Abstract
Over the last 14 years, ichthyological and ecological parameters have been monitored in the Labudovo okno Ramsar site. This area is important for its biodiversity as it is home to many rare and endangered plants and animal species. A total of 3861 fish specimens were sampled and measured at six sampling sites four times during the sampling period. An analysis of biodiversity indexes, relative biomass (kg/ha), and relative annual production (kg/ha) was carried out to assess the effectiveness of existing conservation measures. The results obtained show a trend decline in biodiversity, relative biomass, and relative annual production. This indicates a biodiversity conservation problem that should be addressed through other mechanisms in addition to the principles of the Ramsar Convention.
1. Introduction
Freshwater ecosystems undoubtedly offer a high value-added in the green transition process. They play an essential role in ecosystem services, mainly due to their socio-economic value, provided by ichthyofauna as an inevitable component of functional hydro-ecosystems [1,2,3]. Freshwater resources are rapidly declining in quantity and quality, putting them under pressure and in many cases becoming scarce for human and ecosystem use [4]. Freshwaters are under threat caused by increasing anthropogenic stress, such as nutrient enrichment, urbanization, industrial waste, deforestation, water abstraction, flood prevention engineering, sedimentation, dam construction, climate change, and the spread of invasive species [5]. Protected areas are the cornerstone of biodiversity conservation with the aim of preserving nature from anthropogenic threats [6]. The protection of important biodiversity areas is intended to be an effective measure to restore, conserve, and fulfill the objectives of the EU Oceans, Seas, and Waters mission [7]. One such area is the Special Nature Reserve (SNR) Deliblatska peščara (Eng. Deliblato Sands), located in north-eastern Serbia (44°53′01″ N 21°05′33″ E/44.88361° N 21.09250° E), covering a total area of 34,829.32 ha in the Danube River Basin (DRB) (Figure 1). Historically, the delta and liman systems include numerous interconnected bodies of water, both fresh and brackish, as well as wetlands. The diversity of biotopes created a wide range of ecological conditions and habitats that allowed for high biological productivity and a great diversity of flora and fauna [6]. The SNR Deliblato Sands represents Europe’s largest resort, consisting of vast sand layers with distinct forms of dune relief and characteristic sandstone, steppe, forest, and wetlands, and a unique mosaic of biotic communities. Labudovo okno, as an integral part of the SNR “Deliblato Sands”, was declared a Ramsar site in 2006 according to the Ramsar Convention (The Convention on Wetlands), which refers to the protection of wetlands of international importance, especially as habitats for wetland birds. This area is considered one of the most important nesting sites for wetland birds in Serbia. Along with the significant biodiversity of flora in this area, the biodiversity of fauna also stands out.
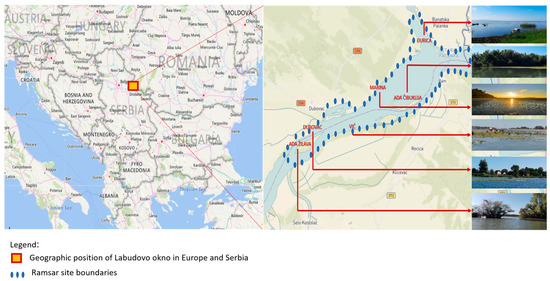
Figure 1.
Geographic position of Labudovo okno ((source: https://rsis.ramsar.org/, accessed on 26 April 2023), modified by authors).
A large number of water bird species nest on Labudovo okno, most of which are on the list of natural rarities or endangered species. An important characteristic of this area is the great variability among the ornitofauna; for example, the greater white-fronted goose and greylag goose, common goldeneye, white-tailed eagle and greater spotted eagle, glossy ibis and pygmy cormorant, and many other water bird species inhabit this Ramsar site. The area of Labudovo okno is an ideal spawning ground for fish species such as carp, pike, catfish, perch, and bream. The fauna of Labudovo okno also consists of various freshwater mollusks such as river clams and leeches, insects such as mosquitoes and water spiders, amphibians and reptiles such as the fire-bellied toad and the Balkan wall lizard, and mammals such as the otter and blind mole rat. From all of the above, it can be concluded that Labudovo okno as a Ramsar site has great biological and socio-economic importance, which implies the preservation of ecosystems whose members are closely connected and conditioned by mutual presence, primarily in terms of the food chain. Numerous and diverse flocks of water birds, numbering over several tens of thousands of individuals, gather every year on the waters of Labudovo okno. This is the most important feeding ground for numerous rare and endangered species. It also represents an important wintering place for water birds in Serbia and one of the most important in the Balkans [8].
The SNR “Deliblato Sands” is one of the most important centers of biodiversity in Europe, and its part Labudovo okno (Figure 1) has been designated as a Ramsar wetland (3733 ha) stretching along the left bank of the Danube River in the extreme south-east area of Vojvodina [9,10].
The purpose of this study arises from two facts: (a) there is a deficit of open scientific data dealing with the species diversity of ichthyofauna, its conservation status, and threats in the protected area Labudovo okno, and (b) Labudovo okno is inhabited by 47.98% of the fish species of the Serbian ichthyofauna [11]. The study provides valuable data for the following categories: (i) ichthyology (new valuable data freely available), (ii) social sphere (knowledge transfer, information provision, and awareness raising on the importance of Labudovo okno from the point of view of protection, use, and welfare), and (iii) policy sector (provision of valuable information needed for the future management of the said area).
2. Materials and Methods
2.1. Sampling Area
The locality Labudovo okno was created by raising the Danube level after the construction of the dam for the hydroelectric power plant “Đerdap I”. The entire Ramsar site was named “Labudovo okno” after this locality [10]. Geographically, this area is located at the south-eastern edge of the Pannonian Plain, in the south-eastern Banat (Figure 1). It stretches along the left bank of the Danube, which is the southern edge of the area known as Deliblatska peščara, between the 1094th and the 1075th rkm (river kilometer). The main types of aquatic biotopes found there are permanent river courses, permanent freshwater wetlands, and occasional flood zones with high groundwater levels under the influence of changes in the water level of the Danube. The area lies in the catchment area of the middle (Pannonian) Danube, which extends from Turn Severin (930 km) to Devinšvrata (1880 km) [10]. From a hydrological point of view, this part of the Danube basin is of great importance as it accounts for more than half of the Danube’s discharge and has the highest concentration of the river’s hydropower [12,13]. In general, the Danube enters the territory of the Republic of Serbia from Hungary at rkm 1433 and leaves it at rkm 845 at the mouth of the Timok River with a total run of 588 km [9].
Sampling localities description:
- (a)
- Site Marina: elevation 60–70 m, GPS 44°48′49″ N 21°16′51″ E. The Marina Channel is in a permanently flooded area on its left bank (1082.5–1078 R-km). The channel is 4.5 km long and about 100 m wide, most of which is at about 65 m above sea level. It is almost completely covered with submerged and floating macrophytes such as Potamogeton perfoliatus, Ceratophylum demersum (the dominant species), Trapa natans, etc. The substrate is muddy and exhibits coarse sedimentation. The trophic composition based on macrophytic vegetation can be described as eutrophic [8,14].
- (b)
- Site Ada Žilava: elevation about 65 m, GPS 44°46′34″ N 21°12′04″ E. The length of the islet is about 2.5 km, and the width is about 1.3 km. The community is mesotrophic to eutrophic and dominated by macrophytes of the genera Potamogeton, Salvinia, and Naias. Cyperus glomeratus and Scirpus lacustris are abundant in the Ada Žilava itself [8,14].
- (c)
- Site Vič: elevation about 70 m, GPS 44°47′35″ N 21°14′55″ E. Geological basis is white, yellow, brown, grey, or black carbonate sand of eolian origin, slightly alkaline reaction. The course of the Danube in this area has the characteristics of a typical potamon. Biocoenosis is eutrophic and dominated by floating and submerged plants [8,14].
- (d)
- Site Đurica: elevation 65 m, GPS 44°50′42″ N 21°18′14″ E. It is characterized by a large area of emergent vegetation (23% of the heath), consisting of typical groups of marsh plants (helophytes). Among the macrophytes, broadleaf cattails (Typha latifolia), reeds (Phragmites australis), and sweet flags (Acorus calamus) dominate on the coast, and Ceratophylidae and Parvopotamidae in the water area. The trophic composition based on macrophytic vegetation can be described as eutrophic [8,14].
- (e)
- Site Ada Čibuklija: elevation ca. 65 m, GPS 44°48′29″ N 21°18′11″ E. It is a floodplain of the Danube near Banatska Palanka. This wetland is elongated, about 4 km long. It is a highly eutrophic community with a high proportion of emergent and submerged macrophytic vegetation consisting of Potamogeton spp., Ceratophylum demersum, C. submersum, and Trapa. natans, Azolla filicauloides, and Lemna sp. [8,14].
- (f)
- Site Dubovac: elevation about 70 m, GPS 44°47′23″ N 21°12′43″ E. The substrate is sandy, fine-grained, and silty, with humus and clay. The macrophyte community structure includes submerged species (Potamogeton crispus, Potamogeton perfoliatus) and floating plants dominated by Trapa natans, Nymphaea alba, and Nuphar luteum. The trophic composition based on macrophytic vegetation can be described as mesotrophic and eutrophic [8,14].
2.2. Sampling, Data Collection and Data Analysis
Between August 2008 and October 2022, 3861 fish were collected using multi-mesh nets with a mesh size of 10–60 mm and electrofishing (HONDA 1.2 kW, 6 A) at the six sampling localities of the Labudovo okno Ramsar site shown in Figure 1. The fish were taxonomically identified according to the available literature [15,16], measured and the parameters of relative biomass (kg/ha) and relative annual production (kg/ha) were calculated. Relative biomass and relative annual production (kg/ha) were calculated based on the total fish samples collected during the study period on the one hand and on the effort of Ricker [17] on the other. Relative annual production was assessed according to the methodology of Huet [18]. Other statistical calculations (one-way ANOVA and chi-square test) were performed using Statistica 12 software.
The analysis of the qualitative and quantitative composition of the ichthyofauna was used to calculate Simpson index, Shannon’s index α-diversity, and Menhinik index according to Biological Diversity Frontiers in Measurement and Assessment [19]. The distribution of biodiversity indexes per localities and investigation period was performed using MANOVA test and chi-square test in statistical software Statistica 12.
3. Results
In the total fish sample collected from 2008 to 2022, 3861 fish individuals belonging to 35 fish species from 13 families were sampled and taxonomically determined. Moreover, the previous reports on fish biodiversity status in research were consulted to identify the overall biodiversity reported in this area (Table 1) [11,16].

Table 1.
Community composition and taxa status after international and national legislation (+ sign represents allochthonous species) [20,21,22].
Most of the sampled species (35) are classified into the family Leuciscidae (10) followed by the family Gobiidae (7), Percidae (4), Cyprinidae (4), Xenocyprinidae (2), Acipenseridae (1), Tincidae (1), Gobionidae (1), Acheilognathidae (1), Centrarchidae (1), Siluridae (1), Ictaluridae (1), and Esocidae (1) (Table 2, Figure 2).

Table 2.
Results of variables analyses.
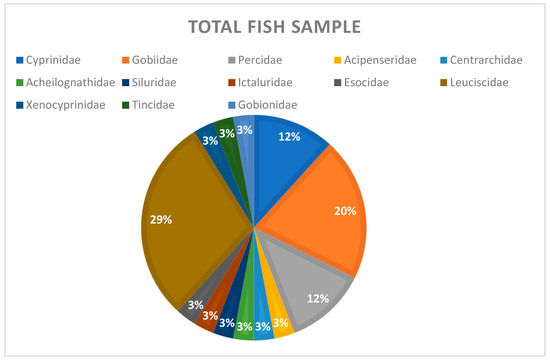
Figure 2.
The percentual share of fish families in the total fish sample.
The percentage of the identified fish families in the total sample is shown in Figure 2.
Besides taxonomic determination, the relative biomass and relative annual production were calculated as presented in Table 2.
When comparing relative biomass and relative annual production between the localities studied, no statistical significance was found using the ANOVA one way test (Figure 3, Wilkins lambda = 0.6157, F = 1.5696, p > 0.05). The test shows no significance in differences between the studied localities, but it is obvious that the highest values of relative biomass were measured at the Đurica and Vič localities. The relative annual production was evenly distributed among the localities. Moreover, relative biomass and relative annual production were evenly distributed over the entire study period (Wilkins lambda = 0.6157, F = 1.7378, p > 0.05), unfortunately with decreasing tendencies (Figure 3D).
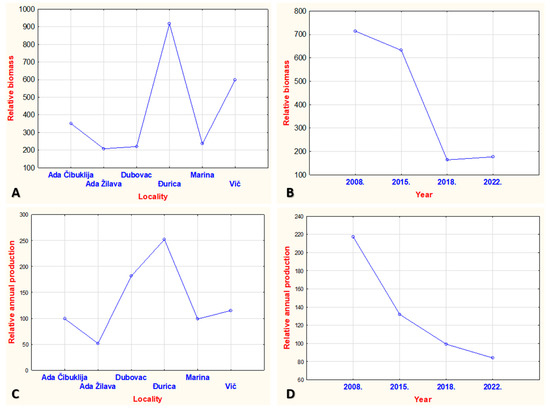
Figure 3.
(A) One-way ANOVA on investigated localities (statistical significance was not detected in distribution of relative biomass in investigated localities: F(5, 18) = 1.6447, p = 0.19907, but it is evident that localities Đurica and Vič have higher values of the relative biomass compared to others. Using chi-square test, the inequality in relative biomass distribution per investigated localities was confirmed: X2 = 3816.512, df = 5, p < 0.05). (B) One-way ANOVA on relative biomass in the period of investigation (statistical significance was not detected, taking into account period of investigation: F(3, 20) = 2.8401, p = 0.06388, but it is evident that in the year 2008 and 2015 higher values of relative biomass were detected. Using chi-square test, the inequality of the relative biomass distribution in investigation period was confirmed: X2 = 3635.791, df = 3, p < 0.05, so it is evident that the relative biomass had a tendency to decrease in the last 14 years). (C) One-way ANOVA on relative annual production per investigated localities (statistical significance was not detected in distribution of relative annual production in investigated localities: F(5, 18) = 1.1067, p = 0.39127, but it is evident that localities Dubovac and Đurica have higher values of relative annual production compared to others. Using chi-square, the inequality in distribution of relative annual production per localities was confirmed: X2 = 775.2887, df = 5, p < 0.05). (D) One-way ANOVA on relative annual production in period of investigation (statistical significance was not detected, taking into account period of investigation: F(3, 20) = 1.1360, p = 0.35852, but it is evident that in the year 2008 and 2015, higher values of relative annual production were detected. Using chi-square, the inequality in relative annual production distribution was confirmed: X2 = 485.8249, df = 3, p < 0.05, so it is possible to state that the relative biomass had a tendency to decrease in the last 14 years).
Statistical significance in the distribution of the biodiversity indexes was tested by the repeated ANOVA/MANOVA test and the X2 test. Both tests revealed no statistical significance in the above distribution (Wilkins Lambda = 0.33731, F = 1, 3446, p > 0.05; Figure 4A). The highest values of the Simpson, Shannon, and Menhinik indexes were obtained (see Table 3).
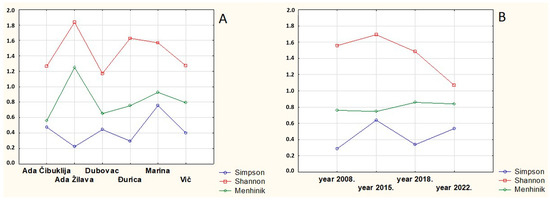
Figure 4.
ANOVA/MANOVA test on the biodiversity indexes per investigated localities (A) and ANOVA/MANOVA test on the biodiversity indexes during the investigation period (B).

Table 3.
Maximum, minimum, and average values of the biodiversity indices.
The fish community studied was also analyzed using selected biotic indices, and the values obtained are shown in Table 3.
During the study period, no statistical significance in differences was found in the biodiversity indexes of each sampling site (Wilkins Lambda = 0.42723, F = 1.9298, p > 0.05; Simpson: X2 = 0.1830718, df = 3, p > 0.05; Shannon: X2 = 0.1501606, df = 3, p > 0.05; Menhinik: X2 = 0.0117812, df = 3, p > 0.05; Figure 4B).
The contribution of predatory fish species (indicated in Table 2) in the total biomass was also calculated and presented in Figure 5.
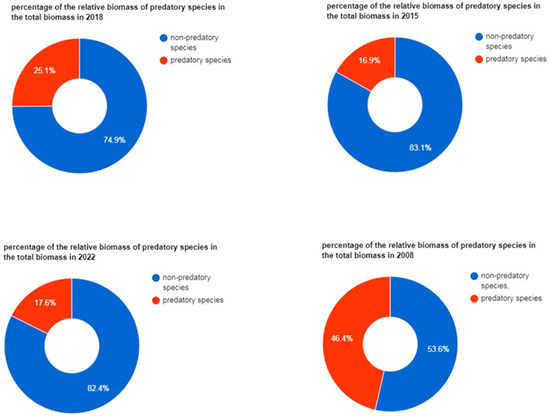
Figure 5.
Changes in contribution of predatory species in fish community over 14 years.
4. Discussion
Our results clearly show the declining trend of the two parameters of fisheries biology—relative biomass and relative annual production. Despite being an area declared a Ramsar site and protected since 2006, it has been evident over the last 14 years that biodiversity and natural production have shown a declining trend (Table 2; Figure 3 and Figure 4). In addition, habitat destruction became evident (Figure 6), which affected the decline in biomass and natural fish production and is a similar prediction for the future. Comparing the state of fish biodiversity in terms of relative biomass and relative annual production with other similar studies, we can see that many other protected areas and Ramsar sites have the same problem [23]. As Figure 1 shows, the locality Đurica is a specific, almost isolated water area that is not subject to direct anthropogenic influence. Sudden changes in the water regime are of low intensity in this locality, which could be the reason for higher values of relative biomass and relative annual production compared to the other five localities [24]. In addition, the terrain at this site is favorable for controlling illegal fishing, which is one of the factors contributing to the decline in biomass in the entire Ramsar site [25].

Figure 6.
The image of habitat destruction.
A comparison of the relative biomass and relative annual production of the studied sites (2008–2022) undoubtedly shows the trend of a decline in both ecological parameters. Encouraging factors are the fact that the relative biomass and relative annual production of alien and invasive species (Prussian carp Carassius gibbelio, black bullhead Ictalurus melas) are significantly lower than before (Table 2). Even though the values of relative biomass and relative annual production of these species varied from site to site and still vary now (depending on the habitat types the sites represent), it can be seen that the values for biomass and production of Prussian carp in the 2018 samples are between three times (Marina) and ten times (Đurica) lower than in those of 2008 (Table 2). A similar ratio can be observed for the brown bullhead [26].
On the other hand, a significant increase in the proportion of autochthonous species, especially common carp (Cyprinus carpio), Wels catfish (Silurus glanis), and both pike-perch species (Sander lucioperca and Sander volgensis), as well as other cyprinids, leuciscids, and perch-like species in the fish communities, can be observed (Table 2). We strongly believe that such a trend is due to the increased influence of competitors within the fish community, especially the Prussian carp, without any direct targeted influence on its population size. This could be the reason for the stimulation of Prussian carp production. In another case, the targeted capture of Prussian carp aimed at reducing its abundance and biomass would stimulate its production and the occurrence of its invasiveness [27]. This effect is known for pioneer and invasive species, especially if the habitats are suitable and do not change over time. Targeted capture of the Prussian carp would only favor clonal reproduction (gynogenesis), increase its production, and affect the existence of autochthonous species. Due to this effect, it is necessary to act in the same direction for at least a while longer by stocking common carp, whose increased production has a significant fishery effect in addition to the ecosystem effect, especially in commercial fisheries, which both commercial and recreational fishermen consider very positive [28].
Wetlands such as the Đurica pond, part of Marina, and Dubovac are ideal habitats for strictly protected species, the crucian carp Carassius carassius and the tench Tinca tinca, which are among the rarest fish species of the Danube ecosystem, not only because of the current limitation of their habitat distribution, but also because of the high abundances of allochthonous and, according to the characteristics of population biology, invasive species such as Prussian carp (Carassius gibelio) and bullhead, both brownhead and bullhead (Ameiurus nebulosus and Ameiurus melas) [29].
The changes in the ecosystem caused by the transformation of the river habitat (Figure 5) into water accumulations affect the relative biomass and relative annual production of carp as well as bream, but certainly also other fish species, especially native benthivores, in addition to crucian carp and tench. The introduction of non-native species such as Prussian carp, bullhead, Amur sleeper, gobies, etc. into a novel habitat undoubtedly leads to a change in fish community structure within the different trophic levels (planktivorous and benthivorous). This must have had an impact on natural production, as new, previously non-existent links in the food chains were created and this natural production was redistributed to new members of these communities [30].
In addition to the consequences for natural production resulting from trophic relationships, the advantages of the biological characteristics of individual non-native species in terms of reproduction should not be forgotten (gynogenesis in the Prussian carp, maintenance of the eggs and offspring of crucian carp, sunfish, and flounder, the absence of a free embryo and larval stage in the individual development of Pontus, variable morphology, the territoriality of the Amur sleeper, etc.) [30]. This leads to the community of these species having a highly invasive character, and it must affect the decrease in abundance or disappearance of steno-valent species, both Potamon and Rhytron, leading to dynamic instability of the population. In such disturbed communities within ecosystems that are themselves disturbed in their abiotic component, a decline in natural production is to be expected, especially for species that are less competitive in the new habitat type [31].
The second part of the study presents the calculation of biodiversity indexes comparing six studied sites during the monitoring period (Figure 4 and Figure 5). The figure shows a decreasing trend of the diversity index, which is a clear indicator of habitat degradation, mainly due to the increase of vegetation cover, silt deposition, and the change of oxygen regime in such sensitive habitats. In addition, overfishing of attractive species can lead to a decline in biodiversity and an increase in the proportion of non-native species in certain locations and generally throughout the area [32]. Climate change and land development are having a major impact on freshwater habitats by altering flow patterns and increasing overall water temperature, which may lead to a decline in fish species that require cold or cool and/or flowing water for part or all of their life cycle [33].
After long-term and thorough research, the conclusion is that Labudovo okno must be preserved as a Ramsar site for numerous reasons, especially for its great biological and socio-economic importance.
For example, fishing was and remains one of the main types of use of the area. In addition to commercial fishing, today within the limits of the protected natural resource of limited capacity, sport fishing is also present, with much greater possibilities than currently used. In the interest of development aligned with the needs of nature protection and the enhancement of ecological tourism, it is necessary to stimulate traditional fishing methods.
The basis of hunting, which is also present in the area of Labudovo okno, is the abundance of water ornitofauna, the use of which is limited by the protection measures of the SNR “Deliblato Sands”.
Agriculture in the area is based on cattle and sheep grazing, for which there are natural conditions and a need in terms of preserving specific natural values. In the future, the focus on the breeding of old, autochthonous breeds may represent the basis of healthy food production, as well as a tourist attraction. Forestry takes place mainly on river islands, limited by protection measures, and agricultural production is present only on small plots and mainly based on extensive cultivation of cereals.
Favorable opportunities for the development of tourism are offered by the Danube with the edge of Deliblato Sands (hunting and fishing, watching and photographing birds, observing the spring and autumn migration of water birds, nautical tourism). Investing in tourist equipment, along with adequate promotion, should contribute to classifying tourism as one of the basic ones in future development aligned with the protection of natural values.
Labudovo okno is also used for scientific research (flora and vegetation, ornithology and herpetofauna, water quality), as well as education for biology students.
Based on the attached results of long-term research, and the established high biodiversity, it is necessary to take all measures for the preservation and sustainable development of Swan’s shaft as one of the most important Ramsar sites, not only in Serbia, but also in the region.
There is a noticeable decrease in the percentage of the biomass of predatory species in relation to the total biomass (Figure 5), and decline of the diversity indexes is also noticeable (Figure 4). Managing eutrophication by restoring wetlands could be much more cost efficient if the wetlands were designed to both reduce nutrients and function as recruitment areas for predatory fish [34]
The reduction of fish biomass of predator species to low levels may compromise the sustainability of fishing and support only relatively low economic yields [35].
5. Conclusions
Protected areas are the cornerstone of biodiversity conservation with the aim of preserving nature from anthropogenic threats. Despite being an area declared a Ramsar site and protected since 2006, it has been evident over the last 14 years that the biodiversity and natural production in the area of Labudovo okno have shown a declining trend. However, other factors are of course also influencing the impoverishment of biodiversity, and these are certainly global warming, habitat destruction due to climate change, and human influence, especially in the form of overfishing. After long-term and thorough research, the conclusion is that Labudovo okno must be preserved as a Ramsar site for numerous reasons, especially for its great biological and socio-economic importance. In addition to the concept of protecting these areas, other mechanisms should also be applied to preserve biodiversity.
Author Contributions
Conceptualization, V.N. and Z.N.; methodology, V.N., Z.N., D.Š.J. and T.K.; investigation, V.N. and Z.N.; formal analysis, V.N., Z.N., A.M. and P.S.; visualization, V.N. and P.S.; writing—original draft preparation, V.N., Z.N. and T.K.; writing—review and editing, V.N., V.D. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (No. of contracts: 451-03-47/2023-01/200178 and No. 451-03-47/2023-01/200007).
Institutional Review Board Statement
There was no need for the approval from an Ethical Committee for this study, 344 because this species is used in commercial fishing. Moreover, authors had approval of the Ministry 345 of Environmental Protection and Environmental Protection Agency for conducting the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in these 341 published articles.
Acknowledgments
Work was granted by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (No. of contracts: 451-03-47/2023-01/200178 and No. 451-03-47/2023-01/200007).
Conflicts of Interest
The authors declare no conflict of interest.
References
- UN Water. Coping with Water Scarcity—Challenge of the Twenty-First Century; FAO: Rome, Italy, 2007. [Google Scholar]
- Grizzetti, B.; Lanzanova, D.; Liquete, C.; Reynaud, A.; Cardoso, A.C. Assessing water ecosystem services for water resource management. Environ. Sci. Policy 2016, 61, 194–203. [Google Scholar] [CrossRef]
- Simonović, P.; Povž, M.; Piria, M.; Treer, T.; Adrović, A.; Škrijelj, R.; Nikolić, V.; Simić, V. Ichthyofauna of the River Sava System. In The Sava River; Milačić, R., Ščančar, J., Paunović, M., Eds.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; Volume 31, pp. 361–400. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Cosgrove, W.J.; Rijsberman, F.R. World Water Vision: Making Water Everybody’s Business; Earthscan Publications Ltd.: London, UK, 2000. [Google Scholar]
- Gell, P.A.; Finlayson, C.M.; Davidson, N.C. Understanding change in the ecological character of Ramsar wetlands: Perspectives from a deeper time—Synthesis. Mar. Freshw. Res. 2016, 67, 869–879. [Google Scholar] [CrossRef]
- Brun, P.; Zimmermann, N.E.; Graham, C.H.; Lavergne, S.; Pellissier, L.; Münkemüller, T.; Thuiller, W. The productivity-biodiversity relationship varies across diversity dimensions. Nat. Commun. 2019, 10, 5691. [Google Scholar] [CrossRef] [PubMed]
- Puzović, S.; Đureković-Tešić, O.; Marić, B.; Stojanović, T.; Vig, L.; Stojnić, N.; Perić, R.; Ham, I.; Lazić, L.; Stojanović, V.; et al. Labudovo Okno Ramsar Site; Provincial Secretariat for Urban Planning and Environmental Protection, Autonomous Province of Vojvodina: Novi Sad, Serbia, 2014. (In Serbian)
- Puzović, S. Ramsar areas in Serbia [Yugoslavia] and their function in preservation of diversity of birds in watery habitats. Prot. Nat. 1998, 283–290. [Google Scholar]
- Stanković, V.; Kuzmanović, N.; Kabaš, E.; Vukojičić, S.; Lakušić, D.; Jovanović, S. Established stands of the highly invasive Echinocystis lobata on the Ramsar sites of the southern part of the Pannonian Plain. Bot. Serbica 2022, 46, 197–207. [Google Scholar] [CrossRef]
- Simonovic, P.; Nikolic, V. Freshwater fish of Serbia: Anannotated check list with some faunistic and zoogeo-graphic considerations. Bios Thessalon. 1997, 4, 137–156. [Google Scholar]
- Lenhardt, M.; Cakic, P.; Kolarevic, J. Influence of the HEPS Djerdap I and Djerdap II dam construction on catch of economically important fish species in the Danube River. Ecohydrol. Hydrobiol. 2004, 4, 499–502. [Google Scholar]
- Lenhardt, M.; Hegedis, A.; Cvijanovic, G.; Jaric, I.; Gacic, Z.; Mickovic, B. Non-native freshwater fishes in Serbia and their impacts to native fish species and ecosystems. Geophys. Res. Abstr. 2006, 8, 07727. [Google Scholar]
- Filipović, D.; Petrović, L. The Significance of the Danube Ecological Corridor in the Proceedings of Implementing Ecological Networks in Serbia Bulletin of the Serbian. Glas. Srp. Geogr. Društva 2015, 95, 109–124. [Google Scholar] [CrossRef]
- Fishbase. Available online: www.fishbase.se (accessed on 22 May 2023).
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germany, 2007; 646p. [Google Scholar]
- Ricker, W.E. Production, reproduction and yield. Veroff. Limnol. 1958, 13, 84–100. [Google Scholar]
- Huet, M.; Timmermans, J.A. Textbook of Fish Culture: Breeding and Cultivation of Fish, 2nd ed.; Fishing News Books Ltd.: Surrey, UK, 1970. [Google Scholar]
- Magurran, A.E.; McGill, B.J. Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2022. Available online: https://www.iucnredlist.org (accessed on 12 April 2023).
- Convention on the Conservation of European Wildlife and Natural Habitats (Bern, Switzerland), September 19, 1979, E.T.S. 104 [Hereinafter Bern Convention]; Council of Europe: Strasbourg, France, 1979.
- Fleurke, F.; Trouwborst, A. European Regional Approaches to the Transboundary Conservation of Biodiversity: The Bern Convention and the EU Birds and Habitats Directives. In Transboundary Governance of Biodiversity; Kotzé, L., Marauhn, T., Eds.; Brill Nijhoff: Leiden, The Netherlands, 2014; pp. 128–162. [Google Scholar]
- Lazar, L.; Rodino, S.; Pop, R.; Tiller, R.; D’Haese, N.; Viaene, P.; De Kok, J.-L. Sustainable Development Scenarios in the Danube Delta—A Pilot Methodology for Decision Makers. Water 2022, 14, 3484. [Google Scholar] [CrossRef]
- Simić, V.; Bănăduc, D.; Curtean-Bănăduc, A.; Petrović, A.; Veličković, T.; Stojkov ić-Piperac, M.; Simić, S. Assessment of the ecological sustainability of river basins based on the modified the ESHIPPO fish model on the example of the Velika Morava basin (Serbia, Central Balkans). Front. Environ. Sci. 2022, 10, 952692. [Google Scholar] [CrossRef]
- Simic, V.; Lujic, J.; Kostic, D.; Cirkovic, M.; Bjelic-Cabrilo, O.; Simic, S.; Markovic, G. Diversity characteristics of the fish species important for fishery in the waters of Serbia. Bulg. J. Agric. Sci. 2013, 19, 77–87. [Google Scholar]
- Mieczan, T.; Płaska, W.; Adamczuk, M.; Toporowska, M.; Bartkowska, A. Effects of the Invasive Fish Species Ameiurus nebulosus on Microbial Communities in Peat Pools. Water 2022, 14, 815. [Google Scholar] [CrossRef]
- Docherty, C.; Ruppert, J.L.W.; Rudolfsen, T.; Hamann, A.; Poesch, M.S. Assessing the spread and potential impact of Prussian carp (Carassius gibelio Bloch, 1782) to freshwater fishes in western North America. BioInvasions. Rec. 2017, 6, 291–296. [Google Scholar] [CrossRef]
- Liasko, R.; Koulish, A.; Pogrebniak, A.; Papiggioti, O.; Taranenko, L.; Leonardos, I. Influence of environmental parameters on growth pattern and population structure of Carassius auratus gibelio in Eastern Ukraine. Hydrobiologia 2011, 658, 317–328. [Google Scholar] [CrossRef]
- Fuad, M.M.H.; Vetešník, L.; Šimková, A. Is gynogenetic reproduction in gibel carp (Carassius gibelio) a major trait responsible for invasiveness? J. Vertebr. Biol. 2021, 70, 21049.1–13. [Google Scholar] [CrossRef]
- Pehlivanov, L.; Uzunova, E.; Pavlova, M. Ichthyofauna of the Vit River (Danube Basin): Composition, Distribution and Conservation Significance. Biotechnol. Biotechnol. Equipment 2014, 23, 337–340. [Google Scholar] [CrossRef]
- Tapkir, S.; Boukal, D.; Kalous, L.; Bartoň, D.; Souza, A.T.; Kolar, V.; Soukalová, K.; Duchet, C.; Gottwald, M.; Šmejkal, M. Invasive gibel carp (Carassius gibelio) outperforms threatened native crucian carp (Carassius carassius) in growth rate and effectiveness of resource use: Field and experimental evidence. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1901–1912. [Google Scholar] [CrossRef]
- Daskalov, G.M.; Grishin, A.N.; Rodionov, S.; Mihneva, V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl. Acad. Sci. USA 2007, 104, 10518–10523. [Google Scholar] [CrossRef] [PubMed]
- Rashleigh, B.; Monroy, E. Freshwater Fish Communities. In Narragansett Bay Estuary Program: State of the Watershed; Narragansett Bay Estuary Program: Providence, RI, USA, 2017; Chapter 20; pp. 376–391. [Google Scholar]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.K.; Ljunggren, L.; Sandström, A.; Johansson, G.; Mattila, J.; Rubach, A.; Råberg, S.; Snickars, M. Declines in Predatory Fish Promote Bloom-Forming Macroalgae. Ecol. Appl. 2009, 19, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).