Enhancement of CO2 Absorption Process Using High-Frequency Ultrasonic Waves
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Titration Method
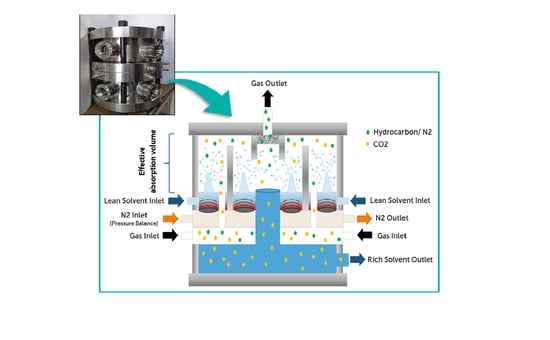
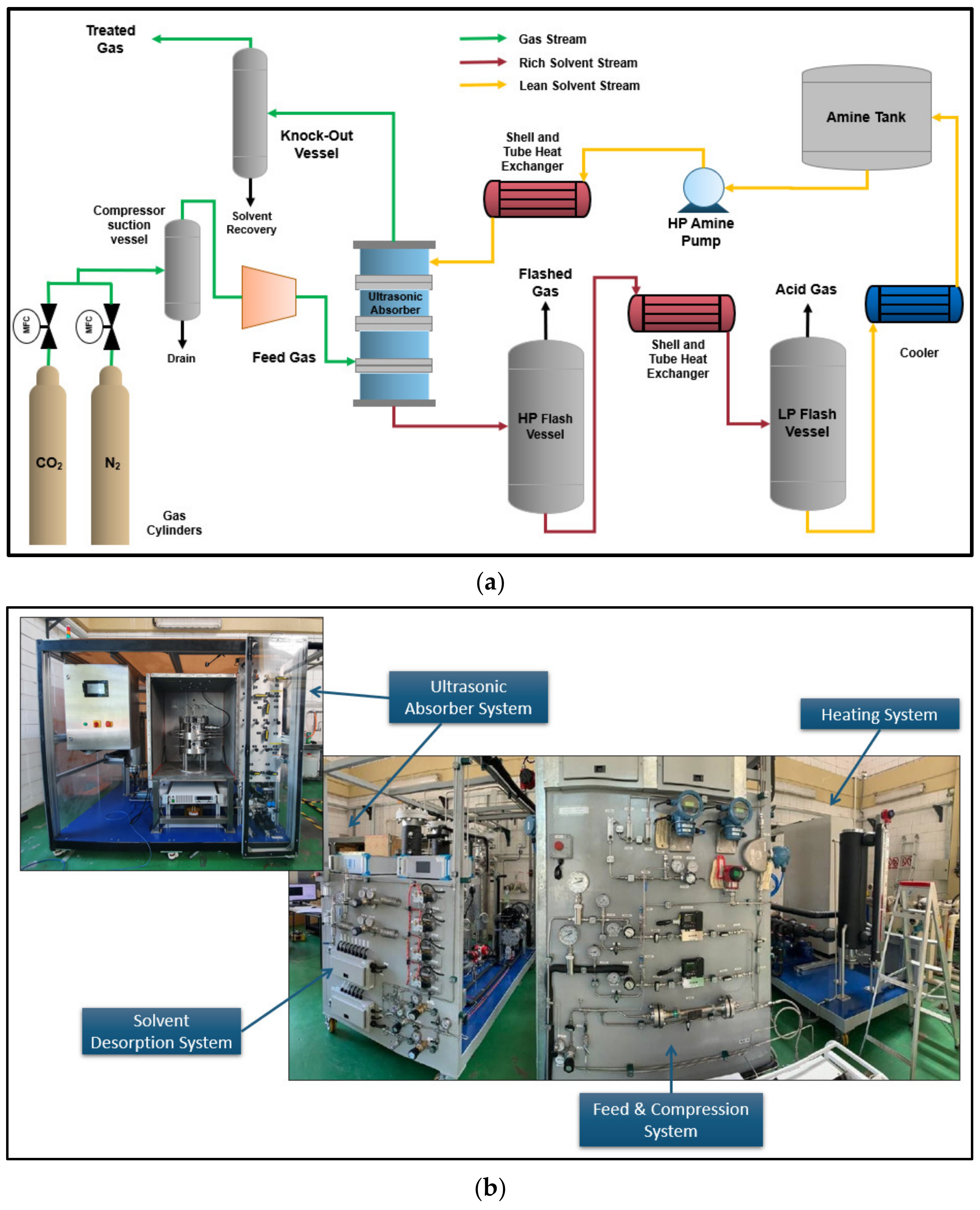
2.3. Lab Scale Setup
2.4. Design of Experiment (DOE)
2.5. Ultrasonic Power Generation
2.6. Liquid-to-Gas Ratio (L/G) Determination
2.7. Mass Transfer Coefficient Determination
3. Results and Discussion
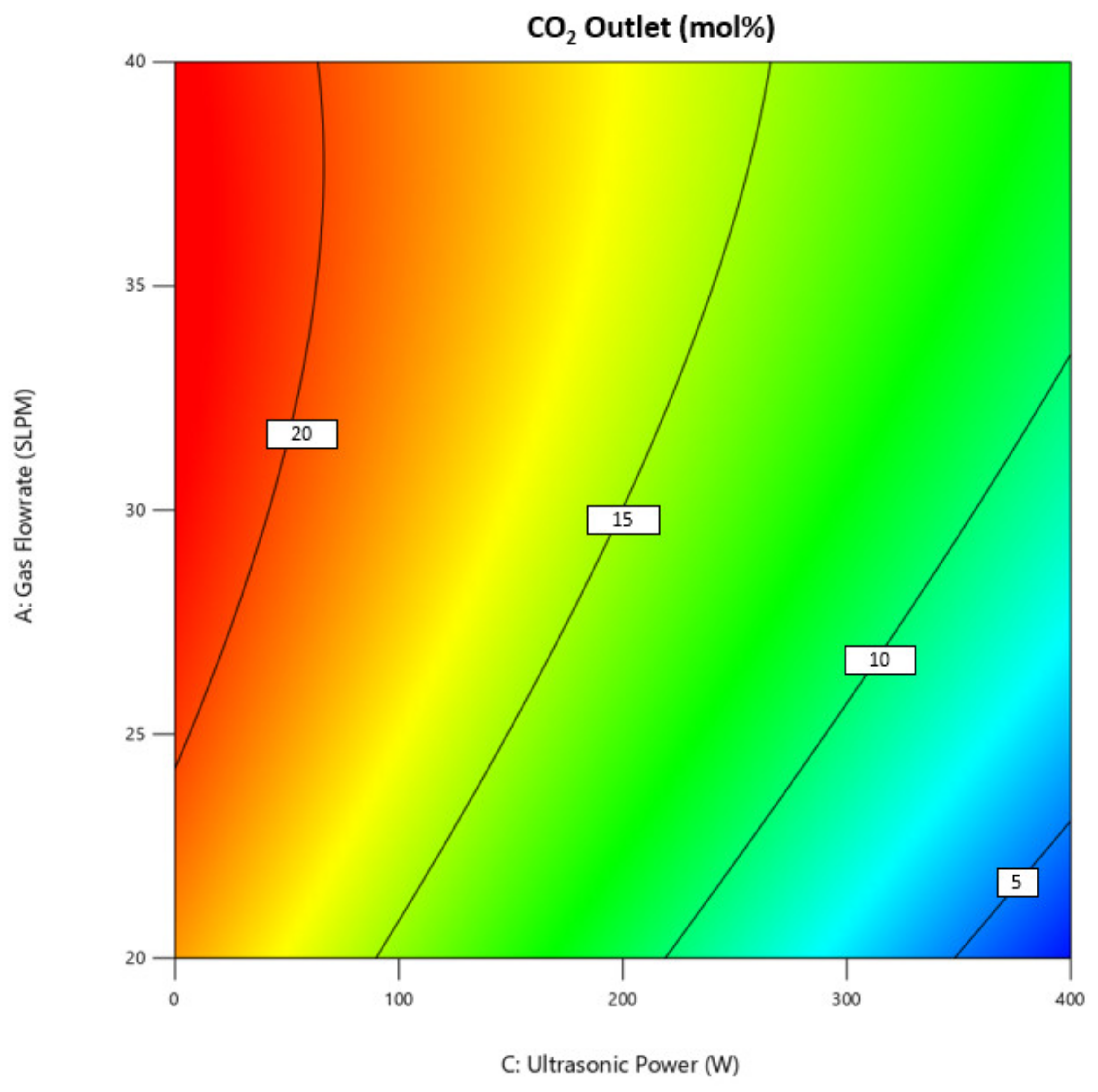
3.1. Effect of Operating Condition on CO2 Outlet Concentration
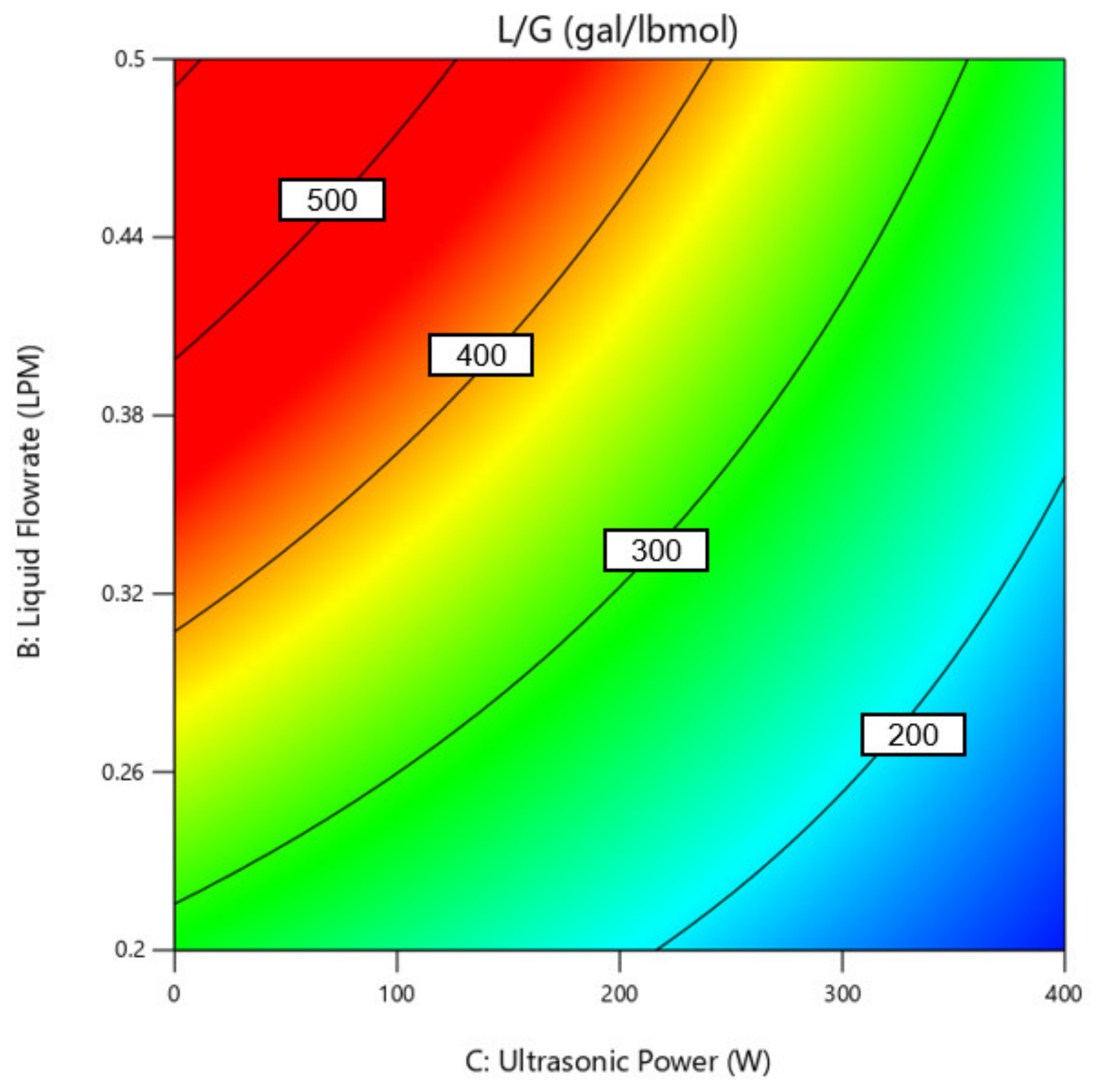
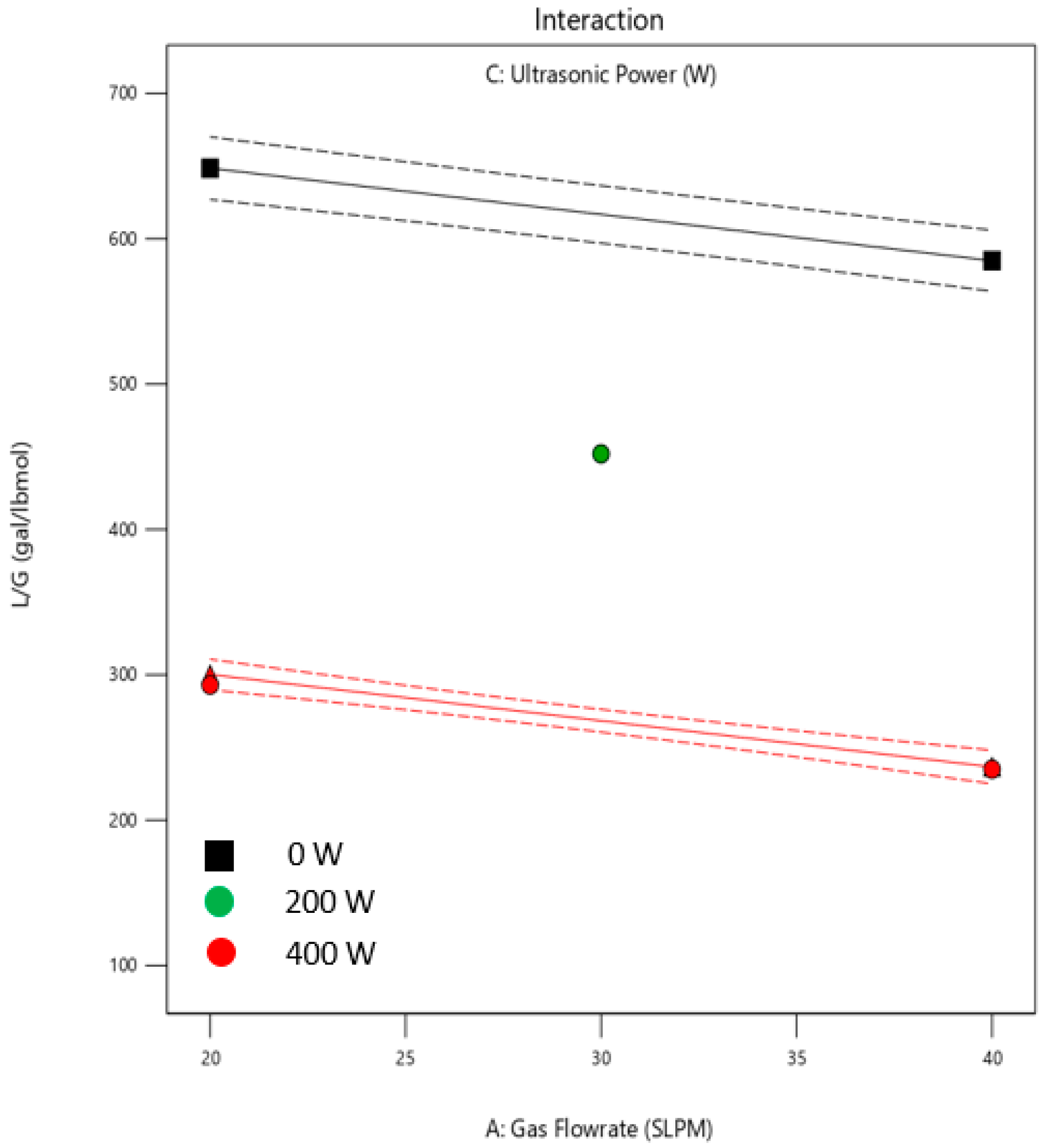
3.2. Effect of Operating Condition on L/G
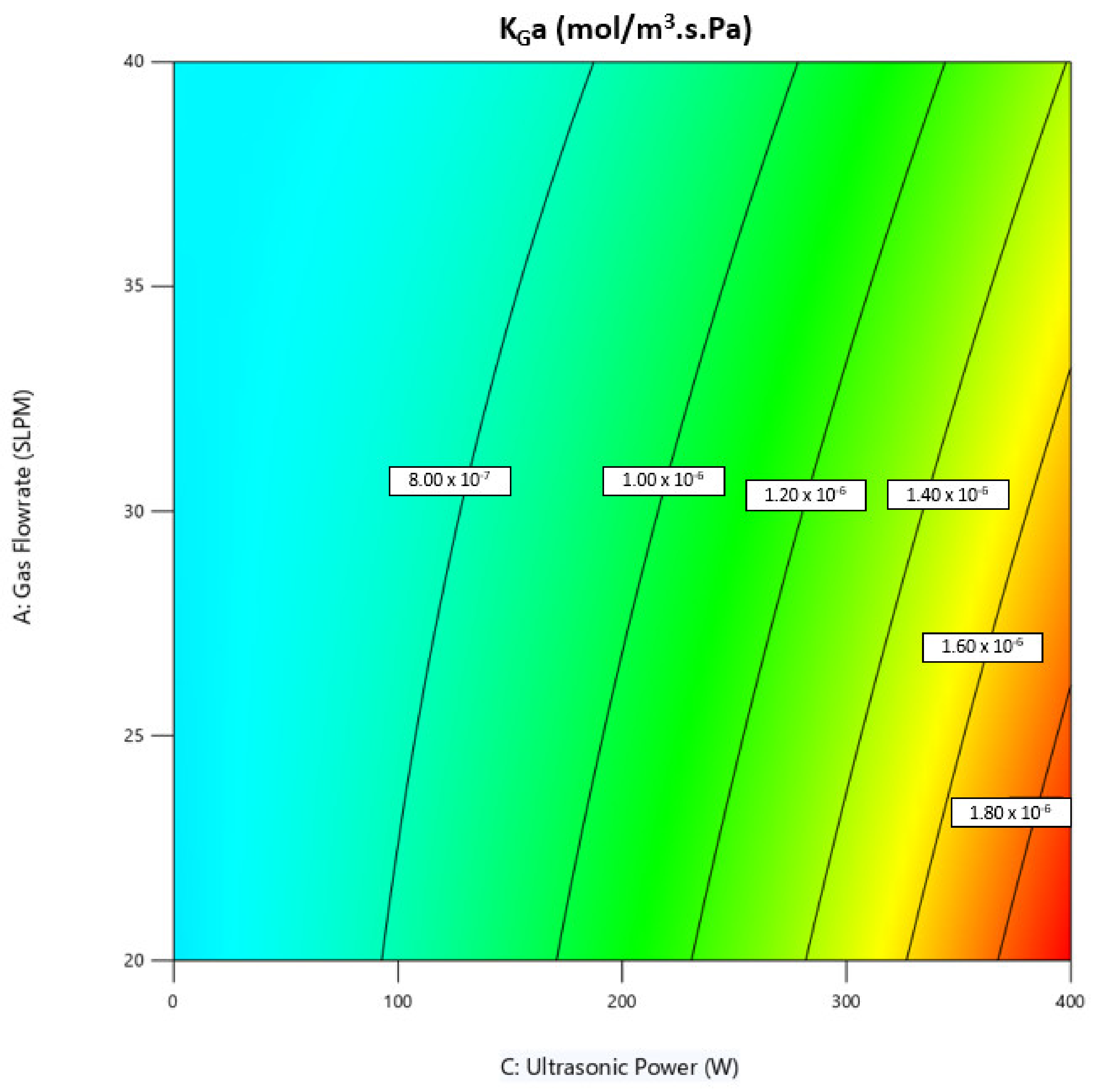
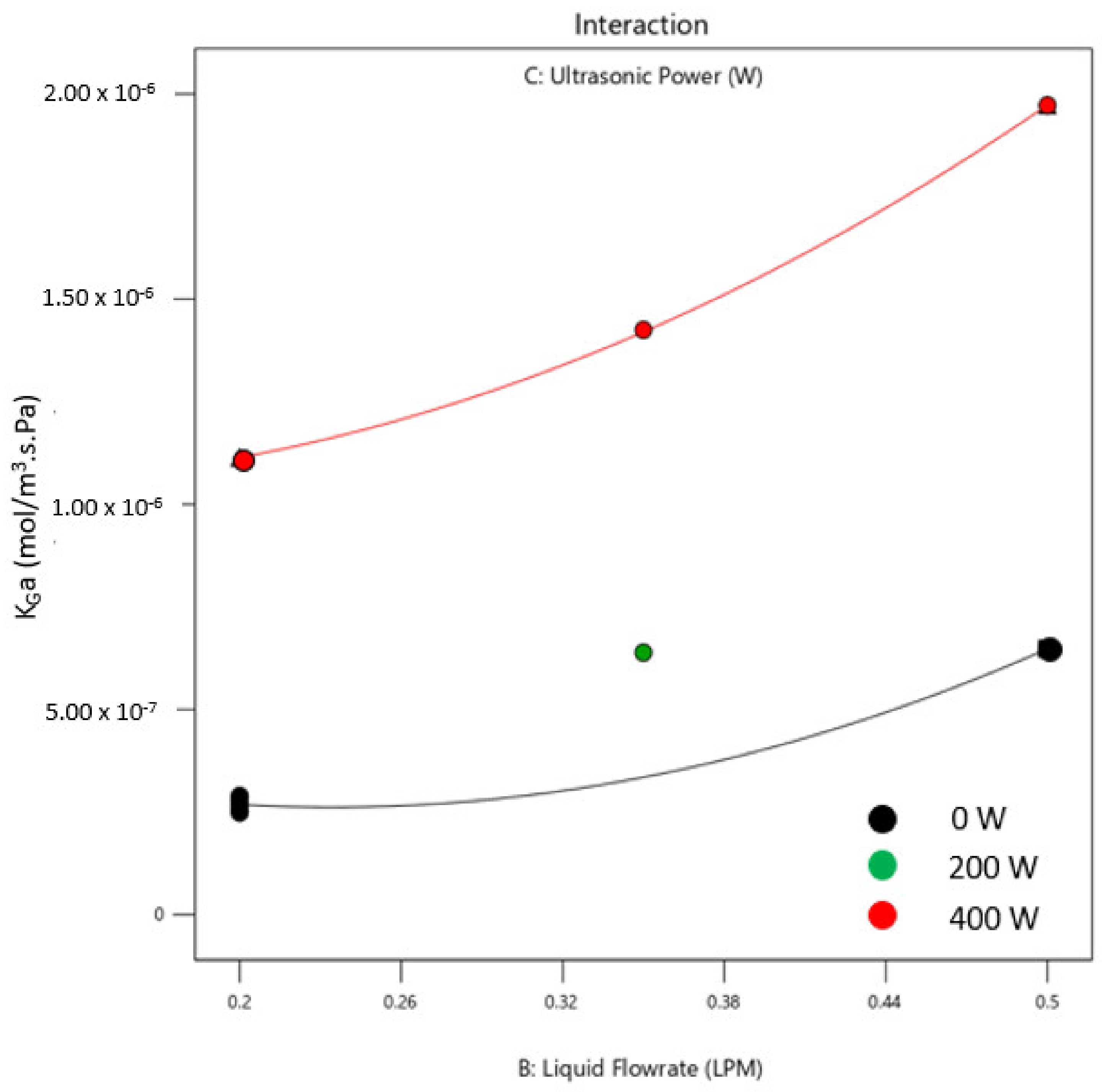
3.3. Effect of Operating Conditions on Mass Transfer Coefficient, KGa
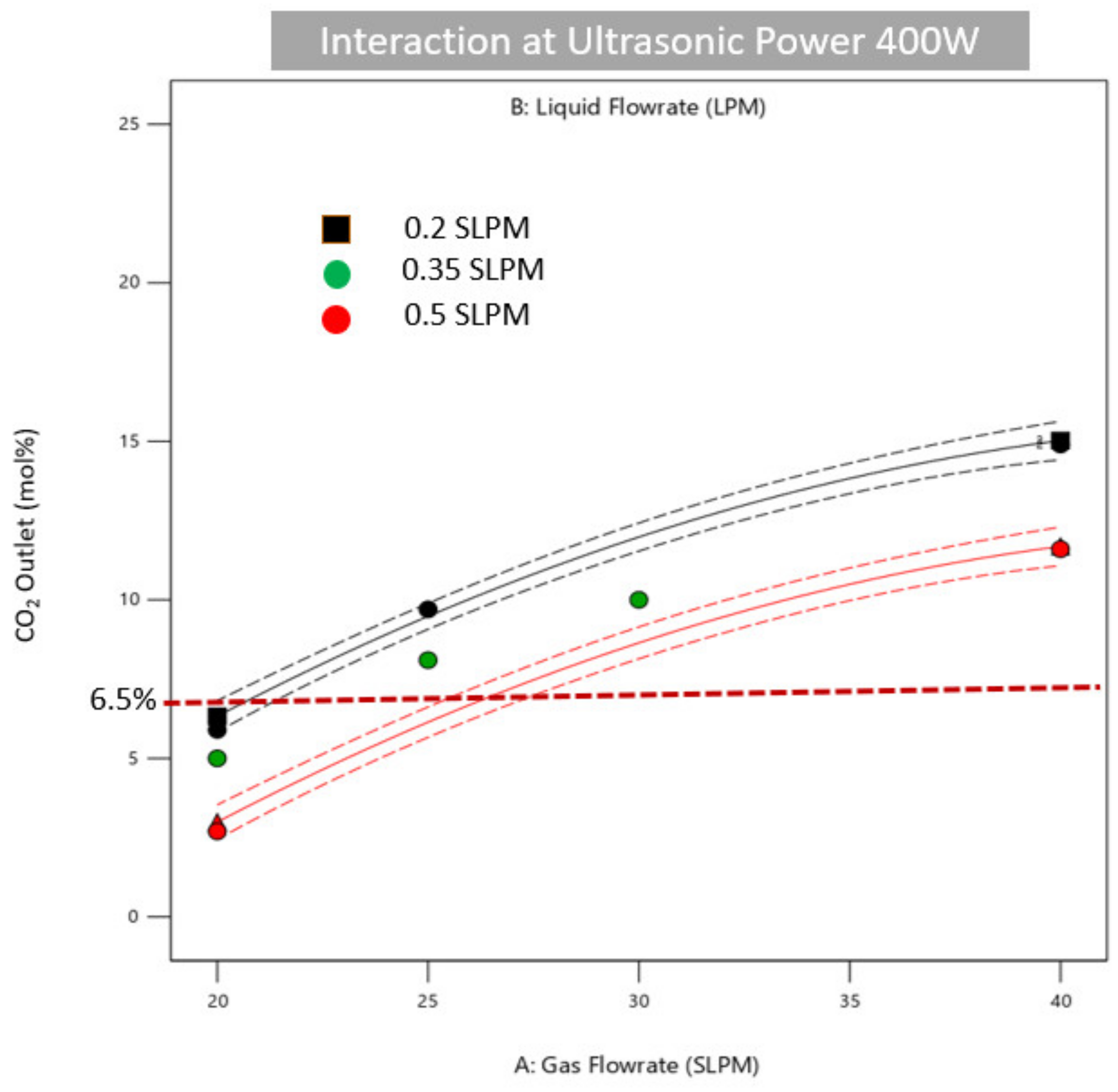
3.4. Ultrasonic-Assisted CO2 Absorption Process Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, N. What Is Environmental Sustainability? Goals with Examples. In South. New Hampsh. Univ. 2022. Available online: https://www.snhu.edu/about-us/newsroom/stem/what-is-environmental-sustainability#:~:text=WhatistheMeaningof,tomeettheirownneeds (accessed on 3 May 2023).
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- United Nations. Emissions Gap Report 2022: The Closing Window; United Nations Environment Programme: Nairobi, Kenya, 2022. [Google Scholar]
- Lin, Q.; Zhang, X.; Wang, T.; Zheng, C.; Gao, X. Technical Perspective of Carbon Capture, Utilization, and Storage. Engineering 2022, 14, 27–32. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Zantye, M.S.; Kazi, M.K. Challenges and opportunities in carbon capture, utilization and storage: A process systems engineering perspective. Comput. Chem. Eng. 2022, 166, 107925. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Isa, F.; Suleman, H.; Zabiri, H.; Maulud, A.S.; Ramasamy, M.; Tufa, L.D.; Shariff, A.M. An overview on CO2 removal via absorption: Effect of elevated pressures in counter-current packed column. J. Nat. Gas Sci. Eng. 2016, 33, 666–677. [Google Scholar] [CrossRef]
- George, G.; Bhoria, N.; Alhallaq, S.; Abdala, A.; Mittal, V. Polymer membranes for acid gas removal from natural gas. Sep. Purif. Technol. 2016, 158, 333–356. [Google Scholar] [CrossRef]
- Solbraa, E. Carbon Capture and Storage Experiences From the Sleipner Field. In Distillation Absorption; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2010; pp. 43–48. [Google Scholar]
- Faiz, R.; Li, K.; Al-marzouqi, M. H2S absorption at high pressure using hollow fibre membrane contactors. Chem. Eng. Process. 2014, 83, 33–42. [Google Scholar] [CrossRef]
- Brau, J.; Neveux, T.; Louis-louisy, M. Assessment of amine solvent flexibility in the OCTAVIUS project. Energy Procedia 2017, 114, 1366–1379. [Google Scholar] [CrossRef]
- Yu, C.; Huang, C.; Tan, C. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Norouzbahari, S.; Shahhosseini, S.; Ghaemi, A. CO2 chemical absorption into aqueous solutions of piperazine: Modeling of kinetics and mass transfer rate. J. Nat. Gas Sci. Eng. 2015, 26, 1059–1067. [Google Scholar] [CrossRef]
- Lin, S.; Chiang, P.; Hsieh, C.; Li, M.; Tung, K. Absorption of carbon dioxide by the absorbent composed of piperazine and 2-amino-2-methyl-1-propanol in PVDF membrane contactor. J. Chin. Inst. Chem. Eng. 2008, 39, 13–21. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for Carbon Dioxide Capture. In Carbon Dioxide Chemistry Capture Oil Recover; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Huttenhuis, P.J.G.; Agrawal, N.J.; Hogendoorn, J.A.; Versteeg, G.F. Gas solubility of H2S and CO2 in aqueous solutions of N-methyldiethanolamine. J. Pet. Sci. Eng. 2007, 55, 122–134. [Google Scholar] [CrossRef]
- Sea, B.; Park, Y.; Lee, K. Comparison of Porous Hollow Fibers as a Membrane Contactor for Carbon Dioxide Absorption. J. Ind. Eng. Chem. 2002, 8, 290–296. [Google Scholar]
- Yeon, S.; Lee, K.; Sea, B.; Park, Y.; Lee, K. Application of pilot-scale membrane contactor hybrid system for removal of carbon dioxide from flue gas. J. Memb. Sci. 2005, 257, 156–160. [Google Scholar] [CrossRef]
- deMontigny, D.; Tontiwachwuthikul, P.; Chakma, A. Comparing the Absorption Performance of Packed Columns and Membrane Contactors. Ind. Eng. Chem. Res. 2005, 44, 5726–5732. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.; Versteeg, G.F. CO2 absorption at elevated pressures using a hollow fiber membrane contactor. J. Memb. Sci. 2004, 235, 99–109. [Google Scholar] [CrossRef]
- Le Grange, P.; Sheilan, M.; Spooner, B. How to Limit Amine Systems Failures. Chem. Eng. 2017, 916. Available online: https://www.thechemicalengineer.com/features/how-to-limit-amine-systems-failures/ (accessed on 3 May 2023).
- Tay, W.H.; Lau, K.K.; Lai, L.S.; Shariff, A.M.; Wang, T. Current development and challenges in the intensified absorption technology for natural gas purification at offshore condition. J. Nat. Gas Sci. Eng. 2019, 71, 102977. [Google Scholar] [CrossRef]
- Tamidi, A.M.; Lau, K.K.; Khalit, S.H. A review of recent development in numerical simulation of ultrasonic-assisted gas-liquid mass transfer process. Comput. Chem. Eng. 2021, 155, 107498. [Google Scholar] [CrossRef]
- Gondrexon, N.; Renaudin, V.; Boldo, P.; Gonthier, Y.; Bernis, A.; Petrier, C. Degassing effect and gas-liquid transfer in a high frequency sonochemical reactor. Chem. Eng. J. 1997, 66, 21–26. [Google Scholar] [CrossRef]
- Kumar, A.; Gogate, P.R.; Pandit, A.B.; Delmas, H.; Wilhelm, A.M. Gas-Liquid Mass Transfer Studies in Sonochemical Reactors. Ind. Eng. Chem. Res. 2004, 43, 1812–1819. [Google Scholar] [CrossRef]
- Kumar, A.; Gogate, P.R.; Pandit, A.B.; Marie, A.; Delmas, H. Investigation of induction of air due to ultrasound source in the sonochemical reactors. Ultrason. Sonochem. 2005, 12, 453–460. [Google Scholar] [CrossRef]
- Laugier, F.; Andriantsiferana, C.; Wilhelm, A.M.; Delmas, H. Ultrasound in gas–liquid systems: Effects on solubility and mass transfer. Ultrason. Sonochem. 2008, 15, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Herran, N.S.; Lopez, J.L.C.; Perez, J.A.S. Gas–liquid Mass Transfer in Sonicated Bubble Columns. Effect of Reactor Diameter and Liquid Height. Ind. Eng. Chem. Res. 2012, 51, 2769–2774. [Google Scholar] [CrossRef]
- Dong, Z.; Yao, C.; Zhang, X.; Xu, J.; Chen, G.; Zhao, Y.; Yuan, Q. A high-power ultrasonic microreactor and its application in gas–liquid mass transfer intensification. Lab Chip 2015, 15, 1145–1152. [Google Scholar] [CrossRef]
- Sajjadi, B.; Asgharzadehahmadi, S.; Asaithambi, P.; Abdul Raman, A.A.; Parthasarathy, R. Investigation of mass transfer intensification under power ultrasound irradiation using 3D computational simulation: A comparative analysis. Ultrason. Sonochem. 2017, 34, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.H.; Barashaid, A.A.B.; Lau, K.K.; Mohd Shariff, A. Effect of Solution Height on Carbon Dioxide Absorption using High Frequency Ultrasonic Irradiation. Chem. Eng. Trans. 2017, 56, 1225–1230. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High frequency ultrasonic assisted mass transfer for water batch reactor. ARPN J. Eng. Appl. Sci. 2016, 11, 1660–1665. [Google Scholar]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High frequency ultrasonic-assisted chemical absorption of CO2 using monoethanolamine (MEA). Sep. Purif. Technol. 2017, 183, 136–144. [Google Scholar] [CrossRef]
- Yusof, S.M.M.; Lau, K.K.; Shariff, A.M.; Tay, W.H.; Mustafa, N.F.A.; Lock, S.S.M. Novel continuous ultrasonic contactor system for CO2 absorption: Parametric and optimization study. J. Ind. Eng. Chem. 2019, 79, 279–287. [Google Scholar] [CrossRef]
- Zare, A.; Mirzaei, S. Removal of CO2 and H2S using aqueous alkanolamine solusions. World Acad. Sci. Eng. Technol. 2009, 37, 194–203. [Google Scholar]
- Qian, Z.; Xu, L.; Li, Z.; Li, H.; Guo, K. Selective absorption of H2S from a gas mixture with CO2 by aqueous N-methyldiethanolamine in a rotating packed bed. Ind. Eng. Chem. Res. 2010, 49, 6196–6203. [Google Scholar] [CrossRef]
- Pal, P.; AbuKashabeh, A.; Al-Asheh, S.; Banat, F. Role of aqueous methyldiethanolamine (MDEA) as solvent in natural gas sweetening unit and process contaminants with probable reaction pathway. J. Nat. Gas Sci. Eng. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Rodríguez, N.; Mussati, S.; Scenna, N. Optimization of post-combustion CO2 process using DEA-MDEA mixtures. Chem. Eng. Res. Des. 2011, 89, 1763–1773. [Google Scholar] [CrossRef]
- Airgas. Methane Safety Data Sheet; Airgas: Radnor, PA, USA, 2020. [Google Scholar]
- Airgas. Nitrogen Safety Data Sheet; Airgas: Radnor, PA, USA, 2021. [Google Scholar]
- Nakhaei-Kohani, R.; Taslimi-Renani, E.; Hadavimoghaddam, F.; Mohammadi, M.R.; Hemmati-Sarapardeh, A. Modeling Solubility of CO2–N2 Gas Mixtures in Aqueous Electrolyte Systems Using Artificial Intelligence Techniques and Equations of State; Nature Publishing Group: London, UK, 2022. [Google Scholar]
- Chan, Z.P.; Wang, T.; Li, L.; Kang, G.; Manan, N.A.; Cao, Y. Discussion on water condensation in membrane pores during CO2 absorption at high temperature. Membranes 2020, 10, 407. [Google Scholar] [CrossRef]
- Yusop Nurida, M.; Norfadilah, D.; Siti Aishah, M.R.; Zhe Phak, C.; Saleh, S.M. Monitoring of CO2 Absorption Solvent in Natural Gas Process Using Fourier Transform Near-Infrared Spectrometry. Int. J. Anal. Chem. 2020, 2020, 9830685. [Google Scholar] [CrossRef]
- Daquan, X. Physics of Ultrasound. In New York Sch. Reg. Anesth. 2022. Available online: https://www.nysora.com/topics/equipment/physics-of-ultrasound/#toc_REFERENCES (accessed on 14 June 2022).
- Gallego-Juarez, J.A. Piezoelectric ceramics and ultrasonic transducers. J. Phys. E 1989, 22, 804–816. [Google Scholar] [CrossRef]
- Edvardsson, M. What Is Piezoelectricity? In Biolin Sci. 2020. Available online: https://www.biolinscientific.com/blog/what-is-piezoelectricity (accessed on 18 February 2023).
- Kang, G.; Chan, Z.P.; Saleh, S.B.M.; Cao, Y. Removal of high concentration CO2 from natural gas using high pressure membrane contactors. Int. J. Greenh. Gas Control 2017, 60, 1–9. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Veawab, A. Characterization and Comparison of the CO2 Absorption Column. Ind. Eng. Chem. Res. 2004, 43, 2228–2237. [Google Scholar] [CrossRef]
- Setameteekul, A.; Aroonwilas, A.; Veawab, A. Statistical factorial design analysis for parametric interaction and empirical correlations of CO2 absorption performance in MEA and blended MEA/MDEA processes. Sep. Purif. Technol. 2008, 64, 16–25. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Lau, K.K.; Tay, W.H. Performance comparison of ultrasonic-assisted and magnetic stirred absorption methods for CO2 separation. SN Appl. Sci. 2020, 2, 1217. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High frequency ultrasonic-assisted CO2 absorption in a high pressure water batch system. Ultrason. Sonochem. 2016, 33, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]






| Parameters | Unit | Symbol | Level | ||
|---|---|---|---|---|---|
| (−1) | (0) | (+1) | |||
| Gas Flowrate (FG) | SLPM | A | 20 | 30 | 40 |
| Liquid Flowrate (FL) | LPM | B | 0.2 | 0.35 | 0.5 |
| Ultrasonic Power (PUS) | W | C | 0 | 200 | 400 |
| Run | Gas Flowrate, FG (SLPM) | Liquid Flowrate, FL (LPM) | Ultrasonic Power, PUS (W) | Response 1: CO2 Outlet (mol%) | Response 2: L/G (gal/lbmol) | Response 3: KGa (mol/m3.s.Pa) |
|---|---|---|---|---|---|---|
| 1 | 40 | 0.2 | 0 | 23.4 | 583.6 | 1.64 × 10−7 |
| 2 | 30 | 0.35 | 0 | 18.1 | 423.2 | 4.51 × 10−7 |
| 3 | 20 | 0.5 | 0 | 18.5 | 919.3 | 2.92 × 10−7 |
| 4 | 30 | 0.35 | 200 | 16.2 | 340.4 | 5.92 × 10−7 |
| 5 | 20 | 0.2 | 400 | 5.9 | 134.2 | 1.30 × 10−6 |
| 6 | 30 | 0.35 | 400 | 10 | 200.8 | 1.24 × 10−6 |
| 7 | 40 | 0.5 | 400 | 11.6 | 234.7 | 1.41 × 10−6 |
| 8 | 30 | 0.2 | 200 | 17.2 | 210.5 | 5.28 × 10−7 |
| 9 | 30 | 0.35 | 200 | 16 | 336.8 | 6.03 × 10−7 |
| 10 | 20 | 0.35 | 200 | 12.5 | 350.6 | 6.39 × 10−7 |
| 11 | 30 | 0.5 | 200 | 15.5 | 451.9 | 6.50 × 10−7 |
| 12 | 40 | 0.2 | 400 | 14.9 | 122.0 | 9.70 × 10−7 |
| 13 | 30 | 0.35 | 200 | 16.4 | 329.7 | 6.01 × 10−7 |
| 14 | 40 | 0.5 | 0 | 21.3 | 745.7 | 3.36 × 10−7 |
| 15 | 40 | 0.35 | 200 | 18.4 | 313.2 | 6.00 × 10−7 |
| 16 | 20 | 0.5 | 400 | 2.7 | 293.1 | 1.97 × 10−6 |
| 17 | 20 | 0.2 | 0 | 18.4 | 372.8 | 2.90 × 10−7 |
| 18 | 30 | 0.35 | 200 | 16.4 | 333.2 | 5.96 × 10−7 |
| 19 | 25 | 0.35 | 400 | 8.1 | 216.0 | 1.26 × 10−6 |
| 20 | 25 | 0.2 | 400 | 9.7 | 132.6 | 1.08 × 10−6 |
| 21 | 20 | 0.35 | 400 | 5 | 230.3 | 1.43 × 10−6 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model—Reduced Quadratic | 521.09 | 6 | 86.85 | 695.29 | <0.0001 | Significant |
| A—Gas Flowrate | 75.30 | 1 | 75.30 | 602.84 | <0.0001 | |
| B—Liq Flowrate | 8.22 | 1 | 8.22 | 65.79 | <0.0001 | |
| C—Ultrasonic Power | 290.31 | 1 | 290.31 | 2324.17 | <0.0001 | |
| AC | 12.61 | 1 | 12.61 | 100.92 | <0.0001 | |
| BC | 3.99 | 1 | 3.99 | 31.95 | <0.0001 | |
| A2 | 7.32 | 1 | 7.32 | 58.57 | <0.0001 | |
| Residual | 1.62 | 13 | 0.1249 | |||
| Lack of fit | 1.19 | 9 | 0.1326 | 1.23 | 0.4510 | Not Significant |
| R2 | 0.9969 | |||||
| Adjusted R2 | 0.9955 | |||||
| Predicted R2 | 0.9905 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model—Reduced 2FI | 1.314 × 105 | 5 | 26,272.56 | 833.68 | <0.0001 | Significant |
| A—Gas Flowrate | 2798.65 | 1 | 2798.65 | 88.81 | <0.0001 | |
| B—Liq Flowrate | 31,174.34 | 1 | 31,174.34 | 989.22 | <0.0001 | |
| C—Ultrasonic Power | 68,031.70 | 1 | 68,031.70 | 2158.78 | <0.0001 | |
| AB | 580.94 | 1 | 580.94 | 18.43 | 0.0016 | |
| BC | 3810.16 | 1 | 3810.16 | 120.90 | <0.0001 | |
| Residual | 315.14 | 10 | 31.51 | |||
| Lack of fit | 257.14 | 7 | 36.73 | 1.90 | 0.3219 | Not Significant |
| R2 | 0.9976 | |||||
| Adjusted R2 | 0.9964 | |||||
| Predicted R2 | 0.9904 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model—Reduced Quadratic | 3.407 × 10−12 | 8 | 4.259 × 10−13 | 1868.64 | <0.0001 | Significant |
| A—Gas Flowrate | 2.574 × 10−15 | 1 | 2.574 × 10−15 | 11.29 | 0.0121 | |
| B—Liq Flowrate | 4.812 × 10−14 | 1 | 4.812 × 10−14 | 211.10 | <0.0001 | |
| C—Ultrasonic Power | 4.006 × 10−13 | 1 | 4.006 × 10−13 | 1757.60 | <0.0001 | |
| AB | 3.209 × 10−14 | 1 | 3.209 × 10−14 | 140.81 | <0.0001 | |
| AC | 4.135 × 10−14 | 1 | 4.135 × 10−14 | 181.41 | <0.0001 | |
| BC | 1.434 × 10−14 | 1 | 1.434 × 10−14 | 62.91 | <0.0001 | |
| B2 | 1.909 × 10−14 | 1 | 1.909 × 10−14 | 83.76 | <0.0001 | |
| C2 | 1.099 × 10−13 | 1 | 1.099 × 10−13 | 482.29 | <0.0001 | |
| Residual | 1.596 × 10−15 | 7 | 2.279 × 10−16 | |||
| Lack of fit | 6.883 × 10−16 | 3 | 2.294 × 10−16 | 1.01 | 0.4750 | Not Significant |
| R2 | 0.9995 | |||||
| Adjusted R2 | 0.9990 | |||||
| Predicted R2 | 0.9977 |
| Parameters | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| A: Gas Flowrate | In range | 20 SLPM | 40 SLPM |
| B: Liquid Flowrate | In range | 0.2 LPM | 0.5 LPM |
| C: Ultrasonic Power | In range | 0 W | 400 W |
| CO2 Outlet | Target: 6.5 mol% | 2.7 mol% | 21.3 mol% |
| L/G | Minimize | 122 (gal/lbmol CO2) | 452 (gal/lbmol CO2) |
| KGa | Maximize | 2.49 × 10−7 (mol/m3.s.Pa) | 1.97 × 10−6 (mol/m3.s.Pa) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamidi, A.M.; Lau, K.K.; Yusof, S.M.M.; Azmi, N.; Zakariya, S.; Patthi, U. Enhancement of CO2 Absorption Process Using High-Frequency Ultrasonic Waves. Sustainability 2023, 15, 11064. https://doi.org/10.3390/su151411064
Tamidi AM, Lau KK, Yusof SMM, Azmi N, Zakariya S, Patthi U. Enhancement of CO2 Absorption Process Using High-Frequency Ultrasonic Waves. Sustainability. 2023; 15(14):11064. https://doi.org/10.3390/su151411064
Chicago/Turabian StyleTamidi, Athirah Mohd, Kok Keong Lau, Siti Munirah Mhd Yusof, Nurulhuda Azmi, Shahidah Zakariya, and Umar Patthi. 2023. "Enhancement of CO2 Absorption Process Using High-Frequency Ultrasonic Waves" Sustainability 15, no. 14: 11064. https://doi.org/10.3390/su151411064
APA StyleTamidi, A. M., Lau, K. K., Yusof, S. M. M., Azmi, N., Zakariya, S., & Patthi, U. (2023). Enhancement of CO2 Absorption Process Using High-Frequency Ultrasonic Waves. Sustainability, 15(14), 11064. https://doi.org/10.3390/su151411064