Production of Hemicellulose Sugars Combined with the Alkaline Extraction Lignin Increased the Hydro-Depolymerization of Cellulose from Corn Cob
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Hydro-Depolymerization of Agricultural Wastes
2.3. Pre-hydro-depolymerization of Hemicellulose in CC
2.4. Alkali Pretreatment of Pre-Hydro-Depolymerized CC Residues (PHR)
2.5. Hydro-Depolymerization of Pre-Hydro-Depolymerized and Delignified CC Residues (PHRD)
2.6. Analysis of Agricultural Wastes and Hydrolysates
2.7. Characterization of CC and Residues
3. Results and Discussion
3.1. Selection of Raw Materials
3.2. Pre-Hydro-Depolymerization of Hemicellulose in CC
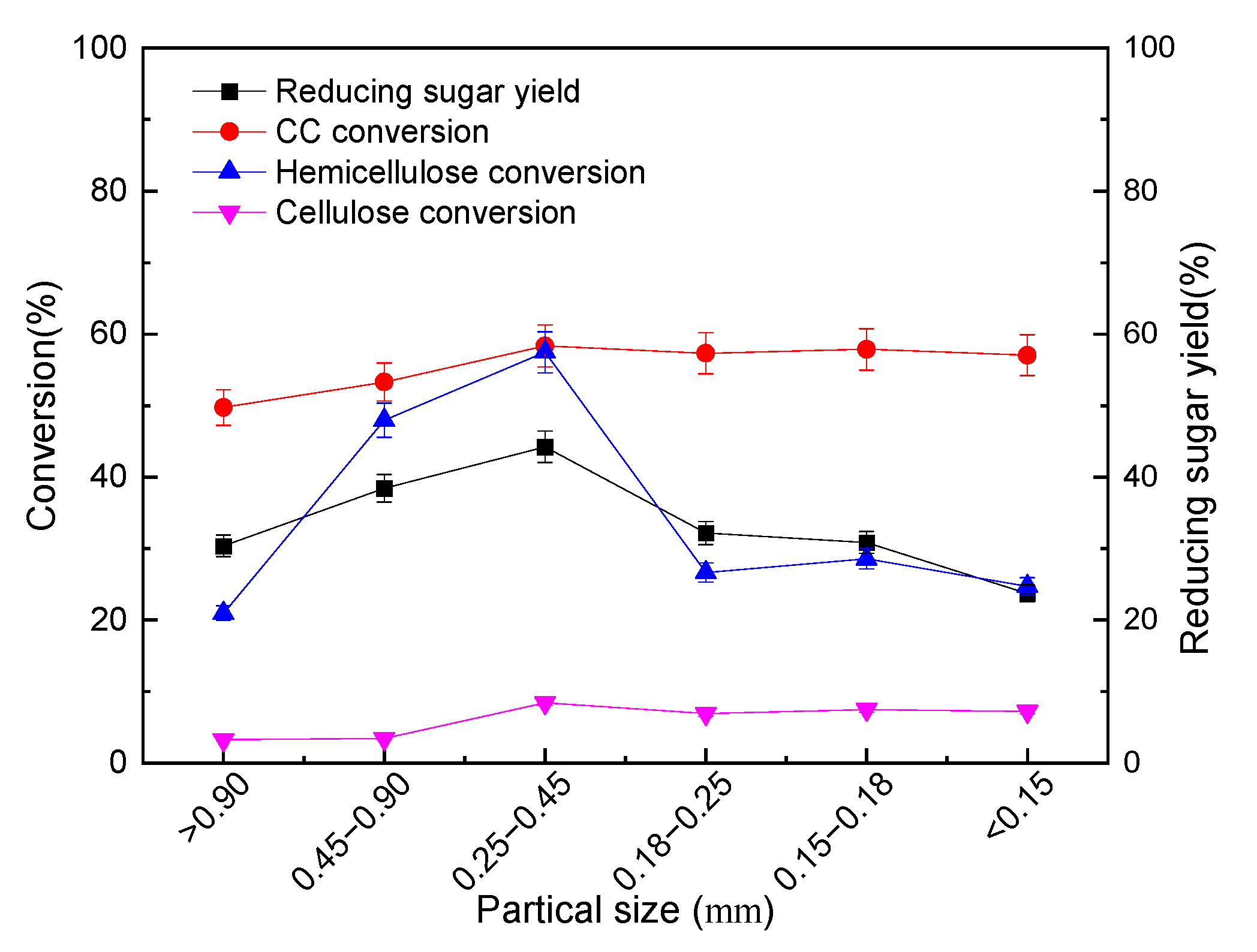
3.2.1. Effects of Particle Size on the Pre-Hydro-Depolymerization of CC
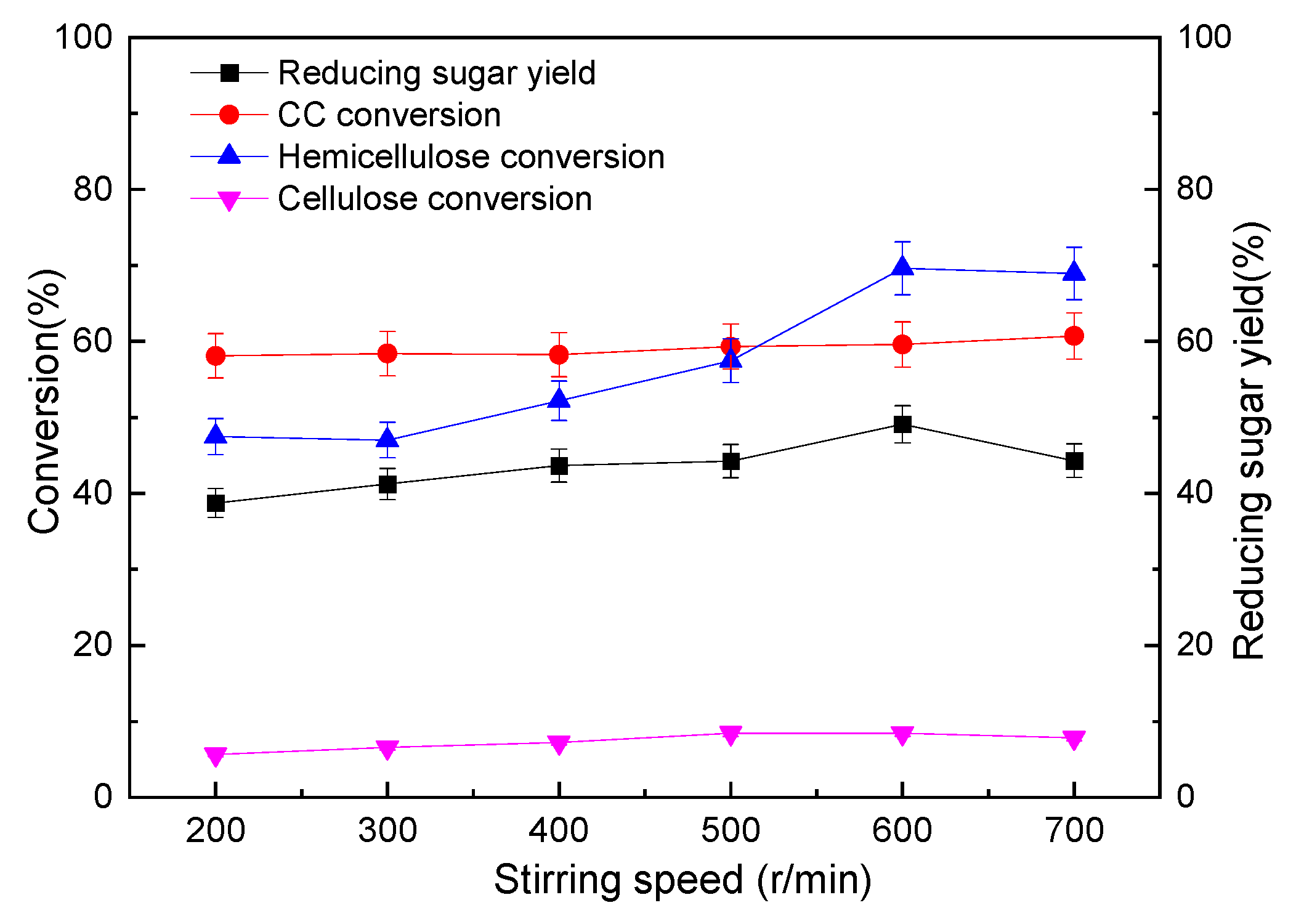
3.2.2. Influences of Stirring Speed on the Pre-Hydro-Depolymerization of CC
3.2.3. Impacts of Temperature on the Pre-Hydro-Depolymerization of CC
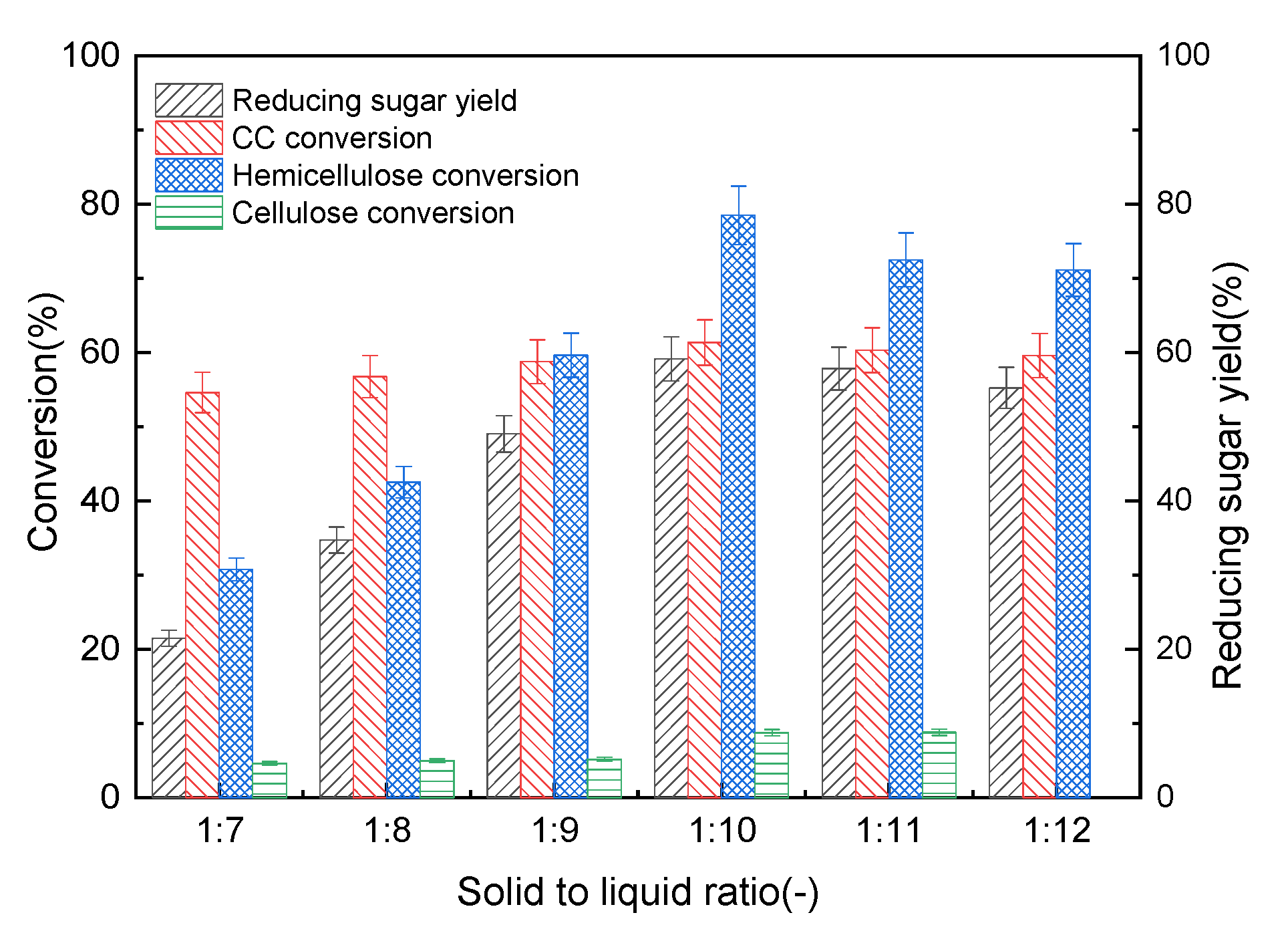
3.2.4. Effects of CAC Solid-to-Liquid Ratio on The Pre-Hydro-Depolymerization of CC
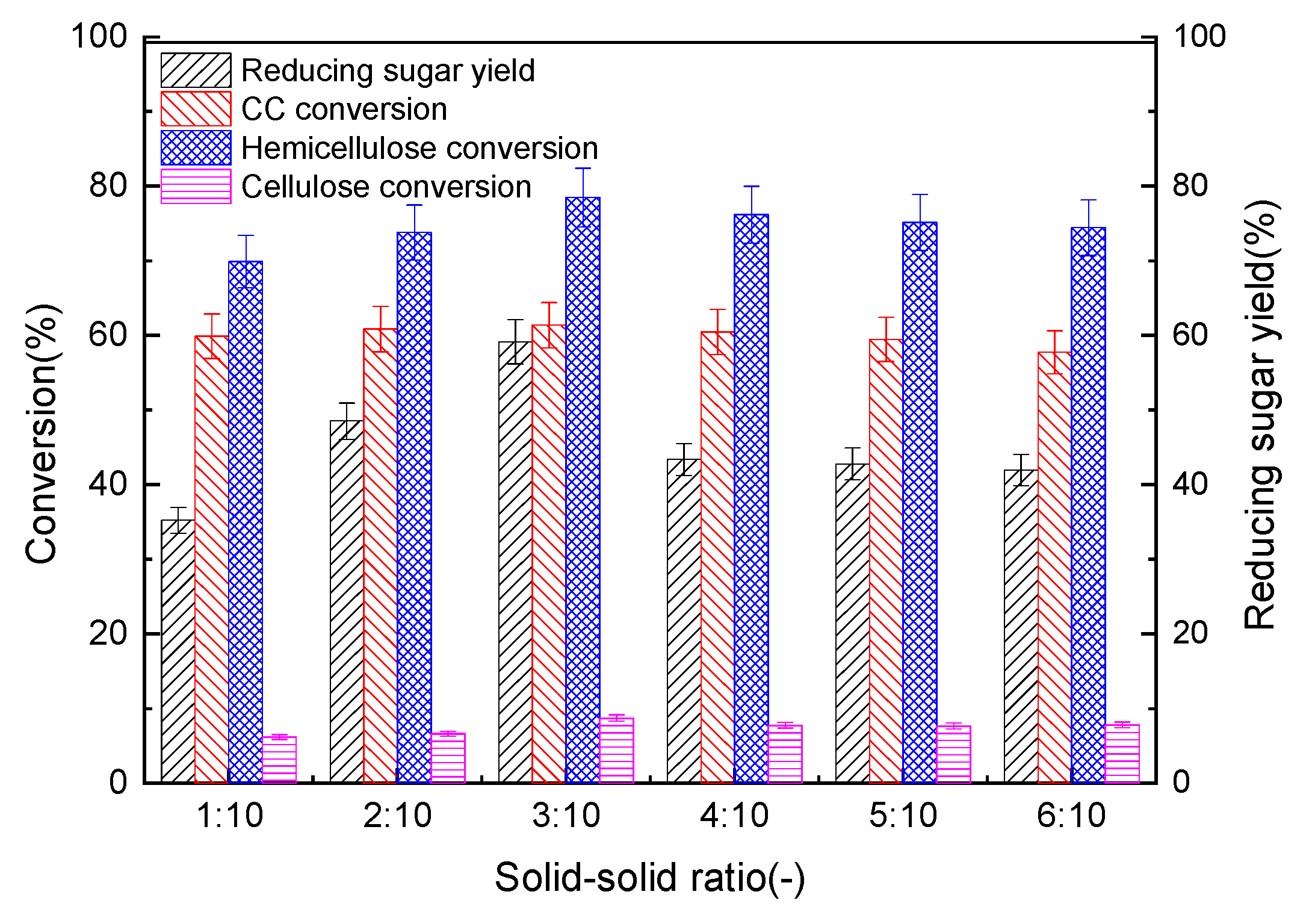
3.2.5. Impacts of CW Solid-to-Solid Ratio on The Pre-Hydro-Depolymerization of CC
3.2.6. Influences of Time on The Pre-Hydro-Depolymerization of CC
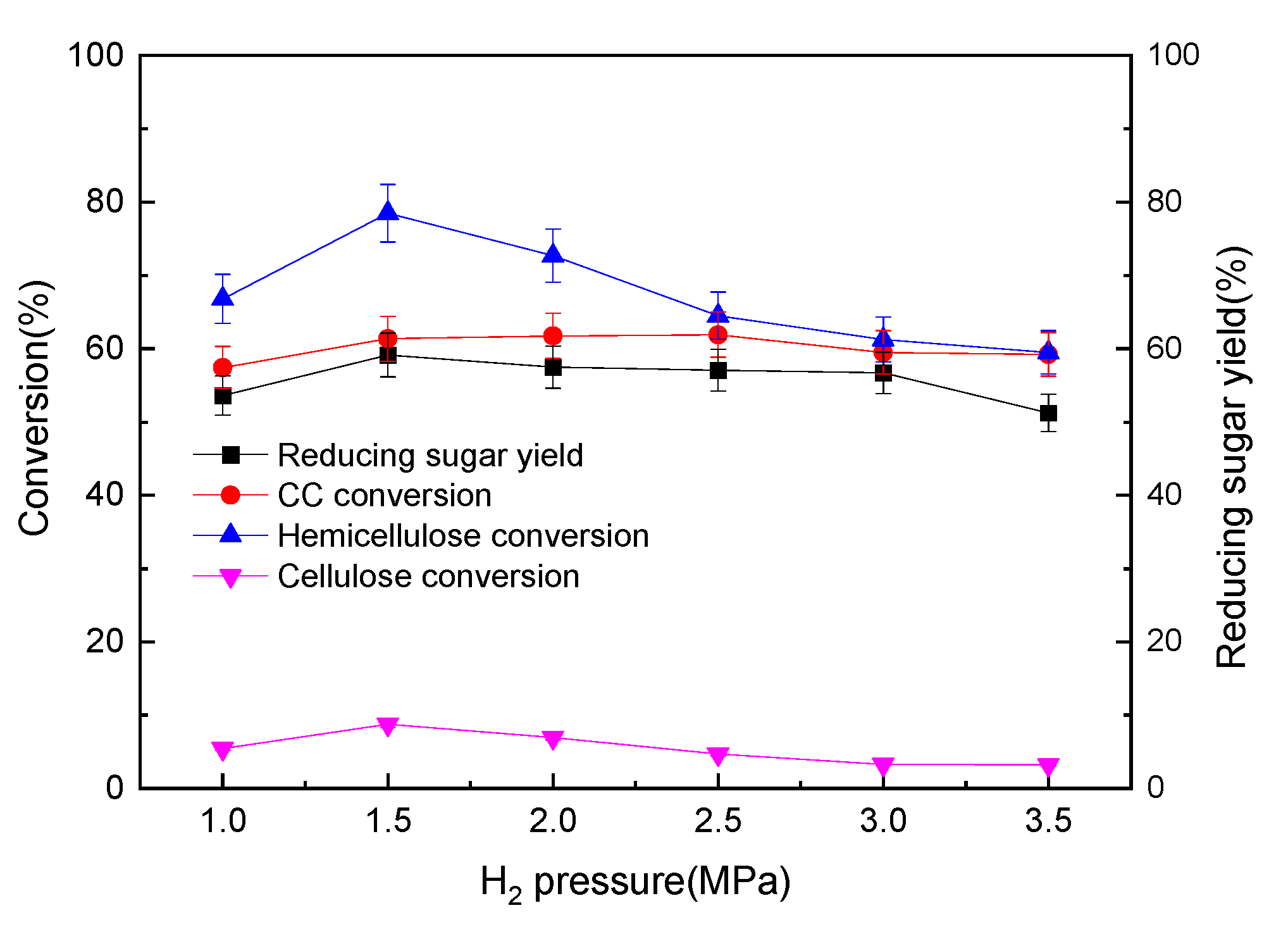
3.2.7. Influences of H2 Pressure on the Pre-Hydro-Depolymerization of CC
3.3. Lignin Extraction from Alkali-Treated PHR
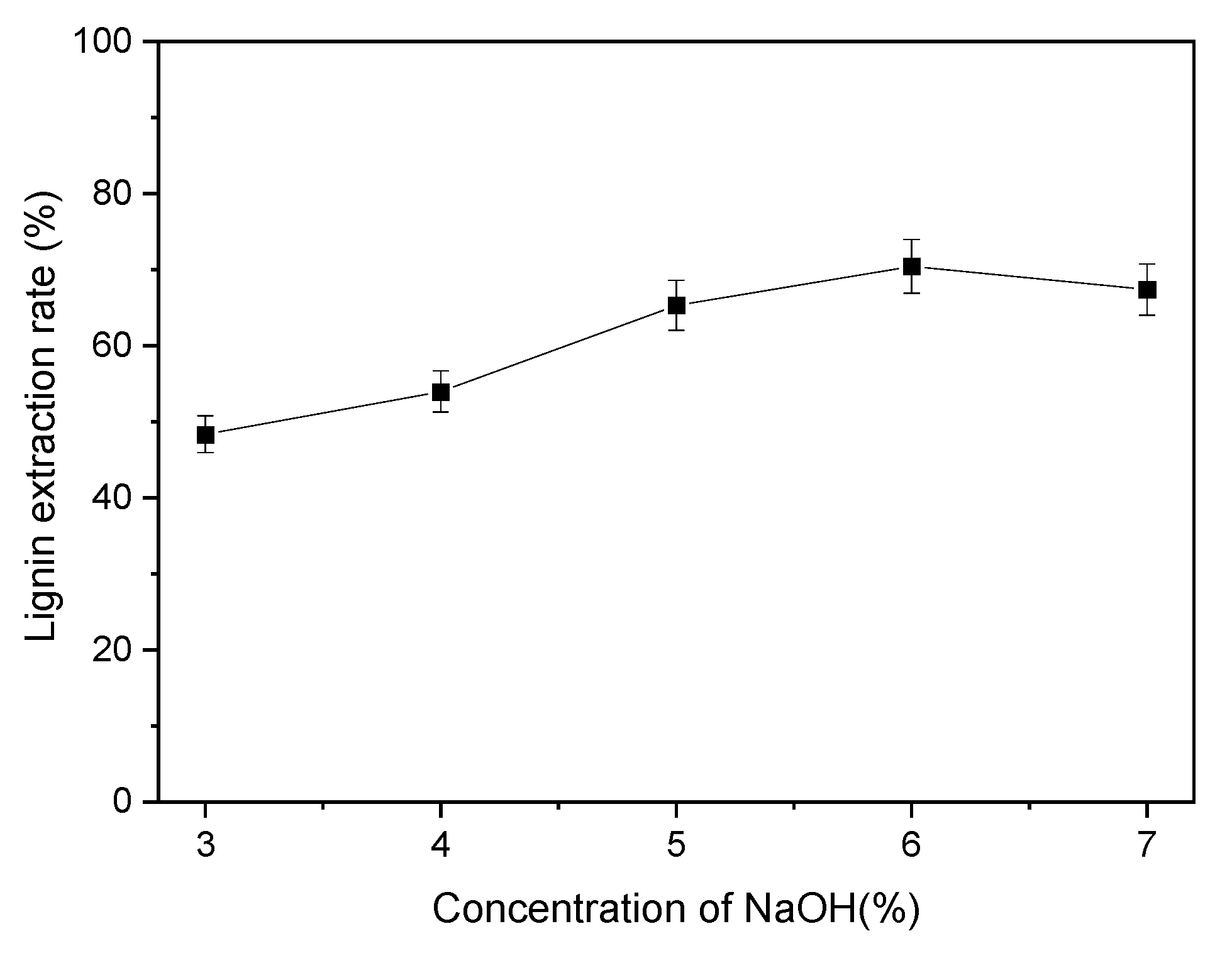
3.3.1. Influences of NaOH Concentration on the LER of PHR
3.3.2. Influences of Temperature on the LER of PHR
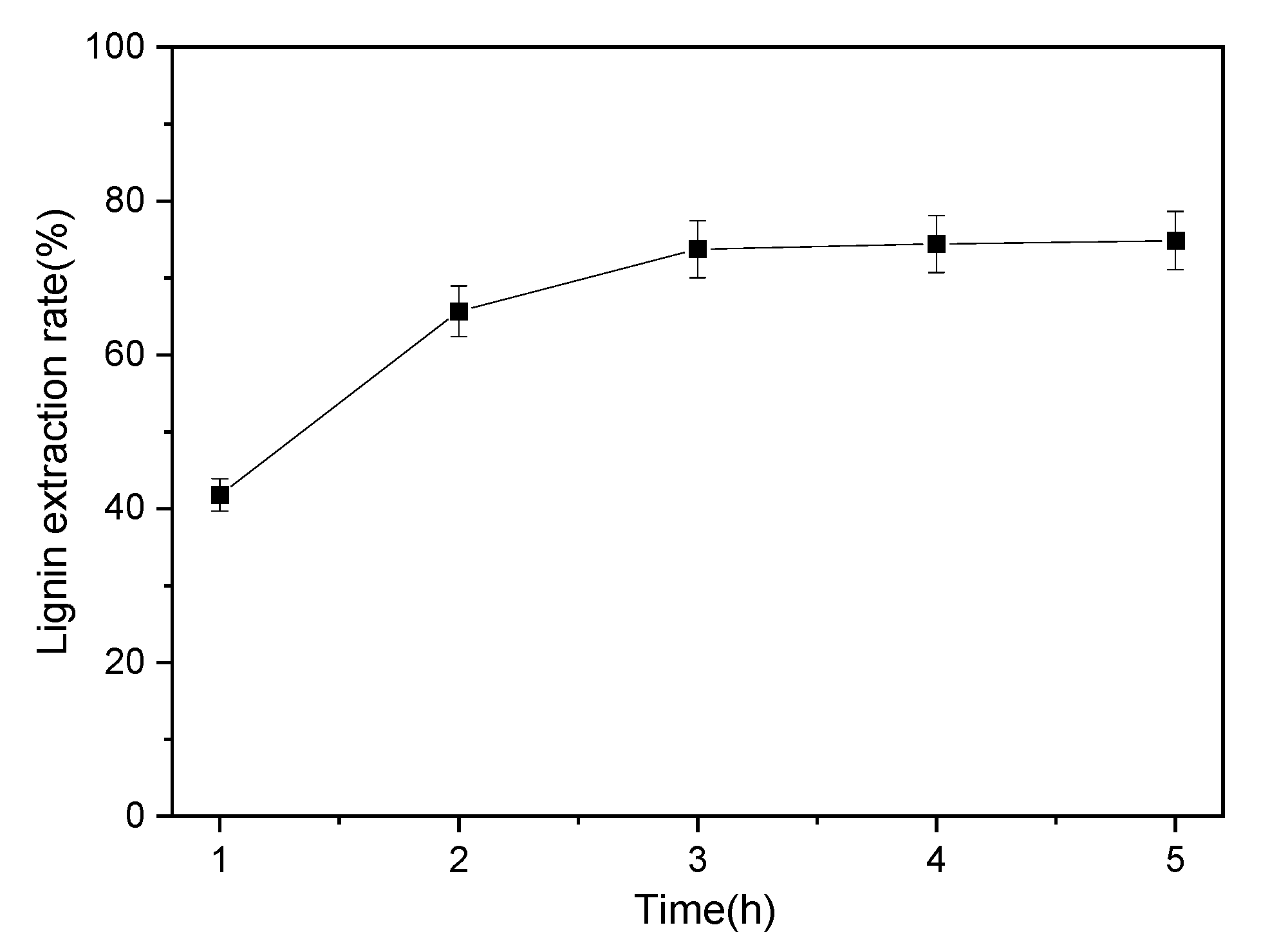
3.3.3. Influences of Time on the LER of PHR
3.4. Characterization Results of CC and Hemicellulos- and Lignin-Related Residues
3.4.1. Components and Specific Surface Area of CC and Hemicellulose- and Lignin-Related Residues
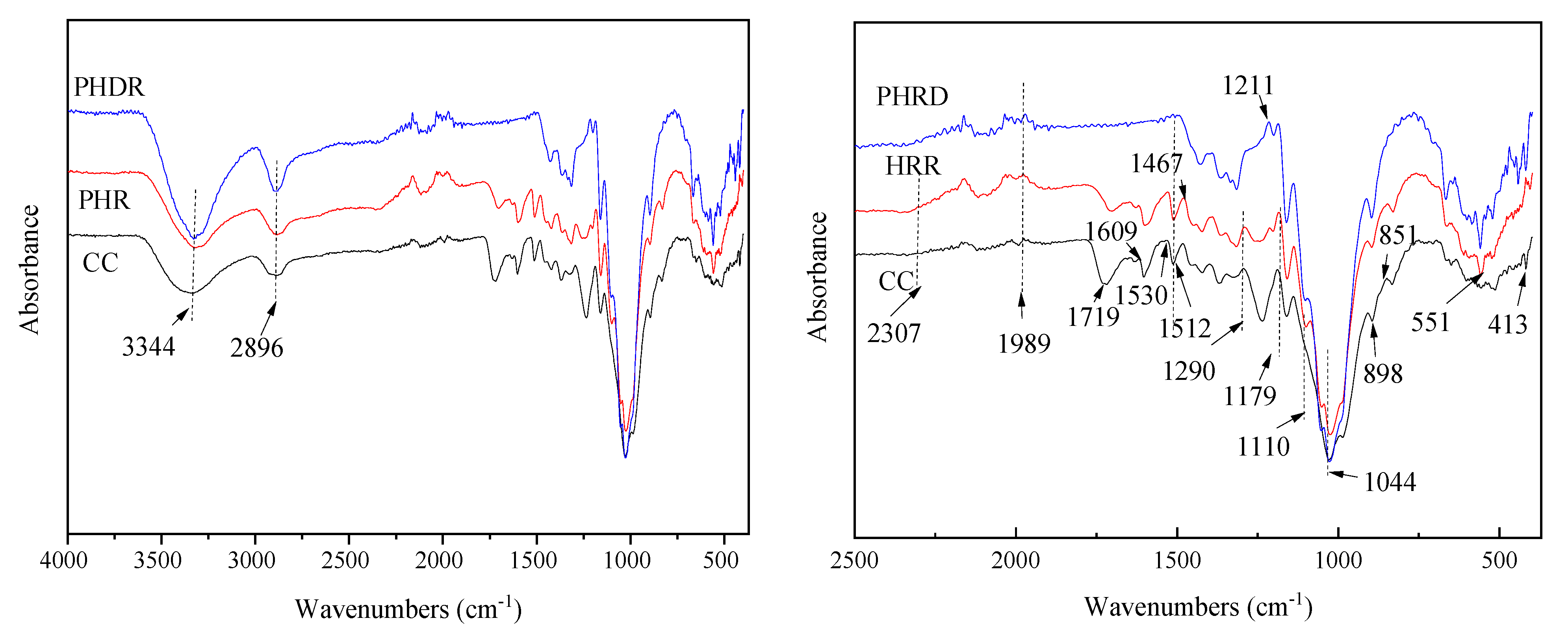
3.4.2. FTIR of CC and Hemicellulose- and Lignin-Related Residues
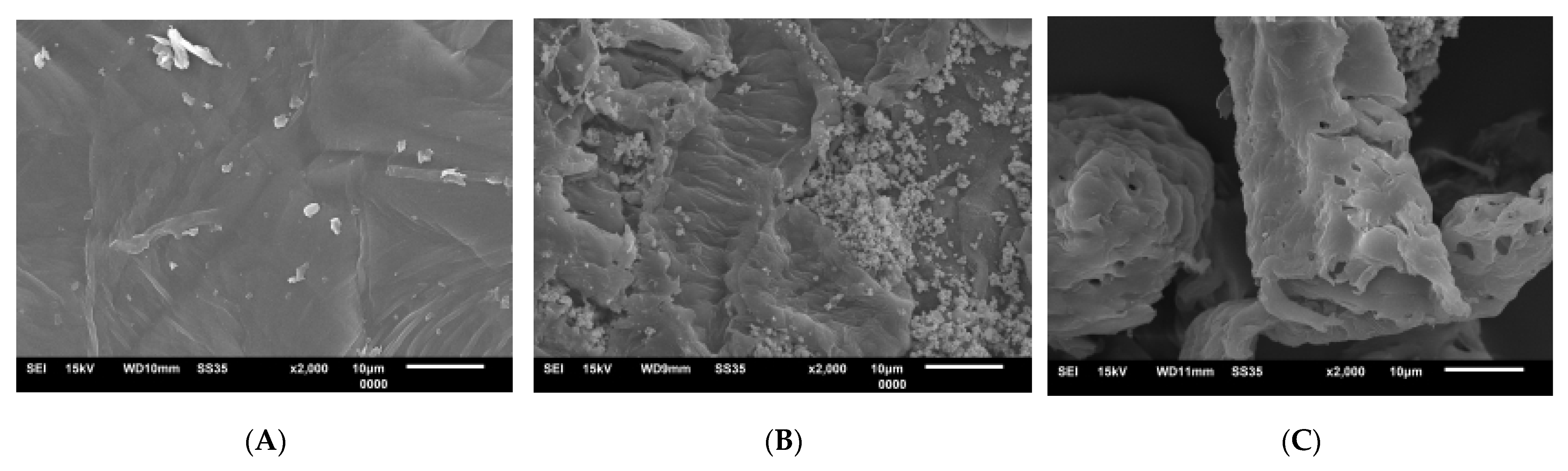
3.4.3. SEM of CC and Hemicellulose- and Lignin-Related Residues
3.5. Optimization of Cellulose Hydro-Depolymerization in CC
3.6. Multiple Hydro-Depolymerizations of PHRD Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, Y.; Wang, Y.; Zhang, M.; Wang, X.; Sun, Q.; Mu, S.; Wang, H.; Fan, M.; Zhang, Y. Selectively chemo–catalytic hydrogenolysis of cellulose to EG and EtOH over porous SiO2 supported tungsten catalysts. Catal. Today 2023, 407, 89–95. [Google Scholar]
- Chen, M.; Wang, Y.; Lu, J.; Du, J.; Tao, Y.; Cheng, Y.; Li, Q.; Wang, H. Combinatorial pretreatments of reed straw using liquid hot water and lactic acid for fermentable sugar production. Fuel 2023, 331, 125916. [Google Scholar]
- Zheng, H.; Wang, Y.; Feng, X.; Li, S.; Leong, Y.K.; Chang, J.S. Renewable biohydrogen production from straw biomass-Recent advances in pretreatment/hydrolysis technologies and future development. Int. J. Hydrog. Energ. 2022, 47, 37359–37373. [Google Scholar]
- Zahoor; Wang, W.; Tan, X.; Imtiaz, M.; Wang, Q.; Miao, C.; Yuan, Z.; Zhuang, X. Rice straw pretreatment with KOH/urea for enhancing sugar yield and ethanol production at low temperature. Ind. Crop. Prod. 2021, 170, 113776. [Google Scholar]
- Zhao, J.; Tao, X.; Li, J.; Jia, Y.; Shao, T. Enhancement of biomass conservation and enzymatic hydrolysis of rice straw by dilute acid-assisted ensiling pretreatment. Bioresour. Technol. 2021, 320, 124341. [Google Scholar] [PubMed]
- Liang, J.; Li, Z.; Dai, S.; Tian, G.; Wang, Z. Production of hemicelluloses sugars, cellulose pulp, and lignosulfonate surfactant using corn stalk by prehydrolysis and alkaline sulfite cooking. Ind. Crop. Prod. 2023, 192, 115880. [Google Scholar]
- He, S.; Wang, J.; Cheng, Z.; Dong, H.; Yan, B.; Chen, G. Synergetic effect and primaryreaction network of corn cob and cattle manure in single and mixed hydrothermal liquefaction. J. Anal. Appl. Pyrol. 2021, 155, 105076. [Google Scholar]
- Mihajlovski, K.; Pecarski, D.; Rajilić-Stojanović, M.; Dimitrijević-Branković, S. Valorization of corn stover and molasses for enzyme synthesis, lignocellulosic hydrolysis and bioethanol production by Hymenobacter sp. CKS3. Environ. Technol. Inno. 2021, 23, 101627. [Google Scholar]
- Maleki, M.; Ariaeenejad, S.; Salekdeh, G.H. Efficient saccharification of ionic liquid- pretreated rice straw in a one-pot system using novel metagenomics derived cellulases. Bioresour. Technol. 2022, 345, 126536. [Google Scholar]
- Laltha, M.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Development of microwave-assisted alkaline pretreatment methods for enhanced sugar recovery from bamboo and corn cobs: Process optimization, chemical recyclability and kinetics of bioethanol production. Ind.Crop. Prod. 2021, 174, 114166. [Google Scholar]
- Qiao, H.; Han, M.; Ouyang, S.; Zheng, Z.; Ouyang, J. An integrated lignocellulose biorefinery process: Two-step, sequential treatment with formic acid for efficiently producing ethanol and furfural from corn cobs. Renew. Energ. 2022, 191, 775–784. [Google Scholar]
- Norazlina, I.; Dhinashini, R.S.; Nurhafizah, I.; Norakma, M.N.; Noor Fazreen, D. Extraction of xylose from rice straw and lemongrass via microwave assisted. Mater. Today Proc. 2022, 48, 784–789. [Google Scholar]
- Liu, C.; Wang, C.; Yao, H.; Chapman, S.J. Pretreatment is an important method for increasing the conversion efficiency of rice straw by black soldier fly larvae based on the function of gut microorganisms. Sci. Total Environ. 2021, 762, 144118. [Google Scholar] [PubMed]
- Woo, W.; Tan, J.; Tan, J.; Indera Luthfi, A.; Abdul, P.; Abdul Manaf, S.; Yeap, S.K. An insight into enzymatic immobilization techniques on the saccharification of lignocellulosic biomass. Ind. Eng. Chem. Res. 2022, 61, 10603–10615. [Google Scholar]
- Lin, Q.; Yan, Y.; Liu, X.; He, B.; Wang, X.; Wang, X.; Liu, C.; Ren, J. Production of xyloolig-osaccharide, nanolignin, and nanocellulose through a fractionation strategy of corncob for biomass valorization. Ind. Eng. Chem. Res. 2020, 59, 17429–17439. [Google Scholar]
- Ratna, A.S.; Ghosh, A.; Mukhopadhyay, S. Advances and prospects of corn husk as a sustainable material in composites and other technical applications. J. Clean. Prod. 2022, 371, 133563. [Google Scholar]
- Zhang, H.; Wu, J. Statistical optimization of aqueous ammonia pretreatment and enzymatic hydrolysis of corn cob powder for enhancing sugars production. Biochem. Eng. J. 2021, 174, 108106. [Google Scholar]
- Lee, K.T.; Chen, W.H.; Sarle, P.; Park, Y.K.; Ok, Y.S. Recover energy and materials from agricultural waste via thermochemical conversion. Earth 2022, 11, 1200–1204. [Google Scholar]
- Zhang, Y.; Guo, S.; Wang, Y.; Liang, X.; Xu, P.; Gao, C.; Ma, C. Production of d-xylonate from corn cob hydrolysate by a metabolically engineered escherichia coli strain. ACS Sustain. Chem. Eng. 2019, 7, 2160–2168. [Google Scholar]
- Ling, R.; Wei, W.; Jin, Y. Pretreatment of sugarcane bagasse with acid catalyzed ethylene glycol-water to improve the cellulose enzymatic conversion. Bioresour. Technol. 2022, 361, 127723. [Google Scholar]
- Sun, J.; Ding, R.; Yin, J. Pretreatment of corn cobs and corn stalks with tetrabutyl phosphate hydroxide ionic liquid to enhance enzymatic hydrolysis process. Biochem. Eng. J. 2022, 177, 108270. [Google Scholar]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Konopacka-Łyskawa, D.; Słupek, E.; Makoś, P.; Cieśliński, H.; Kamiński, M. Influence of alkaline and oxidative pre-treatment of waste corn cobs on biohydrogen generation efficiency via dark fermentation. Biomass Bioenergy 2020, 141, 105691. [Google Scholar]
- Martins-Vieira, J.C.; Lachos-Perez, D.; Draszewski, C.P.; Celante, D.; Castilhos, F. Sugar, hydrochar and bio-oil production by sequential hydrothermal processing of corn cob. J. Supercrit. Fluid. 2023, 194, 105838. [Google Scholar]
- de Mello Captti, C.C.; Pellegrini, V.O.A.; Espirito Santo, M.C.; Cortez, A.A.; Falvo, M.; de Curvelo, A.A.S.; Campos, E.; Filgueiras, J.G.; Guimaraes, F.E.G.; de Azevedo, E.R.; et al. Enzymatic production of xylooligosaccharides from corn cobs: Assessment of two different pretreatment strategies. Carbohydr. Polym. 2023, 299, 12074. [Google Scholar]
- Wang, W.; Cao, X.; Guo, H.; Yang, X.; Guo, N.; Ma, Y. Carbon-based solid acid derived from lignin and polyvinyl chloride for conversion of xylose and crop wastes to furfural. Mol. Catal. 2022, 524, 112329. [Google Scholar]
- Long, Y.; Ma, Y.; Wan, J.; Wang, Y.; Tang, M.; Zheng, Q.; Ma, Y. Hydrolysate from the enzyma-tictreatment of corn cob as a carbon source for heterotrophic denitrification process. Water Process Eng. 2023, 51, 103473. [Google Scholar]
- Sun, J.; Ding, R.; Yin, J. Pretreatment corn ingredient biomass with high pressure CO2 for conversion to fermentable sugars via enzymatic hydrolysis of cellulose. Ind. Crop. Prod. 2022, 177, 114518. [Google Scholar]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Organosolv pretreatment of rice straw followed by microbial hydrolysis for efficient biofuel production. Renew. Energ. 2020, 148, 923–934. [Google Scholar]
- Hussin, M.H.; Rahim, A.A.; Mohamad, I.M.N.; Yemloul, M.; Perrin, D.; Brosse, N. Investigation on the structure and antioxidant properties of modified lignin obtained by different combinative processes of oil palm fronds (OPF) biomass. Ind. Crop. Prod. 2014, 52, 544–551. [Google Scholar]
- Sartika, D.; Firmansyah, A.P.; Junais, I.; Arnata, I.W.; Fahma, F.; Firmanda, A. High yield production of nanocrystalline cellulose from corn cob through a chemical-mechanical treatment under mild conditions. Int. J. Biol. Macromol. 2023, 240, 124327. [Google Scholar]
- Islam, M.T.; Sultana, A.I.; Saha, N.; Klinger, J.L.; Reza, M.T. Pretreatment of biomass by selected type-III deep eutectic solvents and evaluation of the pretreatment effects on hydrothermal carbonization. Ind. Eng. Chem. Res. 2021, 60, 15479–15491. [Google Scholar]
- Chiranjeevi, T.; Mattam, A.J.; Vishwakarma, K.K.; Uma, A.; Peddy, V.C.R.; Gandham, S.; Velankar, H.R. Assisted single-step acid pretreatment (ASAP) process for enhanced delignification of rice straw for bioethanol production. ACS Sustain. Chem. Eng. 2018, 6, 8762–8774. [Google Scholar]
- Zhong, L.; Wang, C.; Xu, M.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Alkali-catalyzed organosolv pretreatment of lignocellulose enhances enzymatic hydrolysis and results in highly antioxidative lignin. Energ. Fuel. 2021, 35, 5039–5048. [Google Scholar]
- Aghaei, S.; Karimi Alavijeh, M.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioenerg. 2022, 161, 106447. [Google Scholar]
- Aggarwal, N.; Pal, P.; Sharma, N.; Saravanamurugan, S. Consecutive organosolv and alkaline pretreatment: An efficient approach toward the production of cellulose from rice straw. ACS Omega 2021, 6, 27247–27258. [Google Scholar]
- Lima, C.S.; Rabelo, S.C.; Ciesielski, P.N.; Roberto, I.C.; Rocha, G.J.M.; Driemeier, C. Multiscale alterations in sugar cane bagasse and straw submitted to alkaline deacetylation. ACS Sustain. Chem. Eng. 2018, 6, 3796–3804. [Google Scholar]
- Khan, M.F.S.; Akbar, M.; Xu, Z.; Wang, H. A review on the role of pretreatment technologies in the hydrolysis of lignocellulosic biomass of corn stover. Biomass Bioenerg. 2021, 155, 106276. [Google Scholar]
- Abdolmaleki, A.; Nabavizadeh, S.S.; Badbedast, M. 1-(Carboxymethyl)pyridinium chloride as an acidic ionic liquid for rice straw effective pretreatment. Renew. Energ. 2021, 177, 544–553. [Google Scholar]
- Saulnier, B.K.; Siahkamari, M.; Singh, S.K.; Nejad, M.; Hodge, D.B. Effect of dilute acid pretreatment and lignin extraction conditions on lignin properties and suitability as a phenol replacement in phenol-formaldehyde wood adhesives. J. Agric. Food Chem. 2023, 71, 592–602. [Google Scholar]
- Yang, X.; Zhao, J.; Liang, J.; Zhu, J. Efficient and selective catalytic conversion of hemicellulose in rice straw by metal catalyst under mild conditions. Sustainability 2020, 12, 106011. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Templeton, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1671, 1–15. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic aid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar]
- Yang, M.; Gao, X.; Lan, M.; Dou, Y.; Zhang, X. Rapid fractionation of lignocellulosic biomass by p-TsOH pretreatment. Energ. Fuel 2019, 33, 2258–2264. [Google Scholar]
- Han, Y.; Xu, J.; Zhao, Z.; Zhao, J. Analysis of enzymolysis process kinetics and estimation of the resource conversion efficiency to corn cobs with alkali soaking, water and acid steam explosion pretreatments. Bioresour. Technol. 2018, 264, 391–394. [Google Scholar] [PubMed]
- Cousins, D.S.; Pedersen, K.P.; Otto, W.G.; Rony, A.H.; Lacey, J.A.; Aston, J.E.; Hodge, D.B. Particle classification by image analysis improves understanding of corn stover degradation mechanisms during deconstruction. Ind. Crop. Prod. 2023, 193, 116153. [Google Scholar]
- Tocco, D.; Carucci, C.; Monduzzi, M.; Salis, A.; Sanjust, E. Recent developments in the delignification and exploitation of grass lignocellulosic biomass. ACS Sustain. Chem. Eng. 2021, 9, 2412–2432. [Google Scholar]
- Zhang, R.; Lin, D.; Zhang, L.; Zhan, R.; Wang, S.; Wang, K. Molecular and biochemical analyses of a novel trifunctional endoxylanase/endoglucanase/feruloyl esterase from the human colonic bacterium bacteroides intestinalis DSM 17393. J. Agric. Food Chem. 2022, 70, 4044–4056. [Google Scholar]
- Wang, Y.; Gong, X.; Hu, X.; Zhou, N. Lignin monomer in steam explosion assist chemical treated cotton stalk affects sugar release. Bioresour. Technol. 2019, 276, 343–348. [Google Scholar]
- Cai, D.; Li, P.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z.; Tan, T. Effect of dilute alkaline pretreatment on the conversion of different parts of corn stalk to fermentable sugars and its application in acetone–butanol–ethanol fermentation. Bioresour. Technol. 2016, 211, 117–124. [Google Scholar]
- Xu, Q.; Yang, W.; Liu, G.; Liang, C.; Lu, S.; Qi, Z.; Hu, J.; Wang, Q.; Qi, W. Enhanced enzymatic hydrolysis of corncob by synthesized enzyme-mimetic magnetic solid acid pretreatment in an aqueous phase. ACS Omega 2019, 4, 17864–17873. [Google Scholar]
- Li, P.; Cai, D.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Zhang, C.; Wang, Z.; Tan, T. Effect of acid pretreatment on different parts of corn stalk for second generation ethanol production. Bioresour. Technol. 2016, 206, 86–92. [Google Scholar]
- Louis, A.C.F.; Venkatachalam, S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020, 163, 260–269. [Google Scholar]
- Sharma, K.; Khaire, K.C.; Thakur, A.; Moholkar, V.S.; Goyal, A. Acacia xylan as a substitute for commercially available xylan and its application in the production of xylooligosaccharides. ACS Omega 2020, 5, 13729–13738. [Google Scholar]
- Yang, X.; Li, X.; Liang, J.; Zhu, J. Comparative study of effective pretreatments on the structural disruption and hydrodepolymerization of rice straw. Sustainability 2023, 15, 4728. [Google Scholar]
- Jackson, M.A.; Price, N.P.J.; Blackburn, J.A.; Peterson, S.C.; Kenar, J.A.; Haasch, R.T.; Chen, C. Partial hydrodeoxygenation of corn cob hydrolysate over palladium catalysts to produce 1-hydroxy-2-pentanone. Appl. Catal. A-Gen. 2019, 577, 52–61. [Google Scholar]
- Hu, H.; Liang, W.; Zhang, Y.; Wu, S.; Yang, Q.; Wang, Y.; Zhang, M.; Liu, Q. Multipurpose use of a corncob biomass for the production of polysaccharides and the fabrication of a biosorbent. ACS Sustain. Chem. Eng. 2018, 6, 3830–3839. [Google Scholar]
- Guo, X.Y.; Zhang, T.; Shu, S.T.; Zheng, W.; Gao, M.T. Compositional and Structural Changes of Corn cob pretreated by electron beam irradiation. ACS Sustain. Chem. Eng. 2016, 5, 420–425. [Google Scholar]
- Sun, Y.; Xu, J.; Xu, F.; Su, R. Efficient separation and physico-chemical characterization of lignin from eucalyptus using ionic liquid-organic solvent and alkaline ethanol solvent. Ind. Crop. Prod. 2013, 47, 277–285. [Google Scholar]
- Zou, H.; Jiang, Q.; Zhu, R.; Chen, Y.; Sun, T.; Li, M.X.; Zhai, J.; Shi, D.; Ai, H.; Gu, L.; et al. Enhanced hydrolysis of lignocellulose in corn cob by using food waste pretreatment to improve anaerobic digestion performance. J. Environ. Manag. 2020, 254, 109830. [Google Scholar]
- Guo, X.; Shu, S.; Zhang, W.; Wang, E.; Hao, J. Synergetic degradation of corn cob with inorganic salt (or hydrogen peroxide) and electron beam irradiation. ACS Sustain. Chem. Eng. 2016, 4, 1099–1105. [Google Scholar]
- Liao, M.H.; Jin, R.T.; Ren, H.W.; Shang, J.Q.; Kang, J.X.; Xin, Q.Y.; Liu, N.; Li, M. Orthogonal experimental design for the optimization of four additives in a model liquid infant formula to improve its thermal stability. LWT–Food Sci. Technol. 2022, 163, 113495. [Google Scholar]



| Materials | Hemicellulose (%) | Cellulose (%) | Lignin (%) | CC Conversion (%) | Cellulose Recovery(%) | Lignin Removal (%) | Hemicellulose Removal (%) | RSY (%) |
|---|---|---|---|---|---|---|---|---|
| Rice straw | 21.50 ± 1.00 | 39.86 ± 0.89 | 25.40 ± 1.17 | 44.87 ± 0.73 | 71.12 | 17.37 | 78.05 | 19.47 ± 0.21 |
| Wheat straw | 28.64 ± 0.93 | 35.13 ± 0.65 | 22.50 ± 1.02 | 46.55 ± 0.95 | 70.19 | 19.66 | 75.97 | 27.58 ± 0.18 |
| Corn stalk | 30.65 ± 0.42 | 36.43 ± 0.72 | 19.42 ± 0.90 | 44.32 ± 1.43 | 69.31 | 18.94 | 77.36 | 19.20 ± 0.62 |
| Corn cob | 32.50 ± 1.22 | 36.92 ± 0.74 | 18.22 ± 0.81 | 48.86 ± 0.93 | 68.54 | 20.38 | 80.29 | 32.42 ± 0.45 |
| Rape stalk | 23.13 ± 1.05 | 47.89 ± 0.99 | 19.07 ± 0.85 | 45.73 ± 1.26 | 72.98 | 18.87 | 77.32 | 20.77 ± 0.36 |
| Sample | Hemicellulose (%) | Cellulose (%) | Lignin (%) | CC Recovery (%) | Hemicellulose Removal (%) | Cellulose Recovery (%) | Lignin Removal (%) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|---|---|---|
| CC | 32.50 ± 0.62 | 36.92 ± 1.34 | 18.22 ± 0.90 | - | - | - | - | 2.23 |
| PHR | 2.53 ± 0.11 | 53.78 ± 1.68 | 30.76 ± 1.53 | 38.65 | 96.18 | 56.30 | 33.06 | 3.50 |
| PHRD | 0 | 78.13 ± 1.90 | 0 | 22.15 | 100 | 46.87 | 100 | 4.81 |
| Number | Factors | Results | ||||
|---|---|---|---|---|---|---|
| A Temperature (°C) | B Solid–Liquid Ratio | C Time (h) | D Solid–Solid Ratio | Cellulose Conversion (%) | CC Conversion (%) | |
| 1 | 1 (140) | 1 (1:8) | 1 (1) | 1 (2:10) | 13.57 | 17.39 |
| 2 | 1 (140) | 2 (1:9) | 2 (2) | 2 (3:10) | 36.63 | 40.42 |
| 3 | 1 (140) | 3 (1:10) | 3 (3) | 3 (4:10) | 27.39 | 44.23 |
| 4 | 2 (150) | 1 (1:8) | 2 (2) | 3 (4:10) | 11.92 | 29.00 |
| 5 | 2 (150) | 2 (1:9) | 3 (3) | 1 (2:10) | 18.53 | 47.06 |
| 6 | 2 (150) | 3 (1:10) | 1 (1) | 2 (3:10) | 9.84 | 21.03 |
| 7 | 3 (160) | 1 (1:8) | 3 (3) | 2 (3:10) | 15.37 | 43.53 |
| 8 | 3 (160) | 2 (1:9) | 1 (1) | 3 (4:10) | 20.75 | 51.16 |
| 9 | 3 (160) | 3 (1:10) | 2 (2) | 1 (2:10) | 18.74 | 47.55 |
| Cellulose conversion | k1 | 25.86 | 13.62 | 14.72 | 16.95 | |
| k2 | 13.43 | 25.30 | 22.43 | 20.61 | ||
| k3 | 18.29 | 18.66 | 20.43 | 20.02 | ||
| Range R | 12.43 | 11.68 | 7.71 | 3.66 | ||
| Primary and secondary order | A > B > C > D | |||||
| Optimal horizontal combination | A1B2C2D2 | |||||
| CC conversion | k1 | 34.01 | 29.97 | 29.86 | 37.33 | |
| k2 | 32.36 | 46.21 | 38.99 | 34.99 | ||
| k3 | 47.41 | 37.60 | 41.46 | 41.46 | ||
| Range R | 15.05 | 16.24 | 11.60 | 6.47 | ||
| Primary and secondary order | A > B > C > D | |||||
| Optimal horizontal combination | A3B2C3D3 | |||||
| Reaction Times | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| CC conversion (%) | 40.42 | 18.95 | 12.72 | 8.37 | 5.24 |
| Cellulose conversion (%) | 36.63 | 16.89 | 10.27 | 8.30 | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, X.; Zhu, L.; Liang, J.; Zhu, J. Production of Hemicellulose Sugars Combined with the Alkaline Extraction Lignin Increased the Hydro-Depolymerization of Cellulose from Corn Cob. Sustainability 2023, 15, 9041. https://doi.org/10.3390/su15119041
Yang X, Li X, Zhu L, Liang J, Zhu J. Production of Hemicellulose Sugars Combined with the Alkaline Extraction Lignin Increased the Hydro-Depolymerization of Cellulose from Corn Cob. Sustainability. 2023; 15(11):9041. https://doi.org/10.3390/su15119041
Chicago/Turabian StyleYang, Xiaorui, Xiaotong Li, Liyan Zhu, Jinhua Liang, and Jianliang Zhu. 2023. "Production of Hemicellulose Sugars Combined with the Alkaline Extraction Lignin Increased the Hydro-Depolymerization of Cellulose from Corn Cob" Sustainability 15, no. 11: 9041. https://doi.org/10.3390/su15119041
APA StyleYang, X., Li, X., Zhu, L., Liang, J., & Zhu, J. (2023). Production of Hemicellulose Sugars Combined with the Alkaline Extraction Lignin Increased the Hydro-Depolymerization of Cellulose from Corn Cob. Sustainability, 15(11), 9041. https://doi.org/10.3390/su15119041





