Production of CO2 Hydrates in Aqueous Mixtures Having (NH4)2SO4 at Different Concentrations; Definition of Consequences on the Process Evolution, Quantification of CO2 Captured and Validation of Hydrates Production as Technique for Ammonium Removal from Waste Water
Abstract
1. Introduction
2. Materials and Methods
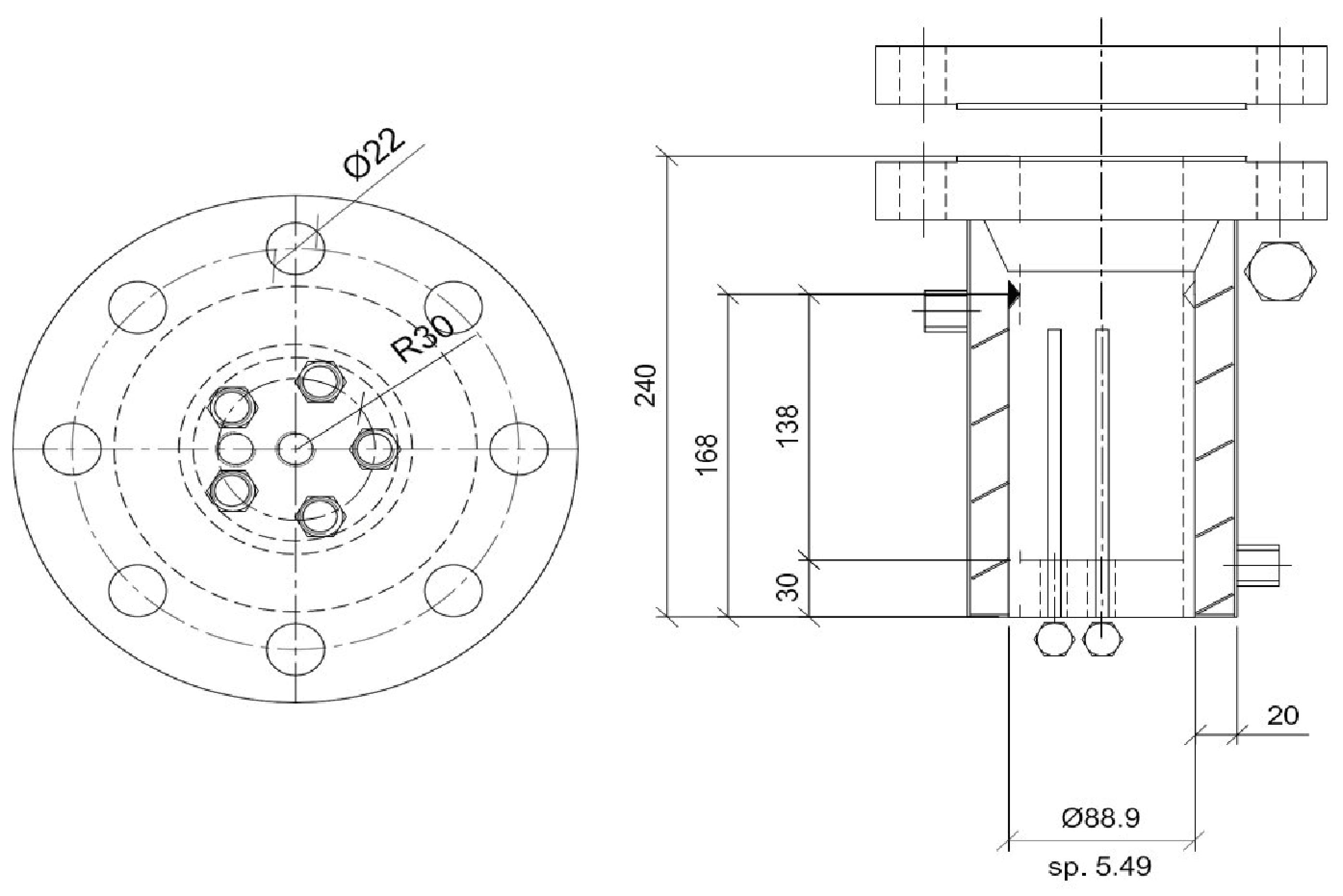
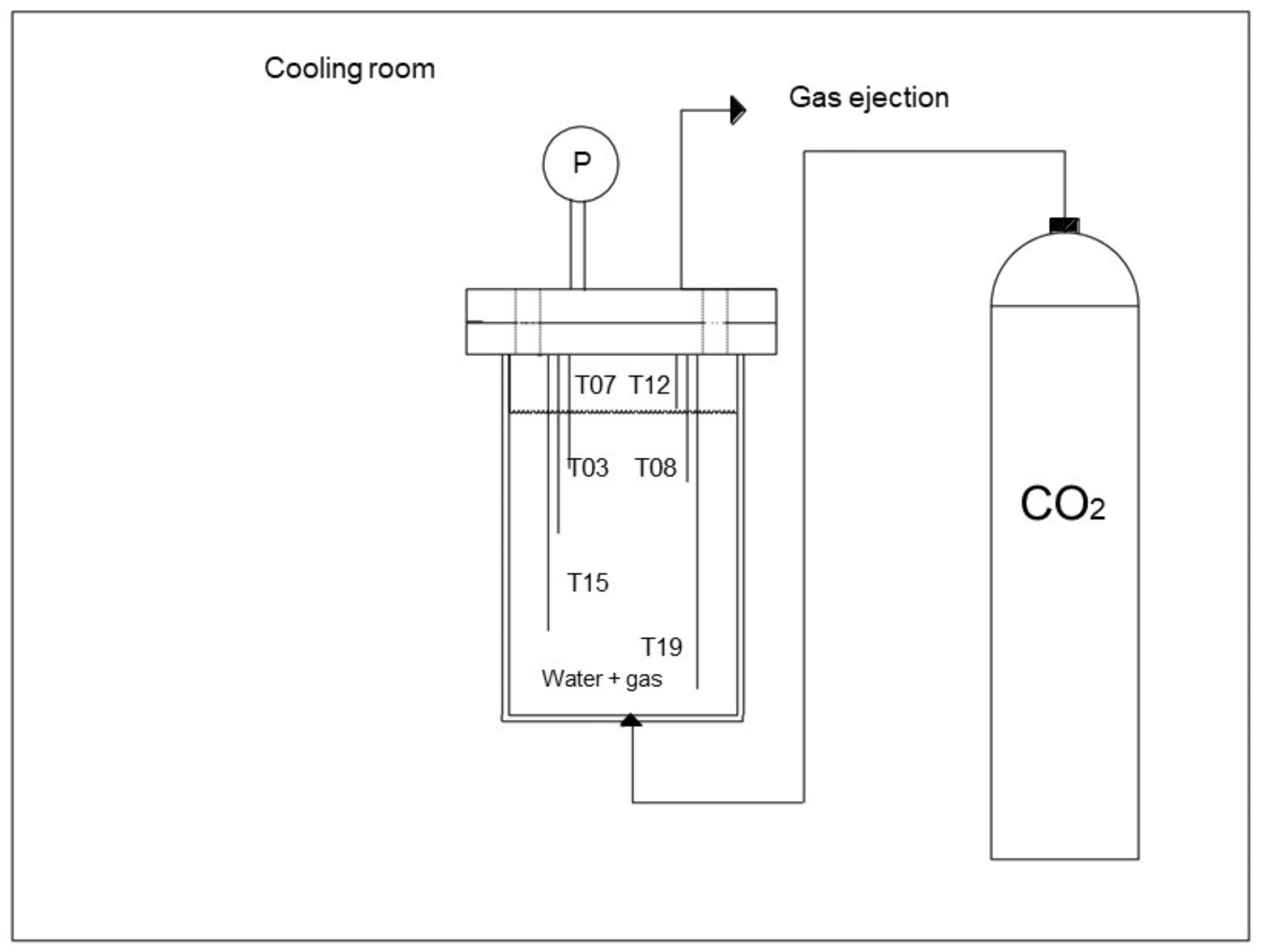
2.1. Experimental Apparatus
2.2. Materials
2.3. Experimental Procedure
- -
- 1.9 wt%, corresponding to 0.3 wt% (NH4)2SO4 in the aqueous solution;
- -
- 6.3 wt%, corresponding to 1.0 wt% (NH4)2SO4 in the aqueous solution;
- -
- 9.5 wt%, corresponding to 1.5 wt% (NH4)2SO4 in the aqueous solution.
3. Results and Discussion
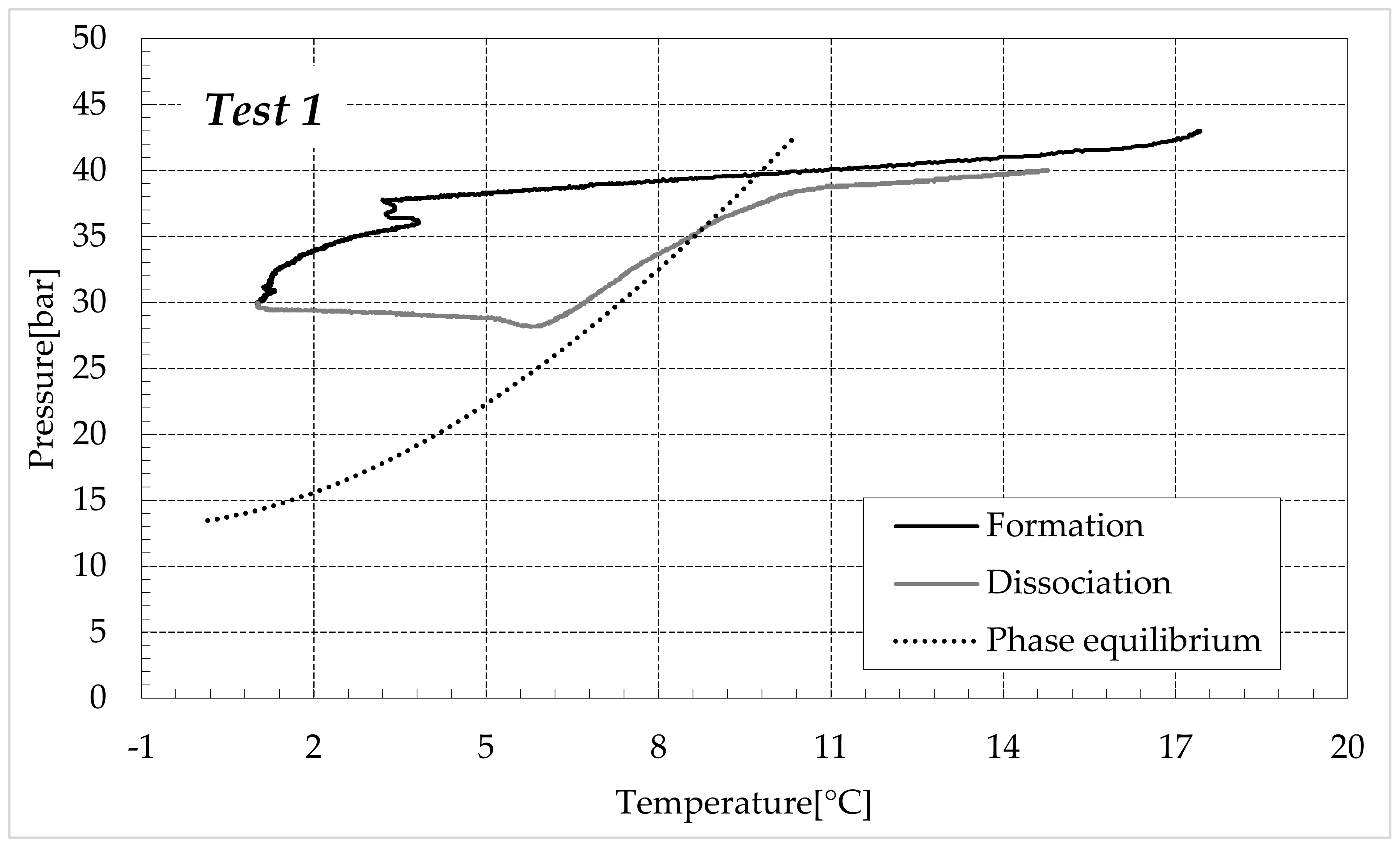
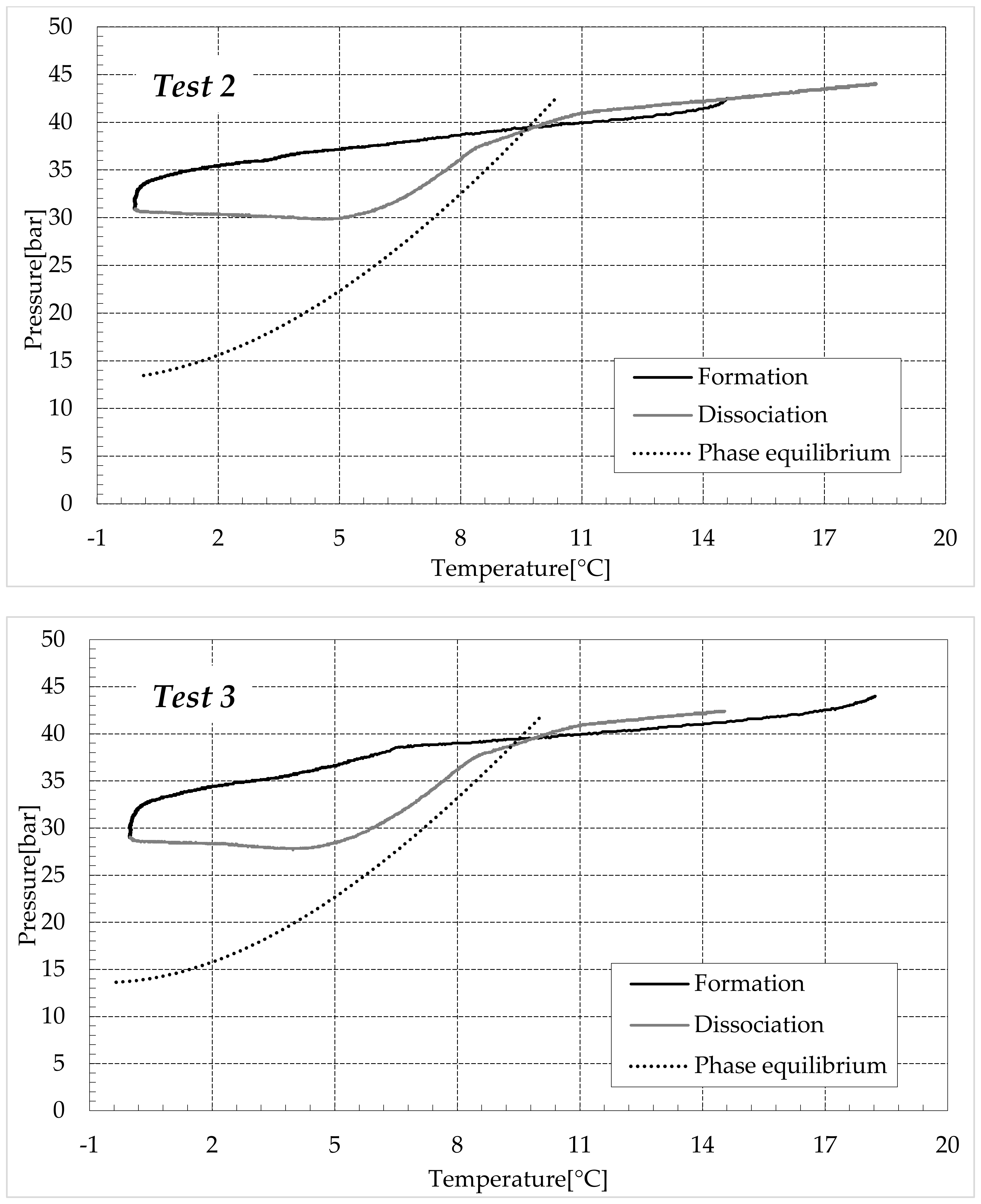
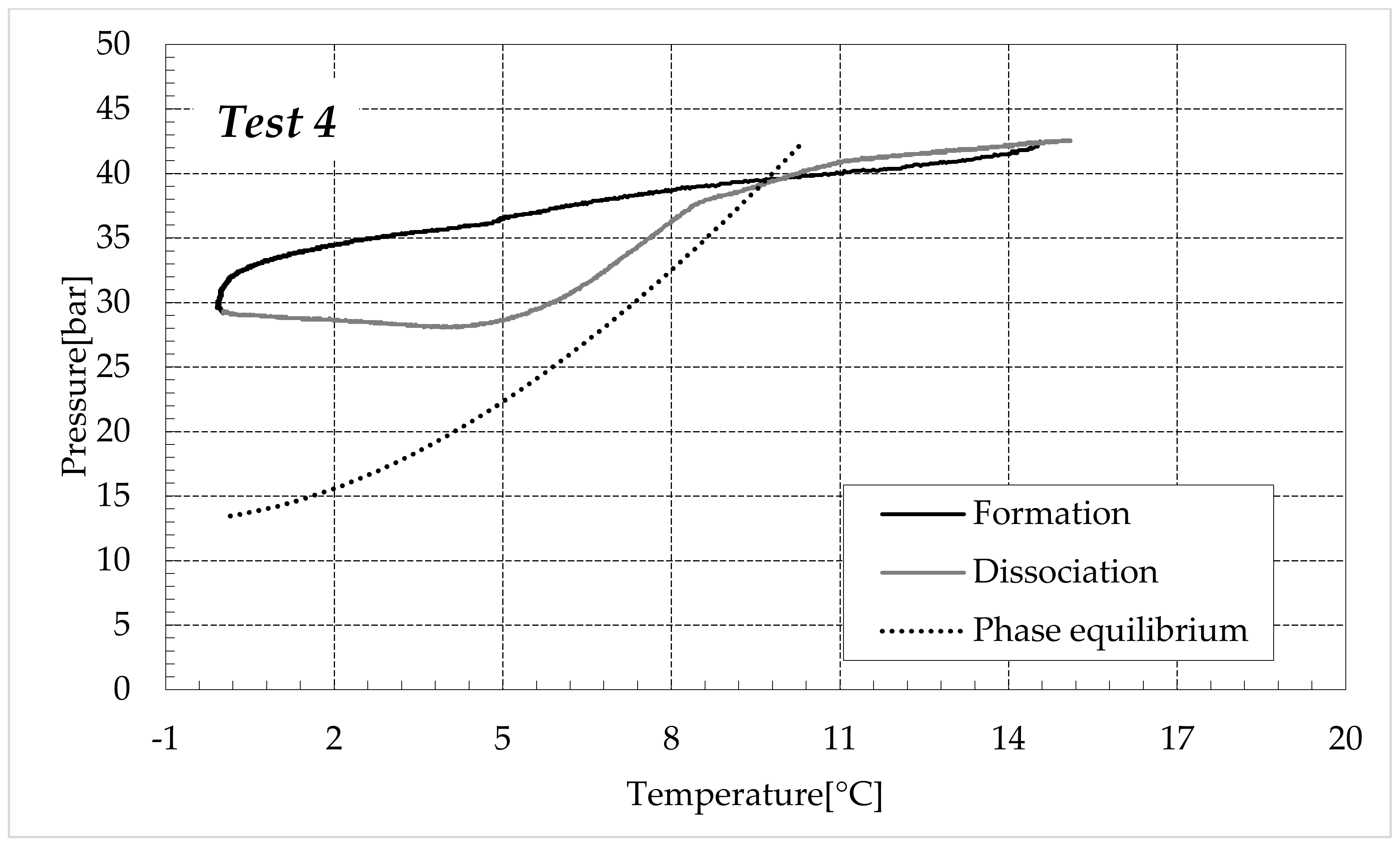
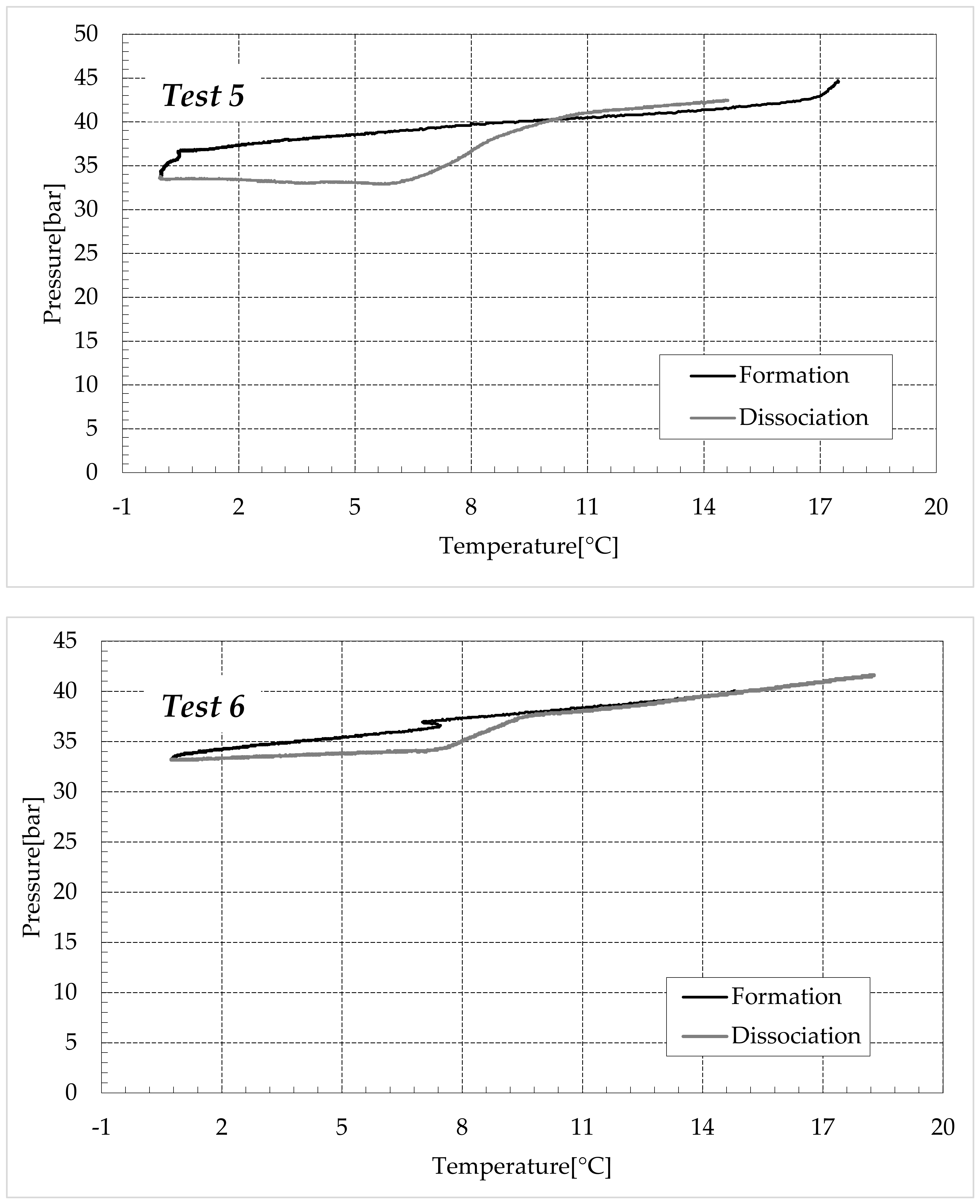
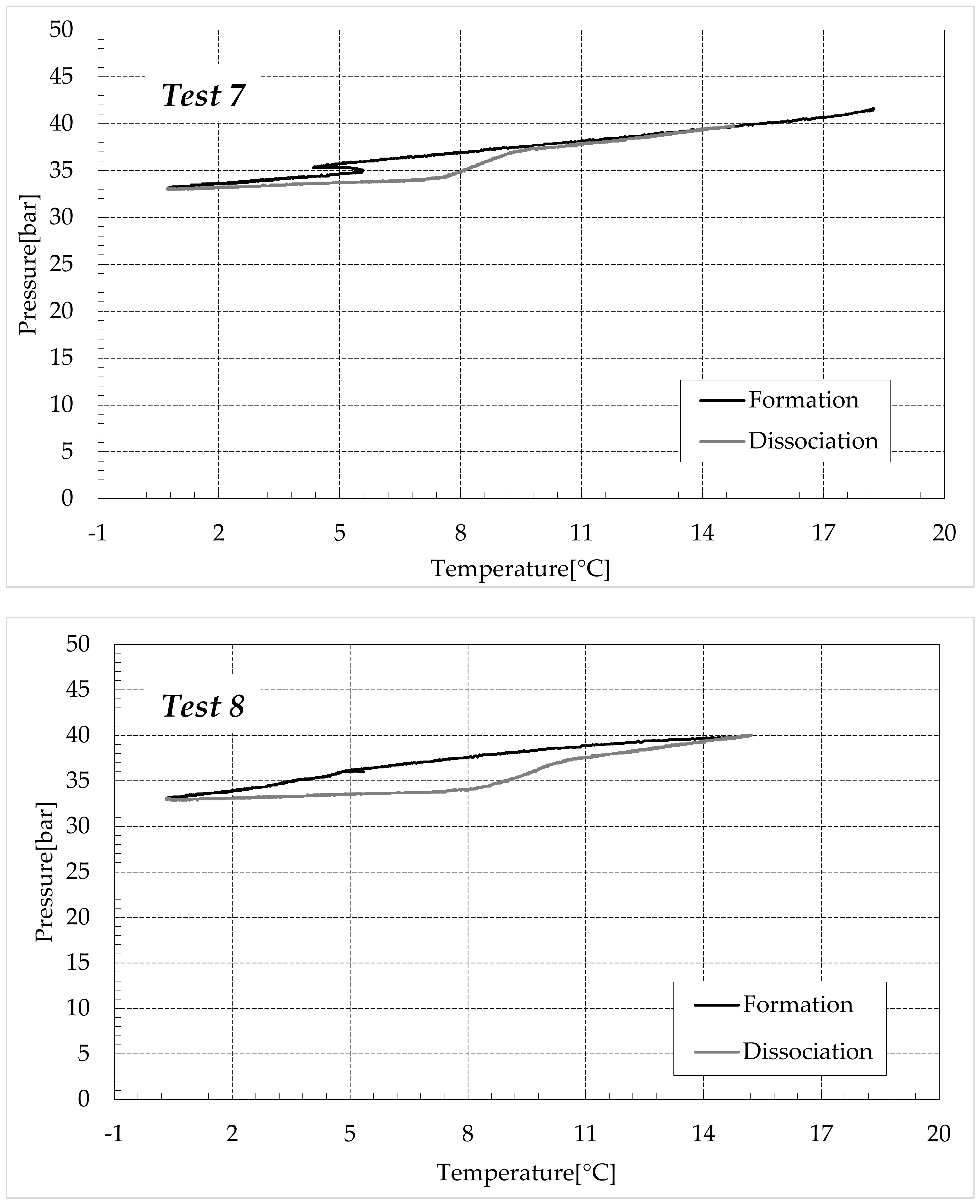
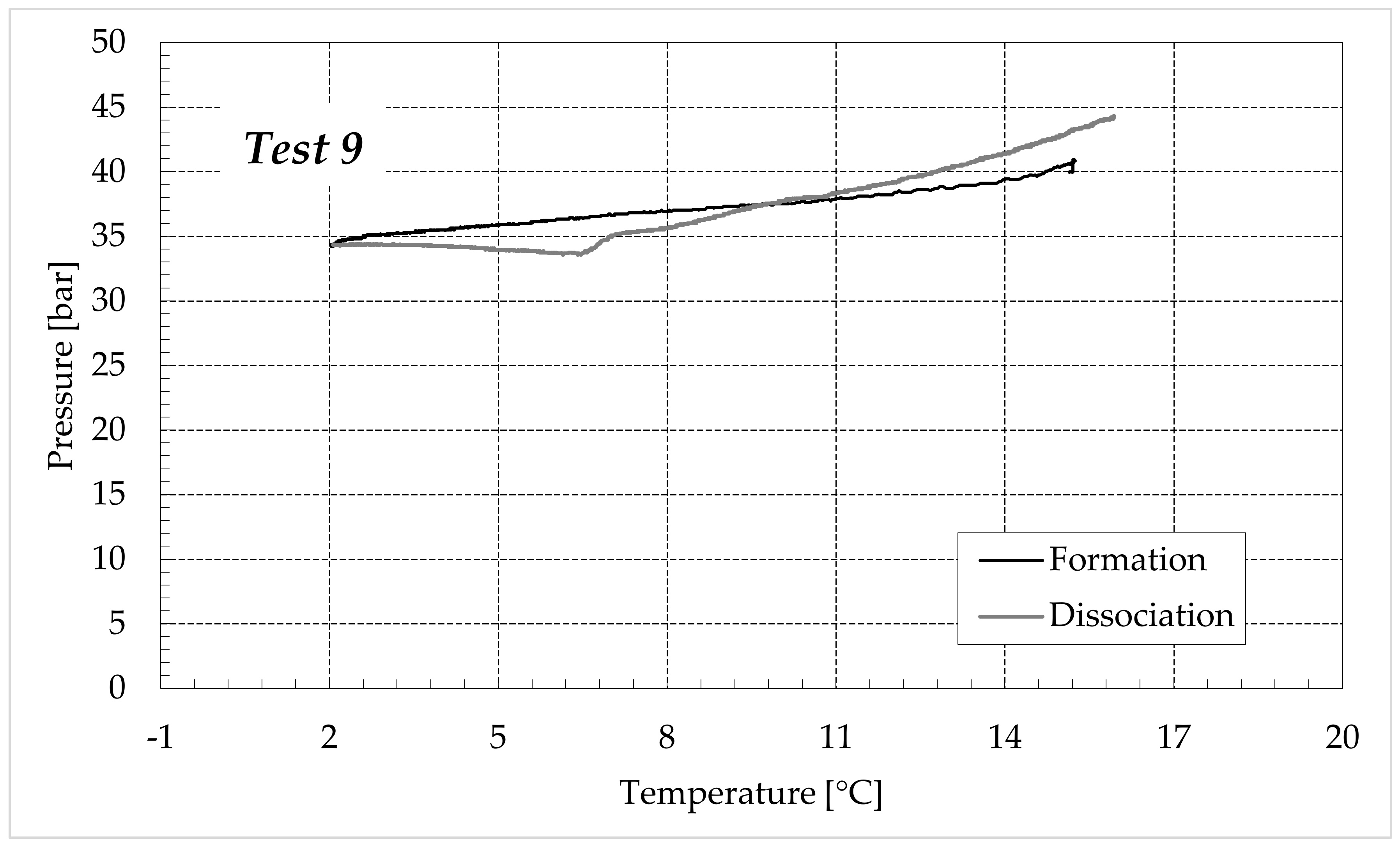
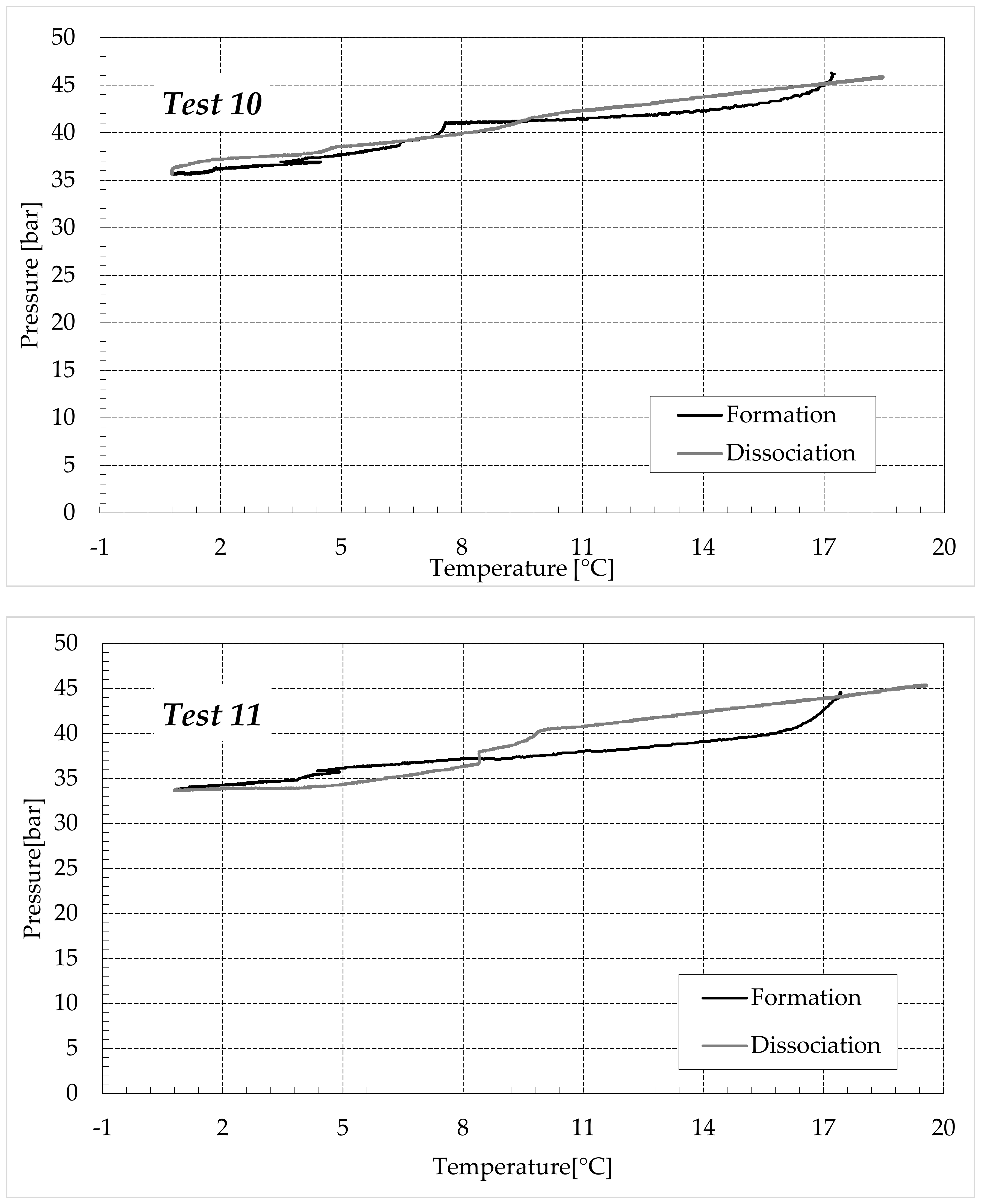
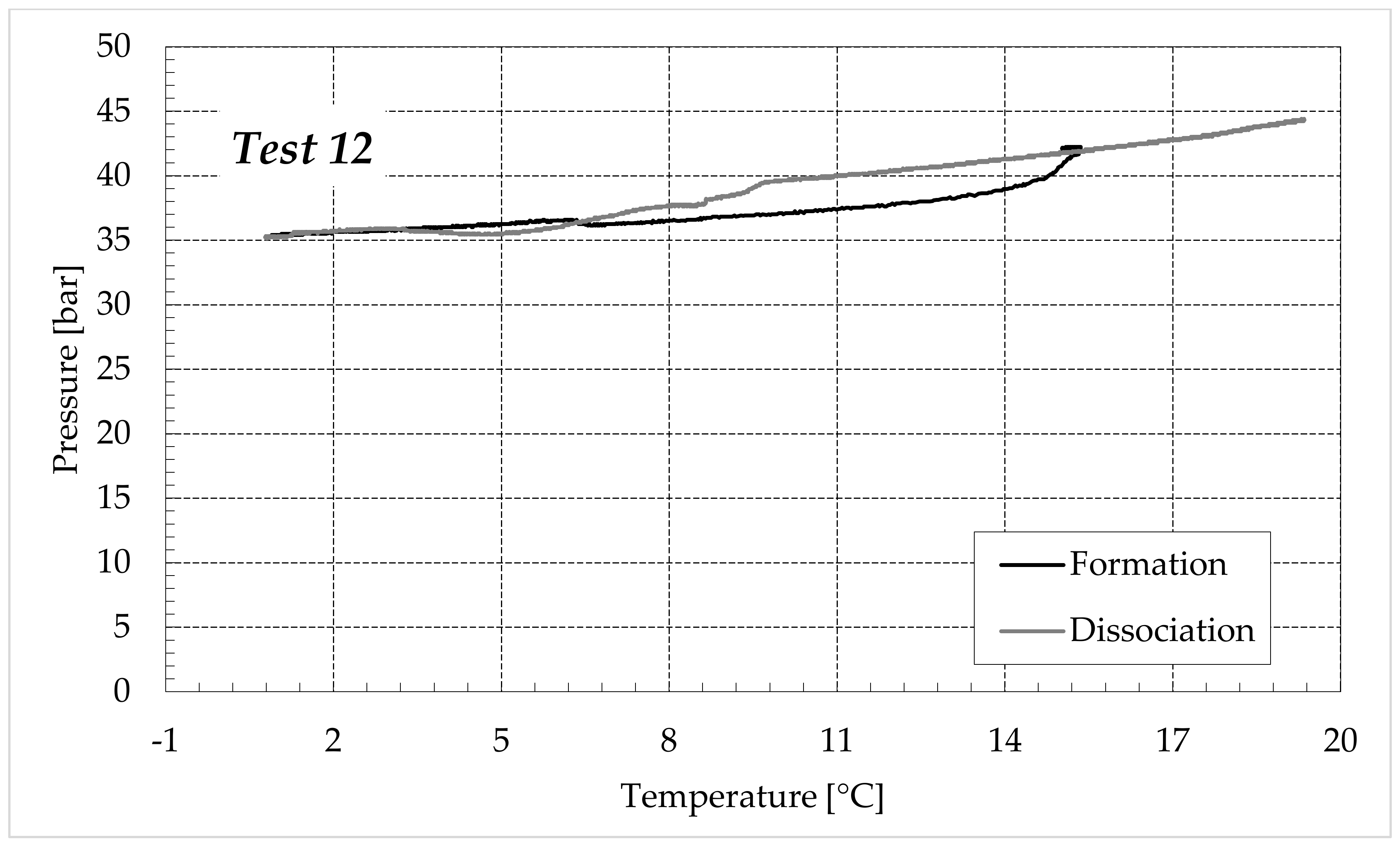
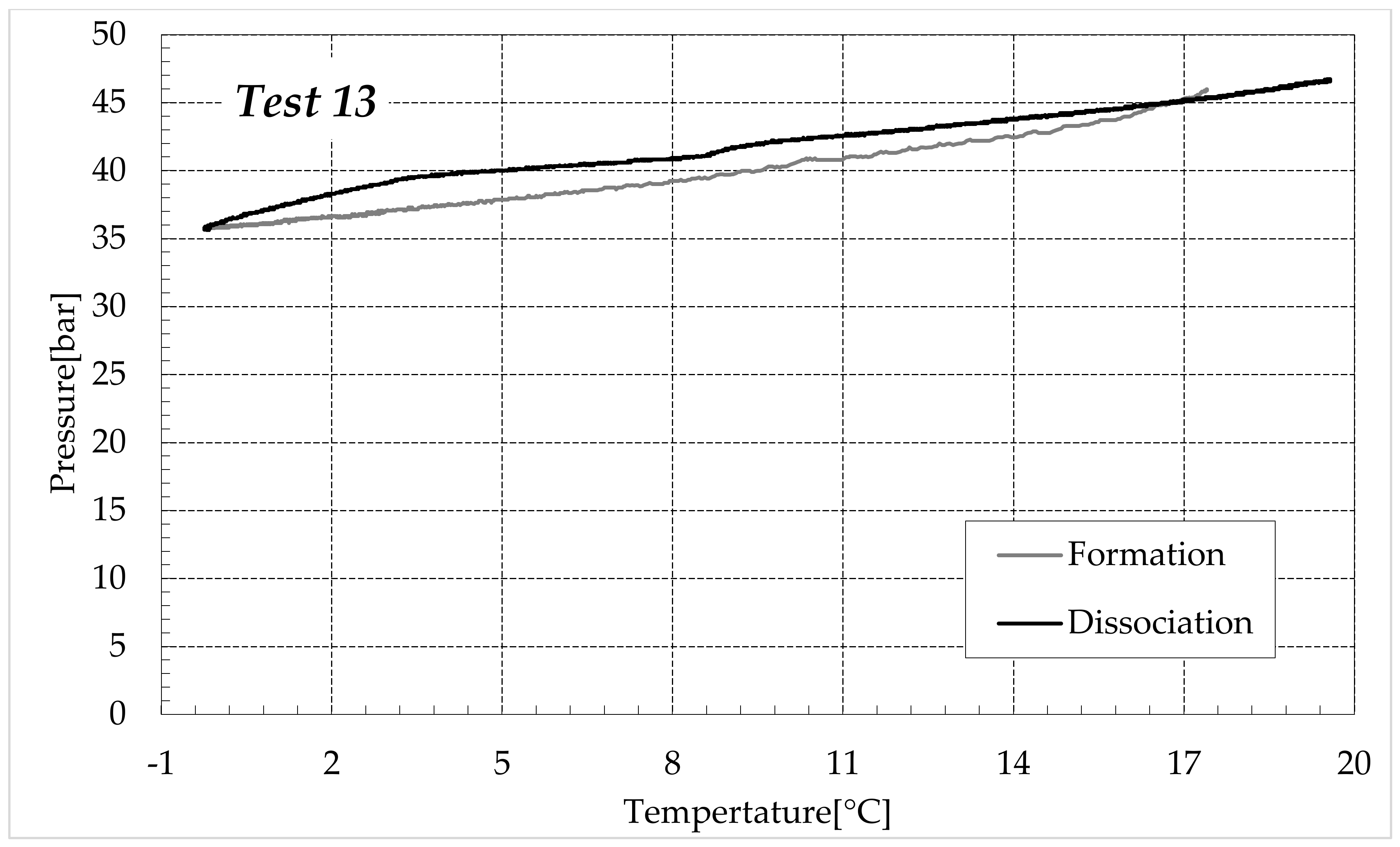
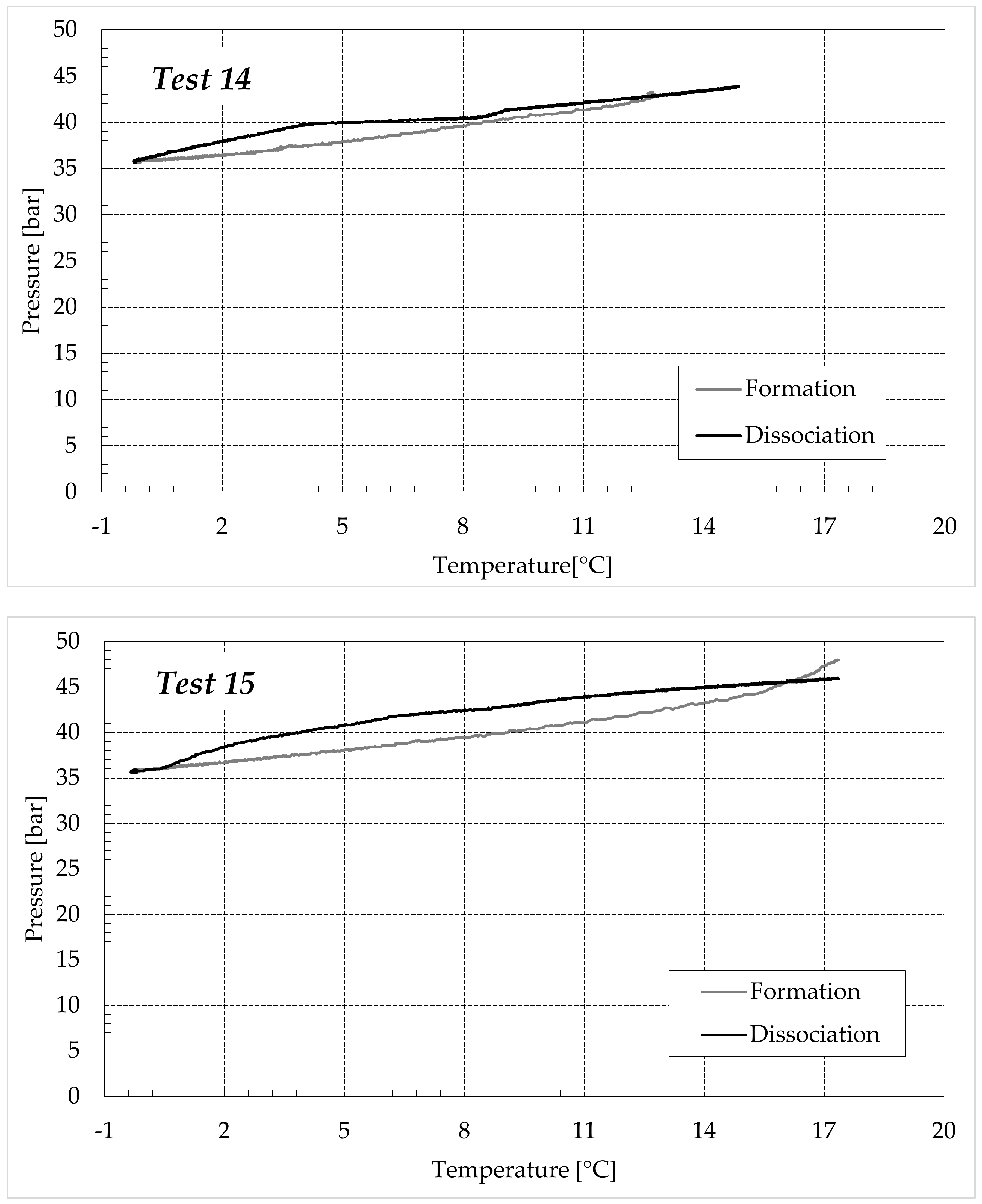
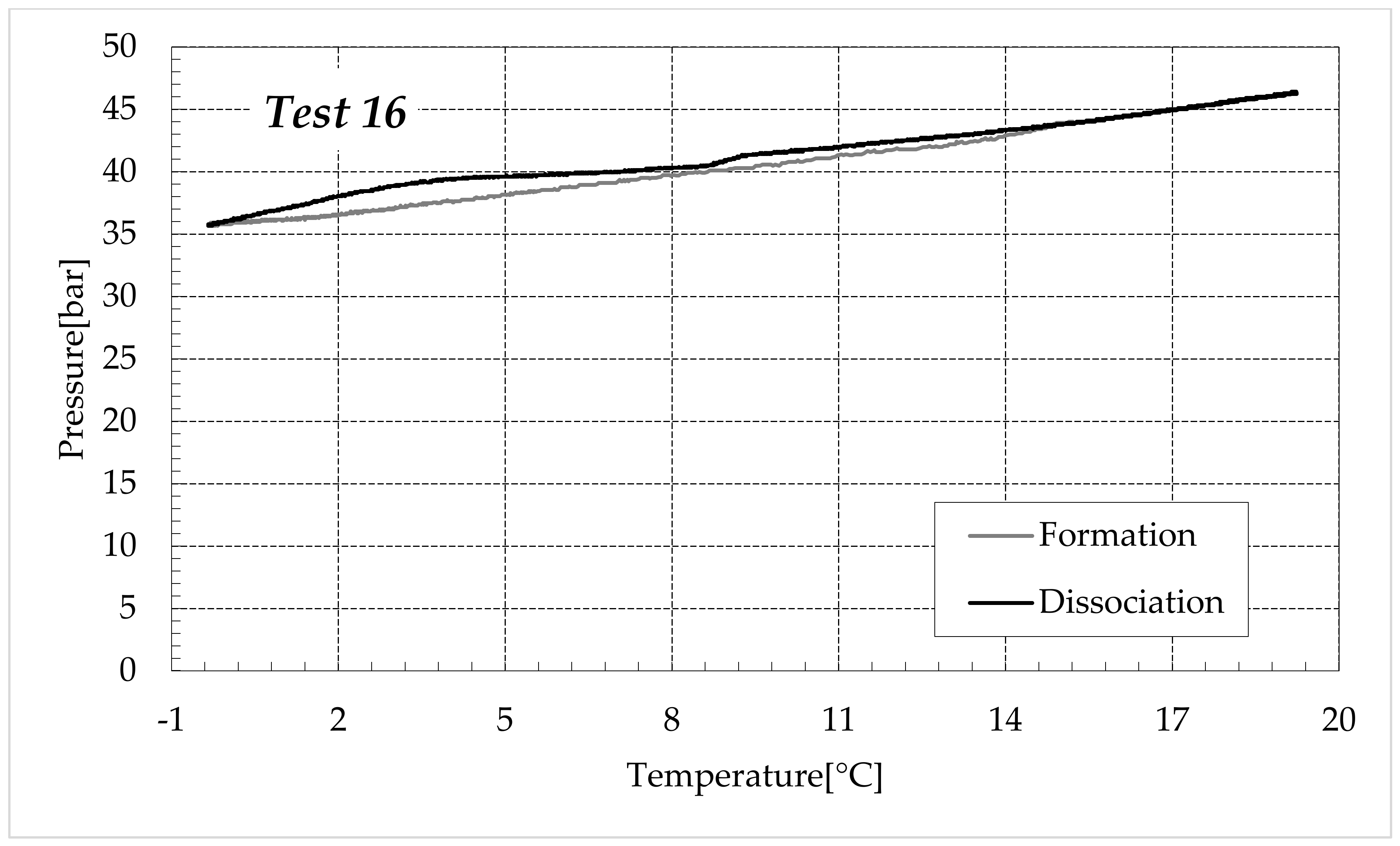
3.1. Formation and Dissociation of CO2 Hydrates in Fresh Water and in Mixtures Containing (NH4)SO4
- -
- Reduction in the presence of 1.9 wt% (NH4)2SO4: from 14.29 to 53.25%;
- -
- Reduction in the presence of 6.3 wt% (NH4)2SO4: from 20.34 to 56.28%;
- -
- Reduction in the presence of 9.5 wt% (NH4)2SO4: from 23.38 to 60.17%.
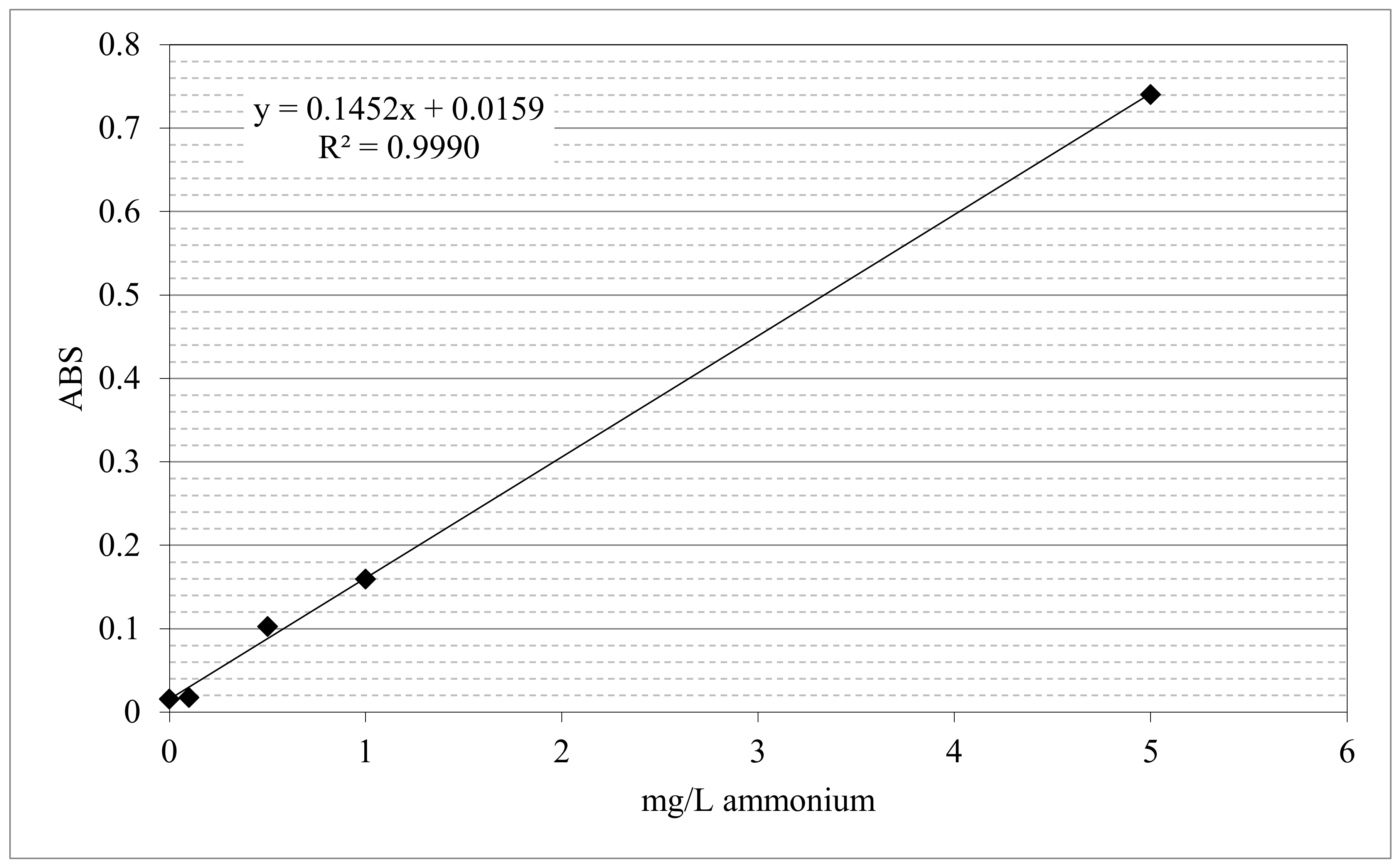
3.2. Determination of (NH4)SO4 Content in Hydrate Phase, via Spectrophotometric Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galloway, T.J.; Ruska, W.; Chappelear, P.S.; Kobayashi, R. Experimental measurement of hydrate numbers for methane and ethane and comparison with theoretical values. Ind. Eng. Chem. Fundam. 1970, 9, 237–243. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates on Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas hydrates—A potential energy source for the 21st Century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F.; Mei, S. Effect of promoters on CO2 hydrate formation: Thermodynamic assessment and microscale Raman spectroscopy/hydrate crystal morphology characterization analysis. Fluid Phase Equilibr. 2021, 550, 113218. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, D.; Yin, J.; Zhou, X.; Li, Y.; Chi, P.; Han, Y.; Ansari, U.; Cheng, Y. Sediment instability caused by gas production from hydrate-bearing sediment in Northern South China Sea by horizontal wellbore: Evolution and mechanism. Nat. Resour. Res. 2023. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J. Factors affecting the lower limit of the safe mud weight window for drilling operation in hydrate-bearing sediments in the Northern South China Sea. Geophys. Geo-Energy Geo-Resour. 2022, 8, 82. [Google Scholar] [CrossRef]
- Wei, W.N.; Li, B.; Gan, Q.; Li, Y.L. Research progress of natural gas hydrate exploitation with CO2 replacement: A review. Fuel 2022, 312, 122873. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Gas hydrates—Geological perspective and global change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Re-definition of the region suitable for CO2/CH4 replacement into hydrates as a function of the thermodynamic difference between CO2 hydrate formation and dissociation. Process Saf. Environ. Prot. 2023, 169, 132–141. [Google Scholar] [CrossRef]
- Rossi, F.; Gambelli, A.M.; Sharma, D.K.; Castellani, B.; Nicolini, A.; Castaldi, M.J. Experiments on methane hydrates formation in seabed deposits and gas recovery adopting carbon dioxide replacement strategies. Appl. Therm. Eng. 2019, 148, 371–381. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, X.S.; Wang, Y.; Liu, J.W.; Hu, H.Q. The optimization mechanism for gas hydrate dissociation by depressurization in the sediment with different water saturations and different particle sizes. Energy 2021, 215, 119129. [Google Scholar] [CrossRef]
- Xu, C.G.; Cai, J.; Yu, Y.S.; Chen, Z.Y.; Li, X.S. Research on micro-mechanism and efficiency of CH4 exploitation via CH4-CO2 replacement from natural gas hydrates. Fuel 2018, 216, 255–265. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, L.J.; Chen, Y.P.; Lin, S.T. In situ methane recovery and carbon dioxide sequestration in methane hydrates: A molecular dynamics simulation study. J. Phys. Chem. B 2011, 115, 15295–15302. [Google Scholar] [CrossRef]
- Baldwin, B.A.; Stevens, J.; Howard, J.J.; Graue, A.; Kvamme, B.; Aspenes, E.; Ersland, G.; Husebo, J.; Zornes, D.R. Using magnetic resonance imaging to monitor CH4 hydrate formation and spontaneous conversion of CH4 hydrate to CO2 hydrate in porous media. Magn. Reson. Imaging 2009, 27, 720–726. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Methane and carbon dioxide hydrates properties in presence of Inconel 718 particles: Analyses on its potential application in gas separation processes to perform efficiency improvement. J. Env. Chem. Eng. 2021, 9, 106571. [Google Scholar] [CrossRef]
- Ren, J.; Zeng, S.; Chen, D.; Yang, M.; Linga, P.; Yin, Z. Roles of montmorillonite clay on the kinetics and morphology of CO2 hydrate in hydrate-based CO2 sequestration. Appl. Energy 2023, 340, 120997. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, J.; Wang, Z.; Gao, Y.; Sun, B.; Liao, Y.; Linga, P. Effect of clay on methane hydrate formation and dissociation in sediment: Implications for energy recovery from clayey-sandy hydrate reservoirs. Appl. Energy 2023, 341, 121064. [Google Scholar] [CrossRef]
- Koide, H.; Shindo, Y.; Tazaki, Y.; Iijima, M.; Ito, K.; Kimura, N.; Omata, K. Deep sub-seabed disposal of CO2—The most protective storage. Energy Convers. Manag. 1997, 38, 253–258. [Google Scholar] [CrossRef]
- Ganteda, R.R.; Burla, S.K.; Boggu, J.M.R.; Prasad, P.S.R. Efficient storage of methane in hydrate from using soybean powder. Methane 2022, 1, 201–209. [Google Scholar] [CrossRef]
- Kumar, S.; Kwon, H.T.; Choi, K.H.; Lim, W.; Cho, J.H.; Tak, K.; Moon, L. LNG: An eco-friendly cryogenic fuel for sustainable development. Appl. Energy 2011, 88, 4264–4273. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, Y.J.; Zheng, T.; Yuan, Q.; Sun, C.Y.; Yang, L.Y.; Chen, G.J. Replacement in CH4-CO2 hydrate below freezing point based on abnormal self-preservation differences of CH4 hydrate. Chem. Eng. J. 2021, 403, 126283. [Google Scholar] [CrossRef]
- Sun, Z.G.; Wang, R.; Ma, R.; Guo, K.; Fan, S. Natural gas storage in hydrates with the presence of promoters. Energy Convers. Manag. 2003, 44, 2733–2742. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef]
- Song, Y.; Wang, S.; Cheng, Z.; Huang, M.; Zhang, Y.; Zheng, J.; Jiang, L.; Liu, Y. Dependence of the hydrate-based CO2 storage processes on the hydrate reservoir environment in high-efficiency storage methods. Chem. Eng. J. 2021, 415, 128937. [Google Scholar] [CrossRef]
- Wang, F.; Guo, G.; Liu, G.Q.; Luo, S.J.; Guo, R.B. Effects of surfactants micelles and surfactant-coated nanospheres on methane hydrate growth pattern. Chem. Eng. Sci. 2016, 144, 108–115. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Review on the usage of small-chain hydrocarbons (C2-C4) as adi gases for improving the efficiency of hydrate-based technologies. Energies 2023, 16, 3576. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsungivit, P.; Linga, P. Rapid methane hydrate formation to develop a cost effective large scale energy storage system. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Montazeri, S.M.; Kolliopoulos, G. Hydrate based desalination for sustainable water treatment: A review. Desalination 2022, 537, 115855. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Qureshi, K.; Maitlo, G.; Ahmed, S. Study of PAN fiber and iron ore absorbents for arsenic removal. Civ. Eng. J. 2020, 6, 548–562. [Google Scholar] [CrossRef]
- Priscilla, S.J.; Judi, V.A.; Daniel, R.; Sivaji, K. Effects of chromium doping on the electrical properties of ZnO nanoparticles. Emerg. Sci. J. 2020, 4, 82–88. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Gambelli, A.M.; Zhao, X.; Gao, Y.; Rossi, F.; Mei, S. In situ experimental study on the effect of mixed inhibitors on the phase equilibrium of carbon dioxide hydrate. Chem. Eng. Sci. 2022, 248, 117230. [Google Scholar] [CrossRef]
- Bates, C.; Gladwin, R.; McGrath, L. Desalination in pilot scale column crystallizers. Desalination 1977, 21, 83–97. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Water desalination using R141b gas hydrate formation. Desalination Water Treat. 2014, 52, 2450–2456. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review on natural gas hydrate as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Simmons, B.A.; Majzoub, E.H.; Clift, W.M.; Dedrick, D.E. Clathrate hydrates for production of potable water. Mater. Res. Soc. Symp. Proc. 2006, 930, 14–19. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Gonfa, G.; Foo Khor, S.; Sharif, A.M. Inhibition effect of amino acids on carbon dioxide hydrate. Chem. Eng. Sci. 2017, 171, 331–339. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Filipponi, M.; Nicolini, A.; Rossi, F. Natural gas hydrate: Effect of sodium chloride on the CO2 replacement process. Int. Multidiscip. Sci. GeoConference SGEM 2019, 19, 333–343. [Google Scholar]
- Holzammer, C.; Finckenstein, A.; Will, S.; Braeuer, A.S. How sodium chloride salt inhibits the formation of CO2 gas hydrates. J. Phys. Chem. B 2016, 120, 2452–2459. [Google Scholar] [CrossRef]
- Cha, J.H.; Seol, Y. Increasing gas hydrate formation temperature for desalination of high salinity produced water with secondary guests. ACS Sustain. Chem. Eng. 2013, 1, 1218–1224. [Google Scholar] [CrossRef]
- Sahu, P.; Krishnaswamy, K.; Ponnani, N.K.; Pande, A. A thermodynamic approach to selection of suitable hydrate formers for seawater desalination. Desalination 2018, 436, 144–151. [Google Scholar] [CrossRef]
- Chapoy, A.; Mazloum, S.; Burgass, R.; Haghighi, H.; Tohidi, B. Clathrate hydrate equilibria in mixed monoethylene glycol and electrolyte aqueous solutions. J. Chem. Thermodyn. 2012, 48, 7–12. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas hydrate-based process for desalination of heavy metal ions from an aqueous solution: Kinetics and rate of recovery. ACS EST Water 2021, 1, 134–144. [Google Scholar] [CrossRef]
- Nam Park, K.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Lu, H.; Matsumoto, R.; Tsuji, Y.; Oda, H. Anion plays a more important role than cation in affecting gas hydrate stability in electrolyte solutions?—A recognition from experimental results. Fluid Phase Equilibr. 2001, 178, 225–232. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P.D.; Bishnoi, P.R. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Shaheen, S.M.; Zhao, Q.; Liu, X.; Rinklebe, J.; Ren, H. Ammonium nitrogen recovery from digestate by hydrothermal pretreatment followed by activated hydrochar sorption. Chem. Eng. J. 2020, 379, 122254. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, H.Y.; Hu, X.H.; Zhu, Q.; Stanislaus, M.S.; Li, S.Y.; Zhao, C.Y.; Wang, Q.H.; Yang, Y.N. Enhanced bio—Methane production from ammonium—Rich waste using eggshell- and lignite- modified zeolite (ELMZ) as a bio—Adsorbent during anaerobic digestion. Process Biochem. 2019, 81, 148–155. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.C.; Ding, L.L.; Ren, H.Q.; Xu, K.; Wu, Y.G.; Sheng, D. Modeling assessment for ammonium nitrogen recovery from wastewater by chemical precipitation. J. Environ. Sci. 2011, 23, 881–890. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar—Induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency be wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Boss, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Ajmal, S.; Kumar, A.; Selvaraj, M.; Alam, M.M.; Yang, Y.; Das, D.K.; Gupta, R.K.; Yasin, G. MXenes and their interfaces for the taming of carbon dioxide & nitrate: A critical review. Coord. Chem. Rev. 2023, 483, 215094. [Google Scholar]
- Ajmal, S.; Yasin, G.; Kumar, A.; Tabish, M.; Ibraheem, S.; Sammed, K.A.; Mushtaq, M.S.; Saad, A.; Mo, Z.; Zhao, W. A disquisition on CO2 electroreduction to C2H4: An engineering and design perspective looking beyond novel choosy catalyst materials. Coord. Chem. Rev. 2023, 485, 215099. [Google Scholar] [CrossRef]
- Rossi, F.; Li, Y.; Gambelli, A.M. Thermodynamic and kinetic description of the main effects related to the memory effect during carbon dioxide hydrates formation in a confined environment. Sustainability 2021, 13, 13797. [Google Scholar] [CrossRef]
- Khan, M.S.; Partoon, B.; Bavoh, C.B.; Lal, B.; Mellon, B.M. Influence of tetramethylammonium hydroxide on methane and carbon dioxide phase equilibrium conditions. Fluid Phase Equilibr. 2017, 440, 1–8. [Google Scholar] [CrossRef]
- Sadeq, D.; Iglauer, S.; Lebedev, M.; Smith, C.; Barifcani, A. Experimental determination of hydrate phase equilibrium for different gas mixture containing methane, carbon dioxide and nitrogen with motor current measurements. J. Nat. Gas Sci. Eng. 2017, 38, 59–73. [Google Scholar] [CrossRef]
- Yu, S.H.; Zhou, S.D.; Li, X.S.; Wang, S.L. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibr. 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Jarrahian, A.; Nakhaee, A. Hydrate-liquid-vapor equilibrium condition for N2 + CO2 + H2O system: Measurement and modelling. Fuel 2019, 237, 769–774. [Google Scholar] [CrossRef]
- Kyung, D.; Lee, K.; Kim, H.; Lee, W. Effect of marine environmental factors on the phase equilibrium of CO2 hydrate. Int. J. Greenh. Gas Control 2014, 20, 285–292. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J.; Zhou, Y. Large scale reactor details and results for the formation and decomposition of methane hydrates via thermal stimulation dissociation. J. Pet. Sci. Eng. 2012, 94, 19–27. [Google Scholar] [CrossRef]



| (NH4)SO4 in Water [wt%] | Corr. NH4+ in Water [wt%] | Test n° |
|---|---|---|
| 0 | 0 | 1 |
| 2 | ||
| 3 | ||
| 4 | ||
| 1.9 | 0.3 | 5 |
| 6 | ||
| 7 | ||
| 8 | ||
| 6.3 | 1.0 | 9 |
| 10 | ||
| 11 | ||
| 12 | ||
| 9.5 | 1.5 | 13 |
| 14 | ||
| 15 | ||
| 16 |
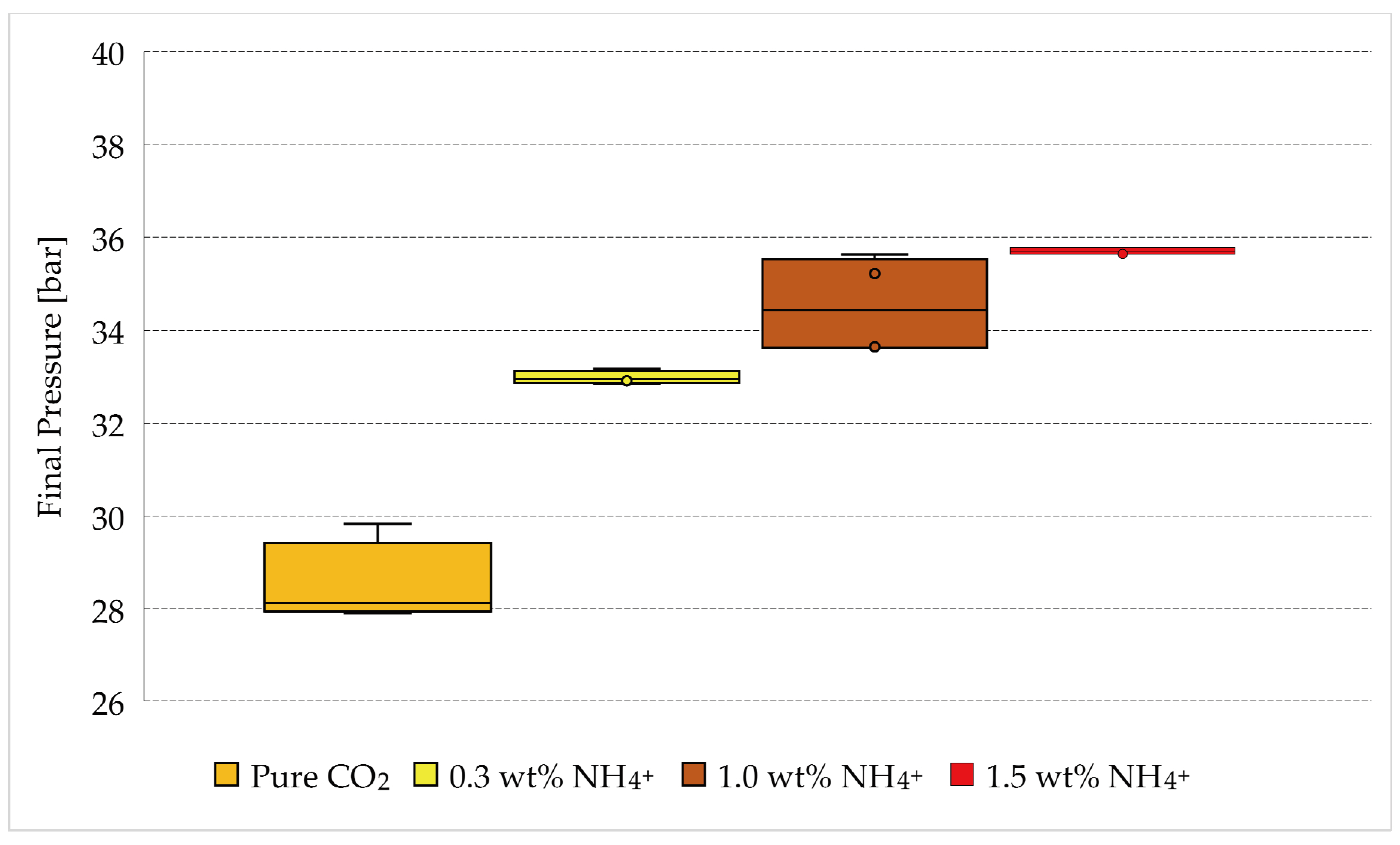
| Final Pressure Values [bar] | |||
|---|---|---|---|
| Tests 1–4 | Tests 5–8 | Tests 9–12 | Tests 13–16 |
| 0 wt% NH4+ | 0.3 wt% NH4+ | 1.0 wt% NH4+ | 1.5 wt% NH4+ |
| 28.18 | 32.91 | 33.62 | 35.77 |
| 29.83 | 33.17 | 35.63 | 35.64 |
| 27.9 | 32.99 | 33.64 | 35.64 |
| 28.09 | 32.85 | 35.22 | 35.78 |
| Moles of Hydrates Formed [mol] | |||
|---|---|---|---|
| Tests 1–4 | Tests 5–8 | Tests 9–12 | Tests 13–16 |
| 0 wt% NH4+ | 0.3 wt% NH4+ | 1.0 wt% NH4+ | 1.5 wt% NH4+ |
| 0.237 | 0.198 | 0.101 | 0.175 |
| 0.204 | 0.108 | 0.183 | 0.092 |
| 0.256 | 0.140 | 0.184 | 0.177 |
| 0.228 | 0.108 | 0.110 | 0.132 |
| Test | Water into Hydrates [mol] | NH4+ in Water [wt%] |
|---|---|---|
| A | 0.624 | 0.428 |
| B | 0.786 | 0.407 |
| C | 0.588 | 0.400 |
| D | 0.702 | 0.449 |
| E | 0.834 | 0.380 |
| F | 0.732 | 0.428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Rushani, X.; Pezzolla, D.; Rossi, F.; Gigliotti, G. Production of CO2 Hydrates in Aqueous Mixtures Having (NH4)2SO4 at Different Concentrations; Definition of Consequences on the Process Evolution, Quantification of CO2 Captured and Validation of Hydrates Production as Technique for Ammonium Removal from Waste Water. Sustainability 2023, 15, 9841. https://doi.org/10.3390/su15129841
Gambelli AM, Rushani X, Pezzolla D, Rossi F, Gigliotti G. Production of CO2 Hydrates in Aqueous Mixtures Having (NH4)2SO4 at Different Concentrations; Definition of Consequences on the Process Evolution, Quantification of CO2 Captured and Validation of Hydrates Production as Technique for Ammonium Removal from Waste Water. Sustainability. 2023; 15(12):9841. https://doi.org/10.3390/su15129841
Chicago/Turabian StyleGambelli, Alberto Maria, Xhino Rushani, Daniela Pezzolla, Federico Rossi, and Giovanni Gigliotti. 2023. "Production of CO2 Hydrates in Aqueous Mixtures Having (NH4)2SO4 at Different Concentrations; Definition of Consequences on the Process Evolution, Quantification of CO2 Captured and Validation of Hydrates Production as Technique for Ammonium Removal from Waste Water" Sustainability 15, no. 12: 9841. https://doi.org/10.3390/su15129841
APA StyleGambelli, A. M., Rushani, X., Pezzolla, D., Rossi, F., & Gigliotti, G. (2023). Production of CO2 Hydrates in Aqueous Mixtures Having (NH4)2SO4 at Different Concentrations; Definition of Consequences on the Process Evolution, Quantification of CO2 Captured and Validation of Hydrates Production as Technique for Ammonium Removal from Waste Water. Sustainability, 15(12), 9841. https://doi.org/10.3390/su15129841









