Adsorption of CO2, CO, H2, and N2 on Zeolites, Activated Carbons, and Metal-Organic Frameworks with Different Surface Nonuniformities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Characterization of Microporous Adsorbents
2.3. Volumetric Adsorption Measurements
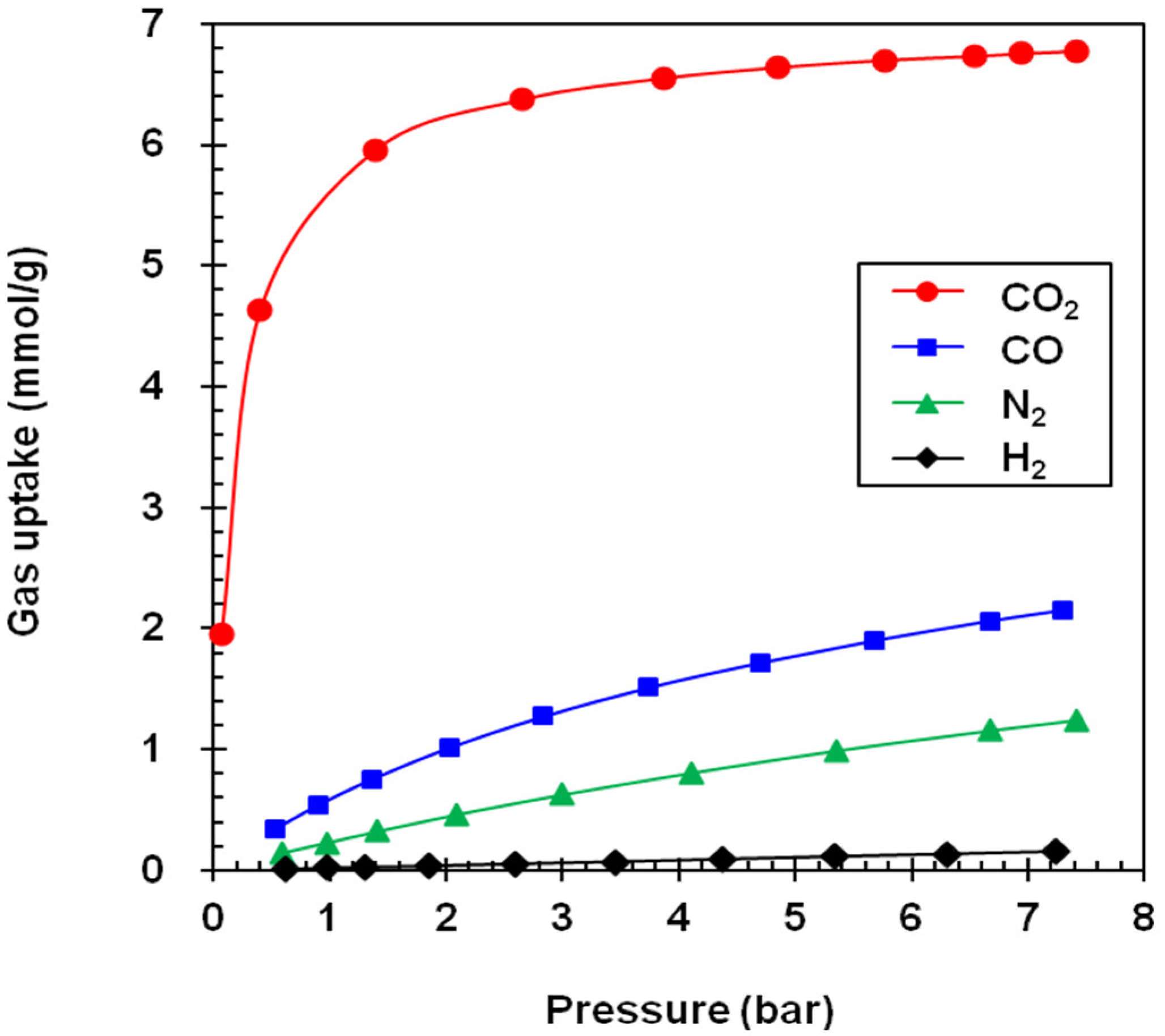
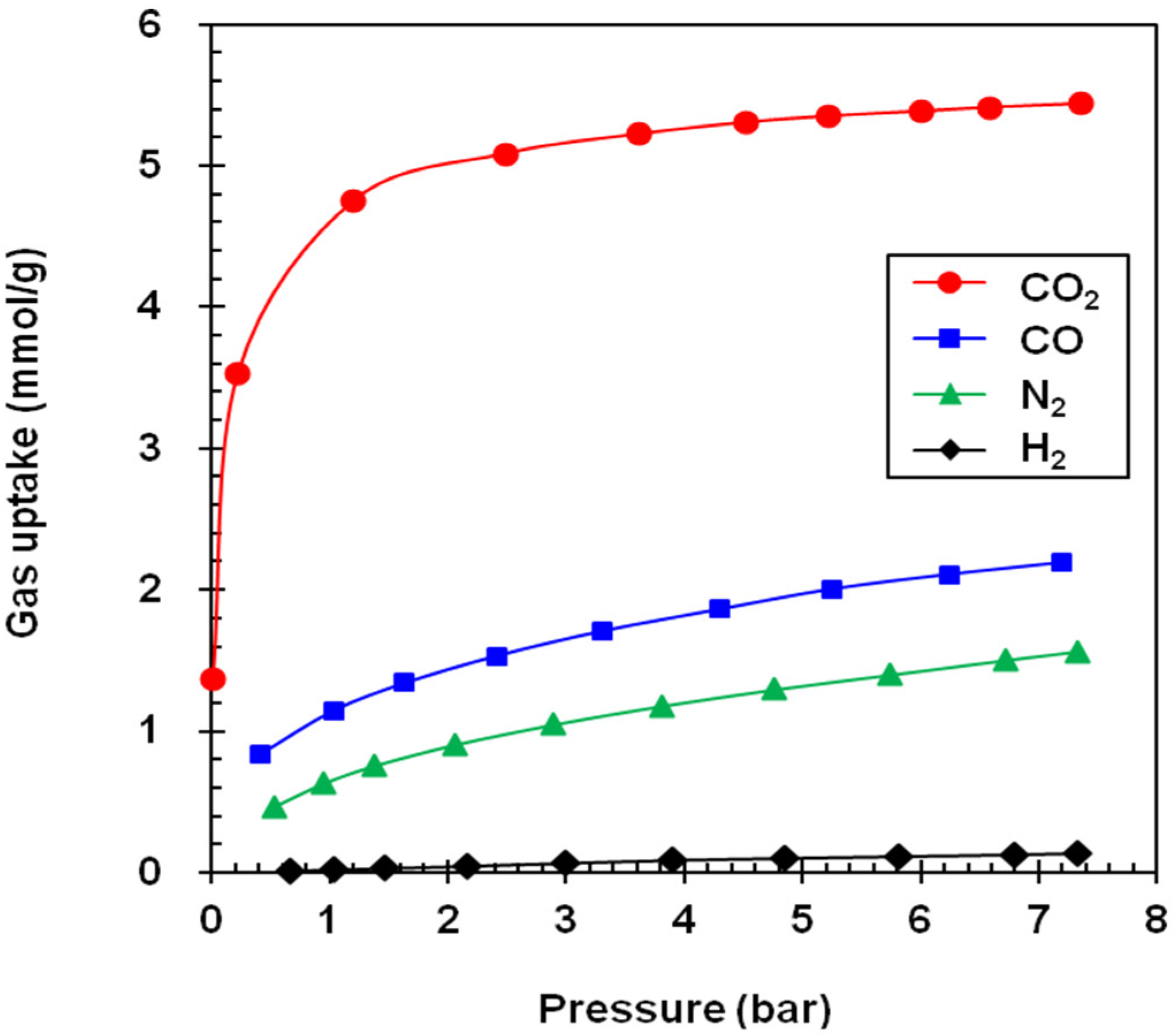
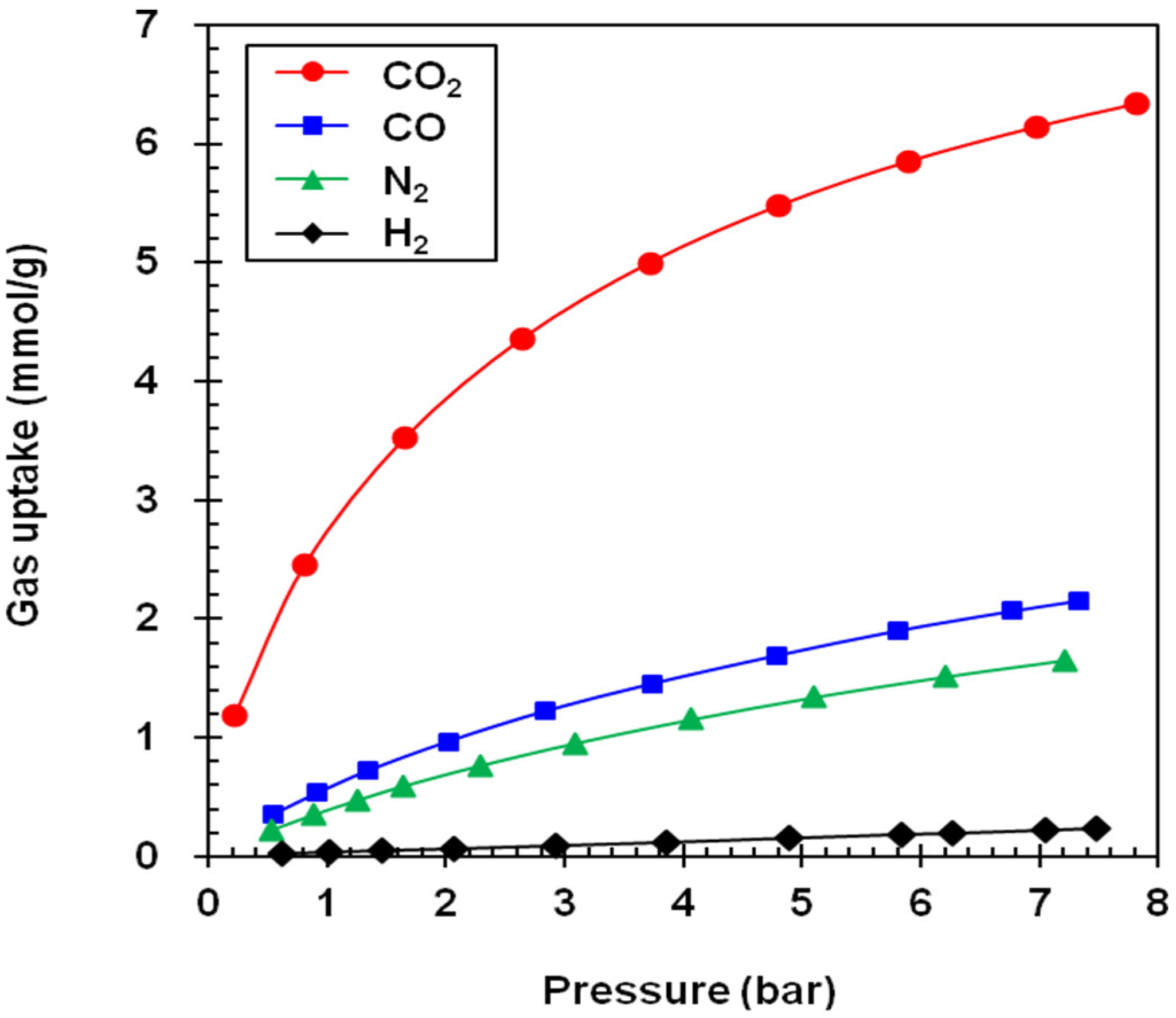
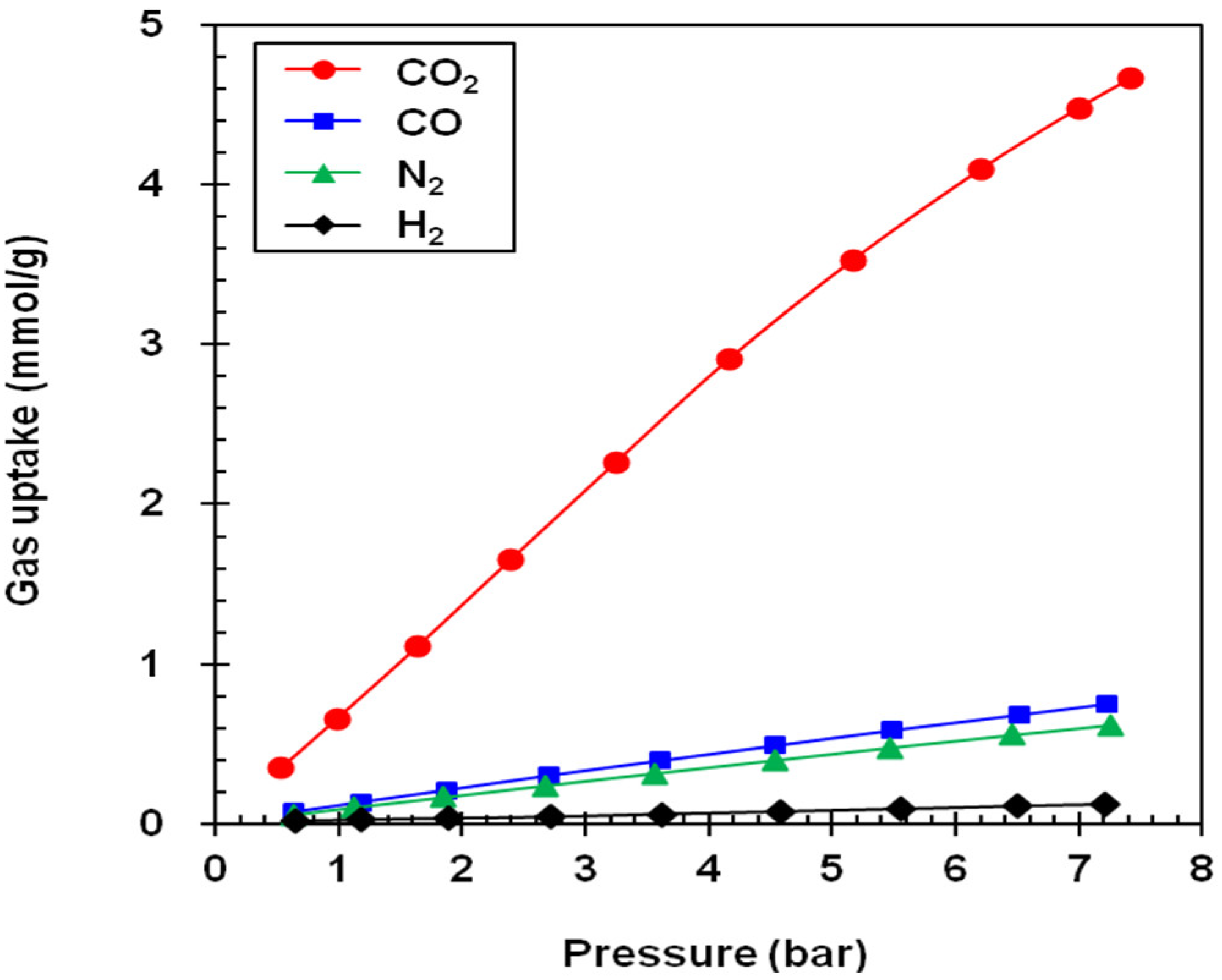
3. Results
4. Discussion
4.1. Effect of the Textural Properties of Adsorbents on the Adsorption of Adsorbates
4.2. Molecular Properties of Adsorbates and Their Adsorption Behaviors
4.3. Surface Nonuniformity of Adsorbents and Their Selectivity of CO2 Adsorption
4.4. Working Capacity of CO2 Adsorption on the Adsorbents
4.5. Regenerability of Adsorbents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campoy, M.; Gómez-Barea, A.; Ollero, P.; Nilsson, S. Gasification of wastes in a pilot fluidized bed gasifier. Fuel Process. Technol. 2014, 121, 63–69. [Google Scholar] [CrossRef]
- Hanaoka, T.; Hiasa, S.; Edashige, Y. Syngas production by CO2/O2 gasification of aquatic biomass. Fuel Process. Technol. 2013, 116, 9–15. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R. Effect of operating conditions on the CO2 recovery from a fine activated carbon by means of TSA in a fluidized bed assisted by acoustic fields. Fuel Process. Technol. 2015, 134, 494–501. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents: Fundamental and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kulprathipanja, S. Aspects of mechanisms, processes, and requirements for zeolite separation. In Zeolites in Industrial Separation and Catalysis; Kulprathipanja, S., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2010; pp. 203–228. [Google Scholar]
- Buckingham, J.; Reina, T.R.; Duyar, M.S. Recent advances in carbon dioxide capture for process intensification. Carbon Capture Sci. Technol. 2022, 2, 100031. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Ferey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Bae, Y.S.; Snurr, R.Q. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef]
- Palomino, M.; Corma, A.; Jorda, J.L.; Rey, F.; Valencia, S. Zeolite Rho: A highly selective adsorbent for CO2/CH4 separation induced by a structural phase modification. Chem. Commun. 2012, 48, 215–217. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Grande, C.A.; Lopes, F.V.S.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Adsorption of off-gases from steam methane reforming (H2, CO2, CH4, CO and N2) on activated carbon. Sep. Sci. Technol. 2008, 43, 1338–1364. [Google Scholar] [CrossRef]
- Sircar, S. Basic research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 2006, 45, 5435–5448. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Jankowska, H.; Swiatkowski, A.; Choma, J. Active Carbon, Ellis Horwood and Wydawnictwa Naukowo-Techniczne; Ellis Horwood: Warsaw, Poland, 1991. [Google Scholar]
- Tagliabue, M.; Farrusseng, D.; Valencia, S.; Aguado, S.; Ravon, U.; Rizzo, C.; Corma, A.; Mirodatos, C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Micropor. Mesopor. Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.B.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, D.; Zhao, D.; Sun, D.; Zhou, H.C. An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. Int. Ed. 2010, 49, 5357–5361. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Bastin, L.; Barcia, P.S.; Hurtado, E.J.; Silva, J.A.C.; Rodrigues, A.E.; Chen, B. A microporous metal organic framework for separation of CO2/N2 and CO2/CH4 by fixed-bed adsorption. J. Phys. Chem. C 2008, 112, 1575–1581. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Ferey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef]
- Mu, B.; Li, F.; Walton, K.S. A novel metal-organic coordination polymer for selective adsorption of CO2 over CH4. Chem. Commun. 2009, 2493–2495. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.S.; Hong, D.Y.; Hwang, Y.K.; et al. High uptakes of CO2 and CH4 in mesoporous metal-organic frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef]
- Garces, S.I.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.A.; Gil, A. Comparative study of the adsorption equilibrium of CO2 on microporous commercial materials at low pressures. Ind. Eng. Chem. Res. 2013, 52, 6785–6793. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.D.; Yazaydin, A.O. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Ribeiro, A.M.; Loureiro, J.M.; Evaggelos, O.; Nikolakis, V.; Rodrigues, A.E. Adsorption of H2, CO2, CH4, CO, N2 and H2O in activated carbon and zeolite for hydrogen production. Sep. Sci. Technol. 2009, 44, 1045–1073. [Google Scholar] [CrossRef]
- Liang, Z.; Marshall, M.; Chaffee, A.L. CO2 adsorption-based separation by metal organic framework (Cu-BTC) versus zeolite (13X). Energy Fuels 2009, 23, 2785–2789. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. A comparison of the CO2 capture characteristics of zeolites and metal-organic frameworks. Sep. Purif. Technol. 2012, 87, 120–126. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H.C. Curvature and parametric sensitivity in models for adsorption in micropores. AIChE J. 1991, 37, 429–436. [Google Scholar] [CrossRef]
- Horvath, G.; Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon. J. Chem. Eng. Jap. 1983, 16, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Ebner, J.R.; Friedman, R.M.; Vannice, M.A. Dissociative N2O adsorption on supported Pt. J. Catal. 2001, 204, 348–357. [Google Scholar] [CrossRef]
- Kim, M.H.; Ebner, J.R.; Friedman, R.M.; Vannice, M.A. Determination of metal dispersion and surface composition in supported Cu–Pt catalysts. J. Catal. 2002, 208, 381–392. [Google Scholar] [CrossRef]
- Yang, W.H.; Kim, M.H. Catalytic reduction of N2O by H2 over well-characterized Pt surfaces. Korean J. Chem. Eng. 2006, 23, 908–918. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. Corrected Horvath-Kawazoe equations for pore-size distribution. AIChE J. 2000, 46, 734–750. [Google Scholar] [CrossRef] [Green Version]
- Maurin, G.; Llewellyn, P.; Poyet, T.; Kuchta, B. Influence of extra-framework cations on the adsorption properties of X-faujasite systems: Microcalorimetry and molecular simulations. J. Phys. Chem. B 2005, 109, 125–129. [Google Scholar] [CrossRef]
- Heymans, N.; Vaesen, S.; De Weireld, G. A complete procedure for acidic gas separation by adsorption on MIL-53 (Al). Micropor. Mesopor. Mater. 2012, 154, 93–99. [Google Scholar] [CrossRef]
- Xuan-Dong, D.; Vinh-Thang, H.; Kaliaguine, S. MIL-53 (Al) mesostructured metal-organic frameworks. Micropor. Mesopor. Mater. 2011, 141, 135–139. [Google Scholar]
- Deniz, E.; Karadas, F.; Patel, H.A.; Aparicio, S.; Yavuz, C.T.; Atilhan, M. A combined computational and experimental study of high pressure and supercritical CO2 adsorption on Basolite MOFs. Micropor. Mesopor. Mater. 2013, 175, 34–42. [Google Scholar] [CrossRef]
- Blanco-Brieva, G.; Campos-Martin, J.M.; Al-Zahrani, S.M.; Fierro, J.L.G. Effectiveness of metal-organic frameworks for removal of refractory organo-sulfur compound present in liquid fuels. Fuel 2011, 90, 190–197. [Google Scholar] [CrossRef]
- Ferey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef]
- Almasoudi, A.; Mokaya, R. Preparation and hydrogen storage capacity of templated and activated carbons nanocast from commercially available zeolitic imidazolate framework. J. Mater. Chem. 2012, 22, 146–152. [Google Scholar] [CrossRef]
- Ramsahye, N.A.; Maurin, G.; Bourrelly, S.; Llewellyn, P.; Loiseau, T.; Ferey, G. Charge distribution in metal organic framework materials: Transferability to a preliminary molecular simulation study of the CO2 adsorption in the MIL-53 (Al) system. Phys. Chem. Chem. Phys. 2007, 9, 1059–1063. [Google Scholar] [CrossRef]
- Ruthven, D.M. Fundamentals of adsorption equilibrium and kinetics in microporous solids. Mol. Sieves 2008, 7, 1–43. [Google Scholar]
- Toth, J. Uniform and thermodynamically consistent interpretation of adsorption isotherms. In Adsorption: Theory, Modeling, and Analysis; Toth, J., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 1–104. [Google Scholar]
- Dunne, J.A.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric heats of adsorption and adsorption isotherms: 2. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on NaX, H-ZSM-5, and Na-ZSM-5 zeolites. Langmuir 1996, 12, 5896–5904. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Raybaud, P.; Belmabkhout, Y.; Cejka, J.; Zukal, A. The role of the extra-framework cations in the adsorption of CO2 on faujasite Y. Phys. Chem. Chem. Phys. 2010, 12, 13534–13546. [Google Scholar] [CrossRef]
- Perez-Pellitero, J.; Amrouche, H.; Siperstein, F.R.; Pirngruber, G.; Nieto-Draghi, C.; Chaplais, G.; Simon-Masseron, A.; Bazer-Bachi, D.; Peralta, D.; Bats, N. Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: Experiments and simulations. Chem. Eur. J. 2010, 16, 1560–1571. [Google Scholar] [CrossRef]
- Couck, S.; Denayer, J.F.M.; Baron, G.V.; Remy, T.; Gascon, J.; Kapteijn, F. An amine-functionalized MIL-53 metal-organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 1999, 131, 6326–6327. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Boddenberg, B.; Rakhmatkariev, G.U.; Wozniak, A.; Hufnagel, S. A calorimetric and statistical mechanics study of ammonia adsorption in zeolite NaY. Phys. Chem. Chem. Phys. 2004, 6, 2494–2501. [Google Scholar] [CrossRef]
- Ward, J.W.; Habgood, H.W. The infrared spectra of carbon dioxide adsorbed on zeolite X. J. Phys. Chem. 1966, 70, 1178–1182. [Google Scholar] [CrossRef]
- Ania, C.O.; Parra, J.B.; Menendez, J.A.; Pis, J.J. Effect of microwave and conventional regeneration on the microporous and mesoporous network and on the adsorptive capacity of activated carbons. Micropor. Mesopor. Mater. 2005, 85, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.; Liu, Q.; Lobo, R.F. Carbon dioxide and nitrogen adsorption on cation-exchanged SSZ-13 zeolites. Langmuir 2013, 29, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Krishna, R.; Long, J.R. CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2(dobdc). Micropor. Mesopor. Mater. 2012, 151, 481–487. [Google Scholar] [CrossRef]
- Sircar, S.; Rao, M.B. Effect of adsorbate size on adsorption of gas mixtures on homogeneous adsorbents. AIChE J. 1999, 45, 2657–2661. [Google Scholar] [CrossRef]
- Sircar, S.; Myers, A.L. Gas separation by zeolites. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 1354–1404. [Google Scholar]
- Radosz, M.; Hu, X.D.; Krutkramelis, K.; Shen, Y.Q. Flue-gas carbon capture on carbonaceous sorbents: toward a low-cost multifunctional carbon filter for “green” energy producers. Ind. Eng. Chem. Res. 2008, 47, 3783–3794. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Effects of acidic treatments on the pore and surface properties of Ni catalyst supported on activated carbon. Carbon 1998, 36, 283–292. [Google Scholar] [CrossRef]
- Kim, J.A.; Park, I.S.; Seo, J.H. A development of high power activated carbon using the KOH activation of soft carbon series cokes. Trans. Electr. Electron. Mater. 2014, 15, 81–86. [Google Scholar] [CrossRef] [Green Version]


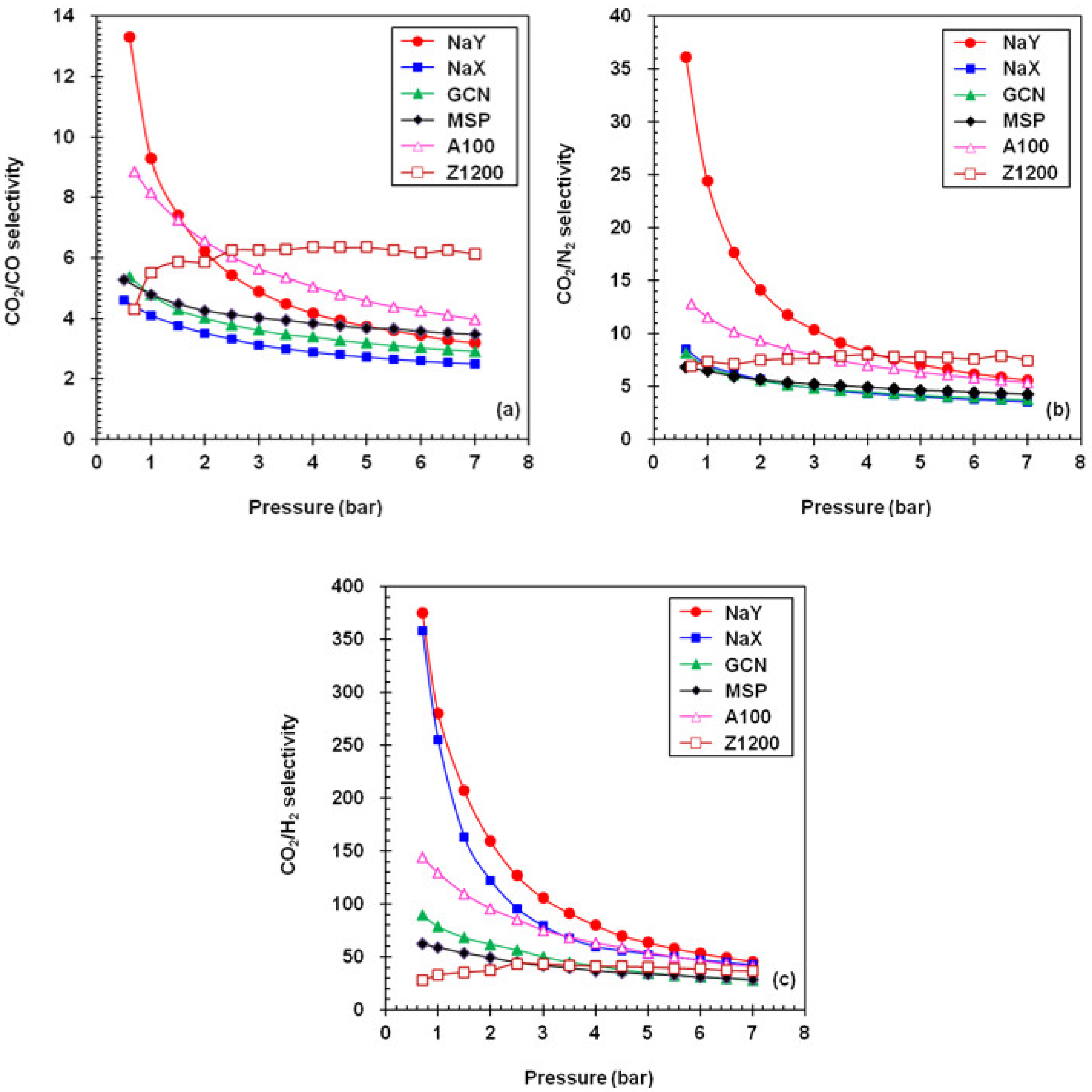
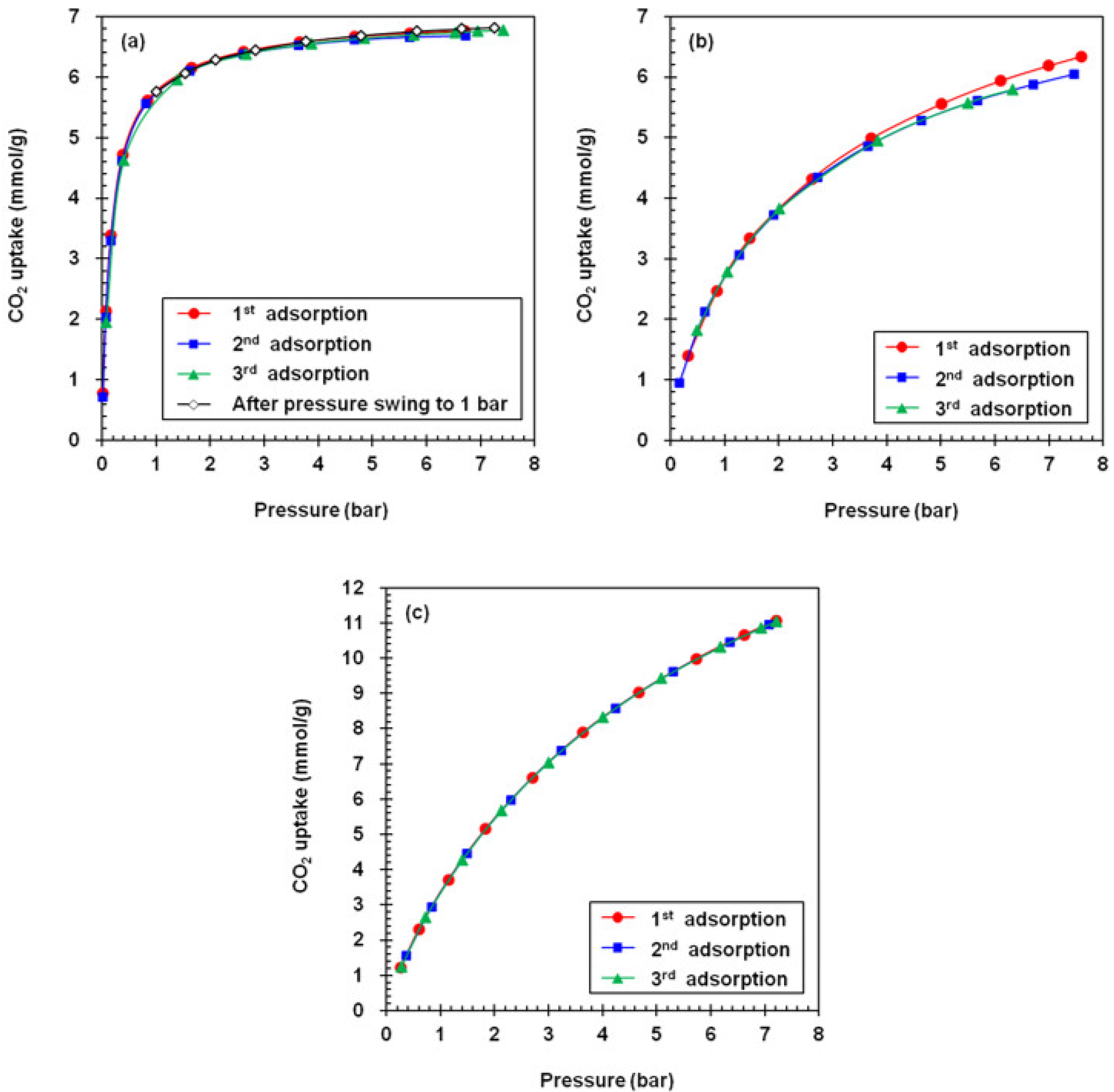



| Adsorbent | Category | SAR a | SBET | dmc b (Å) | Pore Volume (cm3/g) | fmcc | ||
|---|---|---|---|---|---|---|---|---|
| (m2/g) | Vmc | Vms | Vt | |||||
| NaY | Zeolite | 2.3 | 847 | 7.85 | 0.313 | 0.028 | 0.334 | 0.94 |
| NaX d | Zeolite | 1.3 | 724 | 7.58 | 0.272 | 0.097 | 0.357 | 0.76 |
| GCN | AC | - | 1132 | 5.02 e | 0.439 | 0.076 | 0.479 | 0.92 |
| MSP | AC | - | 2508 | 5.53 e | 0.991 | 0.299 | 1.146 | 0.86 |
| A100 f | MOF | - | 838 | 10.96 | 0.361 | 0.899 | 1.109 | 0.33 |
| Z1200 g | MOF | - | 1301 | 12.27 | 0.553 | 0.125 | 0.739 | 0.75 |
| Adsorbent | Gas Uptake (mmol/g) a | Selectivity Factor a,b | Working Capacity (mmol/g) c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | CO | N2 | H2 | CO2/CO | CO2/N2 | CO2/H2 | |||
| NaY | 6.70 (5.60) | 1.95 (1.10) | 1.08 (0.23) | 0.13 (0.02) | 3.4 (5.1) | 6.2 (24.4) | 51.5 (280) | 1.10 | |
| NaX | 5.38 (4.60) | 2.08 (1.12) | 1.43 (0.65) | 0.12 (0.02) | 2.6 (4.1) | 3.8 (4.1) | 44.8 (230) | 0.78 | |
| GCN | 5.87 (2.75) | 1.94 (0.57) | 1.48 (0.40) | 0.19 (0.04) | 3.0 (4.8) | 4.0 (6.9) | 30.9 (68.7) | 3.12 | |
| MSP | 9.75 (3.25) | 2.72 (0.68) | 2.19 (0.50) | 0.31 (0.06) | 3.6 (4.8) | 4.5 (6.5) | 31.4 (54.1) | 6.50 | |
| A100 | 5.18 (2.20) | 1.22 (0.27) | 0.89 (0.19) | 0.11 (0.02) | 4.3 (8.2) | 5.8 (11.6) | 47.1 (110) | 2.98 | |
| Z1200 | 3.95 (0.66) | 0.64 (0.12) | 0.52 (0.09) | 0.10 (0.02) | 6.2 (5.5) | 7.6 (7.3) | 39.5 (33) | 3.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Kim, M.H. Adsorption of CO2, CO, H2, and N2 on Zeolites, Activated Carbons, and Metal-Organic Frameworks with Different Surface Nonuniformities. Sustainability 2023, 15, 11574. https://doi.org/10.3390/su151511574
Kim KH, Kim MH. Adsorption of CO2, CO, H2, and N2 on Zeolites, Activated Carbons, and Metal-Organic Frameworks with Different Surface Nonuniformities. Sustainability. 2023; 15(15):11574. https://doi.org/10.3390/su151511574
Chicago/Turabian StyleKim, Kang Hun, and Moon Hyeon Kim. 2023. "Adsorption of CO2, CO, H2, and N2 on Zeolites, Activated Carbons, and Metal-Organic Frameworks with Different Surface Nonuniformities" Sustainability 15, no. 15: 11574. https://doi.org/10.3390/su151511574
APA StyleKim, K. H., & Kim, M. H. (2023). Adsorption of CO2, CO, H2, and N2 on Zeolites, Activated Carbons, and Metal-Organic Frameworks with Different Surface Nonuniformities. Sustainability, 15(15), 11574. https://doi.org/10.3390/su151511574





