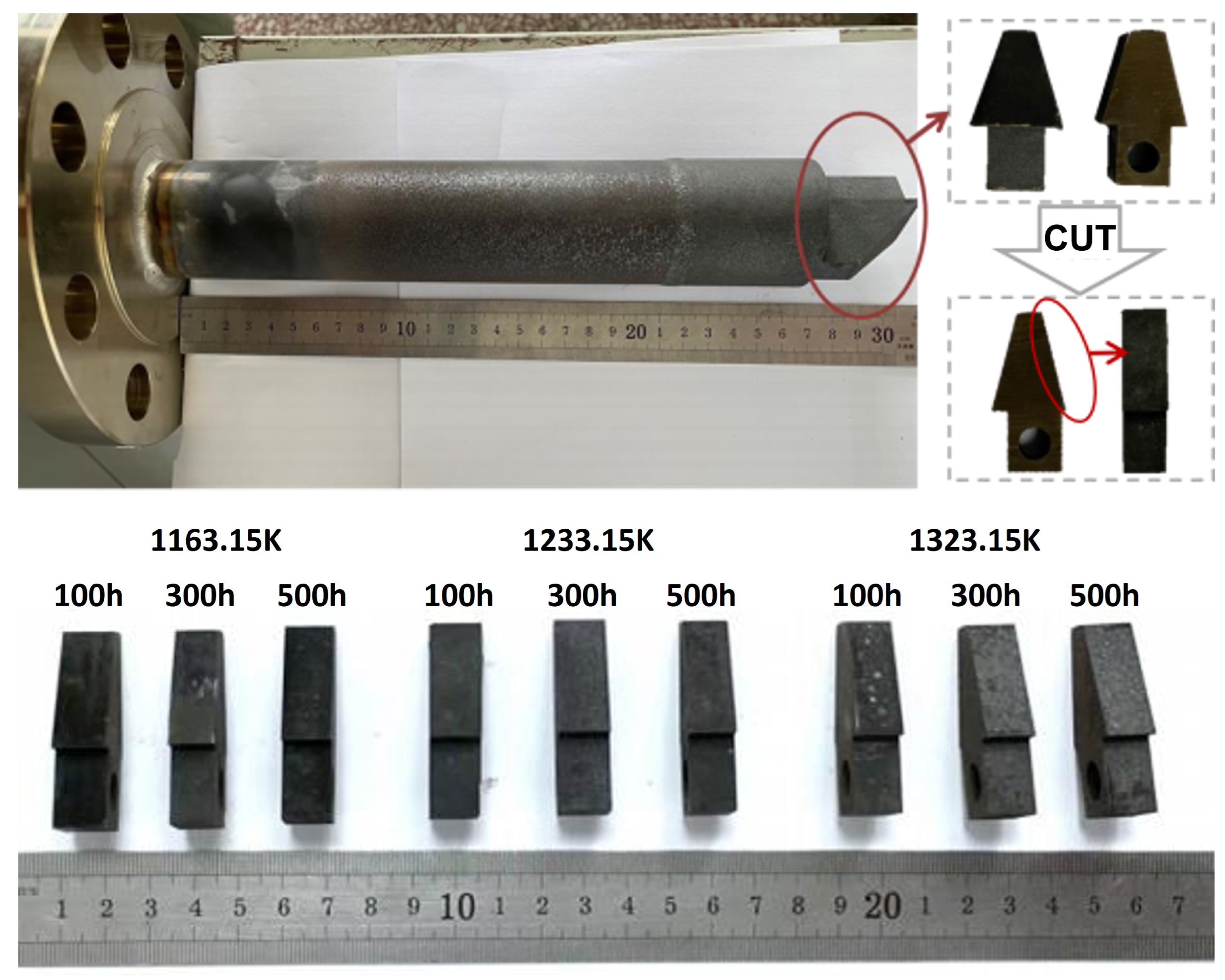
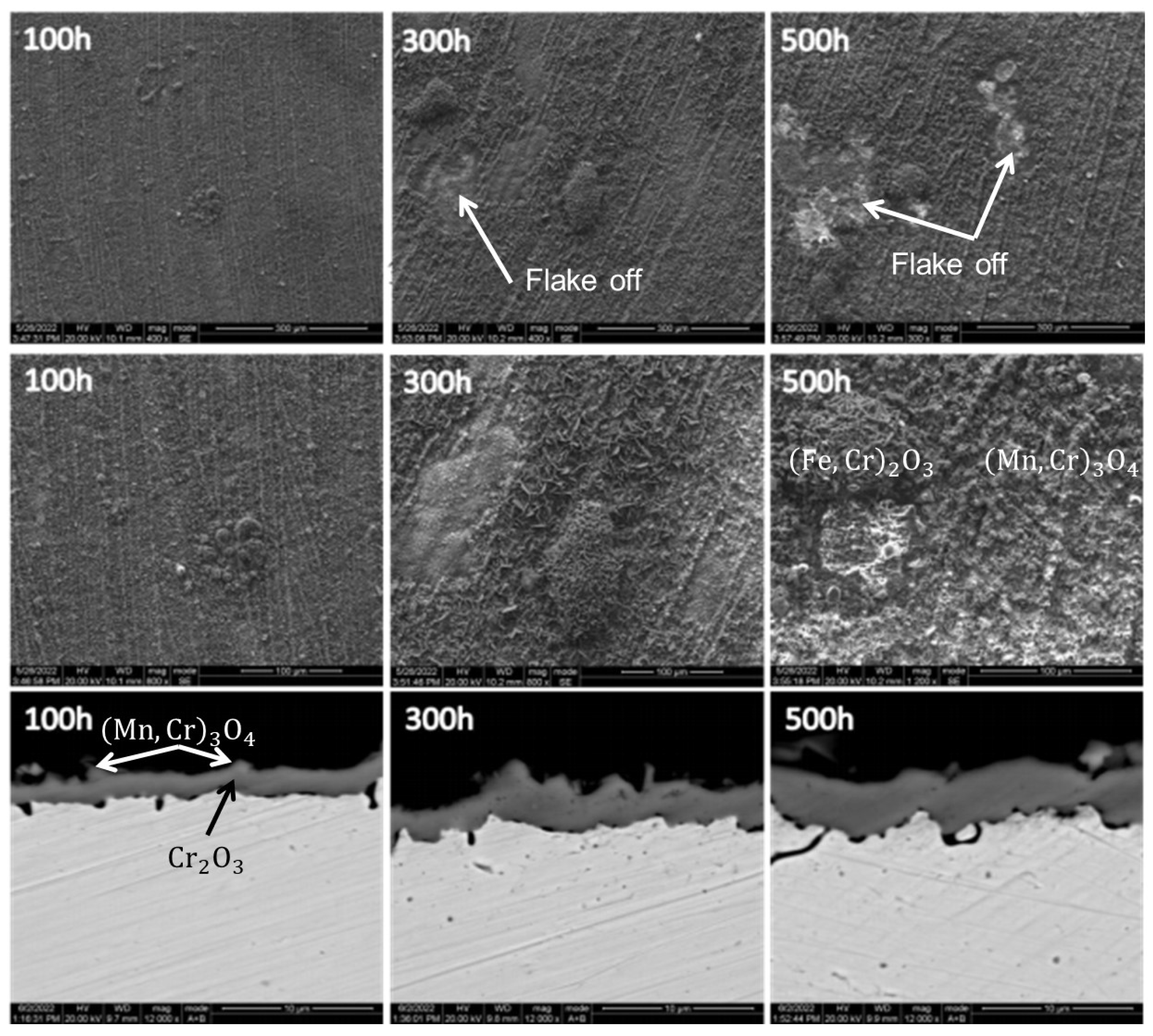
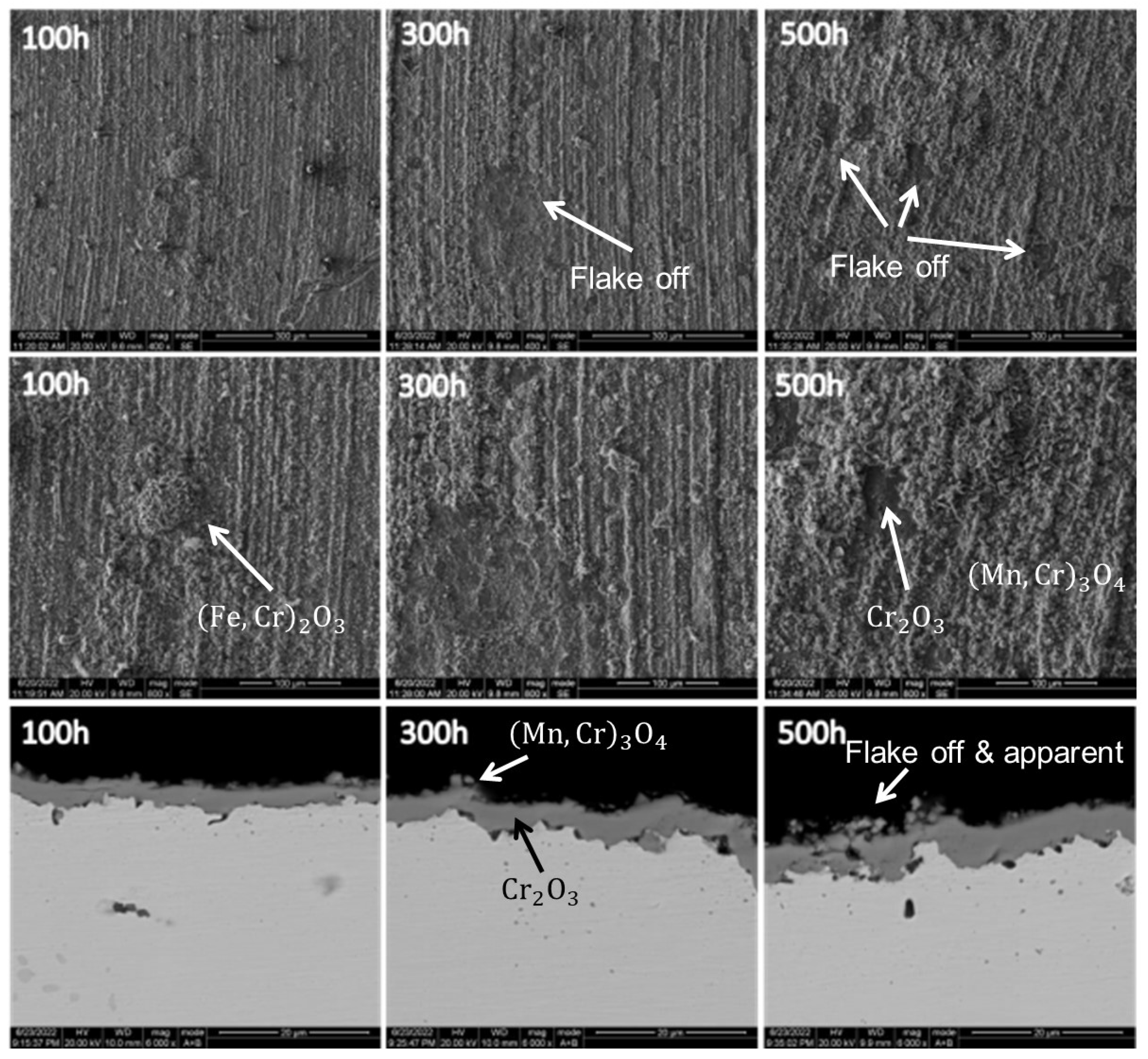
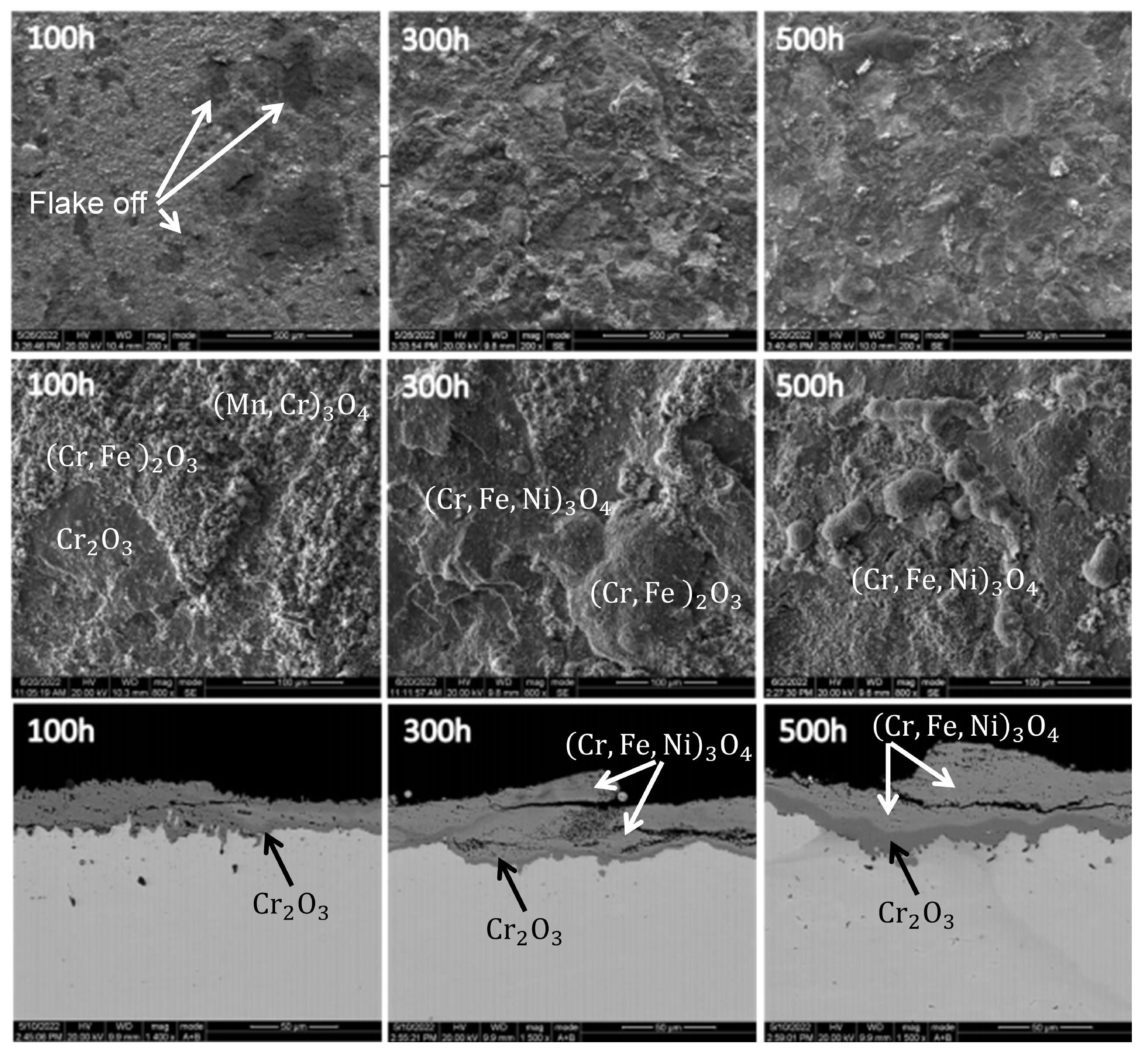
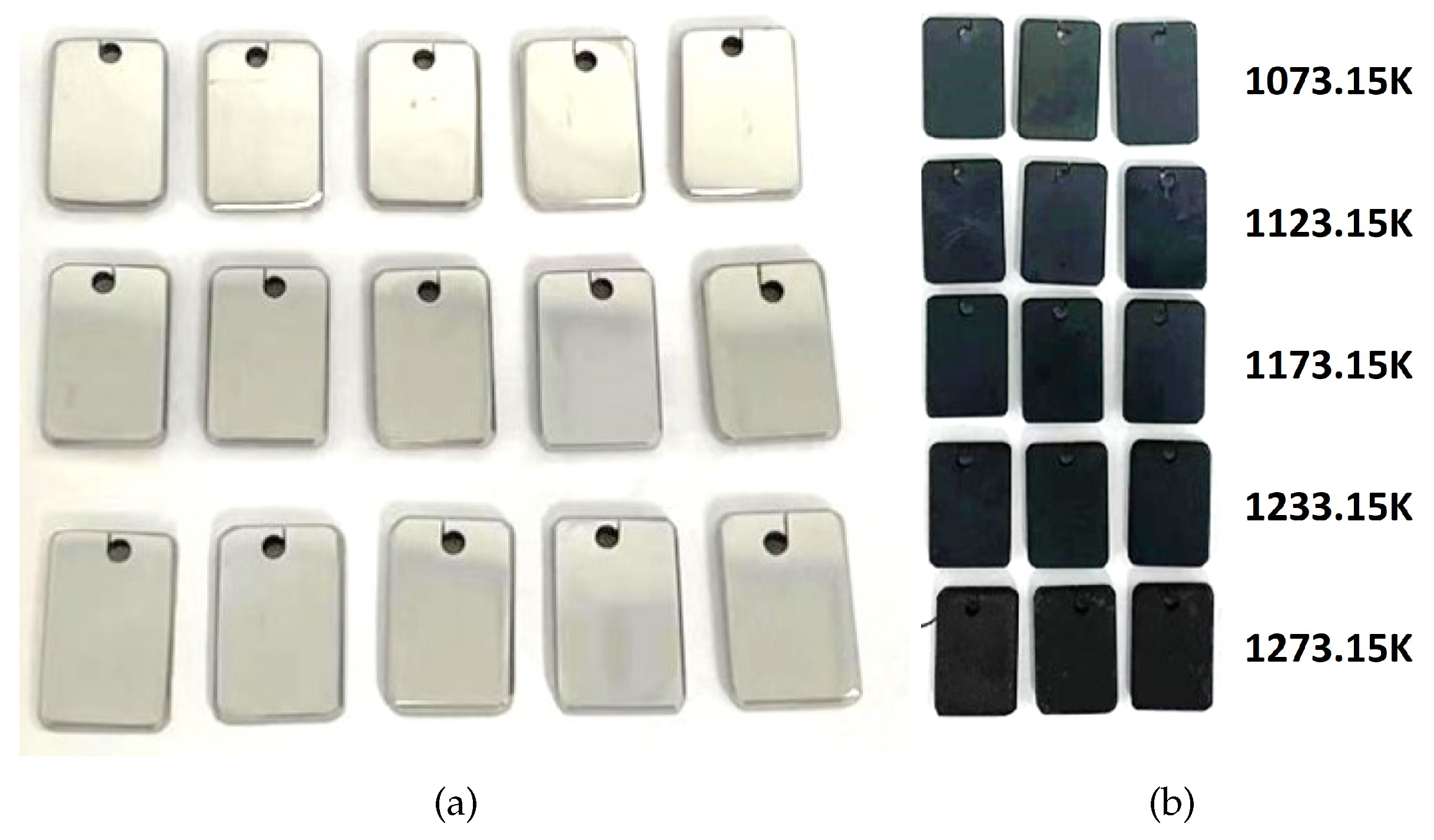
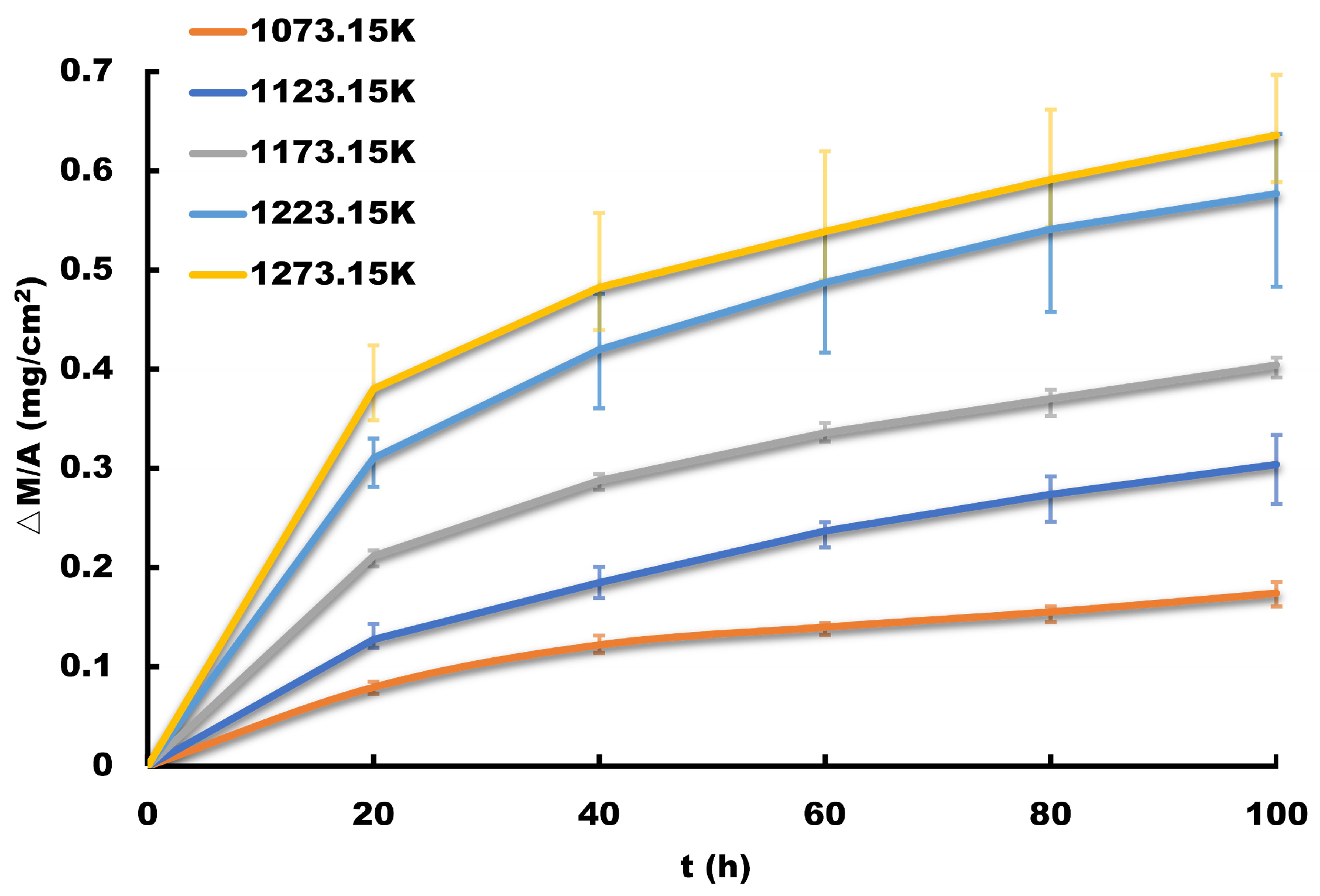
Thermocouples utilized in ethylene cracking furnaces operate within an oxygen-rich environment at 1073.15–1123.15 K. As such, the principal mode of failure for thermocouple protection tubes is high-temperature oxidation corrosion.
2.1. The High-Temperature Oxidation Corrosion Mechanism
In
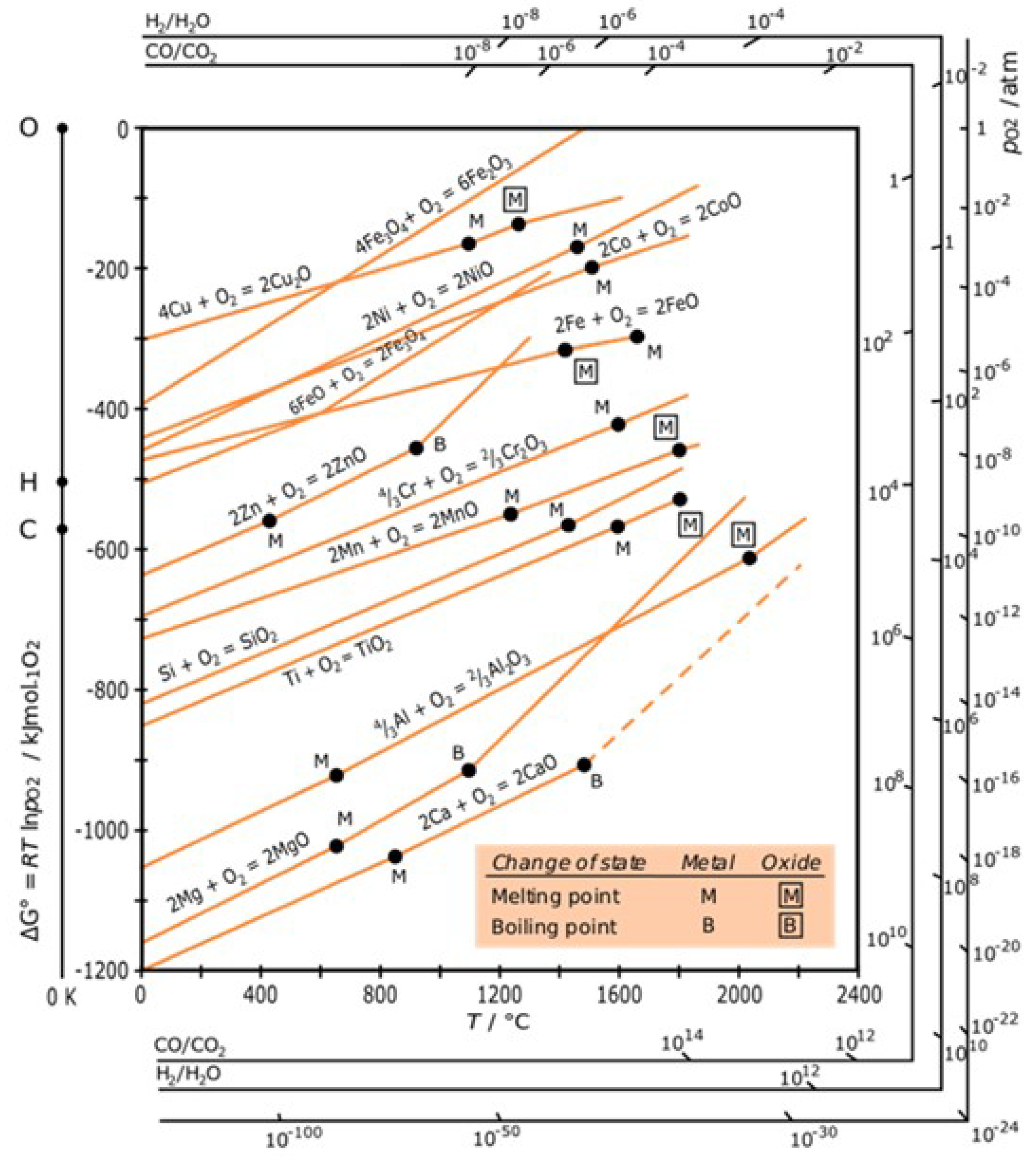
Figure 2, it is demonstrated that the free energy of the oxide formation of typical structural material components is negative at service temperature. This finding suggests that the reaction between these materials and oxygen, which results in the formation of oxides, is thermodynamically spontaneous. Despite this, the materials do not instantaneously transform into oxides when exposed to air due to the presence of a complete oxide layer that functions as an insulator, which allows the oxides to shield the metal surface from oxygen and thereby avoid the rapid oxidation of the material.
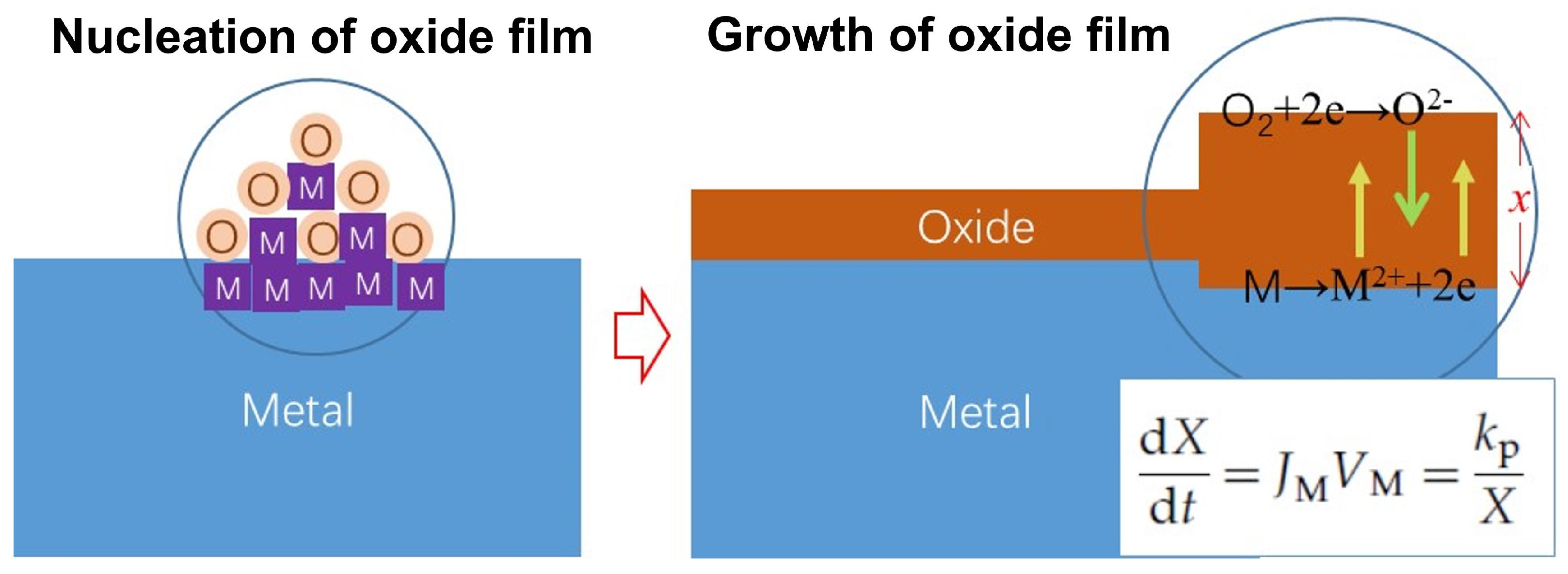
Figure 3 schematically represents the oxidation process of metals in high-temperature air, as observed through various studies [
19,
20,
21,
22,
23]. The initial step involves the rapid growth of oxide nucleation, which is also a swift process at room temperature. Once a complete oxide film forms, oxygen gains electrons at the gas/oxide film interface, while metal loses electrons at the oxide film/metal interface. This process leads to the formation of a concentration gradient of anions, cations, and electrons at the oxide film, which diffuse through the oxide film in opposite directions to complete the entire oxidation process. Multiple studies have demonstrated that the oxides of typical structural material elements behave as p-type semiconductors, with cation vacancies serving as the primary carriers of conductivity. Consequently, the oxidation rate of these materials is generally governed by the diffusion rate of cations in the oxide film.
Both thermodynamic theoretical analyses and experimental studies indicate that dense corrosion products can be described by a parabolic trend in high-temperature oxidation dynamics (corrosion volume versus time) as follows:
in which
denotes the weight gain per unit surface area and
and
t represent the corrosion rate and time, respectively.
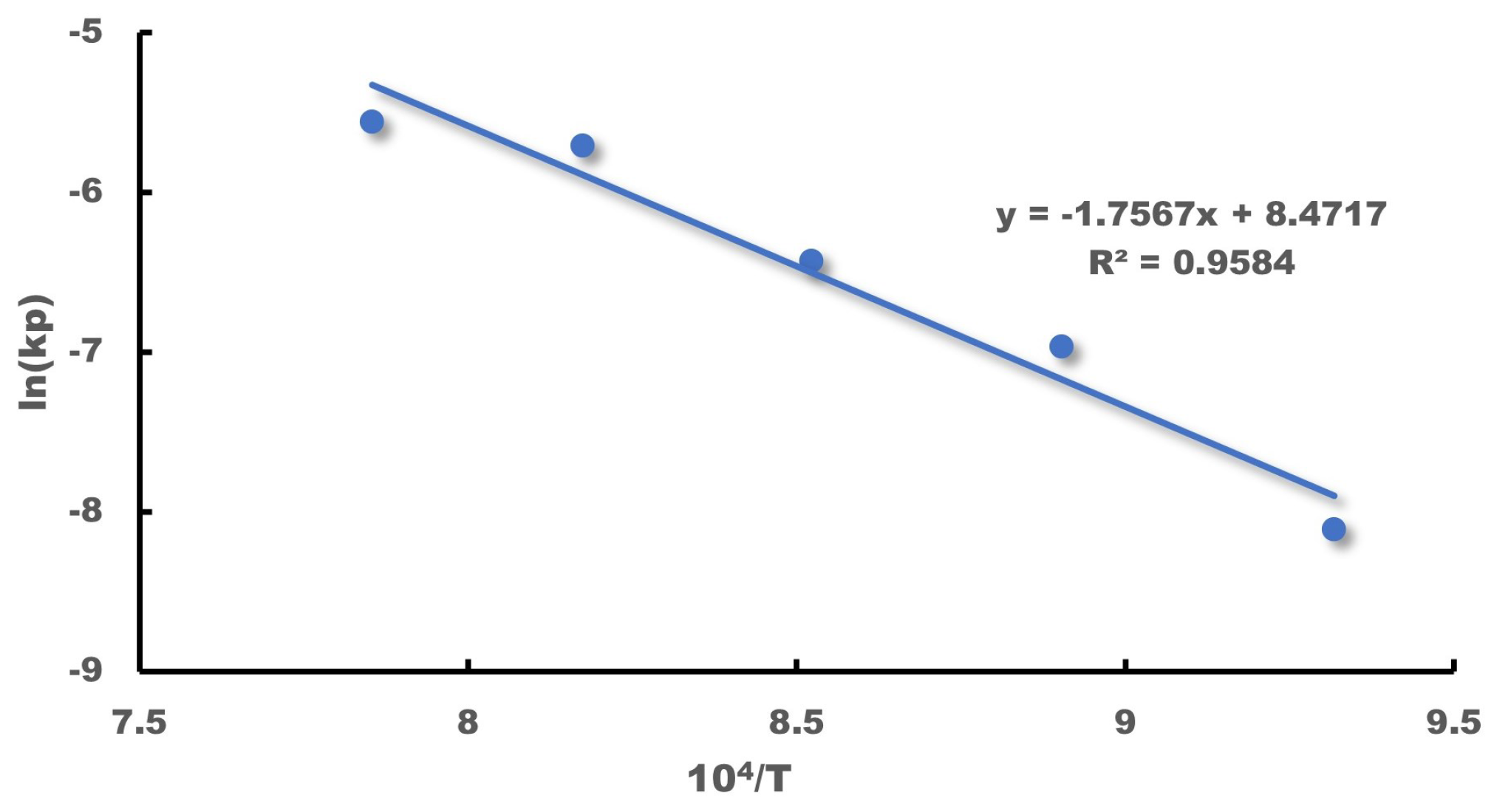
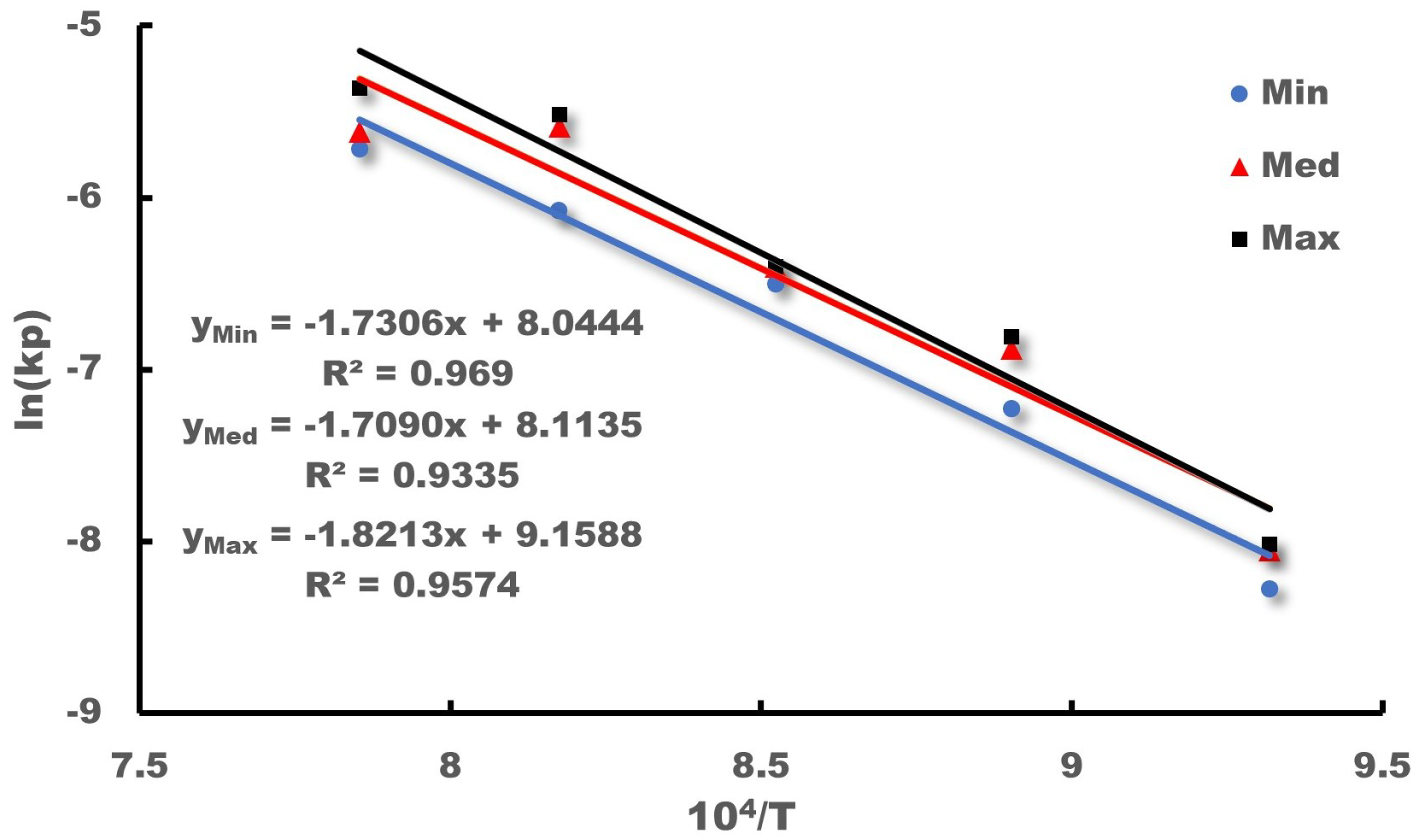
Wagner’s theory posits that the parabolic law indicates a strict dependence of the corrosion rate on the diffusion of the group elements present in corrosion as they move through the corrosion product layer. Additionally, the corrosion rate is inversely proportional to the thickness of the corrosion product layer. The Arrhenius relations are available to express the parameters associated with the diffusion-controlled process and its temperature dependence.
in which
denotes the activation energy and
and
T denote the Boltzmann’s constant and temperature, respectively.
Equation (
1) establishes a quantitative model of high-temperature oxidation corrosion by determining the mass of oxide
generated by a metal at various temperature steps within a given time. Typically, in material corrosion studies, “corrosion rate per year” and “corrosion depth” are used to illustrate the corrosion resistance of metal materials [
24,
25]. Two or three parallel samples are produced at the same temperature step, and their weight gain data are collected to calculate the corrosion rate
of the metal at different temperatures, which can then be used to determine its lifetime value (a definite value) at that temperature if a threshold value is provided. However, it is crucial to acknowledge that both the corrosion rate and lifetime value are approximate mathematical values and that small differences exist between parallel samples due to factors such as surface roughness and processing. From a natural science perspective, each sample represents a unique individual, and a sufficient number of samples undergoing high-temperature oxidation corrosion should follow either the Weibull or normal distribution concerning the corrosion amount, corrosion rate, or life. Therefore, we propose the utilization of the pseudo failure life analysis method for metal corrosion data, which conceptualizes the corrosion process as a degradation process of metal properties or life. We consider different temperature gradients and parallel samples as independent entities, analyze the corrosion rate and life of each parallel sample separately, and ultimately construct a pseudo failure life model of metal material corrosion.
2.2. Pseudo Failure Lifetime Model
Traditional life assessments and predictions primarily rely on theories and methods founded on mathematical statistics and life tests, with the statistical analysis primarily focused on the life data, also known as the “Time to failure”. However, obtaining sufficient failure data, or even any failure data, for highly reliable and long-life products within the limited test time presents significant challenges, which renders traditional life assessment methods insufficient.
The life consumption process of highly reliable long-life products can typically be attributed to potential performance degradation resulting from failure mechanisms, which eventually lead to product failure. If the failure mechanism and degradation magnitude of a product are understood, the product’s reliability can be determined by measuring the time when its performance reaches a critical level of degradation. This approach enables the extrapolation of the reliability of highly reliable long-life products by estimating their degradation pattern under a given stress, even if the actual failure time of the product is not observed. To this end, we propose the use of product performance degradation data to estimate the reliability and lifetime of highly reliable long-life products. This approach involves monitoring the performance state of highly reliable long-life products by using product performance degradation data, which can be employed to model and analyze the product life expectancy.
The slow degradation rate of metal materials during high-temperature oxidation results in an extremely small change in the amount of degradation over a long test time, which renders it challenging to obtain the product life characteristics through testing. In the case of metal materials, the degradation process equates to the corrosion process, with the amount of corrosion serving as the amount of performance degradation. To determine the characteristic life of metal materials, one can extrapolate the corrosion depth at a specific temperature based on the corrosion rate and estimate the time required to reach a critical level. This method provides an estimate of the characteristic life of the metal material.
Equation (
2) characterizes the high-temperature oxidation corrosion process of metallic materials. Within the realm of reliability, the corrosion rate
represents the degradation rate of the metal in this environment. The weight gain
d indicates the amount of degradation. Therefore, we can conclude that:
where
M indicates the mass of the sample.
At time
, the metal is in its initial state, with a mass of
. When
, the mass of the metal changes to
. Assuming that temperature is independent of time, we have
let
and we have
in which
B can be seen as the model parameters. If the metal fails at
, then
t equals the characteristic lifetime
of the metallic material at a particular temperature. Therefore, the
of a single sample can be expressed as
where C is also a model parameter and equals
.
2.3. Acceleration Factor Model Based on Weibull Distribution
Assuming that the product lifetime obeys the two-parameter Weibull distribution, the expression for its cumulative failure probability function
can be established as follows:
in which
is the shape parameter of the Weibull distribution. And we have
Performing a linear fit on the above expression for the same temperature enables us to obtain the Weibull distribution parameters of lifetime and probability at different temperatures, which can be used to analyze the reliability level of the product.
Let
denote the time required for the test to reach the cumulative failure (risk) probability
and
denote the time required for the accelerated life test to reach the same cumulative failure (risk) probability under the other stress condition. Then, the acceleration factor can be derived as