An Optimization Design for the Resource Utilization of Grape Branches Based on the Orthogonal Test and Gray Relational Analysis Method
Abstract
1. Introduction
2. Materials and Methods
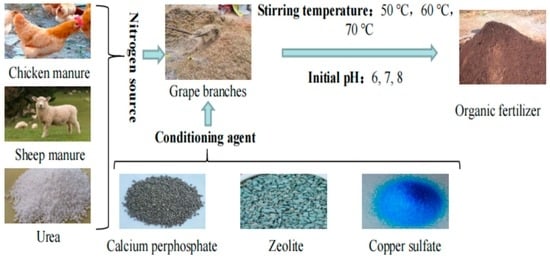
2.1. Collection of Materials
2.2. Experimental Design
2.2.1. Orthogonal Test
2.2.2. Gray Relational Analysis
2.3. Analytical Procedure
2.3.1. Physical Analyses
2.3.2. Chemical Analyses
2.3.3. Seed Germination Test
2.4. Statistical Analysis
3. Results and Discussion
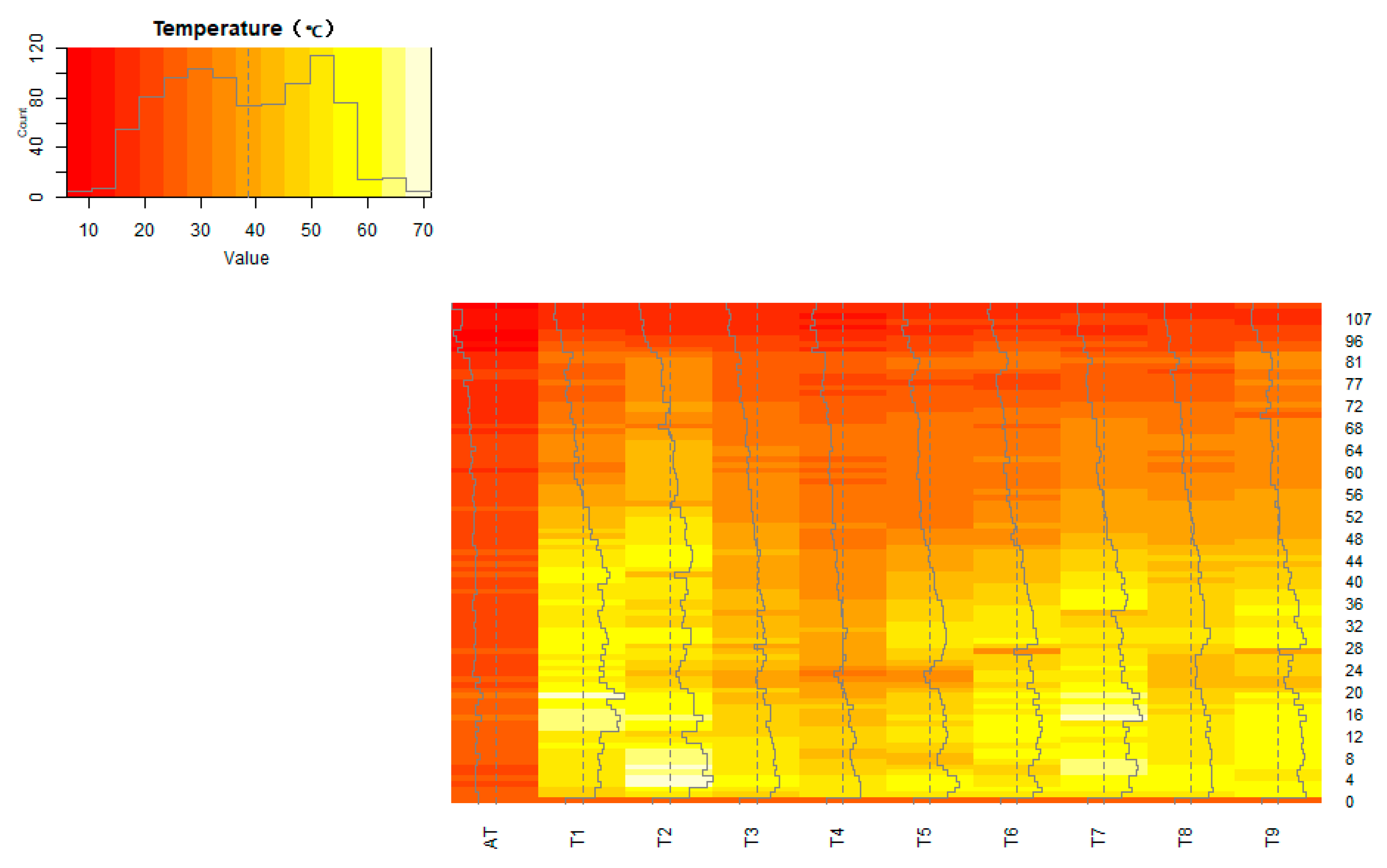
3.1. Effect of Different Conditions on the Temperature during the Composting Process
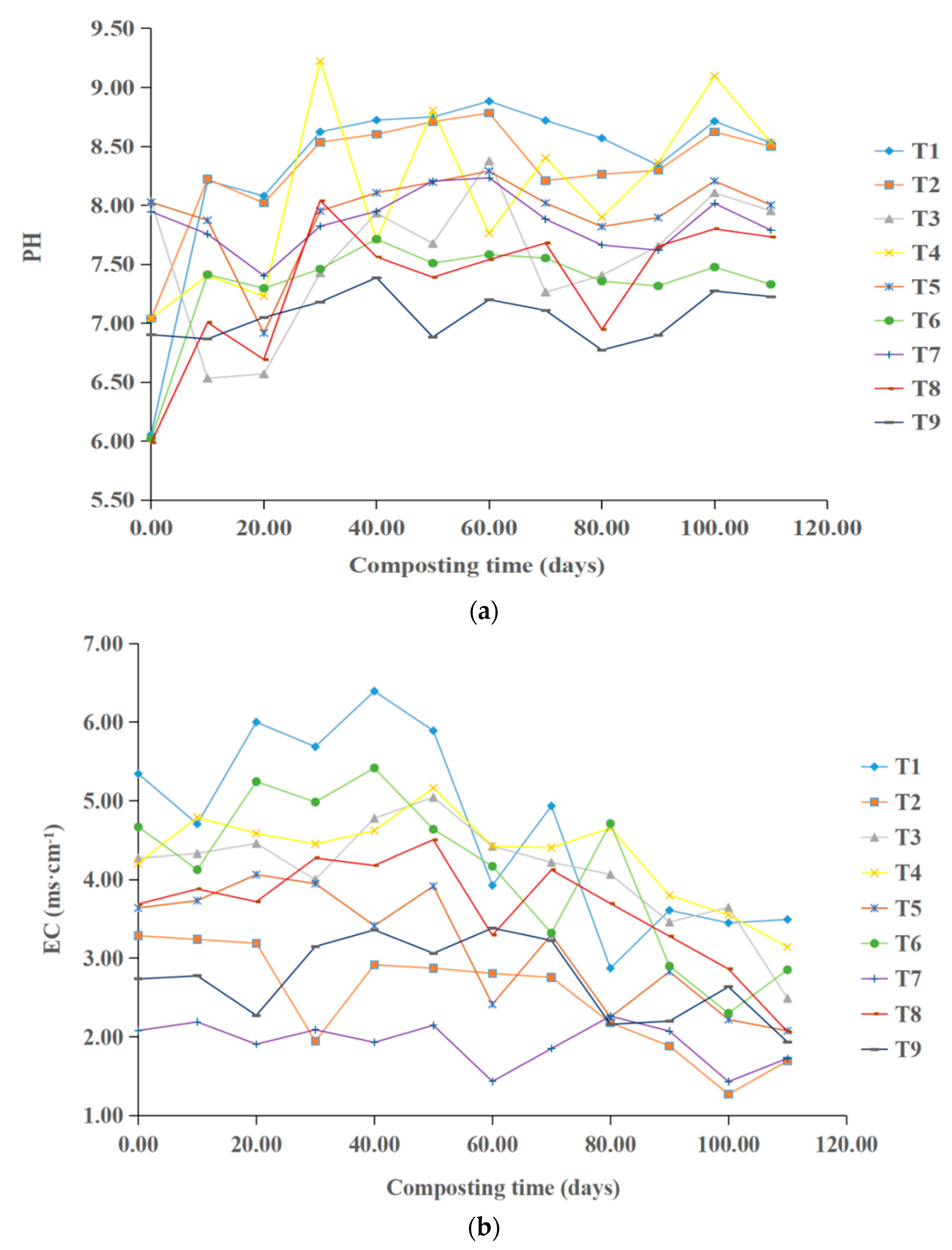
3.2. Effect of Different Conditions on the pH and Electrical Conductivity of the Compost Product
3.3. Effect of Different Conditions on Phytotoxicity of the Compost Product
3.4. Effect of Different Conditions on Organic Matter and Total Humic Acid of the Compost Product
3.5. Effect of Different Conditions on the Particle-Size Distribution and Coarseness Index of the Compost Product
3.6. Effect of Different Conditions on the Mineral Nutrient of the Compost Product
3.7. Analysis of GB Composting Effects
3.7.1. Gray Relational Analysis of GB Composting
3.7.2. Prediction of Optimal Conditions for GB Composting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Zhang, L.; Sun, X. Improvement of two-stage composting of green waste by addition of eggshell waste and rice husks. Bioresour. Technol. 2021, 320, 124388. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.; Guo, X.; Zhao, T.; Zhang, B. Succession and diversity of microorganisms and their association with physicochemical properties during green waste thermophilic composting. Waste Manag. 2018, 73, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, Y.; Kong, Y.; Ma, R.; Yuan, J.; Li, G. Key factors affecting seed germination in phytotoxicity tests during sheep manure composting with carbon additives. J. Hazard. Mater. 2022, 421, 126809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.W.; Meng, Q.R.; Niu, Q.Q.; Wang, S.S.; Yan, H.L.; Li, Q.L. Understanding the key regulatory functions of red mud in cellulose breakdown and succession of β-glucosidase microbial community during composting. Bioresour. Technol. 2020, 318, 124265. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, L.; Li, R. Effects of additives on physical, chemical, and microbiological properties during green waste composting. Bioresour. Technol. 2021, 340, 125719. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage composting of green waste due to the addition of β-cyclodextrin. Compost Sci. Util. 2019, 27, 46–60. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Bai, Z.; Chadwick, D.; Misselbrook, T.; Sommer, S.D.; Qin, W.; Ma, L. Mitigation of ammonia, nitrous oxide and methane emissions during solid waste composting with different additives: A meta-analysis. J. Clean. Prod. 2019, 235, 626–635. [Google Scholar] [CrossRef]
- Ekinci, K.; Keener, H.M.; Elwell, D.L. Composting short paper fiber with broiler litter and additives: Part I: Effects of initial pH and carbon/nitrogen ratio on ammonia emission. Compost. Sci. Util. 2000, 8, 160–172. [Google Scholar]
- Gabhane, J.; William, S.P.; Bidyadhar, R.; Bhilawe, P.; Anand, D.; Vaidya, A.N.; Wate, S.R. Additives aided composting of green waste: Effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour. Technol. 2012, 114, 382–388. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q.; Kida, K. Insight into the microbiology of nitrogen cycle in the dairy manure composting process revealed by combining high-throughput sequencing and quantitative PCR. Bioresour. Technol. 2020, 301, 122760. [Google Scholar] [CrossRef]
- Guo, H.H.; Gu, J.; Wang, X.J.; Yu, J.; Nasir, M.; Zhang, K.Y.; Sun, W. Microbial driven reduction of N2O and NH3 emissions during composting: Effects of bamboo charcoal and bamboo vinegar. J. Hazard. Mater. 2020, 390, 121292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.U.; Sun, X. Using cow dung and spent coffee grounds to enhance the two-stage co-composting of green waste. Bioresour. Technol. 2017, 245, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Fu, Q.Q.; Cao, Y.S.; Wen, Q.X.; Wu, Y.Q. Effects of lime amendment on the organic substances changes, antibiotics removal, and heavy metals speciation transformation during swine manure composting. Chemosphere 2021, 262, 128342. [Google Scholar] [CrossRef]
- Li, X.; Shi, X.S.; Lu, M.Y.; Zhao, Y.Z.; Guo, R.B.; Peng, H. Improved nitrogen conservation capacity during composting of dairy manure amended with oil shale semi-coke as the porous bulking agent. J. Hazard. Mater. 2020, 388, 121742. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Goron, T.L.; Pizarro, T.P.; Sánchez, M.M.Á.; Audette, Y.; Deolu, A.A.O.; Van, D.W.A.; Goitom, T.M.; Shenker, M.; Pombo, S.C.; et al. Application of biostimulant products and biological control agents in sustainable viticulture: A review. Front. Plant Sci. 2022, 13, 932311. [Google Scholar] [CrossRef]
- Winarso, S.; Pandutama, M.H.; Purwanto, L.D. Effectivity of humic substance extracted from palm oil compost as liquid fertilizer and heavy metal bioremediation. Agric. Agric. Sci. Proc. 2016, 9, 146–157. [Google Scholar] [CrossRef]
- Zheng, C.S.; Lan, X.; Tan, Q.L.; Zhang, Y.; Gui, H.P.; Hu, C.X. Soil application of calcium and magnesium fertilizer influences the fruit pulp mastication characteristics of Nanfeng tangerine (Citrus reticulata Blanco cv. Kinokuni). Sci. Hortic. 2015, 191, 121–126. [Google Scholar] [CrossRef]
- Mariano, E.; Sant Ana Filho, C.R.; Bortoletto-Santos, R.; Bendassolli, J.A.; Trivelin, P.C.O. Ammonia losses following surface application of enhanced-efficiency nitrogen fertilizers and urea. Atmos. Environ. 2019, 203, 242–251. [Google Scholar] [CrossRef]
- Wang, X.; Selvam, A.; Wong, J.W. Influence of lime on struvite formation and nitrogen conservation during food waste composting. Bioresour. Technol. 2016, 217, 227–232. [Google Scholar] [CrossRef]
- Wang, X.; Selvam, A.; Lau, S.S.S.; Wong, J.W. Influence of lime and struvite on microbial community succession and odour emission during food waste composting. Bioresour. Technol. 2018, 247, 652–659. [Google Scholar] [CrossRef]
- Liao, C.S.; Yuan, S.Y.; Huang, B.H.; Chang, B.V. Removal of organic toxic chemicals using the spent mushroom compost of Ganoderma lucidum. J. Environ. Monit. 2012, 14, 1983. [Google Scholar] [CrossRef]
- Shivanand, C.; Ashwin, G. Optimization of enzymatic hydrolysis of bamboo biomass for enhanced saccharification of cellulose through Taguchi orthogonal design. J. Environ. Chem. Eng. 2021, 9, 104807. [Google Scholar]
- Chen, Y.; Wen, X.; Liu, S. Analysis of the Factors Influencing the Sludge Aerobic Microbial Compost Using Orthogonal Mode. Inf. Technol. J. 2013, 12, 4715–4719. [Google Scholar]
- Deng, J. Control problem of grey systems. Syst. Control Lett. 1982, 1, 288–294. [Google Scholar]
- Rich, N.; Bharti, A.; Kumar, S. Effect of bulking agents and cow dung as inoculant on vegetable waste compost quality. Bioresour. Technol. 2018, 252, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, L.D.; Hernández, A.M.; Peimbert, M. Exploring the cockatiel (Nymphicus hollandicus) fecal microbiome, bacterial inhabitants of a worldwide pet. Peer J. 2016, 4, e2837. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Kurt, S.C. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Cegarra, J.; Alburquerque, J.A.; Gonzalvez, J.; Tortosa, G.; Chaw, D. Effects of the forced ventilation on composting of a solid olive-mill by-product (“alperujo”) managed by mechanical turning. Waste Manag. 2006, 26, 1377–1383. [Google Scholar] [CrossRef]
- Li, S.; Huang, G.; An, C.; Yu, H. Effect of different buffer agents on in-vessel composting of food waste: Performance analysis and comparative study. J. Environ. Sci. Health Part A 2013, 48, 772–780. [Google Scholar] [CrossRef]
- Biemelt, D.; Schapp, A.; Kleeberg, A.; Grünewald, U. Overland flow, erosion, and related phosphorus and iron fluxes at plot scale: A case study from a non-vegetated lignite mining dump in Lusatia. Geoderma 2005, 129, 4–18. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2008, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.S.; Markovitch, O.; Tarchitzky, J.; Chen, Y. Dissolved organic carbon (DOC) as a parameter of compost maturity. Soil Biol. Biochem. 2005, 37, 2109–2116. [Google Scholar] [CrossRef]
- Jalili, M.; Mokhtari, M.; Eslami, H.; Abbasi, F.; Ghanbari, R.; Ebrahimi, A.A. Toxicity evaluation and management of co-composting pistachio wastes combined with cattle manure and municipal sewage sludge. Ecotoxicol. Environ. Saf. 2019, 171, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, Y.; Wang, G.Y.; Zhang, D.F.; Shen, Y.J.; Ma, R.N.; Li, D.Y.; Li, S.Y.; Li, G.X. Biodrying performance and combustion characteristics related to bulking agent amendments during kitchen waste biodrying. Bioresour. Technol. 2019, 284, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Chang, J.; Kong, Y.; Jiang, T.; Wang, J.; Yuan, J. Enrichment of antibiotic resistance genes after sheep manure aerobic heap composting. Bioresour. Technol. 2021, 323, 124620. [Google Scholar] [CrossRef]
- Kebibeche, H.; Khelil, O.; Kacem, M.; Harche, M.K. Addition of wood sawdust during the co-composting of sewage sludge and wheat straw influences seeds germination. Ecotoxicol. Environ. Saf. 2019, 168, 423–430. [Google Scholar] [CrossRef]
- Guan, T.Y.; Holley, R.A. Pathogen survival in swine manure environments and transmission of human enteric illness—A review. J. Environ. Qual. 2003, 32, 383–392. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Zhang, J.; Zeng, G.; Dong, H.; Cao, W.; Fang, W.; Cheng, Y.; Wang, Y.; Ning, Q. Impacts of iron oxide nanoparticles on organic matter degradation and microbial enzyme activities during agricultural waste composting. Waste Manag. 2019, 95, 289–297. [Google Scholar] [CrossRef]
- Meng, L.Q.; Li, W.G.; Zhang, S.M.; Wu, C.D.; Lv, L.Y. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Bioresour. Technol. 2017, 226, 39–45. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Zhu, Z.; Zhao, Y.; Jia, L.; Lv, P. Humus formation driven by ammonia-oxidizing bacteria during mixed materials composting. Bioresour. Technol. 2020, 311, 123500. [Google Scholar] [CrossRef]
- Nakasaki, K.; Yaguchi, H.; Sasaki, Y.; Kubota, H. Effects of pH control on composting of garbage. Waste Manag. Res. 1993, 11, 117–125. [Google Scholar] [CrossRef]
- Abou-el-Seoud, I.I.; Abdel-Megeed, A. Impact of rock materials and biofertilizations on P and K availability for maize (Zea maize) under calcareous soil conditions. Saudi J. Biol. Sci 2012, 19, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Moni, P.J.K.; Ronit, B.; Prasanna, D.B. Calcium oxalate degrading thermophilic oxalate oxidase from newly isolated Fusarium oxysporum RBP3. Biocatal. Agric. Biotechnol. 2020, 25, 101583. [Google Scholar]
- Wei, Z.Q.; Luo, Y.M.; Wu, S.H.; Sun, Q.P.; Liu, B.S.; Li, J.J. Effects of zeolite addition on the loss of phosphorus and potassium through runoff and leaching in poultry manure composting. J. Agro-Environ. Sci. 2012, 31, 2486–2492. [Google Scholar]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Paul, D.; Sinha, S.N. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrar. Sci. 2016, 15, 130–136. [Google Scholar] [CrossRef]
- McDowell, R.W.; Monaghan, R.M.; Carey, P.L. Potential phosphorus losses in overland flow from pastoral soils receiving long-term applications of either superphosphate or reactive phosphate rock. N. Z. J. Agric. Res. 2003, 46, 329–337. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
| (a) Selected Physicochemical Properties of Raw Materials. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sheep Manure | Urea | Grape Branch | Chicken Manure | ||||||
| Carbon content (g/kg) | 76.18 (0.92) c | 37.61 (3.12) d | 423.09 (20.29) a | 373.75 (13.43) b | |||||
| Nitrogen content (g/kg) | 8.94 (0.77) c | 464.57 (11.35) a | 10.18 (0.04) c | 57.09 (2.96) b | |||||
| C/N | 8.52 (0.69) b | 0.08 (0.01) d | 41.55 (1.82) a | 6.55 (0.58) c | |||||
| Rate of water content | 0.26 (0.01) b | —— | 0.09 (0.01) c | 0.59 (0.02) a | |||||
| (b) Process Parameters (Factors and Levels). | |||||||||
| Process Parameters | Symbol | Level 1 | Level 2 | Level 3 | |||||
| Nitrogen source | A | Chicken manure | Sheep manure | Urea | |||||
| Stirring temperature (°C) | B | 50 | 60 | 70 | |||||
| Initial pH | C | 6 | 7 | 8 | |||||
| Conditioning agent | D | Calcium superphosphate | Zeolite | Copper sulfate | |||||
| (c) Test Treatment Arrangement and Amount of Raw Material Added. | |||||||||
| Treatment | GB (kg) | Nitrogen Source (kg) | Stirring Temperature (°C) | Initial pH | Conditioning Agent (kg) | ||||
| Chicken Manure | Sheep Manure | Urea | Calcium superphosphate | Zeolite | Copper Sulfate | ||||
| T1 | 135 | 9 | 50 | 6 | 7 | ||||
| T2 | 135 | 9 | 60 | 7 | 7 | ||||
| T3 | 135 | 9 | 70 | 8 | 7 | ||||
| T4 | 86 | 59 | 50 | 7 | 7 | ||||
| T5 | 86 | 59 | 60 | 8 | 7 | ||||
| T6 | 86 | 59 | 70 | 6 | 7 | ||||
| T7 | 147 | 1 | 50 | 8 | 7 | ||||
| T8 | 147 | 1 | 60 | 6 | 7 | ||||
| T9 | 147 | 1 | 70 | 7 | 7 | ||||
| Treatment | Seed Germination Rate (%) | Average Root Length (mm) | GI (%) |
|---|---|---|---|
| T1 | 93 (12) a | 14 (5) a | 143 (64) abc |
| T2 | 87 (6) ab | 15 (3) a | 137 (29) abc |
| T3 | 40 (10) d | 16 (5) a | 70 (32) c |
| T4 | 77 (23) ab | 23 (9) a | 175 (12) ab |
| T5 | 50 (10) cd | 23 (5) a | 122 (23) abc |
| T6 | 83 (6) ab | 15 (2) a | 131 (9) abc |
| T7 | 77 (6) ab | 22 (6) a | 188 (61) a |
| T8 | 73 (15) ab | 15 (5) a | 119 (55) abc |
| T9 | 80 (10) ab | 19 (6) a | 162 (58) ab |
| CK | 67 (6) bc | 14 (7) a | 100 (53) bc |
| Treatment | Before Composting | ||||||
|---|---|---|---|---|---|---|---|
| ACa (mg/g) | AK (mg/g) | AMg (mg/g) | AP (mg/g) | N O3−-N (mg/kg) | N H4+-N (mg/kg) | OM (g/kg) | |
| T1 | 12.88 (0.42) b | 18.71 (0.57) b | 1.66 (0.08) c | 1.61 (0.12) b | 95.12 (3) c | 199.76 (1.29) a | 692.96 (0.45) b |
| T2 | 10.91 (0.36) c | 11.38 (0.20) c | 1.89 (0.02) a | 1.33 (0.11) c | 61.31 (3.91) f | 186.43 (7.12) b | 689.17 (1.42) c |
| T3 | 15.45 (1.15) a | 9.43 (0.25) e | 1.86 (0.05) ab | 0.48 (0.02) f | 74.27 (2.25) e | 181.11 (4.91) b | 693.49 (1.04) b |
| T4 | 12.42 (0.74) bc | 10.35 (1.28) d | 1.87 (0.02) ab | 0.70 (0.18) e | 242.54 (3.67) a | 106.31 (7.17) d | 481.79 (0.40) e |
| T5 | 10.6 (1.22) c | 10.46 (0.28) d | 1.84 (0.06) ab | 1.26 (0.06) c | 86.37 (1.52) d | 102.66 (5.89) d | 480.23 (1.18) e |
| T6 | 6.3 (0.38) d | 21.90 (0.29) a | 1.44 (0.06) d | 6.89 (0.05) a | 182.29 (3.04) b | 172.61 (3.43) c | 483.75 (1.80) d |
| T7 | 7.59 (0.78) d | 8.73 (0.09) e | 1.68 (0.08) c | 0.94 (0.05) d | 63.48 (2.51) f | 203.24 (0.33) a | 700.41 (0.99) a |
| T8 | 10.97 (1.39) c | 7.33 (0.17) f | 1.80 (0.04) ab | 0.49 (0.08) f | 61.64 (4.8) f | 205.81 (0.17) a | 699.1 (0.45) a |
| T9 | 10.84 (1.68) bc | 7.59 (0.17) f | 1.76 (0.10) bc | 1.04 (0.07) d | 76.95 (11.62) e | 205.39 (0.93) a | 700.54 (0) a |
| THA (g/kg) | BD (g/cm3) | WHC (%) | VR | Bacteria (×1010CFU/g) | Actinomycetes (×107CFU/g) | Fungi (×105CFU/g) | |
| T1 | - | - | - | - | - | - | - |
| T2 | - | - | - | - | - | - | - |
| T3 | - | - | - | - | - | - | - |
| T4 | - | - | - | - | - | - | - |
| T5 | - | - | - | - | - | - | - |
| T6 | - | - | - | - | - | - | - |
| T7 | - | - | - | - | - | - | - |
| T8 | - | - | - | - | - | - | - |
| T9 | - | - | - | - | - | - | - |
| Treatment | After composting | ||||||
| ACa (mg/g) | AK (mg/g) | AMg (mg/g) | AP (mg/g) | N O3−-N (mg/kg) | N H4+-N (mg/kg) | OM(g/kg) | |
| T1 | 14.45 (0.85) cd, δ | 18.72 (1.18) b, β | 2.02 (0.06) cd, αβ | 2.19 (0.33) b, α | 126.66 (4.41) a, α | 90.61 (8.09) g, γ | 493.95 (12.85) c, α |
| T2 | 15.96 (1.73) bc, αβ | 13.00 (0.44) c, α | 2.26 (0.01) a, αβ | 1.38 (0.07) c, βγ | 92.84 (0.56) b, α | 108.77 (5.00) ef, δ | 582.80 (68.83) b, β |
| T3 | 18.55 (0.52) a, βγδ | 8.08 (0.06) e, γ | 2.18 (0.02) ab, αβ | 0.85 (0.03) d, αβ | 58.64 (4.53) cd, γ | 153.87 (12.04) c, γ | 663.17 (2.27) a, ε |
| T4 | 16.55 (0.92) b, αβγ | 7.25 (0.12) ef, δ | 2.00 (0.03) d, γ | 0.43 (0.10) e, γ | 54.39 (0.99) de, ζ | 120.58 (1.98) de, α | 441.15 (1.37) d, δε |
| T5 | 13.2 (0.46) d, γδ | 7.21 (0.27) ef, δ | 2.07 (0.07) bcd, βγ | 1.30 (0.02) c, βγ | 56.48 (0.27) cd, δ | 117.70 (5.09) de, α | 416.20 (6.44) d, γ |
| T6 | 8.88 (1.41) e, γδ | 21.83 (0.65) a, β | 1.85 (0.12) e, α | 5.83 (0.40) a, δ | 59.47 (3.49) c, ε | 97.67 (6.39) fg, α | 437.10 (5.88) d, δ |
| T7 | 13.37 (0.56) d, α | 10.87 (0.17) d, α | 2.06 (0.06) cd, αβ | 0.93 (0.15) d, βγ | 51.17 (1.29) e, γ | 128.44 (8.51) d, γ | 683.81 (0.60) a, ζ |
| T8 | 17.21 (1.13) ab, α | 6.72 (0.16) f, βγ | 2.11 (0.05) bc, αβ | 0.59 (0.12) de, βγ | 59.06 (2.31) cd, β | 175.00 (1.64) b, β | 655.98 (1.13) a, δε |
| T9 | 12.79 (0.54) d, γδ | 6.99 (0.07) f, βγ | 2.08 (0.02) bcd, αβ | 0.85 (0.04) d, γ | 49.74 (1.16) f, δ | 217.02 (5.38) a, β | 658.33 (1.20) a, δε |
| THA (g/kg) | BD (g/cm3) | WHC (%) | VR | Bacteria (×1010CFU/g) | Actinomycetes (×107CFU/g) | Fungi (×105CFU/g) | |
| T1 | 18.21 (0.63) d | 0.28 (0.02) b | 215 (8) d | 2.78 (0.24) f | 9.23 (0.35) a | 5.17 (0.57) ab | 6.13 (0.71) a |
| T2 | 26.48 (0.45) a | 0.27 (0.01) b | 228 (13) d | 9.83 (0.82) de | 8.47 (0.31) b | 5.27 (0.25) a | 4.83 (0.15) b |
| T3 | 26.38 (0.62) a | 0.14 (0.03) e | 218 (18) d | 56.79 (6.53) a | 5.2 (0.46) c | 2.83 (0.32) d | 1.83 (0.35) d |
| T4 | 21.76 (1.86) c | 0.23 (0.01) c | 151 (9) f | 27.80 (3.65) b | 4.43 (0.45) d | 3.67 (0.32) c | 2.63 (0.31) cd |
| T5 | 17.17 (0.18) d | 0.32 (0.02) a | 165 (19) ef | 11.99 (1.39) d | 3.33 (0.42) e | 3.17 (0.45) cd | 2.33 (0.42) cd |
| T6 | 18.59 (1.66) d | 0.32 (0.01) a | 187 (4.5) e | 7.26 (1.26) def | 3.87 (0.35) de | 3.17 (0.47) cd | 2.93 (0.31) c |
| T7 | 23.86 (1.76) b | 0.23 (0.01) c | 276 (21) c | 8.31 (1.37) de | 5.23 (0.38) c | 5.23 (0.32) a | 5.7 (0.36) a |
| T8 | 23.48 (0.31) bc | 0.14 (0.02) e | 303 (18) b | 19.95 (2.33) c | 3.5 (0.35) e | 4.5 (0.36) b | 3.13 (0.57) c |
| T9 | 26.45 (1.11) a | 0.17 (0.01) d | 330 (15) a | 5.37 (0.81) ef | 4.5 (0.36) d | 5.4 (0.46) a | 4.73 (0.76) b |
| Treatment | >5.00 (mm) | 2.00–5.00 | 1.00–2.00 | 1.00–0.30 | <0.30 | CI (>1.00) |
|---|---|---|---|---|---|---|
| T1 | 8.37 | 10.28 | 11.32 | 16.77 | 3.26 | 59.94 (0.29) g |
| T2 | 10.40 | 22.63 | 6.27 | 7.97 | 2.73 | 78.60 (0.24) e |
| T3 | 16.07 | 22.57 | 5.31 | 2.44 | 3.61 | 87.91 (0.78) b |
| T4 | 9.50 | 22.00 | 7.39 | 8.84 | 2.27 | 77.77 (1.11) e |
| T5 | 15.09 | 18.82 | 6.87 | 7.66 | 1.56 | 81.56 (0.66) d |
| T6 | 8.57 | 25.52 | 7.30 | 5.50 | 3.11 | 82.79 (0.58) c |
| T7 | 13.39 | 26.77 | 4.37 | 4.39 | 1.08 | 89.06 (0.36) a |
| T8 | 7.47 | 27.56 | 6.76 | 7.02 | 1.19 | 83.58 (0.22) c |
| T9 | 8.29 | 22.77 | 4.64 | 9.26 | 5.05 | 71.38 (0.27) f |
| Treatment | Initial Value Transformation | |||||||
|---|---|---|---|---|---|---|---|---|
| ACa | AK | AMg | AP | AN | THA | OM | ||
| Ideal treatment | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| T1 | 0.78 | 0.86 | 0.89 | 0.38 | 0.81 | 0.69 | 0.72 | |
| T2 | 0.86 | 0.60 | 1.00 | 0.24 | 0.76 | 1.00 | 0.85 | |
| T3 | 1.00 | 0.37 | 0.96 | 0.15 | 0.80 | 1.00 | 0.97 | |
| T4 | 0.89 | 0.33 | 0.88 | 0.07 | 0.66 | 0.82 | 0.65 | |
| T5 | 0.71 | 0.33 | 0.92 | 0.22 | 0.65 | 0.65 | 0.61 | |
| T6 | 0.48 | 1.00 | 0.82 | 1.00 | 0.59 | 0.70 | 0.64 | |
| T7 | 0.72 | 0.50 | 0.91 | 0.16 | 0.67 | 0.90 | 1.00 | |
| T8 | 0.93 | 0.31 | 0.93 | 0.10 | 0.88 | 0.89 | 0.96 | |
| T9 | 0.69 | 0.32 | 0.92 | 0.15 | 1.00 | 1.00 | 0.96 | |
| Treatment | Correlation Coefficients | Gray Relational Grade | ||||||
| ACa | AK | AMg | AP | AN | THA | OM | ||
| T1 | 0.6106 | 0.7113 | 0.7582 | 0.4286 | 0.6449 | 0.5267 | 0.5520 | 0.6028 |
| T2 | 0.7113 | 0.4631 | 1.0000 | 0.3796 | 0.5897 | 1.0000 | 0.6970 | 0.7132 |
| T3 | 1.0000 | 0.3538 | 0.8961 | 0.3536 | 0.6330 | 1.0000 | 0.9200 | 0.7424 |
| T4 | 0.7582 | 0.3399 | 0.7419 | 0.3333 | 0.5036 | 0.6571 | 0.4964 | 0.5587 |
| T5 | 0.5433 | 0.3399 | 0.8118 | 0.3735 | 0.4964 | 0.4964 | 0.4694 | 0.5063 |
| T6 | 0.3988 | 1.0000 | 0.6571 | 1.0000 | 0.4570 | 0.5349 | 0.4894 | 0.6514 |
| T7 | 0.5520 | 0.4083 | 0.7931 | 0.3563 | 0.5111 | 0.7753 | 1.0000 | 0.6120 |
| T8 | 0.8313 | 0.3333 | 0.8313 | 0.3407 | 0.7419 | 0.7582 | 0.8961 | 0.6663 |
| T9 | 0.5267 | 0.3366 | 0.8118 | 0.3536 | 1.0000 | 1.0000 | 0.8961 | 0.7110 |
| Treatment | a | b | c | d | Weighted Correlation Degree |
|---|---|---|---|---|---|
| T1 | 1 | 1 | 1 | 1 | 0.6028 |
| T2 | 1 | 2 | 2 | 2 | 0.7132 |
| T3 | 1 | 3 | 3 | 3 | 0.7424 |
| T4 | 2 | 1 | 2 | 3 | 0.5587 |
| T5 | 2 | 2 | 3 | 1 | 0.5063 |
| T6 | 2 | 3 | 1 | 2 | 0.6514 |
| T7 | 3 | 1 | 3 | 2 | 0.6120 |
| T8 | 3 | 2 | 1 | 3 | 0.6663 |
| T9 | 3 | 3 | 2 | 1 | 0.7110 |
| 0.6861 | 0.5912 | 0.6402 | 0.6067 | ||
| 0.5721 | 0.6286 | 0.6610 | 0.6589 | ||
| 0.6631 | 0.7016 | 0.6202 | 0.6558 | ||
| R | 0.1140 | 0.1104 | 0.0408 | 0.0522 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhang, Y.; Wang, X.; Wang, Z.; Li, P.; Shi, X.; Wang, X.; Wang, B.; Li, Y.; Ma, Y.; et al. An Optimization Design for the Resource Utilization of Grape Branches Based on the Orthogonal Test and Gray Relational Analysis Method. Sustainability 2023, 15, 11211. https://doi.org/10.3390/su151411211
Yang M, Zhang Y, Wang X, Wang Z, Li P, Shi X, Wang X, Wang B, Li Y, Ma Y, et al. An Optimization Design for the Resource Utilization of Grape Branches Based on the Orthogonal Test and Gray Relational Analysis Method. Sustainability. 2023; 15(14):11211. https://doi.org/10.3390/su151411211
Chicago/Turabian StyleYang, Minghao, Yican Zhang, Xiaodi Wang, Zhiqiang Wang, Peng Li, Xiangbin Shi, Xiaolong Wang, Baoliang Wang, Yumei Li, Yuquan Ma, and et al. 2023. "An Optimization Design for the Resource Utilization of Grape Branches Based on the Orthogonal Test and Gray Relational Analysis Method" Sustainability 15, no. 14: 11211. https://doi.org/10.3390/su151411211
APA StyleYang, M., Zhang, Y., Wang, X., Wang, Z., Li, P., Shi, X., Wang, X., Wang, B., Li, Y., Ma, Y., Liu, F., & Wang, H. (2023). An Optimization Design for the Resource Utilization of Grape Branches Based on the Orthogonal Test and Gray Relational Analysis Method. Sustainability, 15(14), 11211. https://doi.org/10.3390/su151411211