Antioxidant Performance of Borago officinalis Leaf Essential Oil and Protective Effect on Thermal Oxidation of Fish Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Borago officinalis Essential Oil (BEO) Extraction
2.2. Volatile Compounds of B. officinalis Essential Oil
2.3. Antioxidant Performance of B. officinalis Essential Oil
2.4. Experimental Thermal Oxidation Process
2.5. Determination of the Oxidation Level of the Experimental Groups
2.6. Statistical Analysis
3. Results
3.1. Borago officinalis Essential Oil and Volatile Compounds
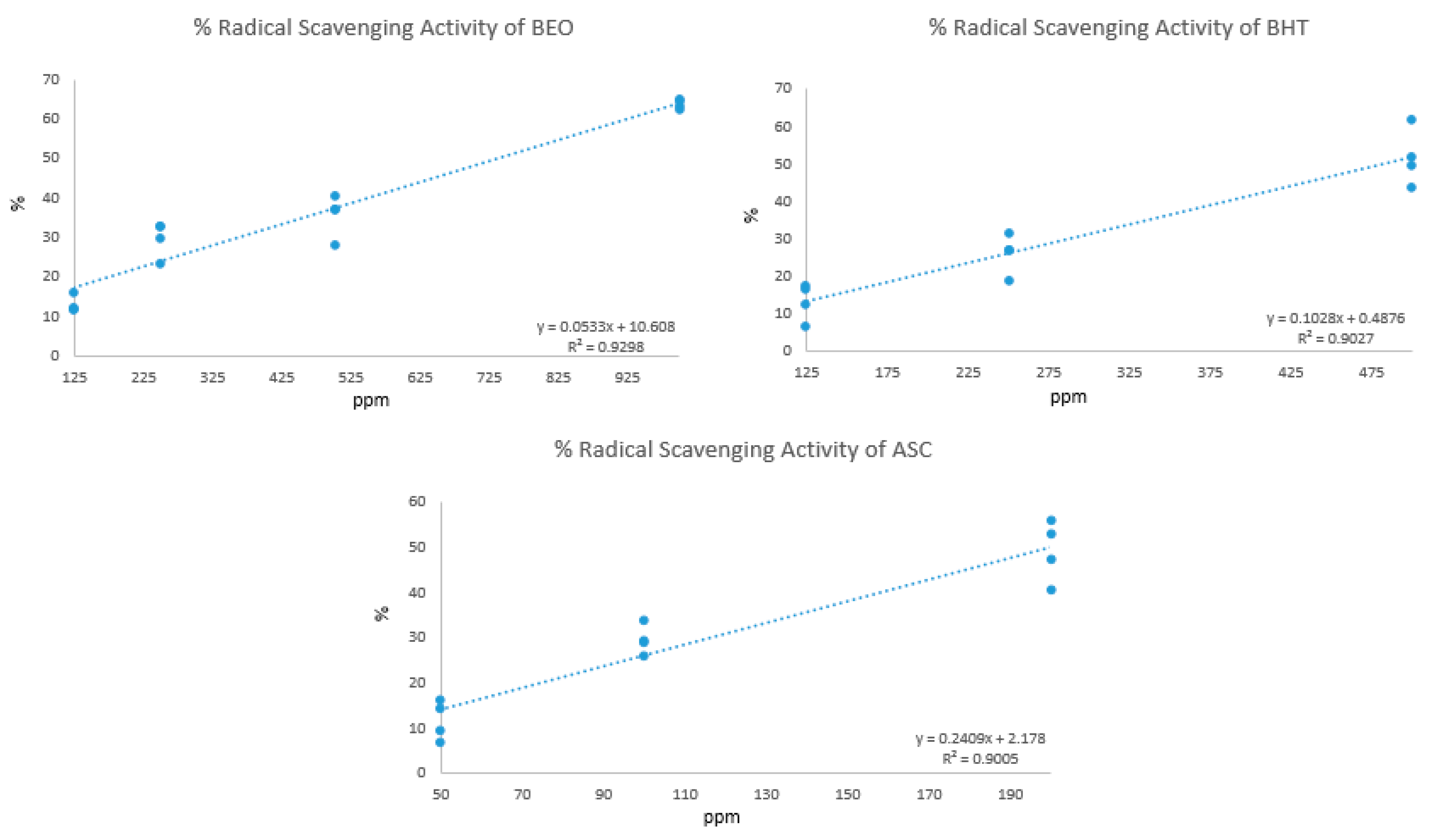
3.2. Antioxidant Activity of Borago officinalis Leaf Essential Oil Volatile Compounds
3.3. Changes in Lipid Oxidation of Experimental Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opstvedt, J. Fish Lipids in Animal Nutrition; International Fishmeal & Oil Manufacturers Association: London, UK, 1985. [Google Scholar]
- Shingfield, K.J.; Ahvenjärvi, S.; Toivonen, V.; Ärölä, A.; Nurmela, K.V.V.; Huhtanen, P.; Griinari, J.M. Effect of dietary fish oil on biohydrogenation of fatty acids and milk fatty acid content in cows. Anim. Sci. 2016, 77, 165–179. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Gulati, S.K.; Ashes, J.R.; Fleck, E.; Scott, T.W.; Nichols, P.D. Utilisation of fish oil in ruminants: I. Fish oil metabolism in sheep. Anim. Feed Sci. Technol. 2001, 89, 189–199. [Google Scholar] [CrossRef]
- Lee, A.V.; You, L.; Oh, S.-Y.; Li, Z.; Code, A.; Zhu, C.; Fisher-Heffernan, R.E.; Regnault, T.R.H.; De Lange, C.F.M.; Huber, L.-A.; et al. Health Benefits of Supplementing Nursery Pig Diets with Microalgae or Fish Oil. Animals 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Benatti, P.; Peluso, G.; Nicolai, R.; Calvani, M. Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties. J. Am. Coll. Nut 2004, 23, 281–302. [Google Scholar] [CrossRef]
- Pike, I. Health benefits from feeding fish oil and fish meal. In The Role of Long Chain Omega-3 Polyunsaturated Fatty Acids in Animal Feeding; IFOMA: Herts, UK, 1999; Volume 28. [Google Scholar]
- Castejón, N.; Señoráns, F.J. Enzymatic modification to produce health-promoting lipids from fish oil, algae and other new omega-3 sources: A review. New Biotechnol. 2020, 57, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Winkler–Moser, J.K.; Hwang, H.-S.; Kerr, B.J. Changes in markers of lipid oxidation and thermal treatment in feed-grade fats and oils. J. Sci. Food Agric. 2020, 100, 3328–3340. [Google Scholar] [CrossRef]
- Dong, G.; Zhu, X.; Ren, H.; Nie, B.; Chen, L.; Li, H.; Yan, B. Effects of oxidized fish oil intake on tissue lipid metabolism and fatty acid composition of channel catfish (Ictalurus punctatus). Aquac. Res. 2014, 45, 1867–1880. [Google Scholar] [CrossRef]
- Tan, L.; Rong, D.; Yang, Y.; Zhang, B. The Effect of Oxidized Fish Oils on Growth Performance, Oxidative Status, and Intestinal Barrier Function in Broiler Chickens. J. Appl. Poult. Res. 2019, 28, 31–41. [Google Scholar] [CrossRef]
- Hamre, K.; Kolås, K.; Sandnes, K. Protection of fish feed, made directly from marine raw materials, with natural antioxidants. Food Chem. 2010, 119, 270–278. [Google Scholar] [CrossRef]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Thompson, D.C.; Trush, M.A. The toxicological implications of the interaction of butylated hydroxytoluene with other antioxidants and phenolic chemicals. Food Chem. Toxicol. 1986, 24, 1189–1195. [Google Scholar] [CrossRef]
- Yang, X.; Song, W.; Liu, N.; Sun, Z.; Liu, R.; Liu, Q.S.; Zhou, Q.; Jiang, G. Synthetic Phenolic Antioxidants Cause Perturbation in Steroidogenesis in Vitro and in Vivo. Environ. Sci. Technol. 2018, 52, 850–858. [Google Scholar] [CrossRef]
- Merel, S.; Regueiro, J.; Berntssen, M.H.G.; Hannisdal, R.; Ørnsrud, R.; Negreira, N. Identification of ethoxyquin and its transformation products in salmon after controlled dietary exposure via fish feed. Food Chem. 2019, 289, 259–268. [Google Scholar] [CrossRef]
- Alabdaly, Y.Z.; Al-Hamdany, E.K.; Abed, E.R. Toxic effects of butylated hydroxytoluene in rats. Iraqi J. Vet. Sci. 2021, 35, 121–128. [Google Scholar] [CrossRef]
- Ji, X.; Yang, M.; Or, K.H.; Yim, W.S.; Zuo, Z. Tissue Accumulations of Toxic Aconitum Alkaloids after Short-Term and Long-Term Oral Administrations of Clinically Used Radix Aconiti Lateralis Preparations in Rats. Toxins 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Felter, S.P.; Zhang, X.; Thompson, C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharmacol. 2021, 121, 104887. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Mitsiopoulou, C.; De Marchi, M.; Righi, F. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins in Livestock Animal Products Yield, Quality, and Oxidative Status: A Review. Antioxidants 2021, 10, 780. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Radünz, M.; Mota Camargo, T.; Santos Hackbart, H.C.d.; Inchauspe Correa Alves, P.; Radünz, A.L.; Avila Gandra, E.; da Rosa Zavareze, E. Chemical composition and in vitro antioxidant and antihyperglycemic activities of clove, thyme, oregano, and sweet orange essential oils. LWT 2021, 138, 110632. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Surai, P.; Deans, S.G. In Vitro Antioxidant Activity of a Number of Plant Essential Oils and Phytoconstituents. J. Essent. Oil Res. 2000, 12, 241–248. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Catalkaya, G.; Capanoglu, E.; Karbancioglu-Guler, F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Paur, I.; Balstad, T.R.; Kolberg, M.; Pedersen, M.K.; Austenaa, L.M.; Jacobs, D.R., Jr.; Blomhoff, R. Extract of Oregano, Coffee, Thyme, Clove, and Walnuts Inhibits NF-κB in Monocytes and in Transgenic Reporter Mice. Cancer Prev. Res. 2010, 3, 653–663. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Rosmarinus officinalis essential oil modulates renal toxicity and oxidative stress induced by potassium dichromate in rats. J. Trace Elem. Med. Biol. 2021, 67, 126791. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Jamali, H.; Abolhasani, M.; Pazoki Toroudi, H. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2019, 110, 9–19. [Google Scholar] [CrossRef]
- Saoudi, M.; Badraoui, R.; Rahmouni, F.; Jamoussi, K.; El Feki, A. Antioxidant and Protective Effects of Artemisia campestris Essential Oil Against Chlorpyrifos-Induced Kidney and Liver Injuries in Rats. Front Physiol. 2021, 12, 618582. [Google Scholar] [CrossRef]
- Jebur, A.B.; El-Sayed, R.A.; El-Demerdash, F.M. Ocimum basilicum Essential Oil Modulates Hematotoxicity, Oxidative Stress, DNA Damage, and Cell Cycle Arrest Induced by β-cyfluthrin in Rat Liver. Front Pharmacol. 2022, 12, 784281. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Rojas-Armas, J.; Arroyo-Acevedo, J.; Ortiz-Sánchez, M.; Palomino-Pacheco, M.; Castro-Luna, A.; Ramos-Cevallos, N.; Justil-Guerrero, H.; Hilario-Vargas, J.; Herrera-Calderón, O. Acute and Repeated 28-Day Oral Dose Toxicity Studies of Thymus vulgaris L. Essential Oil in Rats. Toxicol. Res. 2019, 35, 225–232. [Google Scholar] [PubMed]
- Adokoh, C.K.; Asante, D.-B.; Acheampong, D.O.; Kotsuchibashi, Y.; Armah, F.A.; Sirikyi, I.H.; Kimura, K.; Gmakame, E.; Abdul-Rauf, S. Chemical profile and in vivo toxicity evaluation of unripe Citrus aurantifolia essential oil. Toxicol. Rep. 2019, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Akbar, S. Borago officinalis L. (Boraginaceae). In Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Akbar, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 445–450. [Google Scholar]
- Abu-Qaoud, H.; Shawarb, N.; Hussen, F.; Jaradat, N.; Shtaya, M. Report: Comparison of qualitative, quantitative analysis and antioxidant potential between wild and cultivated Borago officinalis leaves from palestine. Pak. J. Pharm. Sci. 2018, 31, 953–959. [Google Scholar] [PubMed]
- Kesbiç, O.S.; Parrino, V.; Acar, Ü.; Yilmaz, S.; Paro, G.L.; Fazio, F. Effects of Monterey Cypress (Hartw) Leaf Essential Oil as a Dietary Supplement on Growth Performance and Haematological and Biochemical Parameters of Common Carp (L.). Ann. Anim. Sci. 2020, 20, 1411–1426. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Karami, H.; Rasekh, M.; Mirzaee-Ghaleh, E. Comparison of chemometrics and AOCS official methods for predicting the shelf life of edible oil. Chemometr. Intell Lab. Syst. 2020, 206, 104165. [Google Scholar] [CrossRef]
- Yuan, S.-B.; Chen, D.-W.; Zhang, K.-Y.; Yu, B. Effects of Oxidative Stress on Growth Performance, Nutrient Digestibilities and Activities of Antioxidative Enzymes of Weanling Pigs. Asian-Australas J. Anim. Sci. 2007, 20, 1600–1605. [Google Scholar] [CrossRef]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of Marine Omega-3 Supplements and Human Health. BioMed Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef]
- Phung, A.S.; Bannenberg, G.; Vigor, C.; Reversat, G.; Oger, C.; Roumain, M.; Galano, J.-M.; Durand, T.; Muccioli, G.G.; Ismail, A.; et al. Chemical Compositional Changes in Over-Oxidized Fish Oils. Foods 2020, 9, 1501. [Google Scholar] [CrossRef]
- Luo, B.; Chen, D.; Tian, G.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; et al. Effects of Dietary Aged Maize with Oxidized Fish Oil on Growth Performance, Antioxidant Capacity and Intestinal Health in Weaned Piglets. Animals 2019, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Dabrowski, K.; Izquierdo, M.; Hematyar, N.; Imentai, A.; Steinbach, C.; Policar, T. Effects of Vitamin C and E Supplementation on Growth, Fatty Acid Composition, Innate Immunity, and Antioxidant Capacity of Rainbow Trout (Oncorhynchus mykiss) Fed Oxidized Fish Oil. Front. Mar. Sci. 2021, 8, 760587. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Woźny, E.; Mirkiewicz, E.; Nowicka, G.; Szostak, W.B. The effect of thermally oxidized soya bean oil on metabolism of chylomicrons. Increased uptake and degradation of oxidized chylomicrons in cultured mouse macrophages. Atherosclerosis 1987, 66, 45–53. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Chapter One-Natural antioxidants of plant origin. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 1–81. [Google Scholar]
- Abeyrathne, E.D.; Nam, K.; Huang, X.; Ahn, D.U. Plant- and Animal-Based Antioxidants’ Structure, Efficacy, Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Rohfritsch, Z.; Canelli, G.; Pollien, P.; Bel-Rhlid, R. Wheat and Rice Bran as Natural Additives for the Protection of Fish Oil from Oxidation. ACS Food Sci. Technol. 2021, 1, 1160–1168. [Google Scholar] [CrossRef]
- Yu, H.; Yang, G.; Sato, M.; Yamaguchi, T.; Nakano, T.; Xi, Y. Antioxidant activities of aqueous extract from Stevia rebaudiana stem waste to inhibit fish oil oxidation and identification of its phenolic compounds. Food Chem. 2017, 232, 379–386. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop Med. 2014, 7, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Borowy, A.; Chwil, M.; Kapłan, M. Biologically Active Compounds and Antioxidant Activity of Borage (Borago officinalis L.) Flowers and Leaves. Acta Sci. Pol. Hortorum Cultus 2017, 16, 169–180. [Google Scholar] [CrossRef]
- Borowy, A.; KapŁAn, M. Chemical Composition and Antioxidant Activity of Borage (Borago officinalis L.) Seeds. Acta Sci. Pol. Hortorum Cultus 2020, 19, 79–90. [Google Scholar] [CrossRef]
- Bandonienė, D.; Venskutonis, P.R.; Gruzdienė, D.; Murkovic, M. Antioxidative activity of sage (Salvia officinalis L.), savory (Satureja hortensis L.) and borage (Borago officinalis L.) extracts in rapeseed oil. Eur. J. Lipid Sci. Technol. 2002, 104, 286–292. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.-J.; Oh, W.Y.; Lee, J. Antioxidant effects and reaction volatiles from heated mixture of soy protein hydrolysates and coconut oil. Food Sci. Biotechnol. 2023, 32, 309–317. [Google Scholar] [CrossRef]
- Okolo, K.O.; Orisakwe, O.E. In vitro antioxidants and hepatoprotective effects of Pleurotus tuber-regium on carbon tetrachloride–treated rats. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 67–78. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Tanapichatsakul, C.; Monggoot, S.; Gentekaki, E.; Pripdeevech, P. Antibacterial and Antioxidant Metabolites of Diaporthe spp. Isolated from Flowers of Melodorum fruticosum. Curr. Microbiol. 2018, 75, 476–483. [Google Scholar]
- Stanojević, L.P.; Todorović, Z.B.; Stanojević, K.S.; Stanojević, J.S.; Troter, D.Z.; Nikolić, L.B.; Đorđević, B. The influence of natural deep eutectic solvent glyceline on the yield, chemical composition and antioxidative activity of essential oil from rosemary (Rosmarinus officinalis L.) leaves. J. Essent. Oil Res. 2021, 33, 247–255. [Google Scholar] [CrossRef]
- PuvaČA, N.; ČAbarkapa, I.; PetroviĆ, A.; BursiĆ, V.; ProdanoviĆ, R.; SoleŠA, D.; LeviĆ, J. Tea tree (Melaleuca alternifolia) and its essential oil: Antimicrobial, antioxidant and acaricidal effects in poultry production. World’s Poult. Sci. J. 2019, 75, 235–246. [Google Scholar] [CrossRef]
- Usman, L.A.; Oguntoye, O.S.; Ismaeel, R.O. Effect of Seasonal Variation on Chemical Composition, Antidiabetic and Antioxidant Potentials of Leaf Essential Oil of Eucalyptus globulus L. J. Essent. Oil-Bear 2020, 23, 1314–1323. [Google Scholar] [CrossRef]
- Benzaid, C.; Tichati, L.; Djeribi, R.; Rouabhia, M. Evaluation of the Chemical Composition, the Antioxidant and Antimicrobial Activities of Mentha × piperita Essential Oil against Microbial Growth and Biofilm Formation. J. Essent. Oil-Bear 2019, 22, 335–346. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The Yield, Chemical Composition, and Antioxidant Activities of Essential Oils from Different Plant Parts of the Wild and Cultivated Oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Topuz, O.K.; Yerlikaya, P.; Uçak, İ.; Gümüş, B.; Büyükbenli, H.A.; Gökoğlu, N. Influence of pomegranate peel (Punica granatum) extract on lipid oxidation in anchovy fish oil under heat accelerated conditions. J. Food Sci. Technol. 2015, 52, 625–632. [Google Scholar] [CrossRef]
- Raudoniūtė, I.; Rovira, J.; Venskutonis, P.R.; Damašius, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L. Antioxidant properties of garden strawberry leaf extract and its effect on fish oil oxidation. Int. J. Food Sci. 2011, 46, 935–943. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Warnakulasuriya, S.N.; Rupasinghe, H.P.V.; Shahidi, F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013, 140, 189–196. [Google Scholar] [CrossRef]
- Stansby, M. Determination of Peroxide Values for Rancidity in Fish Oils. Ind. Eng. Chem. Anal. Ed. 1941, 13, 627–631. [Google Scholar] [CrossRef]
- Yeşilsu, A.F.; Özyurt, G. Oxidative stability of microencapsulated fish oil with rosemary, thyme and laurel extracts: A kinetic assessment. J. Food Eng. 2019, 240, 171–182. [Google Scholar] [CrossRef]
- Durmus, M.; Özogul, Y.; Ozyurt, G.; Ucar, Y.; Kosker, A.R.; Yazgan, H.; Ibrahim, S.A.; Özogul, F. Effects of citrus essential oils on the oxidative stability of microencapsulated fish oil by spray-drying. Front Nutr. 2022, 9, 978130. [Google Scholar] [CrossRef]
- Kumari, A.; Venkateshwarlu, G.; Choukse, M.; Anandan, R. Effect of essential oil and aqueous extract of ginger (Zingiber officinale) on oxidative stability of fish oil-in-water emulsion. J. Food Process Technol. 2014, 6, 2. [Google Scholar]
- Acar, Ü.; Kesbiç, O.S.; İnanan, B.E.; Yılmaz, S. Effects of dietary Bergamot (Citrus bergamia) peel oil on growth, haematology and immune response of European sea bass (Dicentrarchus labrax) juveniles. Aquac. Res. 2019, 50, 3305–3312. [Google Scholar] [CrossRef]
- Kesbiç, O.S. Effects of The Cinnamon Oil (Cinnamomum verum) on Growth Performance and Blood Parameters of Rainbow Trout (Oncorhynchus mykiss). TURJAF 2019, 7, 370–376. [Google Scholar]
- Harpaz, S.; Glatman, L.; Drabkin, V.; Gelman, A. Effects of Herbal Essential Oils Used To Extend the Shelf Life of Freshwater-Reared Asian Sea Bass Fish (Lates calcarifer). J. Food Prot. 2003, 66, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, Y.; Baygar, T.; Baygar, T.; Hasanhocaoglu, H.; Metin, C. Effects of gelatin-based edible films enriched with laurel essential oil on the quality of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. Food Technol. Biotechnol. 2014, 52, 325–333. [Google Scholar]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; İnanan, B.E.; Zemheri-Navruz, F.; Terzi, F.; Fazio, F.; Parrino, V. Effects of Essential Oil Derived from the Bitter Orange (Citrus aurantium) on Growth Performance, Histology and Gene Expression Levels in Common Carp Juveniles (Cyprinus carpio). Animals 2021, 11, 1431. [Google Scholar] [CrossRef] [PubMed]

| Sn. | Compound | Retention Time (min.) | Concentration (%) |
|---|---|---|---|
| 1 | Benzaldehyde | 10.093 | 3.51 |
| 2 | D-Limonene | 12.692 | 0.83 |
| 3 | 1.8-Cineole (eucalyptol) | 12.806 | 7.22 |
| 4 | Benzeneacetaldehyde | 13.387 | 28.59 |
| 5 | Linalool | 15.667 | 13.60 |
| 6 | Nonanal | 15.772 | 4.09 |
| 7 | 3-Acetylheptane-2,6-dione | 15.906 | 5.19 |
| 8 | α- Terpineol | 19.169 | 7.34 |
| 9 | Undecane | 19.39 | 1.92 |
| 10 | α-Terpinenyl acetate | 24.833 | 7.01 |
| 11 | β-ionone | 29.404 | 3.88 |
| 12 | Caryophyllene oxide | 32.478 | 2.67 |
| 13 | Heptadecane | 35.707 | 2.01 |
| 14 | Limonene dioxide | 36.692 | 6.21 |
| 15 | 2-Pentadecanone, 6, 10, 14-trimethyl | 39.754 | 3.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasdemir, Ö.; Kesbiç, O.S.; Cravana, C.; Fazio, F. Antioxidant Performance of Borago officinalis Leaf Essential Oil and Protective Effect on Thermal Oxidation of Fish Oil. Sustainability 2023, 15, 10227. https://doi.org/10.3390/su151310227
Hasdemir Ö, Kesbiç OS, Cravana C, Fazio F. Antioxidant Performance of Borago officinalis Leaf Essential Oil and Protective Effect on Thermal Oxidation of Fish Oil. Sustainability. 2023; 15(13):10227. https://doi.org/10.3390/su151310227
Chicago/Turabian StyleHasdemir, Özlem, Osman Sabri Kesbiç, Cristina Cravana, and Francesco Fazio. 2023. "Antioxidant Performance of Borago officinalis Leaf Essential Oil and Protective Effect on Thermal Oxidation of Fish Oil" Sustainability 15, no. 13: 10227. https://doi.org/10.3390/su151310227
APA StyleHasdemir, Ö., Kesbiç, O. S., Cravana, C., & Fazio, F. (2023). Antioxidant Performance of Borago officinalis Leaf Essential Oil and Protective Effect on Thermal Oxidation of Fish Oil. Sustainability, 15(13), 10227. https://doi.org/10.3390/su151310227







