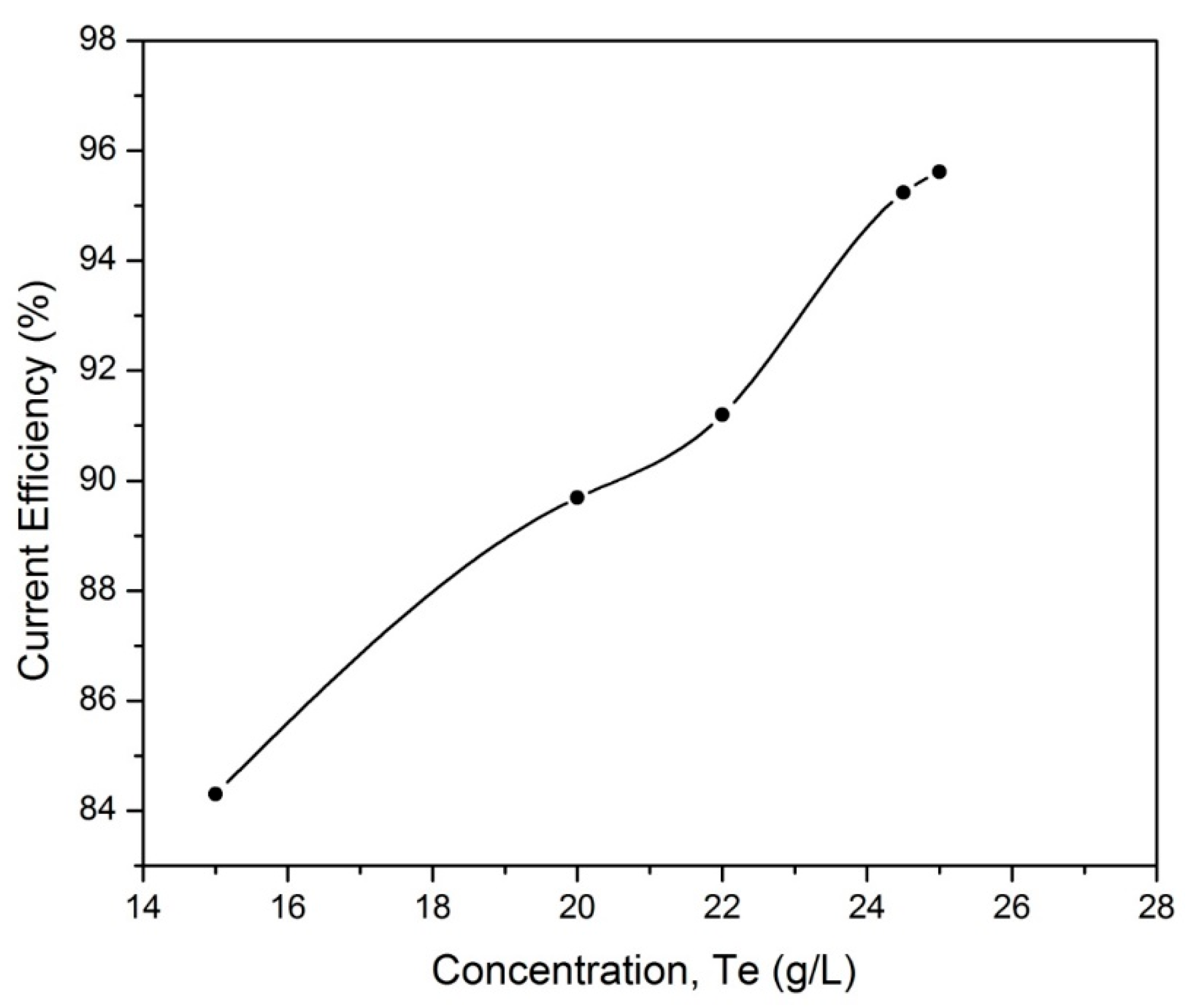
3.2.2. Effect of Tellurium Concentration in Electrolyte
Purity of Te Powder
3.3. Acid Leaching of Residue
4. Conclusions
- The room temperature leaching, as mentioned in method 1, resulted in lower recovery (~37%). High-temperature leaching in the presence of oxygen pressure in 1 M NaOH could give a recovery of ~70%.
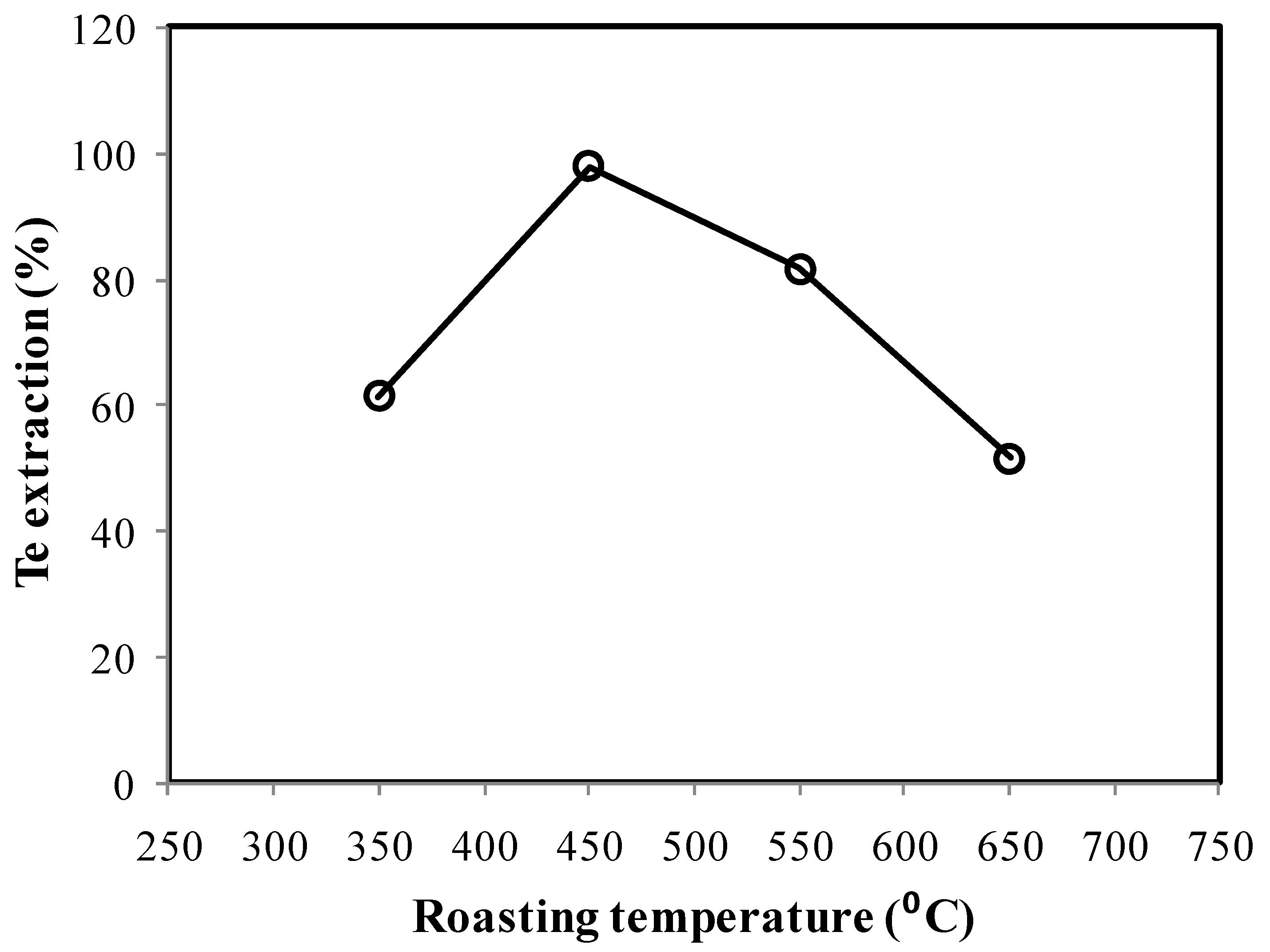
- Roasting of anode slime for 2 h at 450 °C with 20% NaCO3 addition followed by ambient temperature leaching in 1 M NaOH could result in more than 95% Te extraction.
- The most efficient conditions for extraction of Te are air roasting at 450 °C without any addition of soda ash followed by leaching under the conditions: 1 M NaOH, 10% pulp density, 2 h. Te recovery was found to be ~90%.
- The use of spent electrolytes in the leaching of roasted samples has established the possibility of utilization of spent electrolytes in a preferable way if the spent electrolyte contains 5 g/L or less tellurium.
- As the demand for Te is increasing day by day, it will be better to treat anode slimes for the production of Te from secondary resources. Therefore, the findings of the research should lead to commercialization of the process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Qiu, F.; Tian, Q.; Yue, X.; Zhang, T. Production and recovery of tellurium from metallurgical intermediates and electronic waste-A comprehensive review. J. Clean. Prod. 2022, 36, 132796. [Google Scholar] [CrossRef]
- Yu, Y.X. High Storage Capacity and Small Volume Change of Potassium-Intercalation into Novel Vanadium Oxy Chalcogenide Monolayers V2S2O, V2Se2O and V2Te2O: An Ab Initio DFT Investigation. Appl. Surf. Sci. 2021, 546, 149062. [Google Scholar] [CrossRef]
- Borbinha, J.; Romanets, Y.; Teles, P.; Corisco, J.; Vaz, P.; Carvalho, D.; Brouwer, Y.; Luís, R.; Pinto, L.; Vale, A.; et al. Performance Analysis of Geiger–Müller and Cadmium Zinc Telluride Sensors Envisaging Airborne Radiological Monitoring in NORM Sites. Sensors 2020, 20, 1538. [Google Scholar] [CrossRef]
- Shen, H.; Kim, I.Y.; Lim, J.H.; Cho, H.B.; Choa, Y.H. Microstructure Evolution in Plastic Deformed Bismuth Telluride for the Enhancement of Thermoelectric Properties. Materials 2022, 15, 4204. [Google Scholar] [CrossRef]
- Subbaiah, T.; Mishra, B.K.; Ghosh, M.K.; Sanjay, K.; Bhattacharya, I.N.; Sarangi, C.K.; Dash, B.; Sheik, A.R. Hydrometallurgical Process for the Production of Tellurium from High Lead Bearing Copper Refinery Anode Slime. National Patent No: 2459DEC 2013 US 2015 0053572 A1, 25 April 2017. [Google Scholar]
- Hosseinipour, S.; Keshavarz Alamdari, E.; Sadeghi, N. Selenium and Tellurium Separation: Copper Cementation Evaluation Using Response Surface Methodology. Metals 2022, 12, 1851. [Google Scholar] [CrossRef]
- Halli, P.; Wilson, B.P.; Hailemariam, T.; Latostenmaa, P.; Yliniemi, K.; Lundström, M. Electrochemical Recovery of Tellurium from Metallurgical Industrial Waste. J. Appl. Electrochem. 2020, 50, 1–14. [Google Scholar] [CrossRef]
- Rheea, K.-I.; Lee, C.K.; Hab, Y.C.; Jeong, G.-J.; Kim, H.-S.; Sohn, H.-J. Tellurium Recovery from Cemented Tellurium with Minimum Waste Disposal. Hydrometallurgy 1999, 53, 189. [Google Scholar] [CrossRef]
- Yue, X.; Chen, H.; Zhang, T.; Qiu, Z.; Qiu, F.; Yang, D. Controllable Fabrication of Tendril-Inspired Hierarchical Hybrid Membrane for Efficient Recovering Tellurium from Photovoltaic Waste. J. Clean. Prod. 2019, 230, 966. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Xu, Z.; Tian, Q.; Feng, Q. Leaching behavior of metals from copper anode slime using an alkali fusion-leaching process. Hydrometallurgy 2015, 157, 9–12. [Google Scholar] [CrossRef]
- Gomez-Gomez, B.; Sanz-Landaluce, J.; Perez-Corona, M.T.; Madrid, Y. Fate and Effect of in-house synthesized tellurium based nanoparticles on bacterial biofilm biomass and architecture. Challenges for nanoparticles characterization in living systems. Sci. Total Environ. 2020, 719, 137501. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Saha Das, S. Recovery of 131 I from alkaline solution of n-irradiated tellurium target using a tinyDowex-1 column. Appl. Radiat. Isot. 2010, 68, 1967. [Google Scholar] [CrossRef]
- Jin, W.; Su, J.; Chen, S.; Li, P.; Moats, M.S.; Maduraiveeran, G.; Lei, H. Efficient electrochemical recovery of fine tellurium powder from hydrochloric acid media via mass transfer enhancement. Sep. Purif. Technol. 2018, 203, 117. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, Y.; Xiao, Y.; Zhao, Z.; Lei, Y. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: Thermodynamic evaluation and experimental study. Hydrometallurgy 2013, 139, 95–99. [Google Scholar] [CrossRef]
- Fan, J.; Wang, G.; Li, Q.; Yang, H.; Xu, S.; Zhang, J.; Chen, J.; Wang, R. Extraction of tellurium and high purity bismuth from processing residue of zinc anode slime by sulfation roasting-leaching-electrodeposition process. Hydrometallurgy 2020, 194, 105348. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Z.; Li, D.; Tian, Q.; Xu, R.; Zhang, Z. Recovery of tellurium from high tellurium-bearing materials by alkaline sulphide leaching followed by sodium sulphite precipitation. Hydrometallurgy 2017, 171, 355. [Google Scholar] [CrossRef]
- Ren, B.; Zhou, Y.; Ma, H.; Deng, R.; Zhang, P.; Hou, B. Sb release characteristics of the solid waste produced in antimony mining smelting process. J. Mater. Cycles Waste Manag. 2018, 20, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, B.; Hursthouse, A.; Deng, R.; Hou, B. Leaching and releasing characteristics and regularities of Sb and As from antimony mining waste rocks. Pol. J. Environ. Stud. 2019, 28, 4017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, B.; Hursthouse, A.S.; Zhou, S. Antimony ore tailings: Heavy metals, chemical speciation and leaching characteristics. Pol. J. Environ. Stud. 2019, 28, 485. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Li, J.; Liu, T.; Liu, Y.; Yan, M.; Hu, J.; Li, X.; Liu, X.; Liang, Y.; Liu, H.; et al. Effects of redox potential on soil cadmium solubility: Insight into microbial communty. J. Environ. Sci. 2019, 75, 224. [Google Scholar] [CrossRef]
- Mal, J.; Nancharaiah, Y.V.; Maheshwari, N.; van Hullebusch, E.D.; Lens, P.N.L. Continuous removal and recovery of tellurium in an up-flow anaerobic granular sludge bed reactor. J. Hazard. Mater. 2017, 327, 79. [Google Scholar] [CrossRef]
- Shibasaki, T.; Abe, K.; Takeuchi, H. Recovery of tellurium from decopperizing leach solution of copper refinery slimes by a fixed bed reactor. Hydrometallurgy 1992, 29, 399–412. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, Y.; Zhang, G.; Zhang, F.; Yang, Y.; Hua, Z.; Tian, Y.; You, J.; Zhao, Z. An environmental-friendly process for recovery of tellurium and copper from copper telluride. J. Clean. Prod. 2020, 272, 122723. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, Y.; Songa, Y.; Zhang, G.; Zhang, F.; Yang, Y.; Hua, Z.; Tian, Y.; You, J.; Zhao, Z. Recycling of copper telluride from copper anode slime processing: Toward efficient recovery of tellurium and copper. Hydrometallurgy 2020, 196, 105436. [Google Scholar] [CrossRef]
- Yu, H.; Chu, Y.; Zhang, T.; Yu, L.; Yang, D.; Qiu, F.; Yuan, D. Recovery of tellurium from aqueous solutions by adsorption with magnetic nanoscale zero-valent iron (NZVFe). Hydrometallurgy 2018, 177, 1–8. [Google Scholar] [CrossRef]
- Mandal, D.K.; Bhattacharya, B.; Das, R.D. Recovery of tellurium from chloride media using tri-iso-octylamine. Sep. Purif. Technol. 2004, 40, 177. [Google Scholar] [CrossRef]

| S. N. | Leaching Conditions | Te Recovery (%) | ||
|---|---|---|---|---|
| Temperature | Pulp Density (%) | NaOH Conc., M | ||
| 1 | Ambient | 10 | 1 | 36.8 |
| 2 | 80 °C | 10 | 1 | 64.7 |
| S. N. | Leaching Conditions | Te Recovery (%) | |
|---|---|---|---|
| Temperature | Oxidant | ||
| 1 | Ambient | H2O2: 5% (v/v) | 50.3 |
| 2 | Ambient | H2O2: 10% (v/v) | 56 |
| 3 | 80 °C | O2: 2 kg/cm2 | 68.2 |
| 4 | 90 °C | O2: 2 kg/cm2 | 73.1 |
| 5 | 125 °C | O2: 2 kg/cm2 | 73.7 |
| Cycle No. | Te in S.E, g/L | Free Alkali in S.E., g/L | Concentration (g/L) | Te Pick Up from Solid, (g/L) | Te Recovery, (%) | |
|---|---|---|---|---|---|---|
| Te | Se | |||||
| 0 | - | 40 | 24.9 | 2.16 | - | - |
| 1 | 11.2 | 24 | 28.8 | 1.9 | 17.6 | 63.81 |
| 2 | 7.13 | 24 | 29.4 | 1.32 | 22.27 | 80.75 |
| 3 | 5.7 | 22 | 29.1 | 1.12 | 23.4 | 84.84 |
| 4 | 7.1 | 24 | 28.2 | 1.8 | 21.1 | 76.5 |
| Current Density (A/m2) | Voltage (V) | Current Efficiency (%) | Energy Consumption (kWh/kg) |
|---|---|---|---|
| 50 | 1.7 | 85.4 | 1.67 |
| 100 | 2.1 | 85.17 | 2.07 |
| 150 | 2.2 | 84.3 | 2.19 |
| 200 | 2.5 | 70 | 2.93 |
| 250 | 2.25 | 62.23 | 3.03 |
| Current Density (A/m2) | Impurities (%) | Total Impurities (%) | Purity of Te Metal (%) | |||
|---|---|---|---|---|---|---|
| Cu | As | Sb | Se | |||
| 50 | 0.0042 | 0.0 | 0.0081 | 0.055 | 0.067 | 99.93 |
| 100 | 0.003 | 0.0 | 0.066 | 0.065 | 0.134 | 99.86 |
| 150 | 0.0058 | 0.0 | 0.0007 | 0.0071 | 0.0137 | 99.99 |
| 200 | 0.0032 | 0.0332 | 0.004 | 0.0138 | 0.0542 | 99.95 |
| 250 | 0.0039 | 0.0196 | 0.02 | 0.255 | 0.2985 | 99.7 |
| Conc. of Te in Electrolyte, (g/L) | C.V, (V) | E.C, (kWh/kg) |
|---|---|---|
| 15 | 2.20 | 2.10 |
| 20 | 2.10 | 2.00 |
| 22 | 2.10 | 1.95 |
| 24.5 | 2.00 | 1.76 |
| 25 | 2.00 | 1.72 |
| Te Conc. (g/L) | Impurities (%) | Total Impurities (%) | Purity of Te Metal (%) | |||
|---|---|---|---|---|---|---|
| Cu | As | Sb | Se | |||
| 24.9 | 0.0002 | 0.02 | 0.003 | 0.076 | 0.09 | 99.9 |
| 28.81 | 0.0007 | 0.022 | 0.007 | 0.25 | 0.27 | 99.72 |
| 29.4 | 0.0005 | 0.022 | 0.005 | 0.39 | 0.42 | 99.57 |
| 29.14 | 0.0015 | 0.02 | 0.004 | 0.45 | 0.48 | 99.52 |
| 26.21 | 0.0014 | 0.02 | 0.005 | 0.66 | 0.68 | 99.31 |
| S. N. | Leaching Conditions | Cu Recovery (%) | ||
|---|---|---|---|---|
| Residue wt. (g) | Pulp Density (%) | H2SO4 Conc., M | ||
| 1 | 35.5 | 10 | 1.1 | 86 |
| 2 | 70 | 10 | 0.94 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarangi, C.K.; Sheik, A.R.; Marandi, B.; Ponnam, V.; Ghosh, M.K.; Sanjay, K.; Minakshi, M.; Subbaiah, T. Recovery of Tellurium from Waste Anode Slime Containing High Copper and High Tellurium of Copper Refineries. Sustainability 2023, 15, 11919. https://doi.org/10.3390/su151511919
Sarangi CK, Sheik AR, Marandi B, Ponnam V, Ghosh MK, Sanjay K, Minakshi M, Subbaiah T. Recovery of Tellurium from Waste Anode Slime Containing High Copper and High Tellurium of Copper Refineries. Sustainability. 2023; 15(15):11919. https://doi.org/10.3390/su151511919
Chicago/Turabian StyleSarangi, Chinmaya Kumar, Abdul Rauf Sheik, Barsha Marandi, Vijetha Ponnam, Malay Kumar Ghosh, Kali Sanjay, Manickam Minakshi, and Tondepu Subbaiah. 2023. "Recovery of Tellurium from Waste Anode Slime Containing High Copper and High Tellurium of Copper Refineries" Sustainability 15, no. 15: 11919. https://doi.org/10.3390/su151511919
APA StyleSarangi, C. K., Sheik, A. R., Marandi, B., Ponnam, V., Ghosh, M. K., Sanjay, K., Minakshi, M., & Subbaiah, T. (2023). Recovery of Tellurium from Waste Anode Slime Containing High Copper and High Tellurium of Copper Refineries. Sustainability, 15(15), 11919. https://doi.org/10.3390/su151511919







