Abstract
Continuous planting is the primary method for managing Eucalyptus plantations. The “space-replacing time” approach assesses growth parameters of Eucalyptus trees in China across generations, including height, diameter at breast height (DBH), slenderness ratio, trunk oblateness, and longitudinal growth strain. The findings reveal: (1) significant variations in growth strain occur among generations, with average strain increasing noticeably; and (2) growth-linked traits of Eucalyptus urophylla × E. grandis are impacted, with negative correlation between slenderness ratio and growth strain, and positive correlation between height and trunk oblateness. Factors influencing growth strain include height, slenderness, and surface longitudinal growth strain at breast height, with strong correlations observed. These parameters serve as growth strain indicators. Continuous planting affects growth traits and strain in Eucalyptus plantations. It is advisable to select trees with stable or slow growth rates and to avoid continuous planting without limits.
1. Introduction
Eucalyptus is a generic name for the genus Eucalyptus in the Myrtle family, and is one of the best-known fast-growing tree genera in the world [1], with more than 900 species. It is highly adaptable, fast-growing, and productive, and has the shortest rotation period among current plantation species, with a rotation cycle of 6–8 years. Eucalyptus has a high economic value. At present, Eucalyptus is currently planted in more than 100 countries around the world, covering an area of more than 20 hm2 [2]. The plantation area of Eucalyptus in China is more than 4.5 Mhm2, and the commercial timber production of Guangxi, mainly eucalyptus timber, accounts for one-third of total national production, which promotes the development of paper manufacturing and other related industries [3]. At present, Eucalyptus plantation wood is mainly used for paper and wood-based panel production, and less for the production of high-value-added solid wood products such as finger-joined timber, furniture, and construction [4], because Eucalyptus has high growth strain and the wood is prone to cracking and deformation, resulting in a lower yield. Growth strain is an intercellular force generated within the vascular cambium of a tree as the cells differentiate and mature [5], which helps the trunk withstand external loads without being damaged. Growth strain levels are variable among and within tree species and are closely related to growth conditions [6,7,8]. During log sawing and rotary cutting of logs, wood is deformed or suffers longitudinal shrinkage due to the release of growth strain [5,9,10,11,12,13,14,15,16,17,18], which seriously affects wood value. Therefore, growth strain level is an important indicator for forest tree selection and breeding.
Continuous planting is a cycle in which seedlings are grown, harvested, sprouted and renewed to form a new generation of Eucalyptus plantations. Continuous planting can maintain stand fertility, control management costs, and improve economic efficiency [13]. Due to the strong sprouting and regeneration ability, short establishment time, and low silvicultural cost of Eucalyptus, short-cycle multigenerational succession is a common management method for fast-growing Eucalyptus plantations [19], and greatly improves the yield of the plantations. However, in addition to timber yield, it is important to focus on eucalyptus wood properties, which are closely related to wood structure [20]. Studies have shown that Eucalyptus has significant internal growth strain [21,22], which leads to fiber breakage and has a serious impact on wood structure, and that fast-growing plantation species have a higher level of growth strain than natural forests of the same species or secondary forests, due to shorter harvesting periods and inappropriate forestry practices. Secondary forests of the same species have higher levels of growth strain [23,24]. To improve wood utilization, forest species with high growth rates and low growth strain should be selected. Current research on growth strain in Eucalyptus has focused on age and species [25,26], and the question of whether continuous planting affects growth strain in Eucalyptus and whether continuous planting is possible indefinitely has not been addressed.
According to the results of existing studies [5,27,28], the average value of the surface growth strain can usually be used to compare and evaluate the surface growth stress of standing trees and logs. Longitudinal growth stress is substantially greater than lateral growth stress and is the main cause of warping and deformation of lumber [14,21], while lateral growth strain values are relatively small; therefore, in this paper, only the results for longitudinal growth strain are analyzed.
There are three hypotheses about the generation of growth strain: the lignin swelling hypothesis [29], the cellulose tension hypothesis [30], and the unity hypothesis [31]. Growth strain is generated by the deformation of newly formed xylem cells in the trunk. It is unclear whether continuous planting affects the rate of cell differentiation of trees and thus leads to changes in growth strain. Studying the effect of several generations of continuous planting on growth strain will help select trees with low growth strain and fast growth rate, identify the best planting generation, improve land utilization rates, Eucalyptus wood yield and wood quality, and greatly increase the value of Eucalyptus wood.
This paper focuses on the analysis of growth stress variations in relation to continuous planting generations and explores the correlation between stumpage properties and growth stress. By measuring the growth strain and growth traits of 8-year-old trees, we examine this relationship to predict the growth stress of Eucalyptus urophylla × E. grandis. Moreover, we aim to identify the ideal cultivation generation for selecting fast-growing wood.
2. Materials and Methods
2.1. Overview of the Experimental Forest Site
The test forest was located at Dongmen Forestry in Fusui City, Guangxi Zhuang Autonomous Region, China. Eucalyptus plantations were selected from three distinct periods, maintaining consistent stand selection: new plantations (I), first rotation sprout stands (II), and second rotation sprout stands (III). The Eucalyptus plantation standard for each continuous generation was Eucalyptus urophylla × E. grandis of generations 1, 2 and 3. Eucalyptus trees are harvested every 8 years. Forest I is a new plantation, planted with live seedlings and fertilized with basal fertilizer. Forest II is a second-generation forest after harvesting, sprouting, and renewal, and so on. After sprouting, 0.5 kg of Eucalyptus fertilizer is applied, and an additional 0.75 kg plant−1 of compound fertilizer and 1.5 kg plant−1 of organic fertilizer are applied annually, with a planting row spacing of 2 m × 3 m. The basic information for the sample plot is shown in Table 1.

Table 1.
Basic information for sample plot.
2.2. Experimental Method
2.2.1. Measurement of Growth Traits of Sample Trees
For each generation, 8–13 trees without obvious tension were selected (Table 1), and the diameter (the long axis L1, short axis L2) and DBH of each live tree were measured using vernier calipers and diameter-at-breast-height rulers. The tree height measurement tool was an infrared height meter.
2.2.2. Selection of Test Points
To effectively analyze the relationship between different test points in the circumferential direction and the growth strain, the four-equivalent method is used in this paper. The test points are selected as shown in Figure 1.
Figure 1.
Schematic diagram of the location of the test points at the periphery of the trunk.
2.2.3. The Longitudinal Growth Strain Test
The strain gauge method was adopted by peeling off the bark and cambium (size 4 cm × 4 cm) at the circumference test point at breast height to expose the xylem surface, attaching a strain gauge (BE120-10AA-P150, size 9.8 mm × 3 mm, AVIC Electro-Mechanical Instruments Co., Shaanxi, China) to the xylem surface along the fiber direction (four strain gauges per tree), connecting them to an intelligent static resistance strain gauge (YJW-8/16, Beijing Terra Venus Instruments Co., Ltd., Beijing, China), adjusting the zero, and using an electric drill (drill bit diameter 2.5 mm) to make a hole in the strain gauge at the top and bottom along the parallel line at a depth of 10–15 mm. The strain grows when the reading is displayed on the strain gauge. The reading is taken until the value shown on the strain gauge is stable, and the wire is then removed to test another tree. Measurements were determined in the absence of wind, as recommended by Yang et al. [32], to avoid bending strain overlapping with the growth strain. Testing was scheduled for July 2022. The procedure is shown in Figure 2.

Figure 2.
Growth strain measurement: 1. expose xylem and patch; 2. connect strain gauge and set to zero; 3. release growth strain.
2.3. Statistical Analysis
The variables in this study underwent ANOVA with a significance level of p = 0.05. For significant differences, the mean of the variables was further analyzed using the LSD test. The correlation coefficient was employed to evaluate the relationships between growth strain and different tree characteristics. Statistical analysis utilized SPSS software version 26 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Variation of Growth Traits of Eucalyptus spp. in Different Generations
The growth strain in the circumferential position of the tree is influenced by oblateness. Therefore, only the oblateness was analyzed in this section, and the trends of the following four growth traits in continuous planting generations were analyzed by plotting scatter plots of tree height, DBH, slenderness and oblateness by generation (shown in Figure 3). One-way ANOVA and LSD tests (Table 2) were conducted on tree height, DBH, slenderness, and oblateness for different generations to investigate the effects of continuous planting practices on the growth traits of Eucalyptus urophylla × E. grandis.
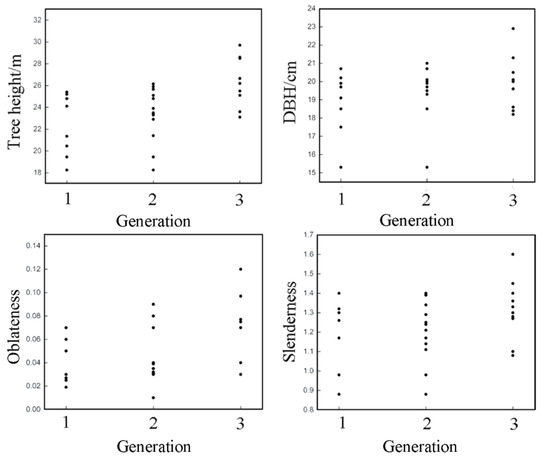
Figure 3.
Relationship between tree height/DBH/oblateness/slenderness and generation.

Table 2.
Relationship between tree height/DBH/oblateness/slenderness and generation.
As can be seen in Figure 3, in general tree height, DBH and oblateness, Eucalyptus urophylla × E. grandis increased over generations, while slenderness remained stable and increased later. As shown in Table 2, the significant p-values of tree height between forest I and forest II and between forest I and forest III were 0.380 and 0.003, respectively. The difference in tree height between forest I and forest III reached a significant level, but the p-values of slenderness of generations 1, 2, and 3 were all greater than 0.05, so it can be assumed that the reason for the difference in slenderness between generations was the change in DBH, which was less related to tree height. The ANOVA showed that the p-values for the oblateness of forest I and forest II and the oblateness of forest I and forest III were 0.489 and 0.035, respectively. The difference between the oblateness of forest I and forest III reached a significant level, and the mean difference between the oblateness of generations 1, 2 and 3 gradually increased.
3.2. Longitudinal Growth Strain on the Trunk Surface of Eucalyptus urophylla × E. grandis Trees in Different Generations of Continuous Planting
In China, some fast-growing timber tree species are planted continuously on the same forestlands, such as Chinese fir and Eucalyptus, which are representative species of successive monocropping that has gone on for multiple generations [33]. Table 3 presents the longitudinal growth strain and mean absolute values for the four directions of the sample trees in the southeast and northwest of the three generations. Overall, all strain values were negative, indicating that tensile strain was generated in the longitudinal direction on the surface of Eucalyptus spp. in all three generations. The ANOVA results showed that differences in growth strain between several generations of Eucalyptus urophylla × E. grandis were significant at the 0.001 level (Table 4), with the largest growth strain in forest III, followed by forest II and then forest I. The distribution of strain (Figure 4) illustrated the wide range of strain values and the gradual increase of growth strain with increasing generations. Forest I had a mean growth strain mainly concentrated in the range of 501 to 950 με, while forest II and forest III had a mean growth strain generally distributed in the range of 801 to 1100 με. Forest II and forest III had an increase in mean growth strain compared with forest I. The average growth strain of live trees in the three generations was 1377 με at the maximum and 616 με at the minimum.

Table 3.
Longitudinal growth strain on the surface of Eucalyptus urophylla × E. grandis in different continuous planting generations.

Table 4.
Analysis of variance (ANOVA) for growth strain of Eucalyptus plantations in several generations.
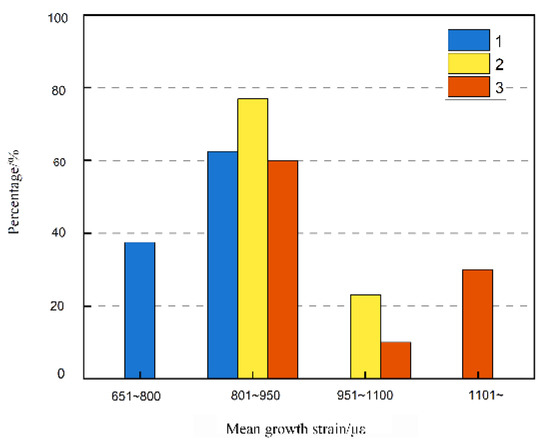
Figure 4.
Frequency distribution of mean longitudinal growth strain for each generation.
Analysis of variance (ANOVA) on the longitudinal growth strain in the circumferential relative position of Eucalyptus urophylla × E. grandis (Table 5) showed that the differences between several generations of continuous planting were not significant in the westward and southward directions, while the differences in longitudinal growth strain in the eastward and northward directions were significant at the 0.05 level and had high variability, indicating that several generations of continuous planting had a large effect on the growth strain in the circumferential relative position of Eucalyptus urophylla × E. grandis.

Table 5.
Analysis of variance (ANOVA) for growth strain of circumferential position of Eucalyptus urophylla × E. grandis over several generations.
3.3. Growth Traits and Longitudinal Growth Strain on the Trunk Surface of Eucalyptus urophylla × E. grandis
3.3.1. Effect of Growth Traits on the Longitudinal Growth Strain of the Trunk Surface of Eucalyptus urophylla × E. grandis
The independent variables affecting the longitudinal surface growth strain of the dependent variable can be divided into two categories. One contains qualitatively described variables such as circumferential relative position (relative position of test points in the circumferential direction, indicated by numbers from 1 to 4), which are collectively referred to as qualitative independent variables in this paper. The other contains quantitatively described growth traits such as tree height, DBH, oblateness, and slenderness. For such continuous independent variables, the visual segmentation function in SPSS 26 software was invoked before ANOVA, and three equal points were inserted into each group of data, i.e., each group of data was transformed into four ordinal values for ANOVA. The resulting independent variables are collectively referred to as quantitative independent variables in this paper. In order to reduce the influence of circumferential relative position on the ANOVA results of other variables, the main effects of all variables and the two-dimensional interaction effects between circumferential relative position and the remaining variables were included in the multi-factor ANOVA model, and the obtained results are shown in Table 3. To further analyze the effects of the respective variables on growth strain, a partial correlation analysis was used for circumferential relative positioning, tree height, and DBH along with ANOVA, and the results are shown in Table 6.

Table 6.
Multi-factor ANOVA (R2 = 0.971, corrected R2 = 0.853).
Table 6 showed that the multi-factor ANOVA model was significant at the 0.001 level. R2 is the coefficient of determination of the multiple linear regression equation with growth strain as the dependent variable, and its value indicates the model analyzed and how much of the total variation in the dependent variable was explained, i.e., the model specified in the multi-factor ANOVA explained 97.1% of the total variation. From the ANOVA analysis, it was concluded that the main effects of tree height, DBH, slenderness, binary interaction effect of circumferential relative position and tree height, DBH, slenderness, binary interaction effect of DBH and slenderness, and ternary interaction effect of circumferential relative position and DBH and slenderness had significant effects on growth strain at the 0.001 level. The main effect of circumferential relative position had significant effects on growth strain at the 0.05 level. The other effects on growth strain were not significant, and the effect of the main effect of oblateness on growth strain was not significant.
3.3.2. Relationship between Different Growth Traits and Longitudinal Growth Strain
Several qualitative descriptive variables were transformed into ordinal variables using the data transformation process in SPSS 26 software, e.g., different circumferential positions were directly transformed from marker numbers to ordinal numbers. In order to accurately illustrate the magnitude of the direct effect of an indicator on longitudinal surface growth strain and to obtain the order of magnitude of the direct effect of each indicator on the degree of longitudinal surface growth strain, this paper introduced partial correlation analysis to investigate the relationship between the respective variables in the morphology of Eucalyptus urophylla × E. grandis and growth strain, and the remaining variables were controlled when calculating the partial correlation coefficient between any of the independent variables and growth strain. The obtained results are shown in Table 7.

Table 7.
Results of partial correlation analysis.
As can be seen from Table 7, the partial correlation coefficients between all independent variables and growth strain were not significant, but the absolute values of the partial correlation coefficients may reflect the direct influence of the independent variables on growth strain, and can be used as a criterion to measure the relative magnitude of the influence of each variable on growth strain. The order of the influence of each independent variable on growth strain was: circumferential relative position > tree height > oblateness > slenderness > DBH. Figure 5 shows the scatter plot of growth traits and surface longitudinal growth strain for Eucalyptus tailed giants.
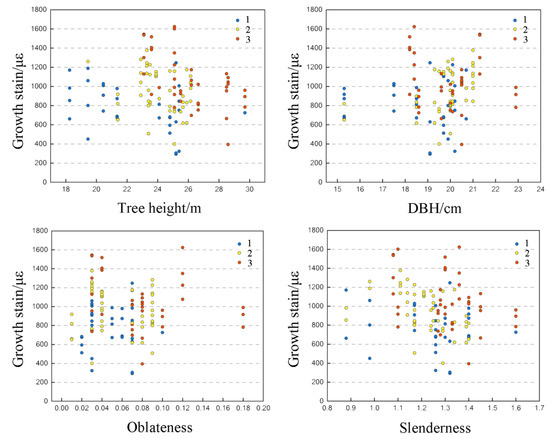
Figure 5.
Growth trait scatter plot and surface longitudinal growth strain.
4. Discussion
4.1. Analysis of Longitudinal Growth Strain on the Surface of Eucalyptus urophylla × E. grandis in Different Continuous Planting Generations
Different scholars have shown that although the trend of growth strain varies, there is significant variation in the level of growth strain among different species and different ages of the same species [25,34,35]. Growth strain can lead to problems such as brittle hearts in standing wood and log splitting. Hii et al. found that log end splitting was the largest source of wood loss, with up to 50% loss of effective log length, through debarking and sawing tests on Eucalyptus logs [36,37,38]. Xu conducted a study on Eucalyptus cloeziana, Eucalyptus dunnis, and Eucalyptus spp. [39]. The results relating to Eucalyptus dunnii and Eucalyptus globulus veneers showed that veneer splitting significantly reduces timber grade. Growth strain has become the main wood quality criterion for selecting good genetic material and assessing the economic value of logs [40]. Previous similar studies have shown that the growth strain of standing timber may be controlled by the genetic characteristics of the tree and that low growth strain levels may be considered an important indicator for species selection [23,41,42]. In the study in Section 2.2, it was found that the mean growth strain in generations 1, 2 and 3 was 789, 935, and 1033 με, respectively, and the difference in growth strain between different generations of Eucalyptus urophylla × E.grandis was significant at the 0.001 level, with the growth strain increasing significantly through generations. Therefore, the number of generations should be controlled.
The reason for the difference in growth strain between forests I, II and III of Eucalyptus spp. may be due to the management model of Eucalyptus plantations. The harvesting and renewal of continuous plantations of Eucalyptus spp. is often performed by coppicing, which means that the harvested Eucalyptus spp. retains the stump [43], so that forests II and III have intact and mature root systems that facilitate nutrient uptake and water transfer, grow actively, have fast growth rates, and have greater average growth strain than forest I.
In different generations of Eucalyptus urophylla × E. grandis, longitudinal growth strain in the east and north directions differed significantly at the 0.05 level. The reasons for the large differences in the east and north directions of sample trees in different generations may be: (1) trees, as organisms, have their own variation that causes this phenomenon; (2) forests II and III sprouts grow from the root base, and as trees respond to environmental factors (light, wind, and slope) and forestry factors (planting density, interplanting, pruning), growth strain gradually accumulates; or (3) forests II and III may have formed cells characteristic of tension wood, i.e., a gelatinous layer, at sites of high strain eastward and northward, and the anatomical characteristics and chemical properties of these cells influence the magnitude of growth strain [44]. The accumulation of phenolic and allelopathic factors in soil may also account for rising growth stress in continuous planting [33].
4.2. Variation in Growth Traits of Eucalyptus Tailored in Different Continuous Planting Generations with Surface Longitudinal Growth Strain
Height and diameter are the most basic trunk factors. Slenderness is the ratio of height and diameter [45], and also reflects the trunk completeness, which directly affects the timber yield and economic value. Slenderness can also be used to determine the growth rate of standing trees, which can control the growth of height and diameter according to different management objectives in forestry work to achieve the best management effect. The oblateness value is also an important trunk morphological factor affecting the quality of forest trees [46,47].
The mean difference in oblateness between generations 1, 2, and 3 gradually decreased from −0.0011 to −0.035, and the oblateness of forests I and III was significant at the 0.05 level, indicating that the trunk became flatter as the generations of Eucalyptus urophylla × E. grandis increased, and the effect of flatness and circumferential relative position on growth strain was not significant. Forests II and III sprouted from forest I and each tree’s standing tree growth and the possibility of the formation of tension wood inside the trunk resulted in large differences in the longitudinal growth strain in the eastward and northward directions between several generations of Eucalyptus urophylla × E. grandis. The relative position of the circumferential direction on the growth strain was influenced by continuous planting.
It has been proposed that growth strain leading to wood splitting increases with increasing age and tree height and varies significantly [1]. As can be seen from Table 7, growth strain was negatively correlated with slenderness and circumferential position, with bias correlation coefficients of −0.031 and −0.146, respectively, and positively correlated with other parameters. Tree height and slenderness have an effect on the longitudinal surface growth strain of standing trees, which can be used for early assessment of growth strain and as a reference standard for growth strain regulation. Increasing the roundness and straightness of standing trees by selecting species and improving cultivation practices can reduce growth strain to some extent, which is similar to the conclusion reached by Chafe in his study of 6-year-old bright-fruited eucalyptus [48]. Eucalyptus Leizhou No. 1 and Eucalyptus citriodora live standing growth strain was correlated with DBH by Hu et al., the former being positively correlated and the latter being negatively correlated [49]. Lv et al. measured five Eucalyptus plantation species in southern China, including Eucalyptus urophylla × E. grandis, Eucalyptus grandis, and Eucalyptus gardenis, and found that Eucalyptus urophylla × E. grandis had the highest growth strain level [50], suggesting that this result may be related to its faster DBH growth rate. Fei, B.H. et al. also studied the relationship between growth strain and DBH in plantation, and concluded that the relationship between growth strain and DBH varied greatly depending on the species source and age of Eucalyptus [28]. The above studies indicate that the relationship between growth strain and DBH is relatively complex and may be influenced by strain and growth conditions. Therefore, the relationship between growth strain and DBH needs to be further investigated.
For rapid vertical growth, trees either require significant wood accumulation or pre-stressing of the external wood to provide rigidity and support to the tree, especially to counteract wind forces. For the same bending moment, larger-diameter trees exhibit less bending stress than smaller-diameter trees. Therefore, larger trees should have lower growth strain compared to those with smaller diameters. In this study, the R2 of the linear regression equation between each growth trait and growth strain reached 97.1%, but the correlation coefficients between individual growth traits and surface circumferential growth strain were not significant, indicating that growth strain is not significantly related to individual growth parameters, but rather to a combination of multiple factors with different tree heights, oblateness rates, and their differential effects, in line with Biechele et al. The same conclusions were proposed by Biechele et al. for the growth strain of different age stands at different tree heights [51].
From the wood volume model V = 0.65671 × 10−4 × D1.769412 × H1.069769, it is known that: tree height and DBH are important factors affecting wood volume; tree wood volume is positively correlated with tree height; oblateness is one of the important factors of tree stem shape [52], which affects wood yield; and oblateness increases with the increase of generations, indicating that although continuous planting can increase wood volume and greatly improve wood yield, it affects the dryness of trees and reduces the wood yield. Growth strain affects the structure of the tree, causing problems such as brittle heart and wood collapse, and the performance of the wood is seriously affected, which greatly reduces the added value of using Eucalyptus solid wood.
5. Conclusions
- (1)
- Tree height, DBH, and oblateness increased with successive generations of continuous planting, while slenderness remained initially stable and then increased.
- (2)
- Generations of continuous planting had a significant impact on growth strain, with a noticeable increase in average growth strain as generations progressed.
- (3)
- None of the individual growth traits had a significant effect on growth strain. However, strong correlations were found between growth strain and tree height as well as oblateness, indicating their potential as indicators of surface longitudinal growth strain.
- (4)
- Over generations, trees underwent changes in shape, including tree height and oblateness, to maintain morphological balance and withstand external factors. This led to an imbalance in growth strain within the trees, resulting in increased overall growth strain. Based on the findings, it is recommended to opt for trees with consistent growth rates and compact dwarf varieties instead of establishing permanent Eucalyptus plantations. Furthermore, it is advisable to maintain these plantations for a maximum of three generations.
Author Contributions
Conceptualization, T.W., Y.F. and P.W.; methodology, T.W.; software, T.W.; validation, T.W. and P.W.; formal analysis, T.W.; investigation, T.W., Q.D. and P.W.; resources, P.W.; data curation, T.W.; writing—original draft preparation, T.W.; writing—review and editing, T.W. and P.W.; visualization, T.W.; supervision, Q.D., Y.F. and P.W.; project administration, T.W., Q.D. and P.W.; funding acquisition, P.W. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangxi Science and Technology Base and Talent Special Project [2022]AC12010, Guangxi Forestry Science and Technology Project: Guilin Scientific Research [2021] No. 20.
Data Availability Statement
Not applicable.
Acknowledgments
The Leika Sub-Field and Guangxi Dongmen Forestry’s assistance is applauded by the authors. Additionally, we would like to express our gratitude to Beijing Terra Venus Instruments Co., Ltd., Huajing Testing 419 (Taizhou) Co., Ltd., and AVIC Electro-Mechanical Instruments Co. for their intellectual assistance.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Tererai, F.; Gaertner, M.; Jacobs, S.M.; Richardson, D.M. Eucalyptus invasions in riparian forests: Effects on native vegetation community diversity, stand structure and composition. For. Ecol. Manag. 2013, 297, 84–93. [Google Scholar] [CrossRef]
- Kong, J.J.; Liu, W.Q.; Huang, F.; Hua, L.; Yu, F.; He, Q.; Su, Y.; Li, J.Y.; Qiu, Q. Spatial Patterns of Non-Structural Carbohydrates in Eucalyptus urophylla × E. grandis under Dry-Season Irrigation with Fertilization. Forests 2021, 12, 1049. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Chen, F.; Li, C.; Wu, L. Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land Degrad. Dev. 2019, 30, 636–646. [Google Scholar] [CrossRef]
- Sembiring, N.; Napitupulu, H.L.; Sembiring, M.T.; Sipahutar, A.I.; Tarigan, C.A. Eucalyptus plantation and its integrated supply chain in pulp and paper mill. IOP Conf. Ser. Earth Environ. Sci. 2021, 912, 12–96. [Google Scholar] [CrossRef]
- Kubler, H. Growth stresses in trees and related wood properties. For. Prod. Abstr. 1987, 10, 74–107. [Google Scholar]
- Nicholson, J.E.; Hillis, W.E.; Ditchburne, N. Some Tree Growth—Wood Property Relationships of Eucalypts. Can. J. For. Res. 1975, 5, 424–432. [Google Scholar] [CrossRef]
- Ferrand, J.C. Growth stresses and silvicuture of Eucalypts. Aust. For. Res. 1983, 13, 75–81. [Google Scholar]
- Wilkins, A.P.; Kitahara, R. Relationship between growth strain and rate of growth in 22 year-old Eucalyptus grandis. Austral. For. 1991, 54, 95–98. [Google Scholar] [CrossRef]
- Arganbright, D.G.; Bensend, D.W.; Manwiller, F.G. Influence of gelatinous fibers on the shrinkage of Silver Maple. Wood Sci. 1970, 3, 83–89. [Google Scholar]
- Yoshida, M.; Hosoo, Y.; Okuyama, T. Periodicity as a factor in the generation of isotropic compressive growth stress between microfibrils in cell wall formation during a twenty-four hour period. Holzforschung 2000, 54, 469–473. [Google Scholar] [CrossRef]
- Benoit, J.; Riboux, A. Comparison of basic density and longitudinal shrinkage in tension wood and opposite wood in young stems of Populus euramericana cv. Ghoy when subjected to a gravitational stimulus. Can. J. For. Res. 2001, 31, 1676–1683. [Google Scholar] [CrossRef]
- Washusen, R.; Ilic, J.; Waugh, G. The relationship between longitudinal growth strain and the occurrence of gelatinous fibers in 10 and 11-year-old Eucalyptus globulus Labill. Eur. J. Wood Wood Prod. 2003, 61, 299–303. [Google Scholar] [CrossRef]
- Murphy, T.N.; Henson, M.; Vanclay, J.K. Growth stress in Eucalyptus dunnii. Austral. For. 2005, 68, 144–149. [Google Scholar] [CrossRef]
- Yang, J.L. The impact of log-end splits and spring on sawn recovery of 32-year-old plantation Eucalyptus globulus Labill. Holz. Roh.-Werkst. 2005, 63, 442–448. [Google Scholar] [CrossRef]
- Malan, F.S. Clonal differences in log end splitting in Eucalyptus grandis in relation to age, parent performance, growth rate and wood density in two even-aged trials in Mpumalanga, South Africa. South For.-J. For. Sci. 2008, 70, 37–43. [Google Scholar] [CrossRef]
- Thomas, D.S.; Henson, M.; Joe, B.; Boyton, S.; Dickson, R.L. Review of growth and wood quality of plantation-grown Eucalyptus dunnii Maiden. Austral. For. 2009, 72, 3–11. [Google Scholar] [CrossRef]
- Hernández, M.; Zaderenko, C.; Monteoliva, S. Growth stress effects on yield and quality of Eucalyptus dunnii timber. Maderas Cienc. Tecnol. 2014, 16, 403–412. [Google Scholar]
- Japarudin, Y.; Lapammu, M.; Alwi, A.; Chiu, K.-C.; Ghaffariyan, M.; Brown, M.; Meder, R. Veneering and sawing performance of plantation-grown Eucalyptus pellita, aged 7–23 years, in Borneo Malaysia. Int. Wood Prod. J. 2021, 12, 116–127. [Google Scholar] [CrossRef]
- Zohar, Y.; Gafni, A.; Morris, J.D.; Shalhevet, S. Eucalyptus plantations in Israel: An assessment of economic and environmental viability. New For. 2008, 36, 135–157. [Google Scholar] [CrossRef]
- Harwood, C.E.; Nambiar, E. Productivity of Acacia and Eucalypt Plantations in Southeast Asia. 2. Trends and Variations. Int. For. Rev. 2014, 16, 249–260. [Google Scholar] [CrossRef]
- Raymond, C.A.; Kube, P.D.; Pinkard, L.; Savage, L.; Bradley, A.D. Evaluation of non-destructive methods of measuring growth stress in Eucalyptus globulus: Relationships between strain, wood properties and stress. For. Ecol. Manag. 2004, 190, 187–200. [Google Scholar] [CrossRef]
- Wu, J.B.; Liang, J.Y.; Chen, M.Y.; Zheng, S.Q.; Xu, J.Y. Study on the Structural Characteristics and Physical and Mechanical Properties of Phoebe bournei Thinning Wood. J. Renew. Mater. 2022, 10, 3025–3039. [Google Scholar] [CrossRef]
- Boyd, J.D. The Key Factor in Growth Stress Generation in Trees Lignification or Crystallisation. IAWA J. 1985, 6, 139–150. [Google Scholar] [CrossRef]
- Malan, F.S. The wood quality of the South African timber resource for high-value solid wood products and its role in sustainable forestry. South For.-J. For. Sci. 2003, 198, 53–62. [Google Scholar] [CrossRef]
- Yang, J.L.; Fife, D.; Matheson, A.C. Growth strain in three provenances of plantation-grown Eucalyptus globulus Labill. Austral. For. 2001, 64, 248–256. [Google Scholar] [CrossRef]
- Yang, J.L.; Waugh, G. Growth stress, its measurement and effects. Austral. For. 2001, 64, 127–135. [Google Scholar] [CrossRef]
- Nicholson, J.E. A rapid method for estimating longitudinal growth stresses in logs. Wood Sci. Technol. 1971, 5, 40–48. [Google Scholar] [CrossRef]
- Fei, B.H.; Jiang, Z.H.; Zhao, R.J.; Wang, X.M. Study on Growth Strain on Plantation Grown Eucalypt Trees. China Wood Ind. 2004, 18. (In Chinese) [Google Scholar]
- Valencia, J.C.; Harwood, C.; Washusen, R.; Morrow, A.; Wood, M.; Volker, P.W. Longitudinal growth strain as a log and wood quality predictor for plantation-grown Eucalyptus nitens sawlogs. Wood Sci. Technol. 2010, 45, 15–34. [Google Scholar] [CrossRef]
- Boyd, J.D. Tree Growth Stresses. V. Evidence of an Origin in Differentiation and Lignification. Wood Sci. Technol. 1972, 6, 251–262. [Google Scholar] [CrossRef]
- Bamber, R.K. The Origin of Growth Stresses: A Rebuttal. IAWA J. 1987, 8, 80–84. [Google Scholar] [CrossRef]
- Yang, J.L.; Bailleres, H.; Okuyama, T.; Muneri, A.; Downes, G. Measurement methods for longitudinal surface strain in trees: A review. Austral. For. 2005, 68, 34–43. [Google Scholar] [CrossRef]
- Ullah, S.; Xu, Y.; Liao, C.; Li, W.; Cheng, F.; Ye, S.; Yang, M. Continuous planting Eucalyptus plantations in subtropical China: Soil phenolic acid accumulation and adsorption physiognomies. Front. For. Glob. Change 2023, 6, 34. [Google Scholar] [CrossRef]
- Okuyama, T.; Yoshida, M.; Yamamoto, H. An estimation of the turgor pressure change as one of the factors of growth stress generation in cell walls: Diurnal change of tangential strain of inner bark. J. Jpn. Wood Res. Soc. 1995, 41, 1070–1078. [Google Scholar]
- Beltrame, R.; de Peres, M.L.; Lazarotto, M.; Gatto, D.A.; Schneid, E.; Haselein, C.R. Growth stress and its relationship with end splits in logs of Eucalyptus spp. Sci. For. 2015, 43, 63–74. [Google Scholar]
- Fang, C.; Guibal, D.; Clair, B.; Gril, J.; Liu, Y.; Liu, S. Relationships between growth stress and wood properties in poplar I-69 (Populus deltoides Bartr. cv. “Lux” ex I-69/55). Ann. For. Sci. 2011, 65, 307. [Google Scholar] [CrossRef]
- Hii, S.Y.; Ha, K.S.; Ngui, M.L.; Ak Penguang, S.; Duju, A.; Teng, X.Y.; Meder, R. Assessment of plantation-grown Eucalyptus pellita in Borneo, Malaysia for solid wood utilisation. Austral. For. 2017, 80, 26–33. [Google Scholar] [CrossRef]
- Davies, N.T. High Throughput Breeding for Wood Quality Improvement. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2019. [Google Scholar]
- Xu, Y.; Li, C.; Zhu, Y.; Wang, Z.; Zhu, W.; Wu, L.; Du, A. The shifts in soil microbial community and association network induced by successive planting of Eucalyptus plantations. For. Ecol. Manag. 2021, 505, 119877. [Google Scholar] [CrossRef]
- McGavin, R.L.; Bailleres, H.; Lane, F.; Fehrmann, J.; Ozarska, B. Veneer Grade Analysis of Early to Mid-rotation Plantation Eucalyptus Species in Australia. BioResources 2014, 9, 6562–6581. [Google Scholar] [CrossRef]
- Swain, T.L.; Gardner, R.A.; Chiappero, C. Final results of three ICFR Eucalyptus dunnii trials in KwaZulu-Natal, South Africa. In ICFR Bulletin Series; Institute for Commercial Forestry Research: Scottsville, South Africa, 2000. [Google Scholar]
- Espey, M.; Tahir, P.M.; Lee, S.H.; Muhammad Roseley, A.S.; Meder, R. Incidence and Severity of End-Splitting in Plantation-Grown Eucalyptus pellita F. Muell. in North Borneo. Forests 2021, 12, 266. [Google Scholar] [CrossRef]
- Pilate, G.; Chabbert, B.; Cathala, B.; Yoshinaga, A.; Leplé, J.-C.; Laurans, F.; Lapierre, C.; Ruel, K. Lignification and tension wood. Comptes Rendus Biol. 2004, 327, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Winarni, W.W.; Susilo, G.S.; Nugroho, A.A.; Safitri, F.R.; Irwan; Ratnaningrum, Y.W.N. Sprouting and rooting ability of the plus trees of Eucalyptus pellita, E. brassiana and its hybrid in Wanagama, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 914, 012051. [Google Scholar] [CrossRef]
- Liu, X.L.; Jiang, X.M.; Yin, Y.F. Surface longitudinal growth strain of plantation Eucalyptus urophylla × E. grandis. Beijing Linye Daxue Xuebao 2005, 27, 99. [Google Scholar]
- Nazari Sendi, M.R.; Navroodi, I.H.; Poorbabaei, H.; Sheikhkanlu Milan, M.; Bakhshandeh, B. Determination of lime tree (Tilia begonifolia Stev.) stems form based on quantitative parameters (Study area: Shafaroud forests of Guilan province, Iran). Folia For. Pol. 2014, 56, 165–170. [Google Scholar] [CrossRef]
- Nieto, V.; Charria, D.L.G.; Oviedo, M.B.S.; Borralho, N.M.G. Effects of provenance and genetic variation on the growth and stem formation of eucalyptus pellita in Colombia. J. Trop. For. Sci. 2016, 28, 227–237. [Google Scholar]
- Chafe, S.C. Variation in longitudinal growth stress with height in trees of Eucalyptus nitens Maiden. Aust. For. Res. 1985, 15, 51–55. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, X.; Hou, Z.; Yin, Y. A preliminary study on variance of longitudinal growth strains in tree trunk of three plantation eucalyptuses. China Wood Ind. 2000, 14, 9–11. [Google Scholar]
- Lv, J.X.; Ying, Y.F.; Zhao, Y.K.; Jiang, X.M. Growth strain evaluation in different species of eucalyptus plantation in south China. Beijing Linye Daxue Xuebao 2005, 27, 69–72. [Google Scholar]
- Biechele, T.; Nutto, L.; Becker, G. Growth Strain in Eucalyptus nitens at Different Stages of Development. Silva Fenn. 2009, 43, 669–679. [Google Scholar] [CrossRef]
- Müller, I.; Finger, C.A.G.; Schneider, P.R. Taper and assortment of wood for Eucalyptus grandis Hill ex Maiden, in the southeast region of Rio Grande Do Sul. Ciência Florest. 2005, 15, 293–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).