Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions
Abstract
1. Introduction
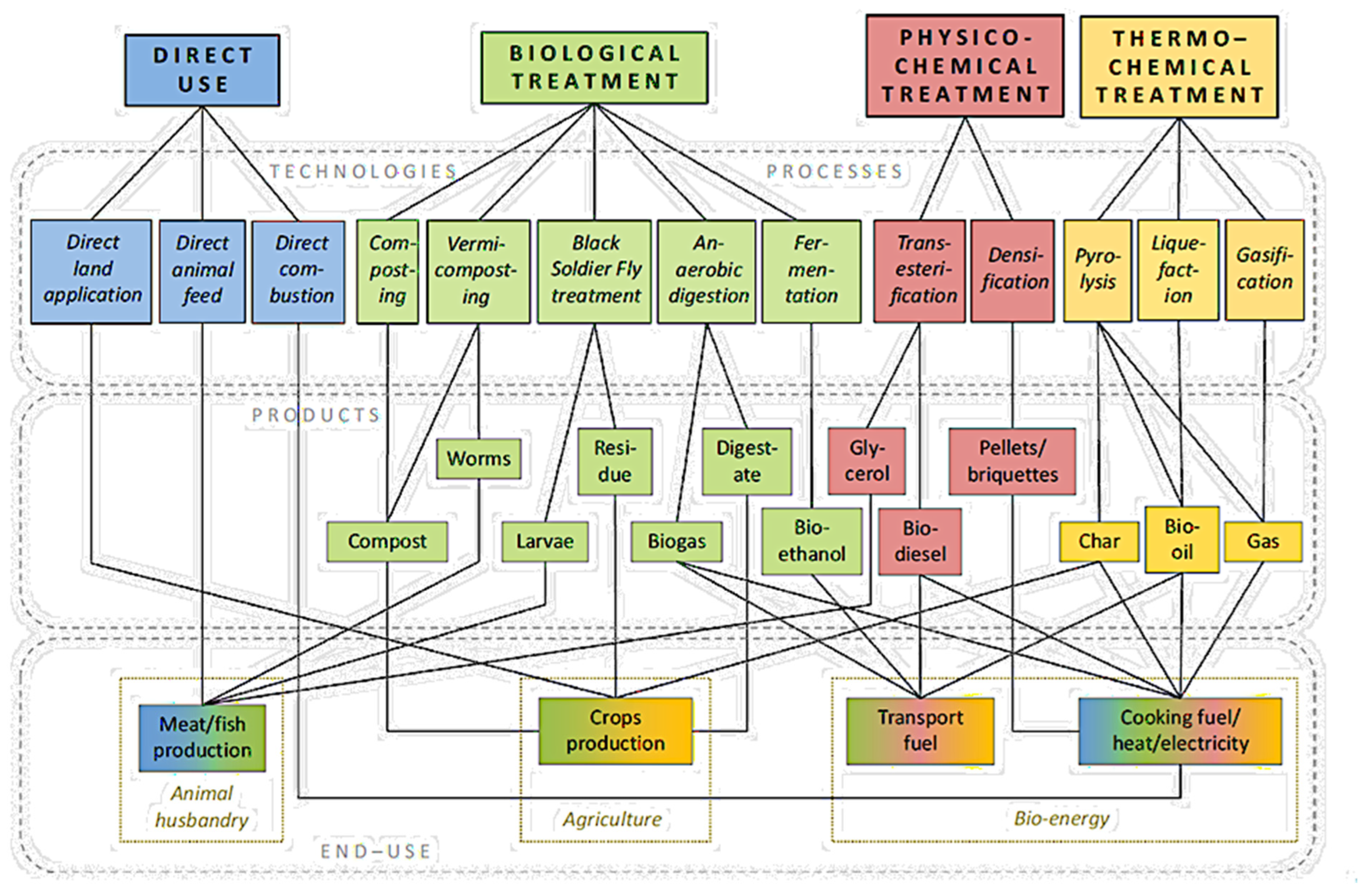
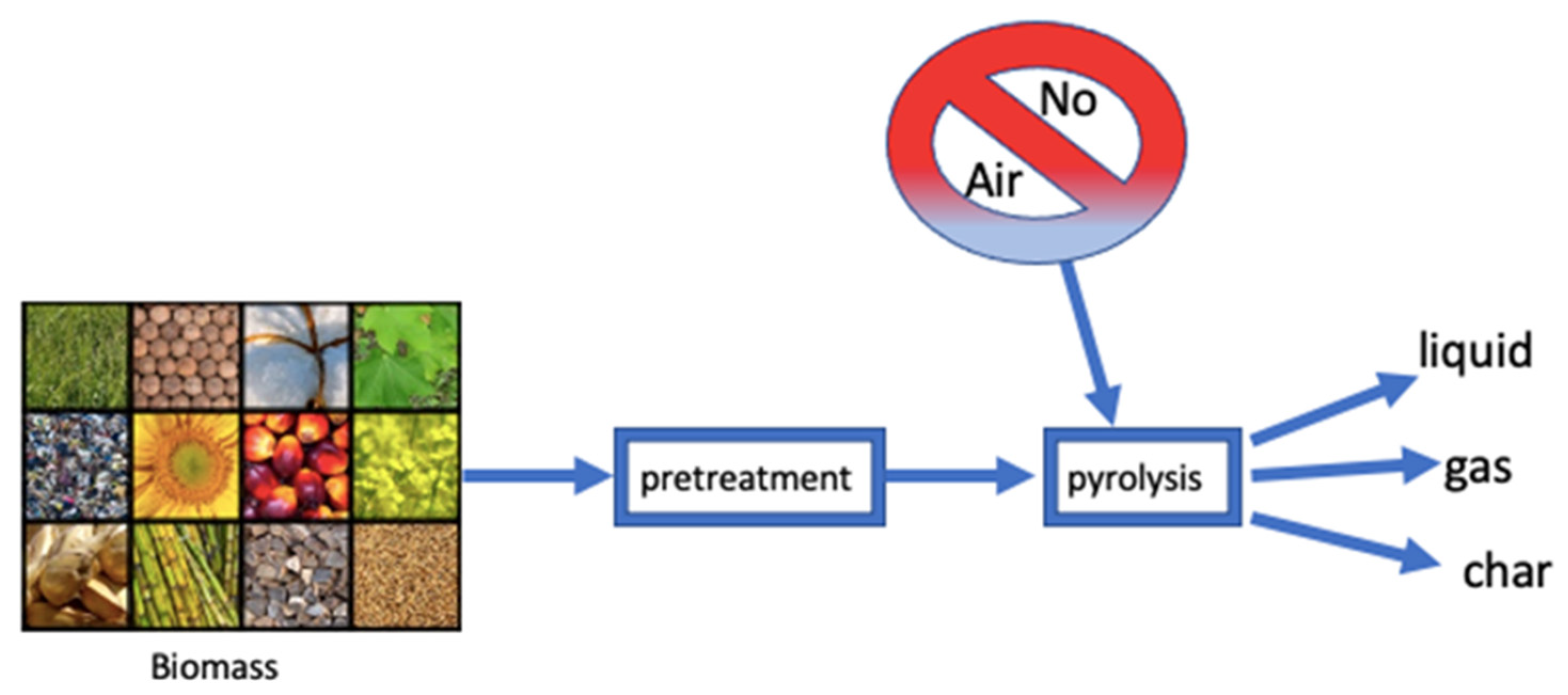
2. Thermal Conversion
3. Pyrolysis Classifications
3.1. Slow Pyrolysis
3.2. Fast Pyrolysis
3.3. Flash Pyrolysis
4. Parameters That Affect the Pyrolysis Process
4.1. Temperature
4.2. Size of Feed Particles
4.3. Residence Time
4.4. Types of Biomass
4.5. Catalyst
4.6. Heating Rate
4.7. Pressure
5. Pretreatment of Biomass
6. Pyrolysis Product Properties
6.1. Bio-Oil
6.2. Bio-Char
6.3. Gaseous (Syngas)
7. Simulation
8. Product Treatment
8.1. Bio-Oil
8.2. Bio-Char
8.3. Gas Treatments and Applications
9. Recent Developments
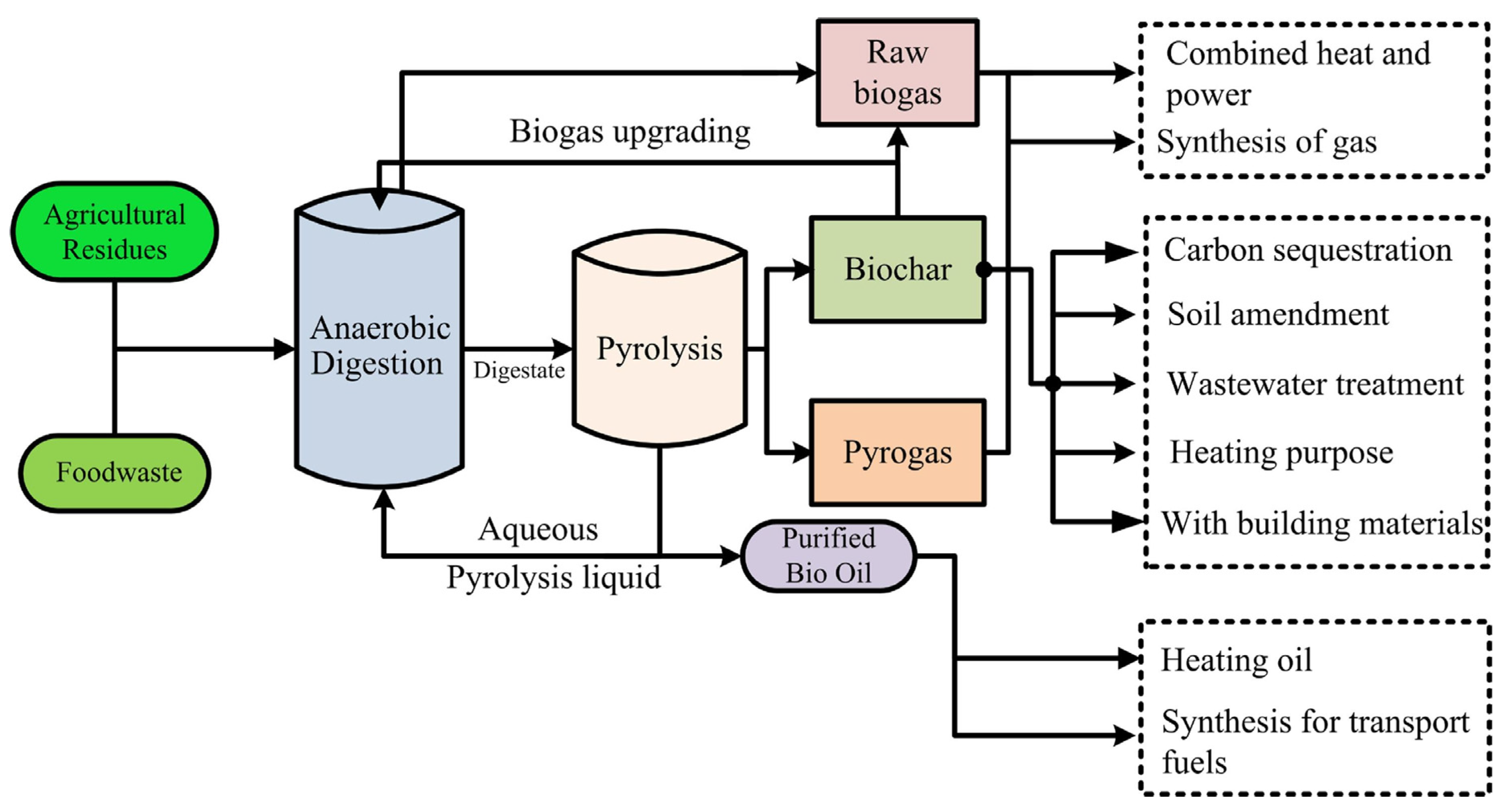
9.1. Pyrolysis and Anaerobic Digestion Integrated Process
9.2. Challenges and Disposal of Recalcitrant Organic Residues Using the Anaerobic Digestion of Food Waste
10. Bio-Oil Using Microalgae
11. Production of Bio-Char from Crop Residues and Its Application for Anaerobic Digestion
12. Economic Studies
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, A.N.M.A.; Naebe, M. Sustainable biodegradable denim waste composites for potential single-use packaging. Sci. Total Environ. 2022, 809, 152239. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.D.; Hossain, M.S.; Islam, M.A.; Haque, A.N.M.A.; Naebe, M. Utilisation of natural wastes: Water-resistant semi-transparent paper for food packaging. J. Clean. Prod. 2022, 364, 132665. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Naebe, M.; Mielewski, D.; Kiziltas, A. Waste wool/polycaprolactone filament towards sustainable use in 3D printing. J. Clean. Prod. 2023, 386, 135781. [Google Scholar] [CrossRef]
- Alhathal Alanezi, A.; Bassyouni, M.; Abdel-Hamid, S.M.S.; Ahmed, H.S.; Abdel-Aziz, M.H.; Zoromba, M.S.; Elhenawy, Y. Theoretical Investigation of Vapor Transport Mechanism Using Tubular Membrane Distillation Module. Membranes 2021, 11, 560. [Google Scholar] [CrossRef]
- Ionel, I.O.A.N.A.; Cioabla, A.E. Biogas production based on agricultural residues. From history to results and perspectives. WSEAS Trans. Environ. Dev. 2010, 8, 591–603. [Google Scholar]
- Faaij, A. Modern biomass conversion technologies. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 343–375. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes—An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Verma, M.; Godbout, S.; Brar, S.K.; Solomatnikova, O.; Lemay, S.P.; Larouche, J.P. Biofuels production from biomass by thermochemical conversion technologies. Int. J. Chem. Eng. 2012, 2012, 542426. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Bassyouni, M. Dynamic mechanical properties and characterization of chemically treated sisal fiber-reinforced polypropylene biocomposites. J. Reinf. Plast. Compos. 2018, 37, 1402–1417. [Google Scholar] [CrossRef]
- Ahmad, H.; Farooq, A.; Bassyouni, M.I.; Sait, H.H.; El-Wafa, M.A.; Hasan, S.W.; Ani, F.N. Pyrolysis of Saudi Arabian date palm waste: A viable option for converting waste into wealth. Life Sci. J. 2014, 11, 667–671. [Google Scholar]
- Sait, H.H.; Hussain, A.; Bassyouni, M.; Ali, I.; Kanthasamy, R.; Ayodele, B.V.; Elhenawy, Y. Hydrogen-rich syngas and biochar production by non-catalytic valorization of date palm seeds. Energies 2022, 15, 2727. [Google Scholar] [CrossRef]
- Bassyouni, M.; Iqbal, N.; Iqbal, S.S.; Abdel-Hamid, S.S.; Abdel-Aziz, M.H.; Javaid, U.; Khan, M.B. Ablation and thermo-mechanical investigation of short carbon fiber impregnated elastomeric ablatives for ultrahigh temperature applications. Polym. Degrad. Stab. 2014, 110, 195–202. [Google Scholar] [CrossRef]
- Bassyouni, M.; ul Hasan, S.W.; Abdel-Aziz, M.H.; Abdel-hamid, S.S.; Naveed, S.; Hussain, A.; Ani, F.N. Date palm waste gasification in downdraft gasifier and simulation using ASPEN HYSYS. Energy Convers. Manag. 2014, 88, 693–699. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Yang, C.W.; Lee, U.D.; Jeong, S.H. Characteristics of the pyrolytic products from the fast pyrolysis of palm kernel cake in a bench-scale fluidized bed reactor. J. Anal. Appl. Pyrolysis 2020, 145, 104708. [Google Scholar] [CrossRef]
- Lynd, L.R.; Cushman, J.H.; Nichols, R.J.; Wyman, C.E. Fuel ethanol from cellulosic biomass. Science 1991, 251, 1318–1323. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- da Silva Trindade, W.R.; dos Santos, R.G. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 642–651. [Google Scholar] [CrossRef]
- Nanda, S.; Golemi-Kotra, D.; McDermott, J.C.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Fermentative production of butanol: Perspectives on synthetic biology. New Biotechnol. 2017, 37, 210–221. [Google Scholar] [CrossRef]
- Yadav, P.; Varma, A.K.; Mondal, P. Production of biodiesel from algal biomass collected from Solani River using Ultrasonic Technique. Int. J. Renew. Energy Res. 2014, 4, 714–724. [Google Scholar]
- Levine, R.B.; Pinnarat, T.; Savage, P.E. Biodiesel production from wet algal biomass through in situ lipid hydrolysis and supercritical transesterification. Energy Fuels 2010, 24, 5235–5243. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Z.; Xu, H.; Yan, K. Selective production of phenol-rich bio-oil from corn straw waste by direct microwave pyrolysis without extra catalyst. Front. Chem. 2021, 9, 700887. [Google Scholar] [CrossRef]
- Solantausta, Y.; Oasmaa, A.; Sipila, K.; Lindfors, C.; Lehto, J.; Autio, J.; Jokela, P.; Alin, J.; Heiskanen, J. Bio-oil production from biomass: Steps toward demonstration. Energy Fuels 2012, 26, 233–240. [Google Scholar] [CrossRef]
- Sutrisno, B.; Hidayat, A. Upgrading of bio-oil from the pyrolysis of biomass over the rice husk ash catalysts. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162, 012014. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ławińska, O.; Korombel, A.; Zajemska, M. Pyrolysis-Based Municipal Solid Waste Management in Poland—SWOT Analysis. Energies 2022, 15, 510. [Google Scholar] [CrossRef]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low-and middle-income settings. Rev. Environ. Sci. Bio/Technol. 2017, 16, 81–130. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Li, S.; Xu, S.; Liu, S.; Yang, C.; Lu, Q. Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Tippayawong, N.; Kinorn, J.; Thavornun, S. Yields and gaseous composition from slow pyrolysis of refuse-derived fuels. Energy Sources Part A 2008, 30, 1572–1580. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Brammer, J.G.; Lauer, M.; Bridgwater, A.V. Opportunities for biomass-derived “bio-oil” in European heat and power markets. Energy Policy 2006, 34, 2871–2880. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Oasmaa, A.; Solantausta, Y. Power generation using fast pyrolysis liquids from biomass. Renew. Sustain. Energy Rev. 2007, 11, 1056–1086. [Google Scholar] [CrossRef]
- Meier, D.; Oasmaa, A.; Peacocke, C.; Piskorz, J.; Radlein, D. Fast Pyrolysis of Biomass: A Handbook; U.S. Department of Energy: Washington, DC, USA, 1999.
- Demirbas, A. Recent advances in biomass conversion technologies. Energy Edu Sci. Technol. 2000, 6, 19–41. [Google Scholar]
- Aguado, R.; Olazar, M.; Gaisán, B.; Prieto, R.; Bilbao, J. Kinetic study of polyolefin pyrolysis in a conical spouted bed reactor. Ind. Eng. Chem. Res. 2002, 41, 4559–4566. [Google Scholar] [CrossRef]
- Cornelissen, T.; Yperman, J.; Reggers, G.; Schreurs, S.; Carleer, R. Flash co-pyrolysis of biomass with polylactic acid. Part 1: Influence on bio-oil yield and heating value. Fuel 2008, 87, 1031–1041. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K. Study on effects of different operating parameters on the pyrolysis of biomass: A review. J. Biofuels Bioenergy 2015, 1, 135. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, S.; Liao, Y.; Zhou, J.; Gu, Y.; Cen, K. Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenergy 2004, 26, 455–462. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef]
- Ozbay, N.; Pütün, A.E.; Pütün, E. Bio-oil production from rapid pyrolysis of cottonseed cake: Product yields and compositions. Int. J. Energy Res. 2006, 30, 501–510. [Google Scholar] [CrossRef]
- Demirbas, A. The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process. Technol. 2007, 88, 591–597. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Uzun, B.B.; Pütün, A.E.; Pütün, E. Fast pyrolysis of soybean cake: Product yields and compositions. Bioresour. Technol. 2006, 97, 569–576. [Google Scholar] [CrossRef]
- Yorgun, S.A.İ.T.; Şensöz, S.; Koçkar, Ö.M. Flash pyrolysis of sunflower oil cake for production of liquid fuels. J. Anal. Appl. Pyrolysis 2001, 60, 1–12. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Chen, L.; Dupont, C.; Salvador, S.; Grateau, M.; Boissonnet, G.; Schweich, D. Experimental study on fast pyrolysis of free-falling millimetric biomass particles between 800 C and 1000 C. Fuel 2013, 106, 61–66. [Google Scholar] [CrossRef]
- Demiral, İ.; Şensöz, S. Fixed-bed pyrolysis of hazelnut (Corylus avellana L.) bagasse: Influence of pyrolysis parameters on product yields. Energy Sources Part A Recovery Util. Environ. Eff. 2006, 28, 1149–1158. [Google Scholar] [CrossRef]
- Onay, O.; Koçkar, O.M. Fixed-bed pyrolysis of rapeseed (Brassica napus L.). Biomass Bioenergy 2004, 26, 289–299. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Haykiri-Acma, H. The role of particle size in the non-isothermal pyrolysis of hazelnut shell. J. Anal. Appl. Pyrolysis 2006, 75, 211–216. [Google Scholar] [CrossRef]
- Guo, X.; Li, S.; Zheng, Y.; Ci, B. Preparation and characterization of bio-oil by microwave pyrolysis of biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 133–139. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Boroson, M.L.; Howard, J.B.; Longwell, J.P.; Peters, W.A. Product yields and kinetics from the vapor phase cracking of wood pyrolysis tars. AIChE J. 1989, 35, 120–128. [Google Scholar] [CrossRef]
- Uddin, M.N.; Daud, W.W.; Abbas, H.F. Effects of pyrolysis parameters on hydrogen formations from biomass: A review. Rsc Adv. 2014, 4, 10467–10490. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Omar, R.; Idris, A.; Yunus, R.; Khalid, K.; Isma, M.A. Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 2011, 90, 1536–1544. [Google Scholar] [CrossRef]
- Jung, S.H.; Kang, B.S.; Kim, J.S. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J. Anal. Appl. Pyrolysis 2008, 82, 240–247. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Ataide, C.H. Analytical pyrolysis of tobacco residue: Effect of temperature and inorganic additives. J. Anal. Appl. Pyrolysis 2013, 99, 49–57. [Google Scholar] [CrossRef]
- Aysu, T. Catalytic pyrolysis of Alcea pallida stems in a fixed-bed reactor for production of liquid bio-fuels. Bioresour. Technol. 2015, 191, 253–262. [Google Scholar] [CrossRef]
- Luo, G.; Resende, F.L. In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees. Fuel 2016, 166, 367–375. [Google Scholar] [CrossRef]
- Mansur, D.; Yoshikawa, T.; Norinaga, K.; Hayashi, J.I.; Tago, T.; Masuda, T. Production of ketones from pyroligneous acid of woody biomass pyrolysis over an iron-oxide catalyst. Fuel 2013, 103, 130–134. [Google Scholar] [CrossRef]
- Smets, K.; Roukaerts, A.; Czech, J.; Reggers, G.; Schreurs, S.; Carleer, R.; Yperman, J. Slow catalytic pyrolysis of rapeseed cake: Product yield and characterization of the pyrolysis liquid. Biomass Bioenergy 2013, 57, 180–190. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhou, G.; Wang, W.; Wang, C.; Komarneni, S.; Wang, Y. Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl. Catal. A Gen. 2014, 470, 115–122. [Google Scholar] [CrossRef]
- Siengchum, T.; Isenberg, M.; Chuang, S.S. Fast pyrolysis of coconut biomass–An FTIR study. Fuel 2013, 105, 559–565. [Google Scholar] [CrossRef]
- Şensöz, S.; Angın, D. Pyrolysis of safflower (Charthamus tinctorius L.) seed press cake in a fixed-bed reactor: Part 2. Structural characterization of pyrolysis bio-oils. Bioresour. Technol. 2008, 99, 5498–5504. [Google Scholar]
- Antal Jr, M.J.; Allen, S.G.; Dai, X.; Shimizu, B.; Tam, M.S.; Grønli, M. Attainment of the theoretical yield of carbon from biomass. Ind. Eng. Chem. Res. 2000, 39, 4024–4031. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Acıkgoz, C.; Onay, O.; Kockar, O.M. Fast pyrolysis of linseed: Product yields and compositions. J. Anal. Appl. Pyrolysis 2004, 71, 417–429. [Google Scholar] [CrossRef]
- Pütün, A.E.; Apaydın, E.; Pütün, E. Rice straw as a bio-oil source via pyrolysis and steam pyrolysis. Energy 2004, 29, 2171–2180. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Gupta, A.K. Pyrolysis and gasification of food waste: Syngas characteristics and char gasification kinetics. Appl. Energy 2010, 87, 101–108. [Google Scholar] [CrossRef]
- Demiral, I.; Şensöz, S. The effects of different catalysts on the pyrolysis of industrial wastes (olive and hazelnut bagasse). Bioresour. Technol. 2008, 99, 8002–8007. [Google Scholar] [CrossRef]
- He, M.; Hu, Z.; Xiao, B.; Li, J.; Guo, X.; Luo, S.; Yang, F.; Feng, Y.; Yang, G.; Liu, S. Hydrogen-rich gas from catalytic steam gasification of municipal solid waste (MSW): Influence of catalyst and temperature on yield and product composition. Int. J. Hydrogen Energy 2009, 34, 195–203. [Google Scholar] [CrossRef]
- Hao, X.H.; Guo, L.J.; Mao, X.A.; Zhang, X.M.; Chen, X.J. Hydrogen production from glucose used as a model compound of biomass gasified in supercritical water. Int. J. Hydrogen Energy 2003, 28, 55–64. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Li, L.; Ma, Y. Microwave-assisted pretreatment of corn stover for enhancing enzymatic hydrolysis and fermentation. Fuel Process. Technol. 2021, 210, 106624. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Wang, Y.; Li, Z. Microwave-assisted pretreatment of corn stover for the production of biofuels: A review. Renew. Sustain. Energy Rev. 2020, 119, 109592. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Wang, Y.; Liu, Y.; Ma, X. Microwave-assisted pretreatment of corn straw for efficient bioethanol production. Bioresour. Technol. 2021, 335, 125246. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Ma, Y.; Li, L.; Yu, J. Optimization of microwave-assisted alkali pretreatment for enhancing enzymatic hydrolysis of corn stover. Fuel 2020, 266, 117080. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Demirbas, A. Production of gasoline and diesel fuels from bio-materials. Energy Sources Part A 2007, 29, 753–760. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Adarsh, K.; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar]
- Çulcuoglu, E.; Ünay, E.; Karaosmanoglu, F.; Angin, D.; Şensöz, S. Characterization of the bio-oil of rapeseed cake. Energy Sources 2005, 27, 1217–1223. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E. Fast pyrolysis of forestry residue. 3. Storage stability of liquid fuel. Energy Fuels 2003, 17, 1075–1084. [Google Scholar] [CrossRef]
- Oasmaa, A.; Meier, D. Norms and standards for fast pyrolysis liquids: 1. Round robin test. J. Anal. Appl. Pyrolysis 2005, 73, 323–334. [Google Scholar] [CrossRef]
- Shihadeh, A.; Hochgreb, S. Diesel engine combustion of biomass pyrolysis oils. Energy Fuels 2000, 14, 260–274. [Google Scholar] [CrossRef]
- Abdullah, N. An Assessment of Pyrolysis for Processing Empty Fruit Bunches. Ph.D. Dissertation, Aston University, Birmingham, UK, 2005. [Google Scholar]
- Rocha, J.; Gomez, E.; Mesa Perez, J.; Cortez, L.; Seye, O.; Brossard Gonzalez, L. The demonstration fast pyrolysis plant to biomass conversion in brazil. In Proceedings of the VII World Renew Energy Congress, Cologne, Germany, 29 June–5 July 2002. [Google Scholar]
- González, J.F.; Román, S.; Encinar, J.M.; Martínez, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrolysis 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan Publications Ltd.: London, UK, 2009; Volume 2, pp. 154–160. [Google Scholar]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 17–31. [Google Scholar]
- Kantarelis, E.; Zabaniotou, A. Valorization of cotton stalks by fast pyrolysis and fixed bed air gasification for syngas production as precursor of second generation biofuels and sustainable agriculture. Bioresour. Technol. 2009, 100, 942–947. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Plasma pyrolysis of biomass for production of syngas and carbon adsorbent. Energy Fuels 2005, 19, 1174–1178. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Hu, Z.; Guo, X.; Luo, S.; Yang, F. Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J. Anal. Appl. Pyrolysis 2010, 87, 181–187. [Google Scholar] [CrossRef]
- Wei, L.; Thomasson, J.A.; Bricka, R.M.; Batchelor, W.D.; Columbus, E.P.; Wooten, J.R. Experimental Study of a Downdraft Gratifier; ASABE Meeting Paper No. 066029; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Dasappa, S.; Paul, P.J.; Mukunda, H.S.; Rajan, N.K.S.; Sridhar, G.; Sridhar, H.V. Biomass gasification technology–a route to meet energy needs. Curr. Sci. 2004, 87, 908–916. [Google Scholar]
- Al Arni, S.; Bosio, B.; Arato, E. Syngas from sugarcane pyrolysis: An experimental study for fuel cell applications. Renew. Energy 2010, 35, 29–35. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Pyrolysis liquids and gases as alternative fuels in internal combustion engines–A review. Renew. Sustain. Energy Rev. 2013, 21, 165–189. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhang, J.; Zhu, Z. Process simulation of preparing biochar by biomass pyrolysis via aspen plus and its economic evaluation. Waste Biomass Valorization 2022, 13, 2609–2622. [Google Scholar] [CrossRef]
- Elkhalifa, S.; AlNouss, A.; Al-Ansari, T.; Mackey, H.R.; Parthasarathy, P.; Mckay, G. Simulation of food waste pyrolysis for the production of biochar: A Qatar case study. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 46, pp. 901–906. [Google Scholar]
- Tan, Y.L.; Abdullah, A.Z.; Hameed, B.H. Product distribution of the thermal and catalytic fast pyrolysis of karanja (Pongamia pinnata) fruit hulls over a reusable silica-alumina catalyst. Fuel 2019, 245, 89–95. [Google Scholar] [CrossRef]
- Lee, X.J.; Lee, L.Y.; Hiew, B.Y.Z.; Gan, S.; Thangalazhy-Gopakumar, S.; Ng, H.K. Valorisation of oil palm wastes into high yield and energy content biochars via slow pyrolysis: Multivariate process optimisation and combustion kinetic studies. Mater. Sci. Energy Technol. 2020, 3, 601–610. [Google Scholar] [CrossRef]
- Sukumar, V.; Manieniyan, V.; Senthilkumar, R.; Sivaprakasam, S. Production of bio oil from sweet lime empty fruit bunch by pyrolysis. Renew. Energy 2020, 146, 309–315. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.H.; Show, P.L. Biochar production via pyrolysis of citrus peel fruit waste as a potential usage as solid biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef] [PubMed]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Souissi-Najar, S.; Ouederni, A.; Fuente, E. Pyrolysis technologies for pomegranate (Punica granatum L.) peel wastes. Prospects in the bioenergy sector. Renew. Energy 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Otoikhian, K.S.; Ighalo, J.O.; Mohammed, I.A. Pyrolysis of different fruit peel waste via a thermodynamic model. ABUAD J. Eng. Res. Dev. 2019, 2, 16–24. [Google Scholar]
- Alves, J.L.F.; Da Silva, J.C.G.; da Silva Filho, V.F.; Alves, R.F.; de Araujo Galdino, W.V.; Andersen, S.L.F.; De Sena, R.F. Determination of the bioenergy potential of Brazilian pine-fruit shell via pyrolysis kinetics, thermodynamic study, and evolved gas analysis. BioEnergy Res. 2019, 12, 168–183. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sheeba, K.N. Combined slow pyrolysis and steam gasification of biomass for hydrogen generation—A review. Int. J. Energy Res. 2015, 39, 147–164. [Google Scholar] [CrossRef]
- Castilla-Caballero, D.; Barraza-Burgos, J.; Gunasekaran, S.; Roa-Espinosa, A.; Colina-Márquez, J.; Machuca-Martínez, F.; Hernández-Ramírez, A.; Vázquez-Rodríguez, S. Experimental data on the production and characterization of biochars derived from coconut-shell wastes obtained from the Colombian Pacific Coast at low temperature pyrolysis. Data Brief 2020, 28, 104855. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Ismail, H.Y.; Abbas, A.; Azizi, F.; Zeaiter, J. Pyrolysis of waste tires: A modeling and parameter estimation study using Aspen Plus®. Waste Manag. 2017, 60, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, M.; Shrikar, B.; Ramanathan, A. Development of computer aided modelling and optimization of microwave pyrolysis of biomass by using aspen plus. IOP Conf. Ser. Earth Environ. Sci. 2019, 312, 012006. [Google Scholar] [CrossRef]
- Kabir, M.J.; Chowdhury, A.A.; Rasul, M.G. Pyrolysis of municipal green waste: A modelling, simulation and experimental analysis. Energies 2015, 8, 7522–7541. [Google Scholar] [CrossRef]
- AlNouss, A.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Pyrolysis study of different fruit wastes using an Aspen Plus model. Front. Sustain. Food Syst. 2021, 5, 604001. [Google Scholar] [CrossRef]
- Treeyawetchakul, C. Preliminary Modified Biodiesel Production by Coupling Reactive distillation with a Steam Reformer via Aspen Plus®. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012064. [Google Scholar] [CrossRef]
- Quintero, J.A.; Cardona, C.A. Process simulation of fuel ethanol production from lignocellulosics using aspen plus. Ind. Eng. Chem. Res. 2011, 50, 6205–6212. [Google Scholar] [CrossRef]
- Oladokun, O.; Nyakuma, B.B.; Ivase, T.J. Gasification of Nigerian Lignite Coals under Air-Steam Conditions using ASPEN Plus for the Production of Hydrogen and Syngas. Pet. Coal 2021, 63, 332–339. [Google Scholar]
- Pilar González-Vázquez, M.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A. Thermodynamic analysis of biomass gasification using aspen plus: Comparison of stoichiometric and non-stoichiometric models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Flagiello, D.; Di Natale, F.; Erto, A.; Lancia, A. Wet oxidation scrubbing (WOS) for flue-gas desulphurization using sodium chlorite seawater solutions. Fuel 2020, 277, 118055. [Google Scholar] [CrossRef]
- Diebold, J.P. Review of the Chemical and Physical Mechanisms of the Storage and Stability of Fast Pyrolysis Bio-Oils; NREL 1; Thermalchemie, Inc.: Lakewood, CO, USA, 2000. [Google Scholar]
- Evans, R.J.; Milne, T.A. Molecular characterization of the pyrolysis of biomass. Energy Fuels 1987, 1, 123–137. [Google Scholar] [CrossRef]
- Stankovikj, F.; McDonald, A.G.; Helms, G.L.; Olarte, M.V.; Garcia-Perez, M. Characterization of the water-soluble fraction of woody biomass pyrolysis oils. Energy Fuels 2017, 31, 1650–1664. [Google Scholar] [CrossRef]
- Koike, N.; Hosokai, S.; Takagaki, A.; Nishimura, S.; Kikuchi, R.; Ebitani, K.; Suzuki, Y.; Oyama, S.T. Upgrading of pyrolysis bio-oil using nickel phosphide catalysts. J. Catal. 2016, 333, 115–126. [Google Scholar] [CrossRef]
- Kadarwati, S.; Oudenhoven, S.; Schagen, M.; Hu, X.; Garcia-Perez, M.; Kersten, S.; Li, C.Z.; Westerhof, R. Polymerization and cracking during the hydrotreatment of bio-oil and heavy fractions obtained by fractional condensation using Ru/C and NiMo/Al2O3 catalyst. J. Anal. Appl. Pyrolysis 2016, 118, 136–143. [Google Scholar]
- Han, Y.; Gholizadeh, M.; Tran, C.C.; Kaliaguine, S.; Li, C.Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar]
- Hew, K.L.; Tamidi, A.M.; Yusup, S.; Lee, K.T.; Ahmad, M.M. Catalytic cracking of bio-oil to organic liquid product (OLP). Bioresour. Technol. 2010, 101, 8855–8858. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Solantausta, Y. Catalytic hydroprocessing of fast pyrolysis bio-oil from pine sawdust. Energy Fuels 2012, 26, 3891–3896. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R. Catalytic hydroprocessing of chemical models for bio-oil. Energy Fuels 2009, 23, 631–637. [Google Scholar] [CrossRef]
- Elliott, D.C.; Lee, S.J.; Hart, T.R. Stabilization of Fast Pyrolysis Oil: Post Processing Final Report (No. PNNL-21549); Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2012.
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014, 16, 491–515. [Google Scholar]
- de Miguel Mercader, F.; Groeneveld, M.J.; Kersten, S.R.; Geantet, C.; Toussaint, G.; Way, N.W.; Schaverien, C.J.; Hogendoorn, K.J. Hydrodeoxygenation of pyrolysis oil fractions: Process understanding and quality assessment through co-processing in refinery units. Energy Environ. Sci. 2011, 4, 985–997. [Google Scholar] [CrossRef]
- Stankovikj, F.; Tran, C.C.; Kaliaguine, S.; Olarte, M.V.; Garcia-Perez, M. Evolution of functional groups during pyrolysis oil upgrading. Energy Fuels 2017, 31, 8300–8316. [Google Scholar] [CrossRef]
- De Miguel Mercader, F.; Koehorst, P.J.J.; Heeres, H.J.; Kersten, S.R.; Hogendoorn, J.A. Competition between hydrotreating and polymerization reactions during pyrolysis oil hydrodeoxygenation. AIChE J. 2011, 57, 3160–3170. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and char quality of flame-curtain “Kon Tiki” kilns for farmer-scale charcoal/biochar production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef] [PubMed]
- Fulton, W.; Gray, M.; Prahl, F.; Kleber, M. A simple technique to eliminate ethylene emissions from biochar amendment in agriculture. Agron. Sustain. Dev. 2013, 33, 469–474. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J. Anal. Appl. Pyrolysis 2013, 103, 60–67. [Google Scholar] [CrossRef]
- Miskolczi, N.; Ates, F.; Borsodi, N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: Contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013, 144, 370–379. [Google Scholar] [CrossRef]
- Pyrolysis. 23 March 2014. Available online: https://proreds.eu/technology/pyrolysis/ (accessed on 5 February 2023).
- Giwa, A.S.; Xu, H.; Chang, F.; Zhang, X.; Ali, N.; Yuan, J.; Wang, K. Pyrolysis coupled anaerobic digestion process for food waste and recalcitrant residues: Fundamentals, challenges, and considerations. Energy Sci. Eng. 2019, 7, 2250–2264. [Google Scholar] [CrossRef]
- Giwa, A.S.; Chang, F.; Xu, H.; Zhang, X.; Huang, B.; Li, Y.; Wu, J.; Wang, B.; Vakili, M.; Wang, K. Pyrolysis of difficult biodegradable fractions and the real syngas bio-methanation performance. J. Clean. Prod. 2019, 233, 711–719. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S. Effect of particle size on pyrolysis of single-component municipal solid waste in fixed bed reactor. Int. J. Hydrogen Energy 2010, 35, 93–97. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, Y. Integrated processes of anaerobic digestion and pyrolysis for higher bioenergy recovery from lignocellulosic biomass: A brief review. Renew. Sustain. Energy Rev. 2017, 77, 1272–1287. [Google Scholar] [CrossRef]
- Song, X.D.; Chen, D.Z.; Zhang, J.; Dai, X.H.; Qi, Y.Y. Anaerobic digestion combined pyrolysis for paper mill sludge disposal and its influence on char characteristics. J. Mater. Cycles Waste Manag. 2017, 19, 332–341. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Wu, J.; Li, Y.; Chang, F.; Zhang, X.; Jin, Z.; Huang, B.; Wang, K. Sustainable recycling of residues from the food waste (FW) composting plant via pyrolysis: Thermal characterization and kinetic studies. J. Clean. Prod. 2018, 180, 43–49. [Google Scholar] [CrossRef]
- Rickwinder, S.; Paritosh, K.; Pareek, N.; Vivekanand, V. Integrated system of anaerobic digestion and pyrolysis for valorization of agricultural and food waste towards circular bioeconomy. Bioresour. Technol. 2022, 360, 127596. [Google Scholar]
- Tang, Z.; Chen, W.; Chen, Y.; Yang, H.; Chen, H. Co-pyrolysis of microalgae and plastic: Characteristics and interaction effects. Bioresour. Technol. 2019, 274, 145–152. [Google Scholar] [CrossRef]
- Zakaria, M.; Sharaky, A.M.; Al-Sherbini, A.-S.; Bassyouni, M.; Rezakazemi, M.; Elhenawy, Y. Water desalination using solar thermal collectors enhanced by nanofluids. Chem. Eng. Tech. 2022, 45, 15–25. [Google Scholar] [CrossRef]
- Pattiya, A.; Titiloye, J.O. Pyrolysis of biomass for biofuels: A review. Sustainability 2019, 11, 2250. [Google Scholar]
- Zhang, S.; Li, J.; Yu, H. Catalytic pyrolysis of biomass for biofuels production: A review. Sustainability 2020, 12, 8939. [Google Scholar]
- Chen, H.; Chen, Y.; Li, M.; Zheng, Y. Fast pyrolysis of biomass for biofuels: A review. Sustainability 2019, 11, 6017. [Google Scholar]
- Xu, J.; Wang, Y.; Ma, X. Catalytic pyrolysis of lignocellulosic biomass for biofuels production: A review. Sustainability 2020, 12, 4435. [Google Scholar]
- Nanda, S.; Dalai, A.K. Catalytic pyrolysis of biomass for biofuels production. Sustainability 2017, 9, 2023. [Google Scholar]
- Chen, S.; Li, Q. Bio-oil production via fast pyrolysis of biomass residues: A review. Sustainability 2016, 8, 1302. [Google Scholar]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Sustainability 2012, 4, 1577–1618. [Google Scholar] [CrossRef]
- Anwar, S.; Zaini, M.A.A.; Kumar, R.; Rashid, U. Pyrolysis of microalgae biomass for bio-oil production. Energy Convers. Manag. 2017, 141, 159–168. [Google Scholar]
- Chia, S.R.; Chew, K.W.; Show, P.L.; Yap, Y.J. Recent advancements in pyrolysis technology for sustainable production of biofuels from microalgae. Renew. Sustain. Energy Rev. 2018, 81, 1853–1872. [Google Scholar]
- Shafeeq, A.; Ahmad, M.; Javed, M.R. Pyrolysis of microalgae biomass for bio-oil production: A critical review. Biomass Convers. Biorefin. 2021, 11, 1095–1132. [Google Scholar]
- Kumar, M.; Sharma, S.; Singh, J.; Kumar, S. Pyrolysis of microalgae for bio-oil production: A critical review. Renew. Sustain. Energy Rev. 2020, 118, 109512. [Google Scholar] [CrossRef]
- Chen, J.; Duan, P.; Li, X.; Ma, X. Hydrothermal liquefaction of microalgae for bio-oil production: A review. Algal Res. 2019, 42, 101614. [Google Scholar]
- Gao, F.; Li, C.; Zhang, X.; Zhang, Y. Hydrothermal liquefaction of microalgae: A review. Renew. Sustain. Energy Rev. 2019, 109, 272–287. [Google Scholar]
- Yen, H.W.; Hu, I.C.; Chen, C.Y. Hydrothermal liquefaction of microalgae for bio-oil production: A review. Bioresour. Technol. 2019, 289, 121639. [Google Scholar]
- Li, S.; Yang, B.; Zhang, Y.; Zhu, Y.; Zhang, Y. Hydrothermal liquefaction of Chlorella vulgaris for bio-oil production: Effects of reaction parameters and kinetic models. Energy 2019, 169, 470–478. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Zhao, X.; Li, S.; Li, J.; Zhang, R. Effect of biochar addition on the performance of anaerobic digestion of corn stover: A comparative study. Bioresour. Technol. 2020, 297, 122469. [Google Scholar]
- Liu, T.; Zhang, L.; Zhang, W.; Li, Y.; Bian, Y. Effects of wheat straw biochar on anaerobic digestion of corn stover and cow dung: Methane production, biodegradability, and microbial community structure. Bioresour. Technol. 2021, 325, 124678. [Google Scholar]
- Chen, Y.; Xie, Y.; Wang, H.; Wei, L.; Chen, Y. Effect of biochar addition on the anaerobic digestion of rice straw: Methane production and carbon transformation. Energy 2019, 168, 1169–1177. [Google Scholar]
- Chawannat, J.; Tippayawong, N. Technical and economic analysis of a biomass pyrolysis plant. Energy Procedia 2015, 79, 950–955. [Google Scholar]
- Karittha, I.-O.; Wiyaratn, W.; Arpornwichanop, A. Technical and economic assessment of the pyrolysis and gasification integrated process for biomass conversion. Energy 2018, 153, 592–603. [Google Scholar]
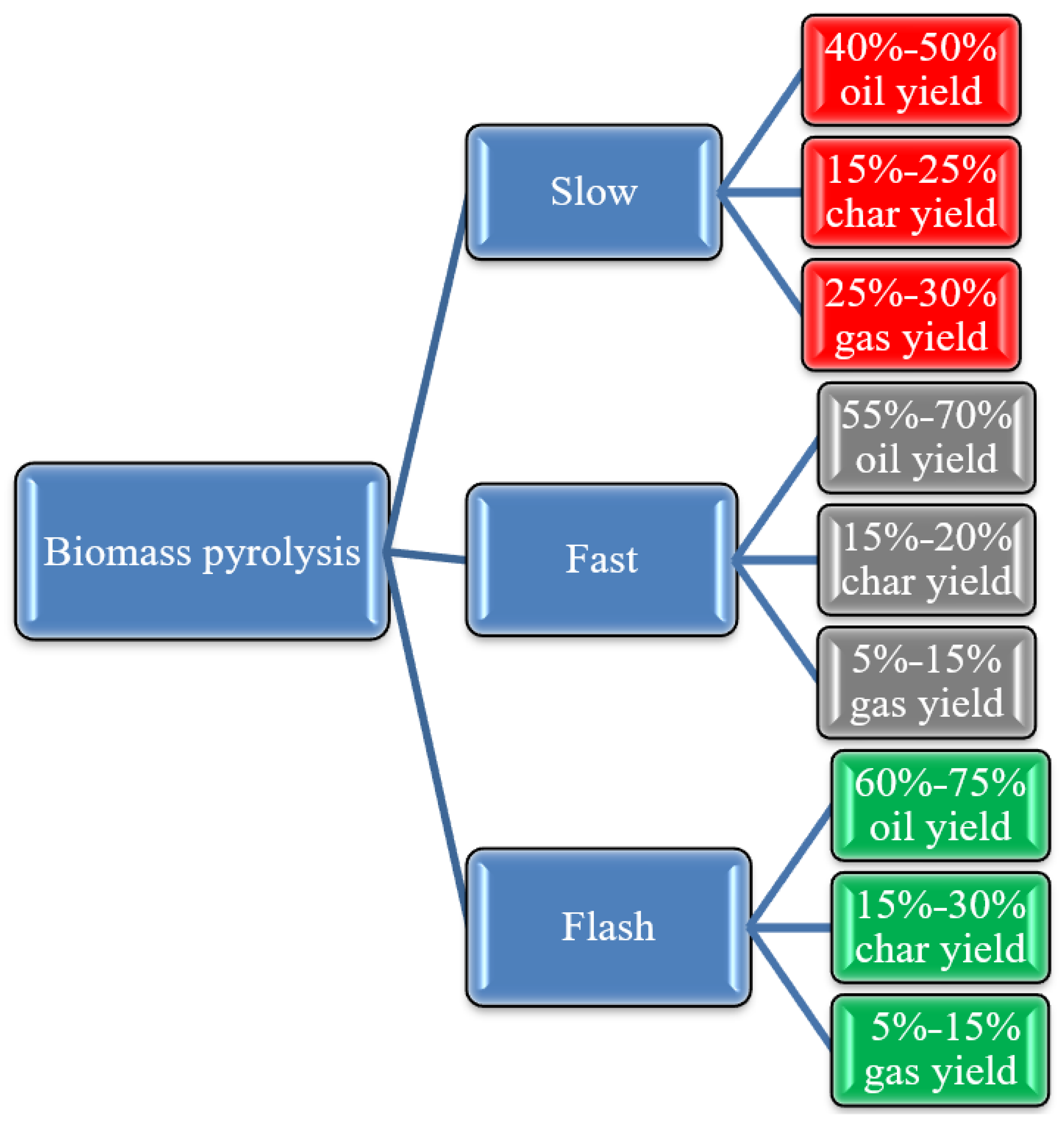
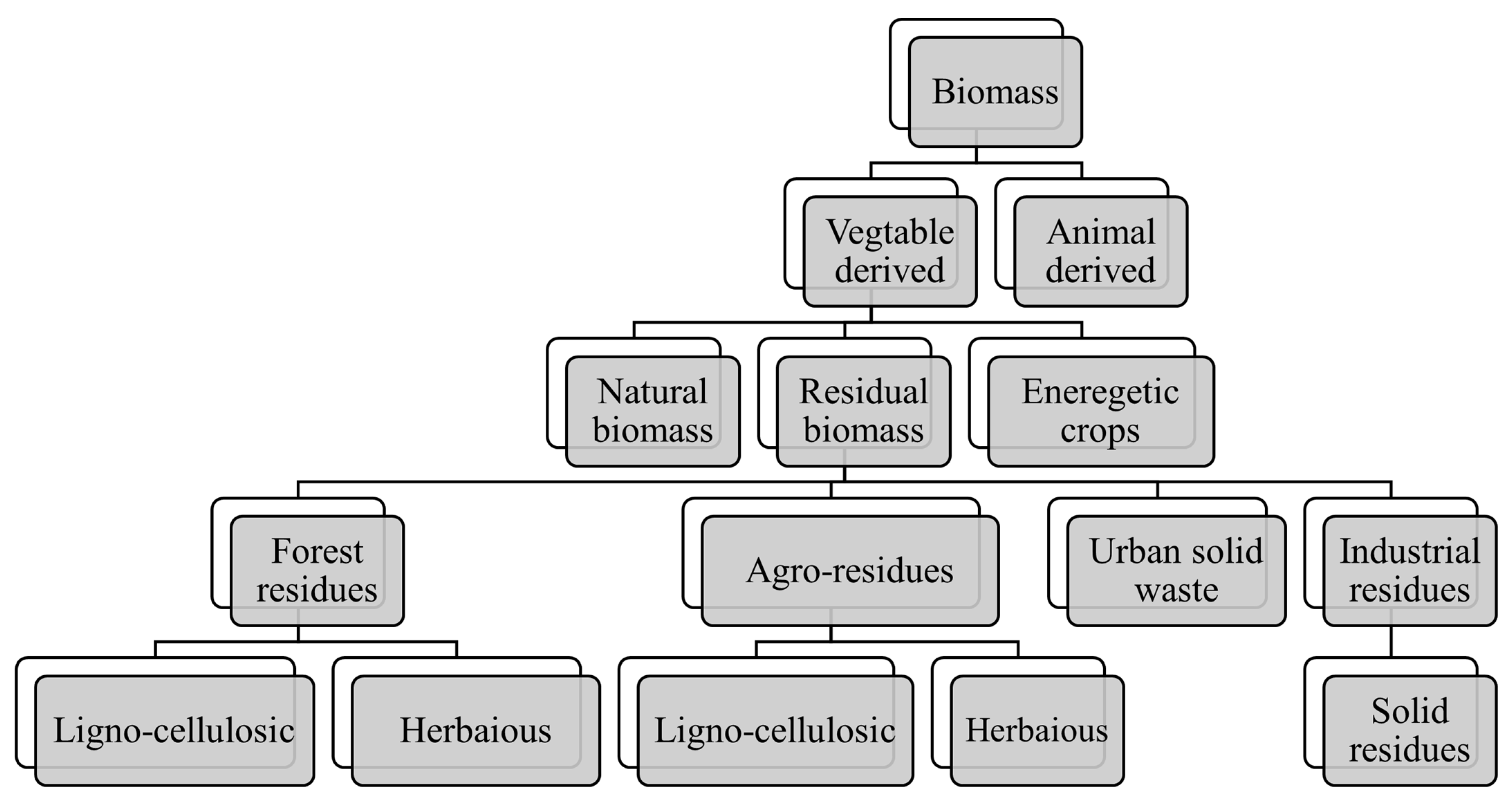
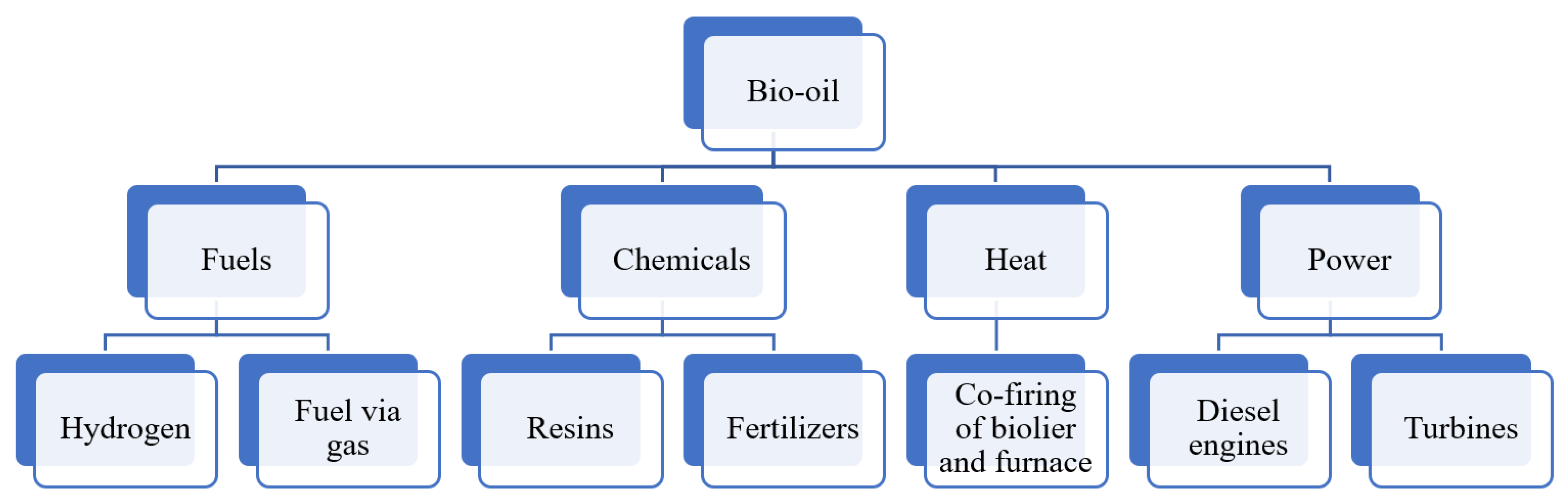
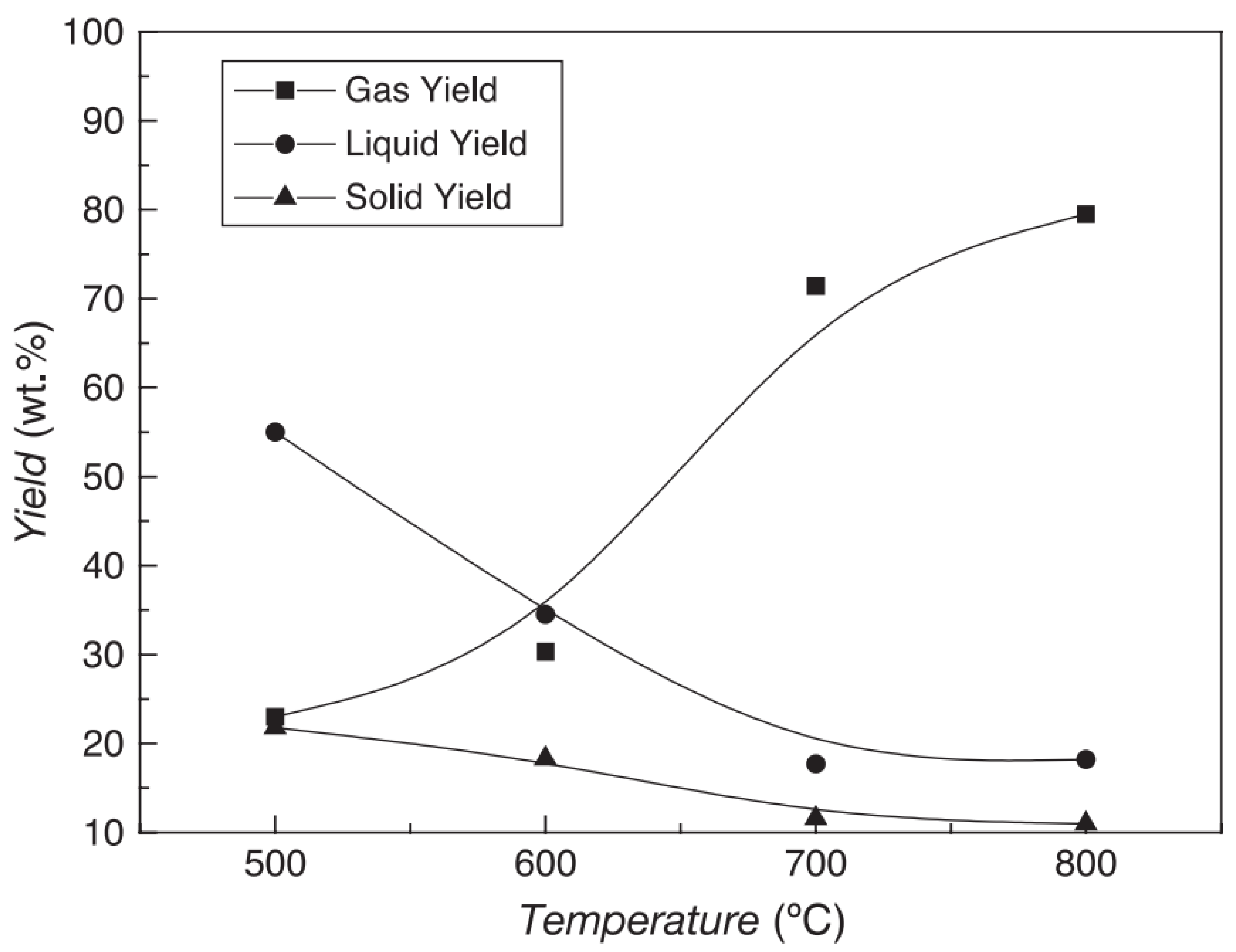
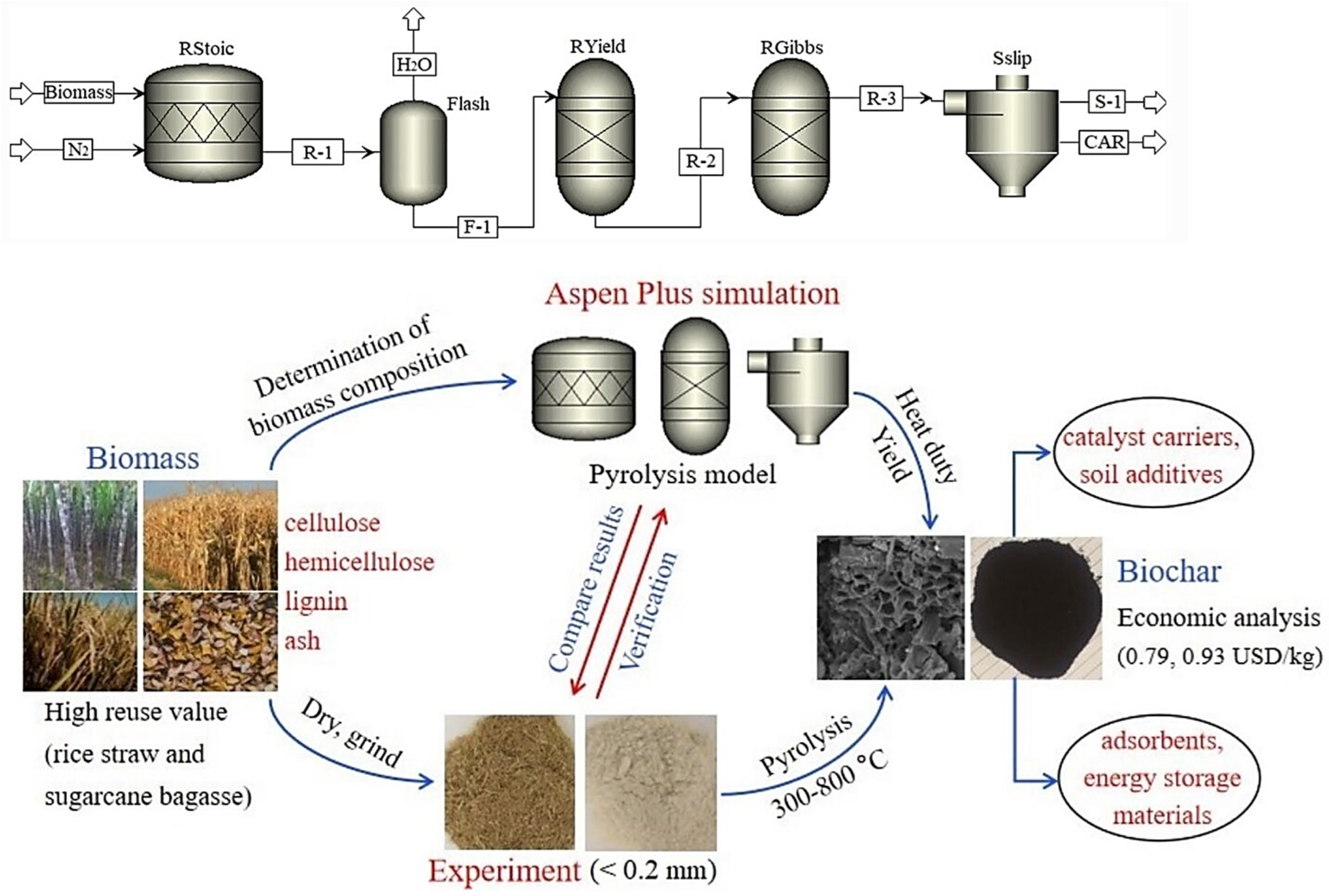
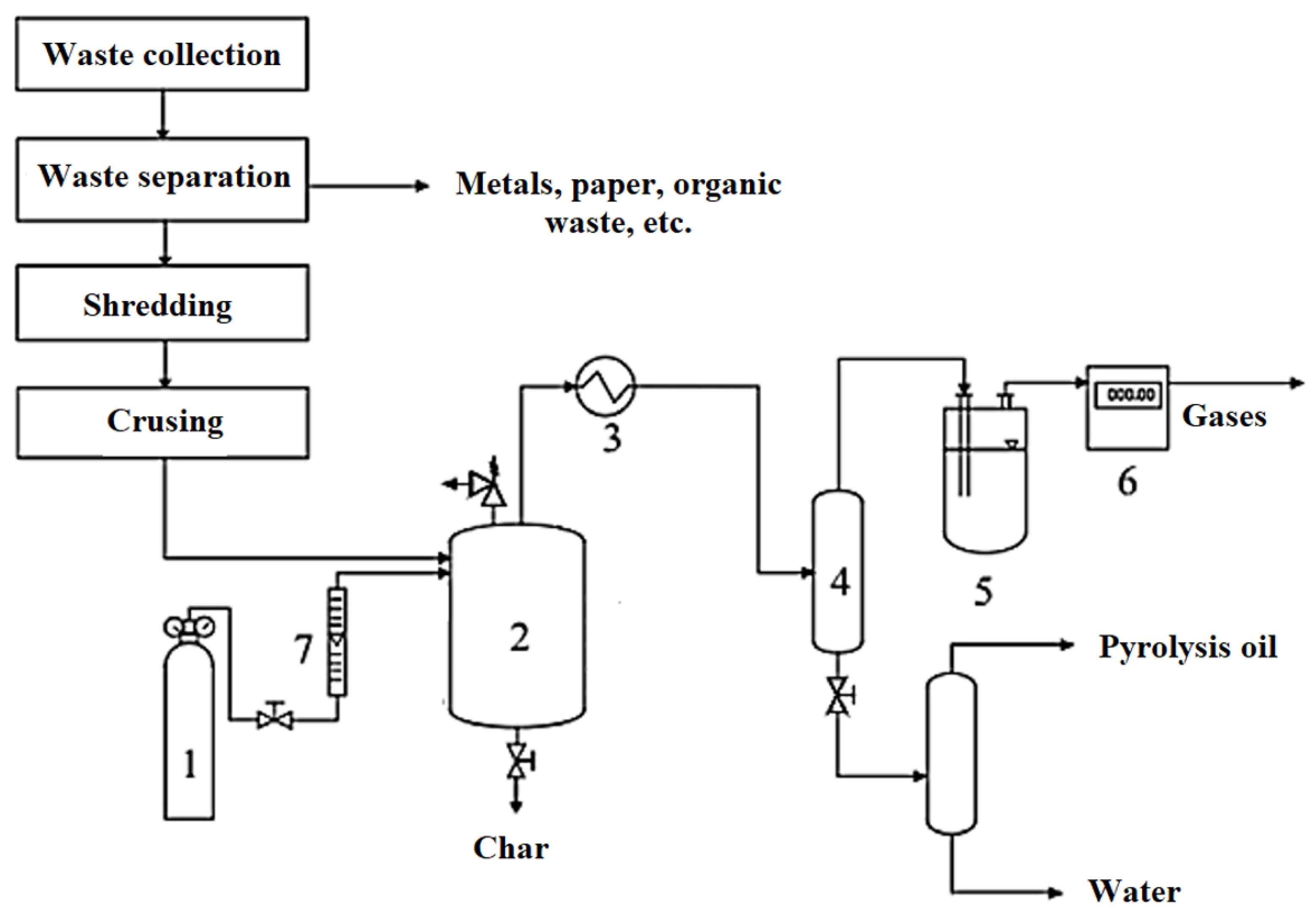


| Parameters | Slow Pyrolysis | Fast Pyrolysis | Flash Pyrolysis | Reference |
|---|---|---|---|---|
| Temperature (°C) | 550–950 | 850–1250 | 900–1200 | [33] |
| Heating rate (°C/s) | 0.1–1.0 | 10–200 | >1000 | |
| Residence time (s) | 300–550 | 0.5–10 | <1 | |
| Particle size (mm) | 5–50 | <1 | <0.5 |
| Biomass | Temperature (°C) | Oil Yield (wt. %) | References |
|---|---|---|---|
| Wheat straw | 600 | 34 | [44,45,46,47] |
| Rice husk | 450 | 70 | |
| Coal | 500 | 42 | |
| Sunflower cake | 550 | 41 | |
| Hardwood samples | 532 | 66.89 | |
| Soybean cake | 530 | 41 | |
| Bagasse | 500 | 66.1 |
| Substrate | Optimal Particle Size | Reference |
|---|---|---|
| Wood particles | 350–800 µm | [52] |
| Hazelnut | 0.225 < dp < 0.425 | [52] |
| Municipal solid waste (MSW) | Uncrushed, 1–2 cm | [53] |
| Rapeseed | <0.4 mm–>1.8 mm | [54] |
| Properties | Oil Characteristics | Interpretation | Ref. |
|---|---|---|---|
| Form | Free-flowing, organic liquid with a dark reddish-brown color | Oil’s chemical composition and the presence of micro-carbon | [31] |
| Odor | Unique scent: a sharp, smokey odor. | Acids and aldehydes with smaller molecular weights | [31] |
| Density | Extremely high in comparison to fossil fuel Bio-oil from pyrolysis: 1.2 kg/L 0.85 kg/L for fossil fuels | High levels of moisture and significant molecular contamination | [31] |
| Viscosity | 40–100 cP | Various feedstock types, water content, and the gathering of several non-heavy ends | [88] |
| Heat value | 26.7 MJ/kg | High oxygen content | [89] |
| Aging | With time, there is an increase in viscosity, a reduction in volatility, phase separation, and gum deposition | A high pH value and complex structure | [31] |
| Miscibility | Petroleum fuel is completely immiscible in non-polar solvents, yet miscible with polar solvents | Polar in nature | [31] |
| Treatment | Advantages | Reference |
|---|---|---|
| Water quenching or leaching |
| [138] |
| Heat treatments/aeration |
| [139] |
| Aging/weathering |
| [140] |
| Activation |
| [141] |
| Particle size reduction |
| [142] |
| Waste | Coupling Technology Process | Remarks | Ref. |
|---|---|---|---|
| Lignocellulosic biomass |
| When comparing the AD–pyrolysis process with the standard AD process, the electricity benefit may be increased by around 42%. Few efforts have been made to combine pyrolysis with AD, and there is a pressing need for more research into the hazardous substances produced during pyrolysis. The decomposition of biomass using AD–pyrolysis–AD is feasible, and the resulting sludge and residues may be put to good use. | [149] |
| Paper mill sludge | AD combined with pyrolysis | The integrated method increases energy independence throughout the treatment phase. | [150] |
| Food waste | Combining AD and pyrolysis | This research shed light on the evaluation of AD pyrolysis for FW treatment and its subsequent transformation into gas, oil, and solid yields for energy generation. This integrated method allows for the efficient concentration of nutrient elements optimized for use in soil conditioning and agronomy. | [151] |
| Recalcitrant organic residues (ROR) | Two-stage pyrolysis coupled with AD | During bio-methanation, ROR’s high H2 to CO ratio (60:20 vol.%) in syngas produces almost 100% more CH4 than the control. With a high CO content, the breakdown rate of H2 is slowed down because of the higher concentration of H2. Conventional ROR treatments have limitations that can be avoided by combining second-stage pyrolysis with the AD process. By using AD to process FW, a byproduct rich in hydrogen (H2) is produced, as well as improved bio-methanation. | [152,153,154,155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability 2023, 15, 11238. https://doi.org/10.3390/su151411238
Aboelela D, Saleh H, Attia AM, Elhenawy Y, Majozi T, Bassyouni M. Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability. 2023; 15(14):11238. https://doi.org/10.3390/su151411238
Chicago/Turabian StyleAboelela, Dina, Habibatallah Saleh, Attia M. Attia, Yasser Elhenawy, Thokozani Majozi, and Mohamed Bassyouni. 2023. "Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions" Sustainability 15, no. 14: 11238. https://doi.org/10.3390/su151411238
APA StyleAboelela, D., Saleh, H., Attia, A. M., Elhenawy, Y., Majozi, T., & Bassyouni, M. (2023). Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability, 15(14), 11238. https://doi.org/10.3390/su151411238









