Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
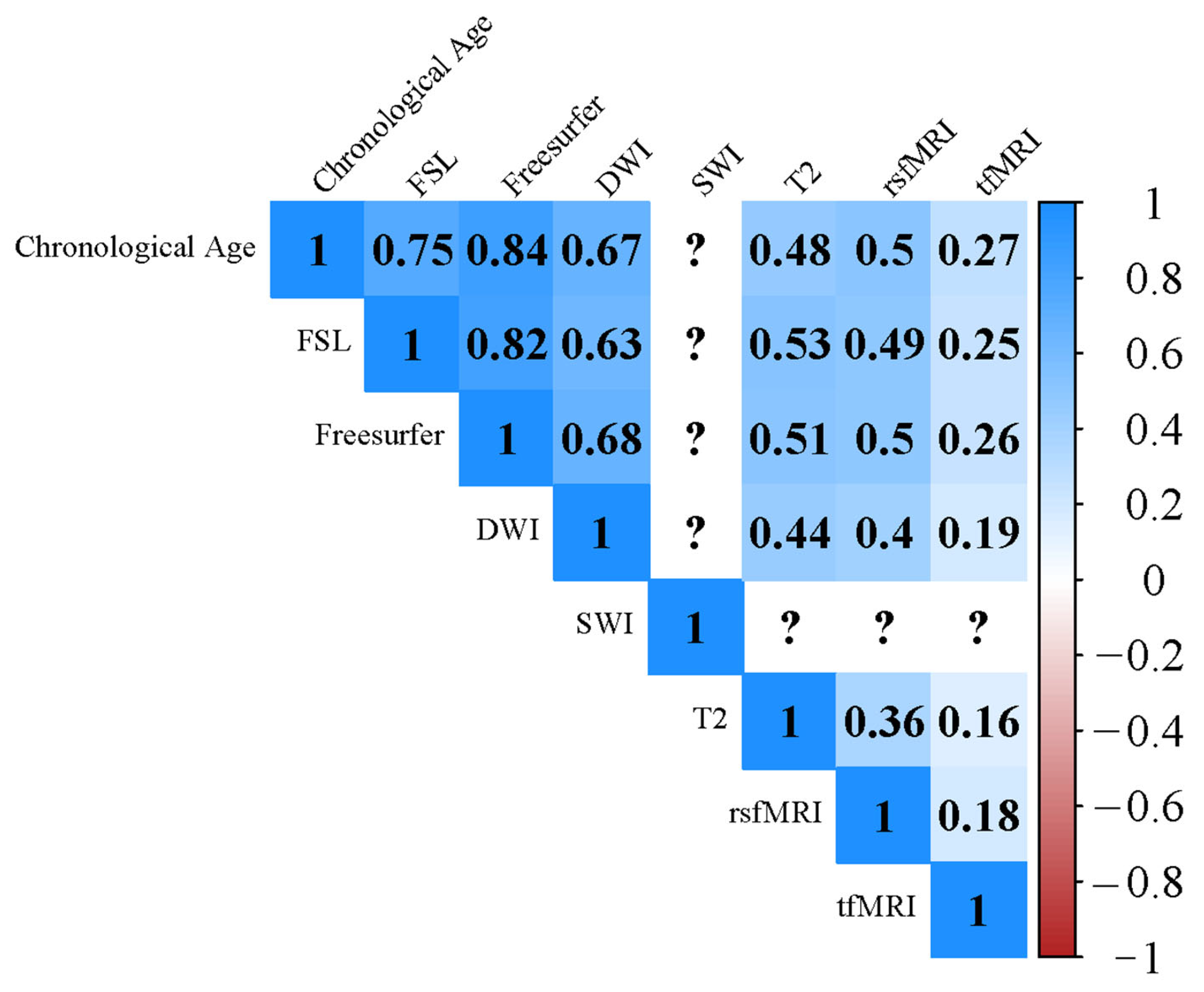
2.2. Imaging Derived Phenotypes (IDPs)
2.3. Neuropsychological Exam
2.4. Lifestyle and Physical Health Determinants
2.5. CR Proxy
2.6. Brain Age Prediction Model
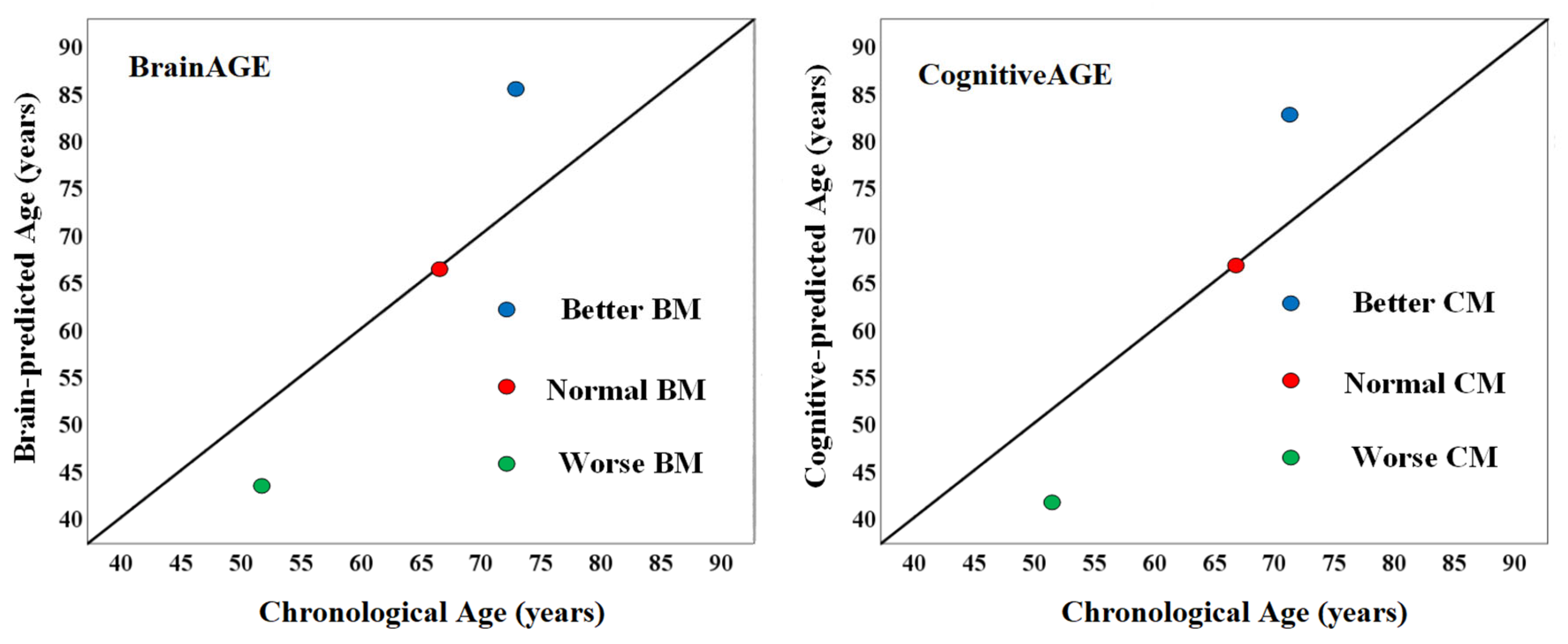
2.7. Quantifying BM and CM
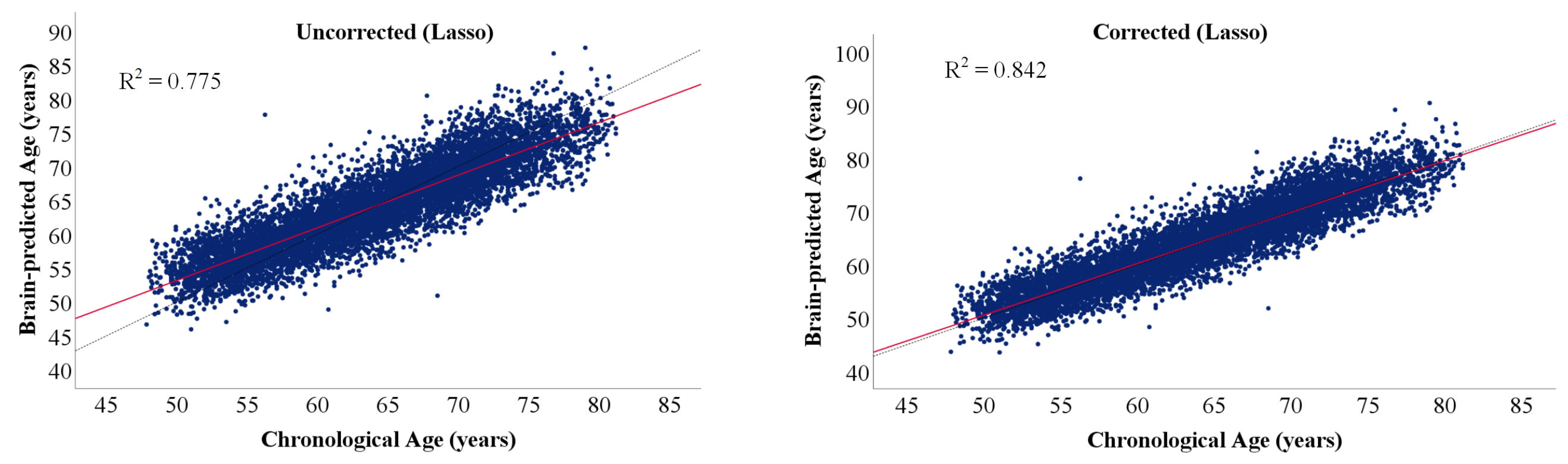
2.8. Bias Correction in Age Prediction
2.9. Statistical Analysis
3. Results
3.1. Brain Age and Cognitive Age Prediction
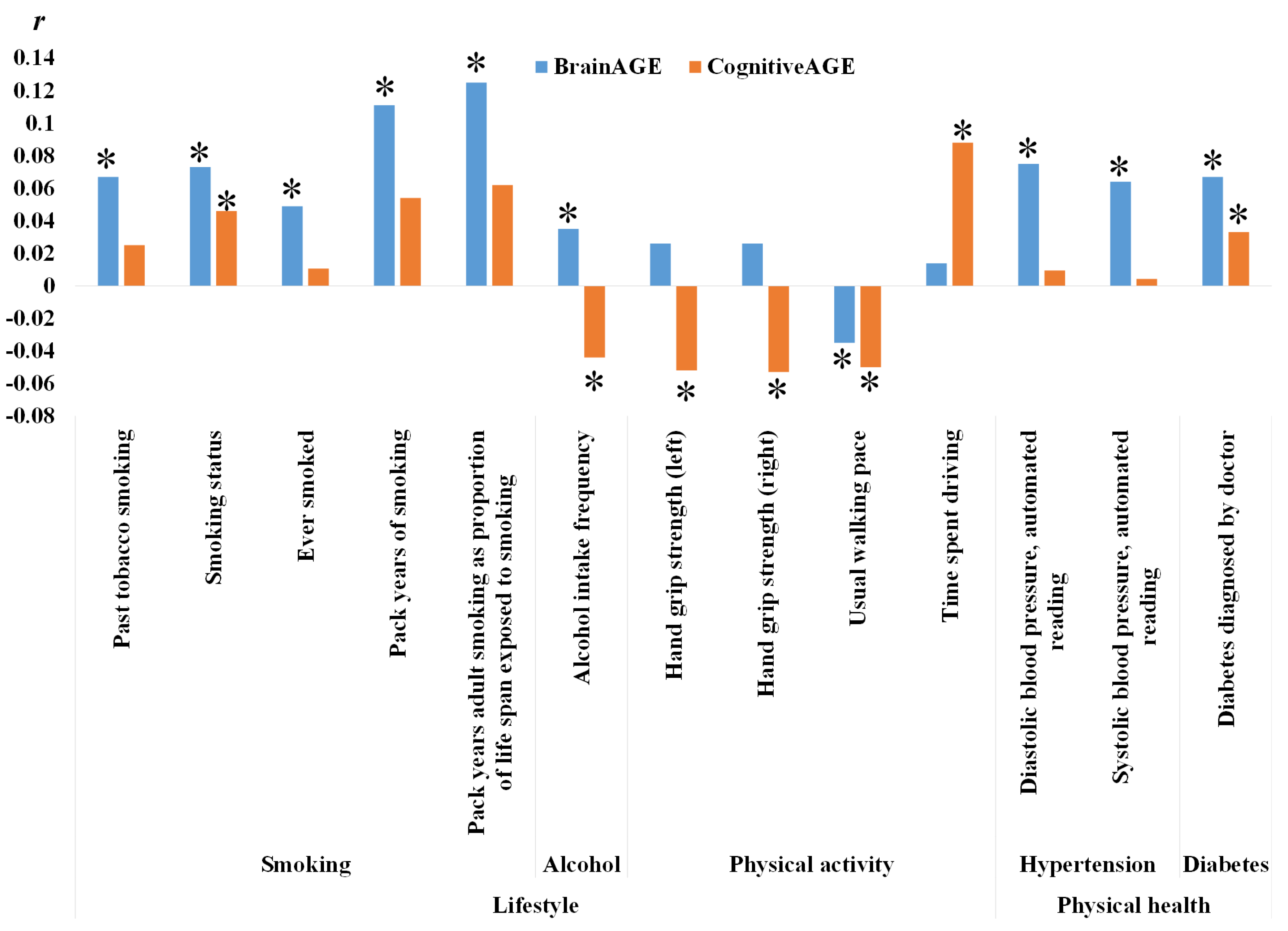
3.2. Relationship between BM, CM and Non-IDPs
3.3. Associations with CR Proxies
4. Discussion
4.1. Residual Approach
4.2. Favorable Lifestyles and Better Healthy Status Are Linked to Higher Maintenance
4.2.1. Smoking
4.2.2. Alcohol Consumption
4.2.3. Physical Activities
4.2.4. Healthy Status
4.3. The Relationship between BM, CM and CR
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Barnes, C.A.; Grady, C.; Jones, R.N.; Raz, N. Brain Reserve, Cognitive Reserve, Compensation, and Maintenance: Operationalization, Validity, and Mechanisms of Cognitive Resilience. Neurobiol. Aging 2019, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Borgeest, G.S.; Henson, R.N.; Shafto, M.; Samu, D.; Kievit, R.A. Greater Lifestyle Engagement Is Associated with Better Age-Adjusted Cognitive Abilities. PLoS ONE 2020, 15, e0230077. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Morris, D.W.; Donohoe, G. Cognitive Genomics: Recent Advances and Current Challenges. Curr. Psychiatry Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Nilsson, H.; Bülow, P.H.; Kazemi, A. Mindful Sustainable Aging: Advancing a Comprehensive Approach to the Challenges and Opportunities of Old Age. Eur. J. Psychol. 2015, 11, 494. [Google Scholar] [CrossRef]
- Katzman, R.; Terry, R.; DeTeresa, R.; Brown, T.; Davies, P.; Fuld, P.; Renbing, X.; Peck, A. Clinical, Pathological, and Neurochemical Changes in Dementia: A Subgroup with Preserved Mental Status and Numerous Neocortical Plaques. Ann. Neurol. 1988, 23, 138–144. [Google Scholar] [CrossRef]
- Satz, P. Brain Reserve Capacity on Symptom Onset after Brain Injury: A Formulation and Review of Evidence for Threshold Theory. Neuropsychology 1993, 7, 273–295. [Google Scholar] [CrossRef]
- Stern, Y. What Is Cognitive Reserve? Theory and Research Application of the Reserve Concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Stern, Y.; Barulli, D. Cognitive Reserve. Handb. Clin. Neurol. 2019, 167, 181–190. [Google Scholar] [CrossRef]
- Jia, F.; Li, Y.; Li, M.; Cao, F. Subjective Cognitive Decline, Cognitive Reserve Indicators, and the Incidence of Dementia. J. Am. Med. Dir. Assoc. 2021, 22, 1449–1455.e4. [Google Scholar] [CrossRef]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and Investigating Cognitive Reserve, Brain Reserve, and Brain Maintenance. Alzheimers Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Nyberg, L.; Lövdén, M.; Riklund, K.; Lindenberger, U.; Bäckman, L. Memory Aging and Brain Maintenance. Trends Cogn. Sci. 2012, 16, 292–305. [Google Scholar] [CrossRef]
- Kremen, W.S.; Elman, J.A.; Panizzon, M.S.; Eglit, G.M.L.; Sanderson-Cimino, M.; Williams, M.E.; Lyons, M.J.; Franz, C.E. Cognitive Reserve and Related Constructs: A Unified Framework Across Cognitive and Brain Dimensions of Aging. Front. Aging Neurosci. 2022, 14, 834765. [Google Scholar] [CrossRef]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, Reserve and Compensation: The Cognitive Neuroscience of Healthy Ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, Capacity, Compensation, Maintenance, Plasticity: Emerging Concepts in Cognitive Reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef]
- Anatürk, M.; Kaufmann, T.; Cole, J.H.; Suri, S.; Griffanti, L.; Zsoldos, E.; Filippini, N.; Singh-Manoux, A.; Kivimäki, M.; Westlye, L.T.; et al. Prediction of Brain Age and Cognitive Age: Quantifying Brain and Cognitive Maintenance in Aging. Hum. Brain Mapp. 2021, 42, 1626–1640. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Sun, S.; Wu, S. A Review of the Application of Three-Dimensional Convolutional Neural Networks for the Diagnosis of Alzheimer’s Disease Using Neuroimaging. Rev. Neurosci. 2023. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Trehearne, A. Genetics, Lifestyle and Environment. UK Biobank Is an Open Access Resource Following the Lives of 500,000 Participants to Improve the Health of Future Generations. Bundesgesundh. Gesundh. Gesundh. 2016, 59, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Alfaro-Almagro, F.; Bangerter, N.K.; Thomas, D.L.; Yacoub, E.; Xu, J.; Bartsch, A.J.; Jbabdi, S.; Sotiropoulos, S.N.; Andersson, J.L.R.; et al. Multimodal Population Brain Imaging in the UK Biobank Prospective Epidemiological Study. Nat. Neurosci. 2016, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.T.; Sharp, K.; Alfaro-Almagro, F.; Shi, S.; Miller, K.L.; Douaud, G.; Marchini, J.; Smith, S.M. Genome-Wide Association Studies of Brain Imaging Phenotypes in UK Biobank. Nature 2018, 562, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Fawns-Ritchie, C.; Deary, I.J. Reliability and Validity of the UK Biobank Cognitive Tests. PLoS ONE 2020, 15, e0231627. [Google Scholar] [CrossRef]
- Meng, X.; D’Arcy, C. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: A Systematic Review with Meta-Analyses and Qualitative Analyses. PLoS ONE 2012, 7, e38268. [Google Scholar] [CrossRef]
- Sun, X.; Nancekivell, S.; Gelman, S.A.; Shah, P. Perceptions of the Malleability of Fluid and Crystallized Intelligence. J. Exp. Psychol. Gen. 2021, 150, 815–827. [Google Scholar] [CrossRef]
- Park, S.; Choi, B.; Choi, C.; Kang, J.M.; Lee, J.-Y. Relationship between Education, Leisure Activities, and Cognitive Functions in Older Adults. Aging Ment. Health 2019, 23, 1651–1660. [Google Scholar] [CrossRef]
- Ihle, A.; Ghisletta, P.; Ballhausen, N.; Fagot, D.; Vallet, F.; Baeriswyl, M.; Sauter, J.; Oris, M.; Maurer, J.; Kliegel, M. The Role of Cognitive Reserve Accumulated in Midlife for the Relation between Chronic Diseases and Cognitive Decline in Old Age: A Longitudinal Follow-up across Six Years. Neuropsychologia 2018, 121, 37–46. [Google Scholar] [CrossRef]
- Baecker, L.; Garcia-Dias, R.; Vieira, S.; Scarpazza, C.; Mechelli, A. Machine Learning for Brain Age Prediction: Introduction to Methods and Clinical Applications. EBioMedicine 2021, 72, 103600. [Google Scholar] [CrossRef]
- Baecker, L.; Dafflon, J.; da Costa, P.F.; Garcia-Dias, R.; Vieira, S.; Scarpazza, C.; Calhoun, V.D.; Sato, J.R.; Mechelli, A.; Pinaya, W.H.L. Brain Age Prediction: A Comparison between Machine Learning Models Using Region- and Voxel-Based Morphometric Data. Hum. Brain Mapp. 2021, 42, 2332–2346. [Google Scholar] [CrossRef]
- More, S.; Antonopoulos, G.; Hoffstaedter, F.; Caspers, J.; Eickhoff, S.B.; Patil, K.R. Brain-Age Prediction: A Systematic Comparison of Machine Learning Workflows. NeuroImage 2023, 270, 119947. [Google Scholar] [CrossRef]
- Lombardi, A.; Diacono, D.; Amoroso, N.; Monaco, A.; Tavares, J.M.R.S.; Bellotti, R.; Tangaro, S. Explainable Deep Learning for Personalized Age Prediction With Brain Morphology. Front. Neurosci. 2021, 15, 674055. [Google Scholar] [CrossRef]
- Tanveer, M.; Ganaie, M.A.; Beheshti, I.; Goel, T.; Ahmad, N.; Lai, K.-T.; Huang, K.; Zhang, Y.-D.; Del Ser, J.; Lin, C.-T. Deep Learning for Brain Age Estimation: A Systematic Review. Infin. Fusion 2023, 96, 130–143. [Google Scholar] [CrossRef]
- Subramaniapillai, S.; Suri, S.; Barth, C.; Maximov, I.I.; Voldsbekk, I.; van der Meer, D.; Gurholt, T.P.; Beck, D.; Draganski, B.; Andreassen, O.A.; et al. Sex- and Age-specific Associations between Cardiometabolic Risk and White Matter Brain Age in the UK Biobank Cohort. Hum. Brain Mapp. 2022, 43, 3759–3774. [Google Scholar] [CrossRef]
- Jawinski, P.; Markett, S.; Drewelies, J.; Düzel, S.; Demuth, I.; Steinhagen-Thiessen, E.; Wagner, G.G.; Gerstorf, D.; Lindenberger, U.; Gaser, C.; et al. Linking Brain Age Gap to Mental and Physical Health in the Berlin Aging Study II. Front. Aging Neurosci. 2022, 14, 791222. [Google Scholar] [CrossRef]
- Jonsson, B.A.; Bjornsdottir, G.; Thorgeirsson, T.E.; Ellingsen, L.M.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Stefansson, K.; Ulfarsson, M.O. Brain Age Prediction Using Deep Learning Uncovers Associated Sequence Variants. Nat. Commun. 2019, 10, 5409. [Google Scholar] [CrossRef]
- Gong, W.; Beckmann, C.F.; Vedaldi, A.; Smith, S.M.; Peng, H. Optimising a Simple Fully Convolutional Network for Accurate Brain Age Prediction in the PAC 2019 Challenge. Front. Psychiatry 2021, 12, 627996. [Google Scholar] [CrossRef]
- Dinsdale, N.K.; Bluemke, E.; Smith, S.M.; Arya, Z.; Vidaurre, D.; Jenkinson, M.; Namburete, A.I.L. Learning Patterns of the Ageing Brain in MRI Using Deep Convolutional Networks. NeuroImage 2021, 224, 117401. [Google Scholar] [CrossRef]
- Xiong, M.; Lin, L.; Jin, Y.; Kang, W.; Wu, S.; Sun, S. Comparison of Machine Learning Models for Brain Age Prediction Using Six Imaging Modalities on Middle-Aged and Older Adults. Sensors 2023, 23, 3622. [Google Scholar] [CrossRef]
- Galton, F. Regression Towards Mediocrity in Hereditary Stature. J. Anthropol. Inst. Great Br. Irel. 1886, 15, 246–263. [Google Scholar] [CrossRef]
- Smith, S.M.; Vidaurre, D.; Alfaro-Almagro, F.; Nichols, T.E.; Miller, K.L. Estimation of Brain Age Delta from Brain Imaging. Neuroimage 2019, 200, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H. Multimodality Neuroimaging Brain-Age in UK Biobank: Relationship to Biomedical, Lifestyle, and Cognitive Factors. Neurobiol. Aging 2020, 92, 34–42. [Google Scholar] [CrossRef] [PubMed]
- De Lange, A.-M.G.; Cole, J.H. Commentary: Correction Procedures in Brain-Age Prediction. Neuroimage Clin. 2020, 26, 102229. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.-C.; Ho, Y.-S.; Wong, S.; Gentleman, S.M.; Ng, H.-K. Neuropathology of Cigarette Smoking. Acta Neuropathol. 2014, 127, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Elbaz, A.; Dugravot, A.; Head, J.; Shipley, M.; Hagger-Johnson, G.; Kivimaki, M.; Singh-Manoux, A. Impact of Smoking on Cognitive Decline in Early Old Age: The Whitehall II Cohort Study. Arch. Gen. Psychiatry 2012, 69, 627–635. [Google Scholar] [CrossRef]
- Campos, M.W.; Serebrisky, D.; Castaldelli-Maia, J.M. Smoking and Cognition. Curr. Drug Abuse Rev. 2016, 9, 76–79. [Google Scholar] [CrossRef]
- Gallinat, J.; Meisenzahl, E.; Jacobsen, L.K.; Kalus, P.; Bierbrauer, J.; Kienast, T.; Witthaus, H.; Leopold, K.; Seifert, F.; Schubert, F.; et al. Smoking and Structural Brain Deficits: A Volumetric MR Investigation. Eur. J. Neurosci. 2006, 24, 1744–1750. [Google Scholar] [CrossRef]
- Swan, G.E.; Lessov-Schlaggar, C.N. The Effects of Tobacco Smoke and Nicotine on Cognition and the Brain. Neuropsychol. Rev. 2007, 17, 259–273. [Google Scholar] [CrossRef]
- Ernst, M.; Heishman, S.J.; Spurgeon, L.; London, E.D. Smoking History and Nicotine Effects on Cognitive Performance. Neuropsychopharmacology 2001, 25, 313–319. [Google Scholar] [CrossRef]
- Zanchi, D.; Brody, A.L.; Montandon, M.-L.; Kopel, R.; Emmert, K.; Preti, M.G.; Van De Ville, D.; Haller, S. Cigarette Smoking Leads to Persistent and Dose-Dependent Alterations of Brain Activity and Connectivity in Anterior Insula and Anterior Cingulate. Addict. Biol. 2015, 20, 1033–1041. [Google Scholar] [CrossRef]
- Peng, P.; Li, M.; Liu, H.; Tian, Y.-R.; Chu, S.-L.; Van Halm-Lutterodt, N.; Jing, B.; Jiang, T. Brain Structure Alterations in Respect to Tobacco Consumption and Nicotine Dependence: A Comparative Voxel-Based Morphometry Study. Front. Neuroanat. 2018, 12, 43. [Google Scholar] [CrossRef]
- Woods, A.J.; Porges, E.C.; Bryant, V.E.; Seider, T.; Gongvatana, A.; Kahler, C.W.; de la Monte, S.; Monti, P.M.; Cohen, R.A. Current Heavy Alcohol Consumption Is Associated with Greater Cognitive Impairment in Older Adults. Alcohol. Clin. Exp. Res. 2016, 40, 2435–2444. [Google Scholar] [CrossRef]
- Yen, F.-S.; Wang, S.-I.; Lin, S.-Y.; Chao, Y.-H.; Wei, J.C.-C. The Impact of Heavy Alcohol Consumption on Cognitive Impairment in Young Old and Middle Old Persons. J. Transl. Med. 2022, 20, 155. [Google Scholar] [CrossRef]
- Rehm, J.; Hasan, O.S.M.; Black, S.E.; Shield, K.D.; Schwarzinger, M. Alcohol Use and Dementia: A Systematic Scoping Review. Alzheimers Res. Ther. 2019, 11, 1. [Google Scholar] [CrossRef]
- Ning, K.; Zhao, L.; Matloff, W.; Sun, F.; Toga, A.W. Association of Relative Brain Age with Tobacco Smoking, Alcohol Consumption, and Genetic Variants. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef]
- Angebrandt, A.; Abulseoud, O.A.; Kisner, M.; Diazgranados, N.; Momenan, R.; Yang, Y.; Stein, E.A.; Ross, T.J. Dose-Dependent Relationship between Social Drinking and Brain Aging. Neurobiol. Aging 2022, 111, 71–81. [Google Scholar] [CrossRef]
- Crome, I.; Crome, P. Moderate Alcohol Consumption in Older Adults Is Associated with Better Cognition and Well-Being than Abstinence. Age Ageing 2008, 37, 120–121. [Google Scholar] [CrossRef]
- Funk-White, M.; Moore, A.A.; McEvoy, L.K.; Bondi, M.W.; Bergstrom, J.; Kaufmann, C.N. Alcohol Use and Cognitive Performance: A Comparison between Greece and the United States. Aging Ment. Health 2022, 26, 2440–2446. [Google Scholar] [CrossRef]
- Piumatti, G.; Moore, S.; Berridge, D.; Sarkar, C.; Gallacher, J. The Relationship between Alcohol Use and Long-Term Cognitive Decline in Middle and Late Life: A Longitudinal Analysis Using UK Biobank. J. Public Health 2018, 40, 313–314. [Google Scholar] [CrossRef]
- Sacanella, E.; Vázquez-Agell, M.; Mena, M.P.; Antúnez, E.; Fernández-Solá, J.; Nicolás, J.M.; Lamuela-Raventós, R.M.; Ros, E.; Estruch, R. Down-Regulation of Adhesion Molecules and Other Inflammatory Biomarkers after Moderate Wine Consumption in Healthy Women: A Randomized Trial. Am. J. Clin. Nutr. 2007, 86, 1463–1469. [Google Scholar] [CrossRef]
- Bektas, A.; Sen, R.; Ferrucci, L. Does a Bit of Alcohol Turn off Inflammation and Improve Health? Age Ageing 2016, 45, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.R. Alcohol’s Effects on the Cardiovascular System. Alcohol Res. 2017, 38, 219–241. [Google Scholar] [PubMed]
- Dare, J.; Wilkinson, C.; Allsop, S.; Waters, S.; McHale, S. Social Engagement, Setting and Alcohol Use among a Sample of Older Australians. Health Soc. Care Community 2014, 22, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, S.; Chikhi, S.; Maltais, D. The Benefits of Physical Activities on Cognitive and Mental Health in Healthy and Pathological Aging. Geriatr. Psychol. Neuropsychiatr. Vieil. 2018, 16, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.; Zhang, Y.-R.; Chen, S.-D.; He, X.-Y.; Huang, S.-Y.; Wu, B.-S.; Deng, Y.-T.; Yang, L.; Ou, Y.-N.; Guo, Y.; et al. Associations of Grip Strength, Walking Pace, and the Risk of Incident Dementia: A Prospective Cohort Study of 340212 Participants. Alzheimers Dement. 2023, 19, 1415–1427. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Chen, F.-T.; Hopman, R.J.; Huang, C.-J.; Chu, C.-H.; Hillman, C.H.; Hung, T.-M.; Chang, Y.-K. The Effect of Exercise Training on Brain Structure and Function in Older Adults: A Systematic Review Based on Evidence from Randomized Control Trials. J. Clin. Med. 2020, 9, 914. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Iadecola, C.; Davisson, R.L. Hypertension and Cerebrovascular Dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C.; Manor, B.; Novak, V. Advanced BrainAGE in Older Adults with Type 2 Diabetes Mellitus. Front. Aging Neurosci. 2013, 5, 90. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C. Ten Years of BrainAGE as a Neuroimaging Biomarker of Brain Aging: What Insights Have We Gained? Front. Neurol. 2019, 10, 789. [Google Scholar] [CrossRef]
- Cherbuin, N.; Walsh, E.I.; Shaw, M.; Luders, E.; Anstey, K.J.; Sachdev, P.S.; Abhayaratna, W.P.; Gaser, C. Optimal Blood Pressure Keeps Our Brains Younger. Front. Aging Neurosci. 2021, 13, 694982. [Google Scholar] [CrossRef]
- Kaufman, A.S.; Kaufman, J.C.; Liu, X.; Johnson, C.K. How Do Educational Attainment and Gender Relate to Fluid Intelligence, Crystallized Intelligence, and Academic Skills at Ages 22-90 Years? Arch. Clin. Neuropsychol. 2009, 24, 153–163. [Google Scholar] [CrossRef]
- te Nijenhuis, J.; van Vianen, A.E.M.; van der Flier, H. Score Gains on G-Loaded Tests: No g. Intelligence 2007, 35, 283–300. [Google Scholar] [CrossRef]
- Tikhomirova, T.; Malykh, A.; Malykh, S. Predicting Academic Achievement with Cognitive Abilities: Cross-Sectional Study across School Education. Behav. Sci. 2020, 10, 158. [Google Scholar] [CrossRef]
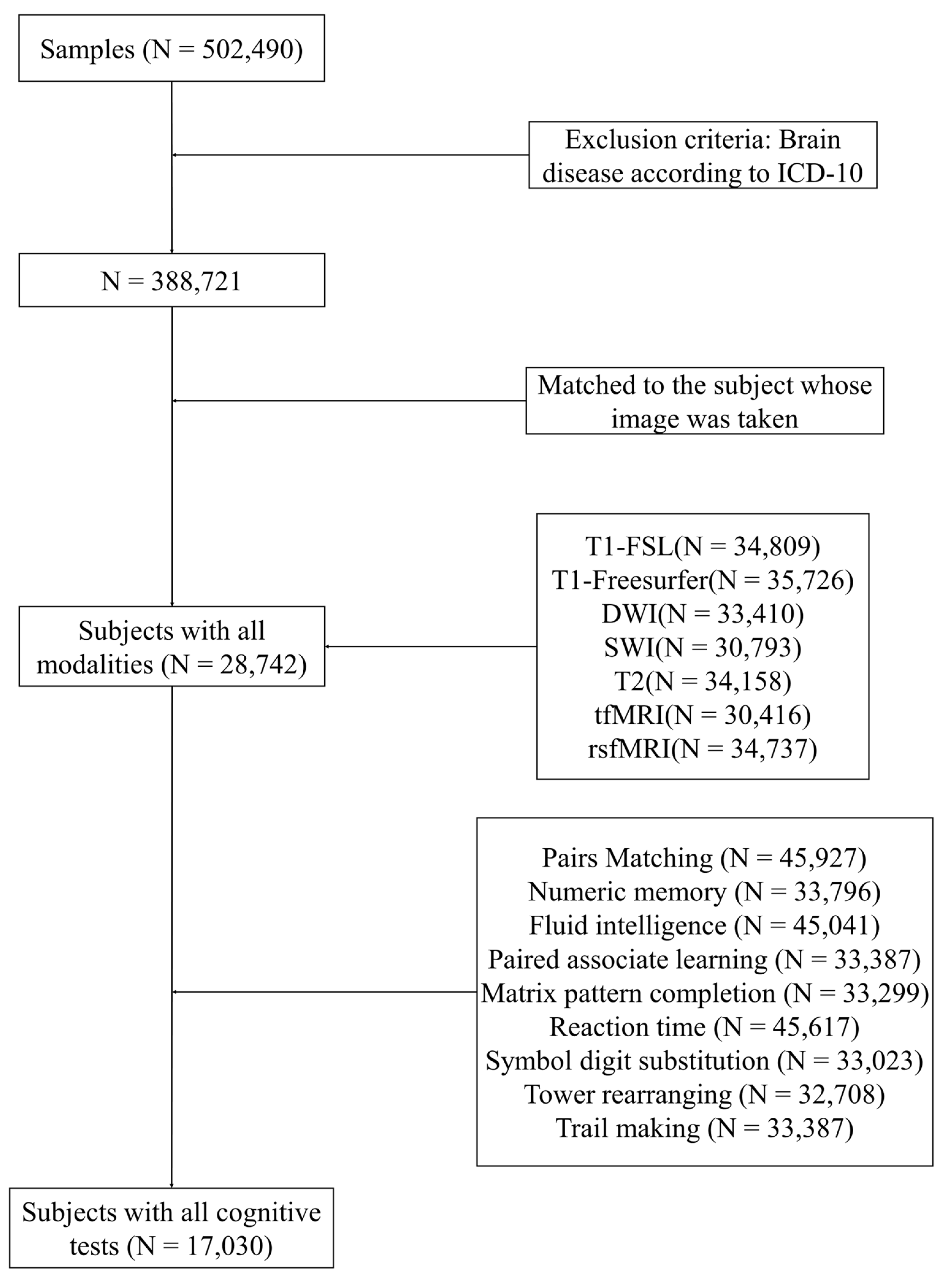

| Demographic Information | Training Set | Test Set | Total |
|---|---|---|---|
| Number of participants | 8587 | 8443 | 17,030 |
| Age (mean (±SD)) | 64.1 (±7.3) | 64.1 (±7.3) | 64.1 (±7.3) |
| Sex (Male/Female) | 4088/4499 | 3954/4489 | 8042/8988 |
| Modality | Description of IDPs | UKB ID | IDPs | |
|---|---|---|---|---|
| T1 | FSL | Anatomical measures of brain anatomy | 25000~25024, 25782~25920 | 164 |
| Freesurfer | 26501~27772 | 1272 | ||
| DWI | Quantification of micro-structural tissue properties and brain connectivity | 25056~25730 | 675 | |
| SWI | Assessment of venous vasculature, microbleeds, and micro-structural aspects | 25026~25039 | 14 | |
| T2 | Volumetric analysis of WM lesions | 25781 | 1 | |
| rsfMRI | Evaluation of inter-regional connectivity, regional spontaneous fluctuation amplitude | 25754~25755 | 76 | |
| tfMRI | The strength of response to the specific task within a given brain mask | 25040, 25042, 25044, 25046, 25048, 25050, 25052, 25054, 25761~25768 | 16 | |
| Total | / | / | 2218 | |
| Testing | UKB ID | Description | Cognitive Domain | Training Set | Test Set |
|---|---|---|---|---|---|
| Range/Mean (SD) | Range/Mean (SD) | ||||
| Pairs matching | 399 | Number of incorrect matches made in round | Visual declarative memory | −1.58~0.00/−0.59 (0.26) | −1.43~0.00/−0.59 (0.26) |
| Numeric memory | 4282 | Maximum number of digits remembered correctly | Working memory | 2~12/6.82 (1.26) | 2~12/6.83 (1.23) |
| Fluid intelligence | 20016 | Fluid intelligence score assessment | Verbal and numerical reasoning | 0~13/6.65 (2.02) | 0~13/6.65 (1.99) |
| Paired associate learning | 20197 | Number of correctly associated word pairs | Verbal declarative memory | 0~10/7.13 (2.48) | 0~10/7.15 (2.49) |
| Matrix pattern completion | 6373 | Number of correctly solved puzzles | Non-verbal reasoning | 0~15/8.10 (2.10) | 1~14/8.14 (2.06) |
| Reaction time | 20023 | Mean time taken to correctly identify matches | Processing speed | −3.14~−2.56/−2.77 (0.072) | −3.19~−2.54/−2.77 (0.073) |
| Symbol digit substitution | 23324 | Number of correct symbol digit matches made | Processing speed | 0~37/19.33 (5.17) | 0~36/19.37 (5.10) |
| Tower rearranging | 21004 | Number of correctly solved puzzles | Executive function | 0~18/10.06 (3.19) | 0~18/10.07 (3.18) |
| Trail making | 6350 | Duration to complete alphanumeric path | Executive function | −3.58~−2.20/−2.72 (0.15) | −3.62~−2.31/−2.71 (0.15) |
| Category | Subclass | UKB Field | UKB ID | Participants/N |
|---|---|---|---|---|
| Lifestyle | Smoking | Past tobacco smoking | 1249 | 8310 |
| Smoking status | 20116 | 8404 | ||
| Ever smoked | 20160 | 8404 | ||
| Pack years of smoking | 20161 | 1804 | ||
| Pack years adult smoking as proportion of life span exposed to smoking | 20162 | 1804 | ||
| Alcohol | Alcohol intake frequency | 1558 | 8425 | |
| Former alcohol drinker | 3731 | 453 | ||
| Alcohol drinker status | 20117 | 8425 | ||
| Physical activity | Hand grip strength (left) | 46 | 8103 | |
| Hand grip strength (right) | 47 | 8108 | ||
| Usual walking pace | 924 | 8423 | ||
| Time spent watching television | 1070 | 8415 | ||
| Time spent driving | 1090 | 8376 | ||
| Physical health | Hypertension | Diastolic blood pressure, automated reading | 4079 | 6505 |
| Systolic blood pressure, automated reading | 4080 | 6505 | ||
| diabetes | Diabetes diagnosed by doctor | 2443 | 8404 |
| UKB ID | CR Proxy | Participants/N | Mean (SD) | Range |
|---|---|---|---|---|
| 1031 | Social interactions | 8418 | 2.7 (1.1) | 1~7 |
| 6138 | Educational attainment | 8050 | 3.1 (1.1) | 1~4 |
| 6160 | Leisure activities | 6559 | 1.6 (0.7) | 1~4 |
| 20016 | Early fluid intelligence | 2723 | 6.9 (2.0) | 0~13 |
| MAE (Years) | |||||||
|---|---|---|---|---|---|---|---|
| T1 | DWI | SWI | T2 | rsfMRI | tfMRI | All Modality | |
| FSL | Freesurfer | ||||||
| 3.906 | 3.123 | 3.511 | 6.159 | 5.276 | 5.191 | 5.878 | 2.767 |
| UKB ID | CR Proxies | BrainAGE | CognitiveAGE | ||
|---|---|---|---|---|---|
| r | p | r | p | ||
| 1031 | Social activities | 0.016 | 0.154 | 0.007 | 0.519 |
| 6138 | Educational attainment | −0.026 | 0.021 | −0.096 | <0.001 |
| 6160 | Leisure activities | −0.030 | 0.014 | −0.045 | <0.001 |
| 20016 | Early fluid intelligence | −0.034 | 0.075 | −0.214 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Xiong, M.; Jin, Y.; Kang, W.; Wu, S.; Sun, S.; Fu, Z. Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank. Sustainability 2023, 15, 9620. https://doi.org/10.3390/su15129620
Lin L, Xiong M, Jin Y, Kang W, Wu S, Sun S, Fu Z. Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank. Sustainability. 2023; 15(12):9620. https://doi.org/10.3390/su15129620
Chicago/Turabian StyleLin, Lan, Min Xiong, Yue Jin, Wenjie Kang, Shuicai Wu, Shen Sun, and Zhenrong Fu. 2023. "Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank" Sustainability 15, no. 12: 9620. https://doi.org/10.3390/su15129620
APA StyleLin, L., Xiong, M., Jin, Y., Kang, W., Wu, S., Sun, S., & Fu, Z. (2023). Quantifying Brain and Cognitive Maintenance as Key Indicators for Sustainable Cognitive Aging: Insights from the UK Biobank. Sustainability, 15(12), 9620. https://doi.org/10.3390/su15129620






