Abstract
In light of pollinator decline, plant species suitable for the restoration and conservation of pollinators need to be selected. In this systemic review, we concentrated on the importance of NFWV (non-forest woody vegetation, i.e., linear or grouped trees/shrubs) for pollinators across agricultural landscapes. In the temperate climate zone, flowering trees and shrubs provide nectar sugar (energy) and pollen (nutrients) for managed and wild pollinators. They also create nesting niches and serve as host plants that support the full life cycle of wild pollinators. The creation of woodland strips/groups is a cost-effective and time-saving strategy to improve self-repeatable nectar and pollen resources on a landscape scale. The spatial distribution of NFWV can help to create an entire network of pollinator-friendly habitats. NFWV can support the continuity of food resources outside of the crop flowering season, i.e., during seasonal bottlenecks. This concept also offers other environmental benefits (e.g., water and air quality improvement, climate mitigation). However, future work should address the usefulness of trees/shrubs for different crops and regions to develop a network of flower-rich corridors. Likewise, more advanced and detailed studies are necessary to determine the qualitative characteristics of nectar and pollen, which may result in optimization of pollinator nutrition.
1. Introduction
Pollinators (mainly insects) are crucial for sexual reproduction of ca. 87% of wild angiosperm flora occurring in terrestrial ecosystems [1,2]. They are also critical for commercial production, as ca. 35% of the global crop production volumes worldwide depend on pollination [1,3]. In Europe, 85% of 264 cultivated crops benefit from insect pollination [4]. The annual economic value of the insect pollination service is estimated at ca. USD 235–580 [5]. The demand for pollinator service is expected to increase over the coming decades due to the expansion of entomophilous crop areas associated with the rising food demands of the growing human population [4,5,6,7,8]. However, populations of both wild and managed pollinators are facing serious challenges and are under threat in many parts of the world, which poses potential risks for human food security [9].
There are multiple contributors to the pollinator decline, i.e., the intensification of agricultural production (the use of pesticides, herbicides) [6,9], the spread of diseases and parasites [10], climate change [11,12], invasive species, and even light pollution [13]. However, the degradation and fragmentation of natural habitats and the consequent shortages of nesting and mating sites as well as food scarcities and inadequate nutrient composition are mentioned as the leading factors in limiting pollinator populations [8,14,15].
In Europe, multiple actions were taken to balance the negative environmental effects caused by intensive agricultural practices on biodiversity and ecosystems. Agri-environment schemes (AES) were introduced as early as in the 1980s [16,17,18]. Initially, the AES were focused on the protection of species (e.g., Birds Directive) and habitats (Habitats Directive) [19]. Subsequent strategies emphasized the need to improve ecosystem services (e.g., pollination) [3,15]. With regard to the pollination service, local management practices on farms (e.g., organic farming) and management of habitats in the surroundings of fields (e.g., implementation of multi-flower strips in field margins, restoration of meadows) were implemented as part of large-scale programs [3,20]. However, due to great flexibility in the agri-environment strategies and differences in priorities between countries, the effectiveness of the introduced programs varied substantially [18]. In general, environmental improvement has been restricted to local farms/habitats, whereas the advantages in the landscape scale were less pronounced [21,22].
In 2018, the Pollinator Initiative was launched in the EU, focusing on the integration of policies as part of the EU biodiversity strategy [23]. Three main directions were highlighted, i.e., maintenance/restoration of pollinator-friendly habitats, rational use of pesticides, and control of invasive species in order to tackle the decline of pollinators [24].
Currently, the environmental policy (EU Farm to Fork Strategy; European Green Deal) addresses the support for long-term sustainability and focuses on integration of environmental, economic, and social goals [25]. It is highlighted that cost-effective and time-saving actions are required to restore the connectivity of ecosystem services across landscapes [15].
To support pro-ecological systems and goals, restoration and management of non-forest woody vegetation (NFWV) are proposed to improve the structural and functional diversity of agricultural landscapes [17,26,27,28,29,30,31,32,33,34,35,36,37]. Various studies have indicated that NFWV located in mid-fields, meadows, and pastures and along roads, field margins, and river banks can fulfill multiple ecosystem functions (e.g., improvement of air and water quality, capture of soil pollutants, carbon storage, climate regulation, biocontrol) and enhance crop/livestock productivity [19,28,35,38,39,40,41,42,43]. NFWV can also considerably contribute to pollinator abundance and diversity by securing the food supply and ensuring its continuity and complementarity with the resources available in other habitats or crops [30,44]. Moreover, trees and shrubs provide diversity of nesting and mating sites [35,38,45,46,47,48,49,50,51,52].
However, the use of trees and shrubs to improve pollinator food resources requires information on the quantity and quality of nectar and pollen available in their flowers [14,22,44,53,54]. Such knowledge will allow rational management of resources and help to implement strategies for pollinator conservation [9,55,56,57].
The aim of this paper was to investigate the importance of woody species (trees and shrubs) for supporting insect pollinator food resources in agricultural areas. In particular, we focused on the quality and quantity of nectar and pollen supplied by non-forest woody vegetation. We also revised other benefits of trees/shrubs for pollinators. This information can be used by landscape planners, land-use decision makers, government agencies, landowners, and farmers who can be involved in creation and implementation of pollinator-friendly agri-environmental strategies. We also tried to outline knowledge gaps to be addressed in future experimental studies.
2. Methods
We followed the instruction and recommendations described by Mengist et al. [58], identifying records through database screening, obtaining full-text articles and text analysis characteristics for the systematic review. Research papers published in English and in Polish were browsed using electronic databases (Web of Science, Scopus, ResearchGate, and Google Scholar). The search was carried out from September 2022 to January 2023. The papers were searched using the following keyword combinations: ‘non-forest woody vegetation’, ‘woody vegetation’, ‘trees and shrubs’, ‘agroforestry, ‘shelterbelts’, ‘hedgerows or hedge’, and ‘conservation’, in combination with the following keywords: ‘nectar and pollen’, ‘floral resources’, ‘food resources’, ‘phenology’, and ‘pollinators’ (honey bees, bees, flies, moths, wasps, beetles or Apis mellifera, Bombus, Diptera, Syrphidae, Coleoptera). Original research papers and review papers were included. Papers presenting relevant research identified based on the authors’ knowledge were included as well. These papers presented data collected in our research unit. We also included key articles related to the impact of climate change on nectar and pollen resources that can be important for plant–pollinator relationships. Moreover, key papers focused on the environmental impacts of trees/shrubs were incorporated. For the introduction section, to show changes in the approaches to environmental services, we screened crucial articles that discussed the European programs designed and performed to counteract pollinator decline.
The following steps were performed when collecting the data: (i) search in databases and other sources, (ii) initial screening of records for data, (iii) article qualification (eligibility/exclusion) for the review purpose, (iv) analysis of articles necessary for the synthesis, (v) synthesis of the information and writing the article.
The database searching and screening involved: (i) reading abstracts to identify irrelevant articles, (i) assessment of the full-text version of articles for their fulfillment of the inclusion criteria.
We tried to identify the widest possible variety of relevant studies with the goal of reducing bias. The inclusion criteria covered: (i) field studies on nectar and pollen production in native tree/shrub species, and all information on their attractiveness to insect pollinators that might have an impact on plant selection for food resources management, (ii) data relevant to the temperate climate zone in Europe, (iii) language—articles in English and Polish were considered, (iv) articles published between 1972–2023 were included. Such a wide time range was considered due to the lack of more contemporary studies in relation to nectar/pollen qualitative/quantitative characteristics in woody species. However, the articles published before 2010 constituted only 22.8% of the entire set of the articles reviewed in this study. The exclusion criteria comprised: (i) studies on nectar and pollen production by exotic tree/shrub species/cultivars, (ii) data from non-European countries.
Initially, we searched 521 papers. However, we only considered papers on NFWV characteristics for the temperate climate zone. Therefore, we finally included 176 papers in the synthesis.
3. Food Resources
3.1. Nectar and Pollen
Several studies have documented that NFWV provides food for managed and wild pollinators [39,59,60,61]. In terms of the type of resources, trees and shrubs that develop entomophilous flowers provide nectar and pollen (e.g., willow—Salix L., plum—Prunus L., lime tree—Tilia L., maple—Acer L., horse chestnut—Aesculus L., and locust—Robinia L.) or produce only pollen (e.g., roses—Rosa spp.) [62,63,64,65]. However, many insect pollinator taxa also feed on wind-pollinated plants, where they collect floral pollen, or use non-floral resources (resins) [66]. The role of anemophilous woody taxa is limited; nevertheless, wind-pollinated plants in some regions and seasons provide essential food resources, e.g., hazelnut (Corylus avellana L.), common alder—Alnus glutinosa or elms (Ulmus spp.) (reviewed in Saunders) [62,67,68,69]. However, there are also reports that woody wind-pollinated taxa are avoided by pollinators, e.g., elder—Sambucus nigra L. [31].
Nectar is a sugar-rich water solution consisting mainly of sugars (glucose, fructose, sucrose) and serving as an energy source for insects [23,29,36,48]. The nectar sugar composition and the ratio of sucrose to hexoses (sucrose/(glucose + fructose)) differ between plant species [67,68]. However, the data on the nectar composition in tree/shrub species are very scarce. As reported by [70], sucrose-rich nectar (S/G + F ratio between 0.5–0.99) is produced by the flowers of Acer spp. Aesculus, Tilia cordata, and Robinia pseudoacaccia.
Nectar may also contain small amounts of amino acids, mineral salts, dyes, and fragrance compounds [67,68]. Naef et al. [71] identified monoterpenes (ethers, aldehydes, acids), isoprenoids, and phenols among the volatile compounds in the nectar of lime—T. cordata.
Nectar sugar concentrations differ considerably in the flowers of woody plants, e.g., ca. 15–35% of sugars was detected in lime tree (Tilia spp.), ca. 15–60% in bird cherry (Prunus padus Mill.), and ca. 50–70% in horse chestnut (Aesculus spp.) nectar [72,73,74].
Woody plant species are known to offer abundant amounts of sugars in their nectar, e.g., [67,69,70,73,74,75,76,77,78,79,80,81,82]. For example, the total sugar content was 10–40 mg per floral unit in willow (Salix spp.), 0.57–0.65 mg per flower in maple (Acer spp.) [80], 0.61–0.78 mg per flower in plum (Prunus spp.) [76], 1.28–2.2 mg per flower in black locust (Robinia pseudoaccacia L.) [70,73], and 5.7–6.6 mg per flower in Tilia platyphyllos Scop. [74]. These values are higher than those noted in the flowers of herbaceous plants. For comparison, the sugar content in common herbaceous species was 0.07–0.14 mg per flower in red clover (Trifolium pratense L.) [78] or ranged from 0.15 to 0.48 mg per flower in oilseed rape (Brassica napus L.) [83].
At the species level, the total amount of food resources available is related to the nectar/pollen amount per floral unit (=flower/inflorescence) and abundance of flowering (=number of developed flowers) of an individual tree/shrub [63]. Woody plant species are known to flower abundantly; however, they show considerable year-to-year differences related to alternating flowering [63,64,75,77,78,84]. For instance, Tilia cordata can develop from 121,000 to 1,473,000 flowers per tree [85]. Accordingly, one Tilia tree can produce 0.3–2.0 kg of sugars [78]. Data on the nectar sugar productivity of particular tree species are presented in Figure 1.
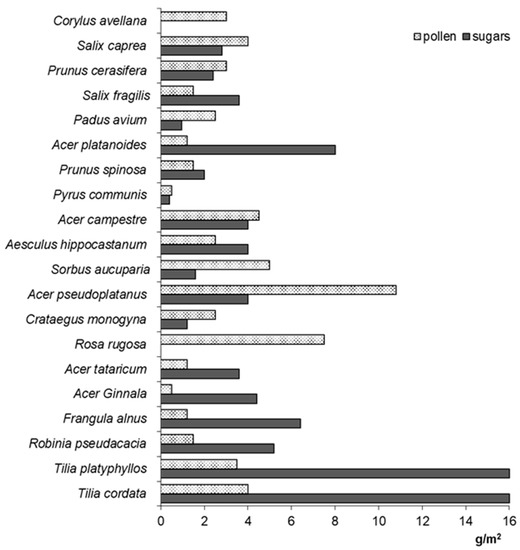
Figure 1.
Sugar and pollen production in several woody plant species; based on [8,9,62,63,64,65,67,69,70,74,75,76,77,78,79,80,81,83,85].
Pollen is regarded as a determinant of pollinator development, immunocompetence, and health [86,87]. It is a critical component in the diet of all life stages of bees and several wasp species [88]. Another category includes wasps, beetles, flies, butterflies, and moths feeding on pollen only in the larval or adult stage [55,67].
Researchers agree that both the quantity and quality of pollen resources play an important role in proper pollinator nutrition [14,85]. The amount of pollen available in flowers varies considerably between plant species and is positively correlated with the mass of pollen produced in anthers and the number of developed anthers [63]. For example, the number of anthers in small-leaved linden—T. cordata L.—was 21–30, while the pollen content ranged from 0.05 to 0.5 mg per flower [89]. The flowers of Crimean linden—T. x euchlora K. Koch—with ca. 40 developed anthers produced ca. 0.8 mg of pollen per flower [85]. Multi-anther rose flowers (with 82–260 anthers per flower) are known to be very effective pollen producers, e.g., 3.3 mg of pollen per flower was found in dog rose—Rosa canina L.—and even 26.7 mg of pollen per flower was produced by beach rose—R. rugosa Thunb. [90]. For comparison, pollen production in common herbaceous species ranged from 0.01 to 0.02 mg per flower in red clover—Trifolium pratense—or from 0.3 mg to 0.5 mg per flower in wild mustard—Sinapis arvensis L. [63]. However, although woody species produce high amounts of pollen, some of them are not attractive to bees. For example, elder (Sambucus nigra) pollen contains a high concentration of cyanogenic glycosides, which repel bee pollinators but attract flies [91].
Total pollen resources calculated per tree fluctuate between years, which is significantly related to the high variability in blooming abundance [63,64,77,85]. Year-to-year differences of blooming varied from 473,000 to 2,892,000 flowers per Tilia insularis tree; consequently, the total pollen production ranged from 167 g to 1.032 g per one tree [85]. The pollen production by horse chestnut—A. hippocastanum—may be in the range of 12–25 g per tree between growing seasons [77]. Pollen production in several wood species is shown in Figure 1.
In terms of quality, pollen is rich in protein, lipids, macro- and microelements, vitamins (mainly D, E, A), and hormones [72,92]. The nutritional value of pollen is mostly based on its crude protein content [63,92,93]. Depending on the botanical origin, the protein content in pollen ranges from 2.9% to 60% [94]. Typically, pollen from different plants has a similar composition of amino acids and does not differ significantly in their proportion [70,92,95]. In general, the data on the pollen quality in woody plants are rather scarce. According to protein content, Di Pasquale [87] ranked analyzed species as follows: Cistus > Erica > Castanea > Rubus (with the protein content of 12–22%). High-quality pollen (with high protein and lipid concentrations) is produced by willow (Salix spp.), maple (Acer spp.), plum (Prunus spp.), chestnut (Castanea spp.), and ash (Fraxinus spp.) [63,87].
The quantity of macro- and microelements in pollen is related to the botanical origin [96]. As reported by Filipiak et al. [14], the concentration and proportion of elements in pollen play a great role in balancing pollinator diets. In particular, the scarcity of Na, S, Cu, P, and K (and possibly Zn and N) in pollen has an adverse effect on insect growth and development [53,55]. Recently, it has been emphasized that ‘key pollen species’ rather than pollen diversity are required for proper development, growth, and survival of pollinator individuals and colonies [55,97]. Among woody plant species, blackberry (Rubus ulmifolius Schott) and walnut (Juglans nigra L.) are suggested to be used in order to promote the nutritional balance of the insect pollinator diet [55].
3.2. Seasonal Availability of Food Resources during the Flowering Season
In addition to the quantity and quality of food resources, the continuity of the food supply over the foraging season is important [84]. In the temperate climate zone, seasonal bottlenecks in food continuity are noted, in particular in early spring (March–April) [32,33,98], early summer (June/July) [44,99], and late summer (August) [34,100,101]. In particular, the first seasonal food bottleneck is dangerous, as insects establish their nests (e.g., bumblebee queens, solitary bees, Osmia, Andrena), and the lack of resources results in high mortality rates [32,44].
It is accepted that patches of non-forest woody vegetation with adequate spatial distribution can support seasonal continuity of floral resources [32,102,103,104]. In Switzerland, the combination of crops and grasslands with tree species (e.g., fruit trees, woody vegetation) is [105] recommended. In Poland, six to nine patches (0.025–0.3 ha each) of diverse vegetation types (natural, seminatural, non-cropped vegetation) within an area of 100 ha is suggested to obtain food resource seasonal stability [99]. It has been highlighted that trees and shrubs provide the first nectar and pollen resources in Central Europe (Poland) and England [44,84,106,107]. In these regions, early spring pollen (February–March) is provided by hazelnut (Coryllus avellana L.). Its pollen is willingly collected by honey bees and Osmia spp. [20,102,108] Willows (e.g., Salix caprea L.), maple (Acer platanoides L.), and wild plums (Prunus spinosa L. and P. cerasifera Ehrh.) are recommended for nectar and pollen enrichment in April [63,78]. In the end-April–May period (during mass blooming of rapeseed/fruit trees in orchards), food resources are sufficient to meet pollinator demands [84,106]. However, to support food resource diversity, white willow—Salix alba L., crack willow—S. fragilis L., field maple—Acer campestre L., sycamore—A. pseudoplatanus L., bird cherry—Padus avium Mill., apple—Malus Mill., cotoneaster—Cotoneaster Medic., and horse chestnut—Aesculus hippocastanum L. are proposed to be used in mid-Europe [77,80,106], Scotland [109], and England [110]. Common hawthorn—Crataegus monogyna Jacq., black locust—R. pseudoaccacia, and alder buckthorn—Frangula alnus Mill. are suggested to fill the late-spring gap (end of May–June), related to the end of flowering of orchard species/rape [111]. Buckthorn is particularly valuable, as it blooms even until August [65]. Species from the genus Tilia (native and exotic) are recommended to support sugar and pollen resources in the summer period (July–August) [78,85]. However, the list is open and there are also other woody species that are not included in the reviewed literature but possibly provide forage resources.
4. Nectar and Pollen Insect Collectors
In tropical ecosystems, lizards, birds (hummingbirds, sunbirds), or mammals (bats, monkeys) are insect pollinators. In turn, insects, e.g., bees, flies, wasps, butterflies, moths, and beetles, are the main native pollinators in temperate regions [62].
The plant–pollinator relationships are multi-factorial. Briefly, flower morphology (corolla depth), color, and scent and the characteristics of floral rewards (nectar, pollen, oils) determine the accessibility to the flower rewards and their attractiveness [62,112]. Consequently, pollinators have evolved diverse strategies, and their behavior is focused on resource exploitation in a temporally and spatially changing habitat [62]. Their adaptations are related to morphological traits (e.g., proboscis length, body size) and behavioral (night vs. day time activity; preferences towards flower color, scent) and/or physiological adaptations (e.g., temperature tolerance, energy expenditure) [45,52,62,86,91,97,98,101,112]. Typically, in making feeding decision, bees prefer yellow and blue flowers and choose nectar with a 50–60% sugar concentration, while butterflies favor red flowers with a 35% concentration of nectar sugar [62,67,113]. With regard to pollen reward, there is evidence suggesting that bumble bees preferably collect pollen with higher protein than lipid content (P:L ratio 5:1 or 10:1), reviewed in [112,114].
Most insects collect nectar and pollen from a wide range of plant species and flower types (generalist pollinators) [54,115]. There are also specialized (oligolectic) pollinators, which entirely depend on specific plant species [116]. Considering generalist pollinators, honey bees and bumble bees use floral resources available in entomophilous trees and shrubs [35,60,117,118,119]. In particular, flowers of willows—Salix, plum—Prunus, linden—Tilia, maple—Acer, horse chestnut—Aesculus, hawthorn—Crataegus, and locust—Robinia are willingly visited by bees [32,52,68,89]. Additionally, pollinators can use pollen from anemophilous woody species, e.g., hazelnut—Corylus avellana, oak—Quercus L., and ash—Fraxinus L. pollen, is collected by honey bees [108,120], while Osmia spp. collect Betula, Juglans, Populus, and Quercus pollen [34,90,91,121,122]. Many studies confirm that butterflies [36,37,46,103,123] and flies [104,124,125,126,127] feed on nectar produced by entomophilous flowers of trees and shrubs. It is known that syrphid flies willingly collect pollen from wind-pollinated taxa, e.g., common alder—Alnus glutinosa L. and C. avellana [127,128]. Based on the results obtained in England, Donkersley [22] indicated that tree-derived pollen was overrepresented in the honey bee diet, compared to the vegetation cover noted across the landscape (e.g., in the landscape dominated by grasslands). Therefore, high preference of honey bees for tree pollen is suggested.
As reported by Fowler [129], ca. 15% of wild bee species are pollen specialists. The specialization can be very narrow (oligolecty) with preference for feeding on a single family/genus of flowering plants [130]. Such specialization can refer to ‘floral constancy’. In particular, wild bee specialists are associated with entomophilous species, e.g., Andrena apicata (Andrenidae) feeds on species from the genera Salix, while Colletes hederae (Colletidae) prefers collecting pollen from Hedera helix (Araliaceae) [131]. Specialized insect pollinators are more susceptible to the habitat or limited by losses of floral resources than polylectic species [132]. The destruction of their nutritional, nesting, or mating niches may even lead to their extinction. The failure of the mutualistic plant–pollinator interaction has a negative impact on the plant population strength and can result in their disappearance [21].
5. Nesting Niches
Besides the food niche component, nesting, mating, and sheltering niches are crucial for wild pollinators [47]. Generally, insect pollinators benefit from the location of their nesting sites close to abundantly flowering vegetation patches, including woody vegetation [7,133,134,135]. It is estimated that ca. 80% of wild pollinators are ground-nesting species. Other insect pollinators nest above the ground, often forming their nests in tunnels made in soft woody plant stems [48,136].
Ground-nesting insect pollinators benefit from the limitation of soil disturbance in the surroundings of hedgerows/woodlots [45,46,47].
Considering above-ground pollinators, the diversity of woody plant species and the presence of tree holes and dead trees increase the number of their nesting sites [137]. Especially, Japanese wax tree (Rhus spp.), black elder (Sambucus nigra), and Rubus spp. (raspberries, blackberries) have a beneficial effect on nesting sites and consequently are advantageous for pollinator abundance [48]. It has been shown that NFWV even has a positive impact on the availability of nesting sites found in adjacent meadows [49,50,51,52]. Moreover, trees occurring in the vicinity of crops were found to improve indirectly the efficiency of pollination services in agroecosystems [105]. However, opposite results showing no positive effects of NFWV on the occurrence of nesting places and pollinator abundance were reported as well [47,138]. In addition to nesting niches, non-forest woody vegetation gives an opportunity for hibernation and overwintering [47].
In addition to pollinators, native woody plant species exert positive effects on other organisms important for agroecosystems. They serve as host plants for natural enemies of pests (e.g., syrphid flies, beetles, moths, and butterflies) and contribute to their good potential to be used as biological control of crops [30,139,140].
6. Refuge Areas and No-Spray Buffer Zones
All insecticides are potentially poisonous although their toxicity depends on the type, timing, and doses of application [141]. Particularly dangerous are systemic insecticides, e.g., imidacloprid [142]. Pesticides reduce insect pollinator survival and have a negative impact on pollinator food plants. Their droplets can get into nectar or adhere to pollen [143]. Systemic pesticides can also contaminate nectar and pollen via the vascular system in plants [144]. Evidence suggests that contaminants from nectar and pollen negatively influence the immunocompetence and health of pollinators [145].
At the landscape scale, an increase in the proportion of ecological islands (=non-cropped areas) can buffer the effect of pesticides on pollinators in agricultural areas [146]. Among ecological islands, non-forest woody vegetation can act as no-spray buffer zones providing pollinators with a refuge and protecting them from pesticide spraying [117]. One of the widespread opinions is that NFWV can help reduce exposure of pollinators to pesticides [35]. However, appropriate timing and location of spraying as well as environmental monitoring are especially required to minimize the exposure effect [141]. Spray deposition can differ with distance, air humidity, and wind speed [146]. As reported by Kjaer et al. [142], buffer zones made of ca. 12 m long hedgerows can reduce spray deposition in nearby areas by ca. 70%. It is recommended that trees and/or shrubs chosen for buffer zones should be less attractive for pollinators or should not flower during the pesticide application time [144,145].
7. Environmental Effects
7.1. Mitigation of Climate Warming
Climate change refers to the air temperature rise and extension of drought periods noted across many areas worldwide [147]. These changes can generate a threat for the plant–pollinator interaction [12,67,112,148,149,150]. Firstly, phenological acceleration of flowering of plant species can potentially escalate the incompatibilities between plants and pollinators [150,151]. This may exert an impact on changes in the structure of mutualistic networks and may aggravate the food scarcity, especially in early spring [12,151]. Secondly, the temperature rise trend combined with precipitation shortages induces changes in the characteristics of floral rewards [63,67,148,151,152,153]. It is widely known that the nectar volume, nectar sugar concentration, and sugar composition are influenced by temperature [68,149]. Investigations carried out in climatic chambers have documented that heat stress decreases nectar and sugar production in perennial forbs [152]. Similar effects can be expected in tree species, although there are very scarce data on the impact of temperatures higher than optimal on nectar/sugar productivity in woody species. In Tilia flowers, the nectar sugar production may differ in the range of 100–300% between seasons; moreover, at high temperatures combined with low air humidity, the nectar of Tilia species may be highly concentrated (up to 70%) and may thus be avoided as unattractive and difficult to collect [74]. Data from Denmark revealed that the lack of rainfall before flowering diminished the sugar secretion rate in heather (Calluna vulgaris) [57]. Elevated air temperature can also exert a negative effect on pollen performance, i.e., anthers produce less pollen (decrease of ca. 30–80%) that is less viable and/or less likely to germinate [63,154].
Another unfavorable aspect of climate change is drought caused by prolonged periods of precipitation shortages and lack of snow cover [147]. Mainly, the reduction of photosynthetic resources related to the decrease in water availability has a negative effect on plant characteristics vital for plant–pollinator interactions [153]. It is reported that drought stress substantially reduces the flower life-span and floral display size (size of flowers, inflorescences) [150], has an impact on the composition and proportions of floral volatiles, and reduces the abundance of blooming (=number of developed flowers) [63,155]. Consequently, flower attractiveness for pollinators may decrease, and, as a result, the pollination effectiveness may decline [112,150]. This may have negative consequences for the maintenance of plant populations [21]. The reduction in total nectar sugars and pollen productivity may also exacerbate nutrition problems and exert immediate effects on insect pollinator behavior (activity, energetics, health) [156].
Non-forest woody vegetation provides shade under the canopy; hence, it plays an important role in reducing heat stress in plant populations and pollinators [40]. Mitigation of pollinator heat stress is particularly important in the scenario of global warming (temperature rise, humidity decrease) [148]. Under the shade of shrubs/trees, the activity of pollinators increases; therefore, this type of vegetation may have a positive effect on pollination services during periods of extreme temperatures [151]. The shade provided by non-forest woody vegetation also has a positive effect on the nectar production by herbaceous annuals and perennials (both wild species and crops) [152].
7.2. Landscape Scale Connectivity
It is accepted that patches of non-forest woody vegetation can increase landscape connectivity by providing habitat for feeding, nesting, and other life activities [157]. One widespread option is that NFWV across the landscape supports the abundance, richness, and diversity of pollinators as well as their long-term population dynamics [158,159]. These vegetation types support creation of a network of ecological corridors that can enhance movements of pollinators and natural enemies across fragmented habitats [160]. It is also underlined that, in a patchy landscape, pollinators can fly between the patches more easily and thus promote long-distance pollen dispersal [161,162]. For example, the corridor effect of hedgerow infrastructure on the movement of diverse pollinator groups, e.g., bees, including less-common wild native bees [159,163,164], butterflies [165], flies [166,167], and carabid beetles [168], has been documented. In the case of syrphid flies, a network of hedgerows is known to act as a functional biological corridor if the hedgerows grow in connection with forests [167], but see [47].
The corridor effect of NFWV within the modern landscape can enhance pollination services, e.g., increase the frequency of pollinator visits and pollination efficiency [169]. In the Mediterranean region, an increase in hedgerows to 6% of the landscape cover resulted in a considerable increase in pollination of entomophilous crops and increased their yield (by approx. 70%) [41]. An increase in the commercial crop value (up to 60%) in strawberry plantations adjacent to hedgerows was reported by Castle et al. [42]. As suggested by these authors, such a result is related to increased pollinator abundance and flight mobility. Hedgerows have also been reported to exert a positive effect on pollen transfer and reproductive success of wild plant species (e.g., Salvia pratensis L., Lamiaceae) [163].
However, a barrier effect of NFWV (e.g., linear hedgerows) has also been documented. A dense hedgerow network has been shown to reduce pollen transfer [43]. The barrier effect of hedgerows was documented in the case of movements of butterflies, beetles, dipterans [43], and highly mobile bees [46,164]. Impairment of pollen transfer is presumably associated with blockade of pollinator movements and pollen dispersal in the landscape matrix [46,164]. It is emphasized that especially tall and dense woody vegetation patches are likely to act as a barrier to pollinator flights and pollen dispersal within the landscape matrix [41]. The <2 m height of hedgerows can minimize their barrier effect. The orientation of woody species rows is also of great importance, as it can enhance or limit the mobility of insect pollinators [165]. Unfavorable effects on pollinator communities and subsequent crop pollination service delivery have been documented for conifer hedgerows, which not only create barriers but intensify the effect of food deficiency [127].
8. Management Strategies
The data on total available food resources and their seasonal distribution are necessary for an optimal management strategy to support pollinators at the landscape scale. However, effective management of food resources should consider the complexity of the plant–pollinator relationship [45,52,62,86,97,98,100,101,112]. Optimal food resource management for pollinators in the agricultural landscape has to deal with pollinator behavior, their nutritional requirements, nutritional preference, flight energetics, and flight distance [115,116,170,171].
Planting trees/shrubs is a cost-effective and time-saving strategy providing long-term effects [15,44,103,172]. An advantageous trait of trees and shrubs is their year-to-year spatial resource repeatability [44,63].
Traditionally, native plant species are regarded to be best adapted to local pollinators and environmental conditions [173]. In view of the current climate change, enrichment of local flora with woody species/cultivars adapted to expected changes is recommended for improvement of resources [174]. In turn, alien species with invasive potential should be avoided or introduced with caution, especially when they cannot be replaced [175]. The extensive use of wind-pollinated conifers with nectarless flowers (e.g., Thuja spp., Monterey cypress—Hesperocyparis macrocarpa) should be systematically replaced by more attractive food species, in particular by native woody species. In areas that are particularly neglected and poor in food flora, trees and shrubs should be planted systematically, bearing in mind that the effects will be distant in time, as most tree species bloom for the first time after 5–10 years and reach full flowering potential after ca. 20 years. Therefore, the beneficial assumptions of NFWV should be expanded to include herbaceous vegetation.
The selection of trees and shrubs for improvement of food resources should also consider the regional character of the landscape. Among woody species, willows (Salix spp.), maple (Acer spp.), sour cherry (Prunus spp.), horse chestnut (Aesculus spp.), hawthorn (Crataegus spp.), European bird cherry (Prunus padus), locust (Robinia spp.), and lime tree (Tilia sp.) are especially recommended for England and Central and Eastern Europe [22,28,44,106]. Additionally, Japanese wax tree (Rhus spp.), black elder (Sambucus nigra), and Rubus spp. (raspberries, blackberries) have to be retained within the landscape, as these species have an advantageous effect on pollinator abundance through the provision of nesting sites [48].
Moreover, when choosing nectar and pollen-yielding woody species, other qualities should be considered in order to maximize ecological benefits (e.g., improvement of air and water quality and retention, carbon sequestration, reduction of wind speed) as well as improvement of the esthetic appearance of the landscape [176] (Table 1).

Table 1.
Importance of non-forest woody vegetation for insect pollinators and pollination service in the agricultural landscape of the temperate zone.
9. Concluding Remarks
In conclusion, maintenance of the diversity of entomophilous plant species and sustainable agriculture needs pollinators. The decline in pollinator abundance requires continued efforts to stop this unfavorable trend recorded worldwide. Establishing woody species is a cost-effective concept that may help to increase the quality of the habitat for bees and non-bee pollinators by providing key food resources with year-to-year repeatability. Trees may also contribute to mitigation of food gaps over pollinator seasonal activity. Planting trees/shrubs together with other management types (restoration of semi-natural meadows, implementation of multi-flower strips in field margins, organic farming) is expected to lead to cumulative advantageous effects and reduction of pollinator malnutrition. Simultaneously, non-forest woody vegetation provides multiple environmental benefits (climate mitigation, water retention improvement, removal of pollutants from air and soil) and enhances crop/livestock productivity.
However, there is still much to be learned about the interaction between pollinators and woody plant species. Further studies should be conducted to determine which tree/shrub species/cultivars should be prioritized for pollinator conservation. In particular, we need more phenological knowledge to indicate trees/shrubs species that can ensure floral continuity with a reduced overlap with flowering crops and suitability for different climate regions. Moreover, more advanced and detailed research is necessary to determine the qualitative characteristics of nectar and pollen in native and alien woody species, which will allow optimization of pollinator nutrition.
Author Contributions
M.B.—investigation, B.D.—conceptualization, methodology, supervision, writing; M.S.-A.—investigation, visualization; E.C.—investigation; K.W.—investigation, writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Education and Science of Poland, LKR/S/49/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Bugin, G.; Lenzi, L.; Ranzani, G.; Barisan, L.; Porrini, C.; Zanella, A.; Bolzonella, C. Agriculture and Pollinating Insects, No Longer a Choice but a Need: EU Agriculture’s Dependence on Pollinators in the 2007–2019 Period. Sustainability 2022, 14, 3644. [Google Scholar] [CrossRef]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee Pollination Improves Crop Quality, Shelf Life and Commercial Value. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132440. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.G.; Martins, H.L.; Pinto, B.C.; Franco, A.L.; Amaral, L.S.; de Castro, C.V. The Significance of Pollination for Global Food Production and the Guarantee of Nutritional Security: A Literature Review. Environ. Sci. Proc. 2022, 15, 7. [Google Scholar]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J. The Assessment Report on Pollinators, Pollination and Food Production: Summary for Policymakers; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; ISBN 92-807-3568-3. [Google Scholar]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop Pollination from Native Bees at Risk from Agricultural Intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Giannini, T.C.; Maia-Silva, C.; Acosta, A.L.; Jaffe, R.; Carvalho, A.T.; Martins, C.F.; Zanella, F.C.; Carvalho, C.A.; Hrncir, M.; Saraiva, A.M. Protecting a Managed Bee Pollinator against Climate Change: Strategies for an Area with Extreme Climatic Conditions and Socioeconomic Vulnerability. Apidologie 2017, 48, 784–794. [Google Scholar] [CrossRef]
- Hoiss, B.; Krauss, J.; Steffan-Dewenter, I. Interactive Effects of Elevation, Species Richness and Extreme Climatic Events on Plant–Pollinator Networks. Global Chang. Biol. 2015, 21, 4086–4097. [Google Scholar] [CrossRef]
- Owens, A.C.; Cochard, P.; Durrant, J.; Farnworth, B.; Perkin, E.K.; Seymoure, B. Light Pollution Is a Driver of Insect Declines. Biol. Conserv. 2020, 241, 108259. [Google Scholar] [CrossRef]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological Stoichiometry of the Honeybee: Pollen Diversity and Adequate Species Composition Are Needed to Mitigate Limitations Imposed on the Growth and Development of Bees by Pollen Quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A. A Global-Scale Expert Assessment of Drivers and Risks Associated with Pollinator Decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef]
- CEC. Council Regulation EEC No. 797/85 of 12 March 1985 on Improving the Efficiency of Agricultural Structures. Off. J. 1985, 93, 1–18. [Google Scholar]
- Busch, G. Future European Agricultural Landscapes—What Can We Learn from Existing Quantitative Land Use Scenario Studies? Agric. Ecosyst. Environ. 2006, 114, 121–140. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The Role of Agri-environment Schemes in Conservation and Environmental Management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F. Landscape Moderation of Biodiversity Patterns and Processes-eight Hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Lundberg, J.; Moberg, F. Mobile Link Organisms and Ecosystem Functioning: Implications for Ecosystem Resilience and Management. Ecosystems 2003, 6, 0087–0098. [Google Scholar] [CrossRef]
- Brown, M.J.; Dicks, L.V.; Paxton, R.J.; Baldock, K.C.; Barron, A.B.; Chauzat, M.-P.; Freitas, B.M.; Goulson, D.; Jepsen, S.; Kremen, C. A Horizon Scan of Future Threats and Opportunities for Pollinators and Pollination. PeerJ 2016, 4, e2249. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P. Trees for Bees. Agric. Ecosyst. Environ. 2019, 270, 79–83. [Google Scholar] [CrossRef]
- Geppert, C.; Hass, A.; Földesi, R.; Donkó, B.; Akter, A.; Tscharntke, T.; Batáry, P. Agri-environment Schemes Enhance Pollinator Richness and Abundance but Bumblebee Reproduction Depends on Field Size. J. Appl. Ecol. 2020, 57, 1818–1828. [Google Scholar] [CrossRef]
- Underwood, E.; Darwin, G.; Gerritsen, E. Pollinator Initiatives in EU Member States: Success Factors and Gaps; Report Under Contract for Provision of Technical Support Related to Target 2 of the EU Biodiversity Strategy to 2020; Institute for European Environmental Policy: Brussels, Belgium, 2017. [Google Scholar]
- Stevenson, P. Turning the Commission’s Farm to Fork Strategy into a Far-Reaching Reform of EU Agriculture. In dA Derecho Animal: Forum of Animal Law Studies; Universitat Autonoma de Barcelona: Bellaterra, Spain, 2020; Volume 11, pp. 177–187. [Google Scholar]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D. A Global Quantitative Synthesis of Local and Landscape Effects on Wild Bee Pollinators in Agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Carré, G.; Roche, P.; Chifflet, R.; Morison, N.; Bommarco, R.; Harrison-Cripps, J.; Krewenka, K.; Potts, S.G.; Roberts, S.P.; Rodet, G. Landscape Context and Habitat Type as Drivers of Bee Diversity in European Annual Crops. Agric. Ecosyst. Environ. 2009, 133, 40–47. [Google Scholar] [CrossRef]
- Beck, T. Principles of Ecological Landscape Design; Island Press: Washington, DC, USA, 2013; ISBN 1-59726-702-3. [Google Scholar]
- DemKová, K.; LipsKý, Z. Changes in the Extent of Non-Forest Woody Vegetation in the Novodvorsko and Žehušicko Region (Central Bohemia, Czech Republic). AUC Geogr. 2013, 48, 5–13. [Google Scholar] [CrossRef]
- Ruttan, A.; Lortie, C.J.; Haas, S.M. Shrubs as Magnets for Pollination: A Test of Facilitation and Reciprocity in a Shrub-Annual Facilitation System. Curr. Res. Insect Sci. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Macdonald, K.J.; Kelly, D.; Tylianakis, J.M. Do Local Landscape Features Affect Wild Pollinator Abundance, Diversity and Community Composition on Canterbury Farms? N. Z. J. Ecol. 2018, 42, 262–268. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gibbs, J.; Gratton, C. Diverse Landscapes Have a Higher Abundance and Species Richness of Spring Wild Bees by Providing Complementary Floral Resources over Bees’ Foraging Periods. Landsc. Ecol. 2016, 31, 1523–1535. [Google Scholar] [CrossRef]
- Martins, K.T.; Albert, C.H.; Lechowicz, M.J.; Gonzalez, A. Complementary Crops and Landscape Features Sustain Wild Bee Communities. Ecol. Appl. 2018, 28, 1093–1105. [Google Scholar] [CrossRef]
- Persson, A.S.; Smith, H.G. Seasonal Persistence of Bumblebee Populations Is Affected by Landscape Context. Agric. Ecosyst. Environ. 2013, 165, 201–209. [Google Scholar] [CrossRef]
- Garratt, M.P.; Senapathi, D.; Coston, D.J.; Mortimer, S.R.; Potts, S.G. The Benefits of Hedgerows for Pollinators and Natural Enemies Depends on Hedge Quality and Landscape Context. Agric. Ecosyst. Environ. 2017, 247, 363–370. [Google Scholar] [CrossRef]
- Varah, A.; Jones, H.; Smith, J.; Potts, S.G. Enhanced Biodiversity and Pollination in UK Agroforestry Systems. J. Sci. Food Agric. 2013, 93, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Hannon, L.E.; Sisk, T.D. Hedgerows in an Agri-Natural Landscape: Potential Habitat Value for Native Bees. Biol. Conserv. 2009, 142, 2140–2154. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for Ecosystem Services and Environmental Benefits: An Overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Burel, F. Hedgerows and Their Role in Agricultural Landscapes. Crit. Rev. Plant Sci. 1996, 15, 169–190. [Google Scholar] [CrossRef]
- Nikolova, I.; Georgieva, N.; Kirilov, A.; Mladenova, R. Dynamics of Dominant Bees-Pollinators and Influence of Temperature, Relative Humidity and Time of Day on Their Abundance in Forage Crops in Pleven Region, Bulgaria. J. Global Agric. Ecol. 2016, 5, 200–209. [Google Scholar]
- Dainese, M.; Montecchiari, S.; Sitzia, T.; Sigura, M.; Marini, L. High Cover of Hedgerows in the Landscape Supports Multiple Ecosystem Services in M Editerranean Cereal Fields. J. Appl. Ecol. 2017, 54, 380–388. [Google Scholar] [CrossRef]
- Castle, D.; Grass, I.; Westphal, C. Fruit Quantity and Quality of Strawberries Benefit from Enhanced Pollinator Abundance at Hedgerows in Agricultural Landscapes. Agric. Ecosyst. Environ. 2019, 275, 14–22. [Google Scholar] [CrossRef]
- Campagne, P.; Affre, L.; Baumel, A.; Roche, P.; Tatoni, T. Fine-Scale Response to Landscape Structure in Primula Vulgaris Huds.: Does Hedgerow Network Connectedness Ensure Connectivity through Gene Flow? Popul. Ecol. 2009, 51, 209–219. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Wrzesień, M.; Ziółkowska, E. The Need for Weeds: Man-Made, Non-Cropped Habitats Complement Crops and Natural Habitats in Providing Honey Bees and Bumble Bees with Pollen Resources. Sci. Total Environ. 2022, 840, 156551. [Google Scholar] [CrossRef]
- Esther Julier, H.; Roulston, T.H. Wild Bee Abundance and Pollination Service in Cultivated Pumpkins: Farm Management, Nesting Behavior and Landscape Effects. J. Econ. Entomol. 2009, 102, 563–573. [Google Scholar] [CrossRef]
- Kremen, C.; M’Gonigle, L.K.; Ponisio, L.C. Pollinator Community Assembly Tracks Changes in Floral Resources as Restored Hedgerows Mature in Agricultural Landscapes. Front. Ecol. Evol. 2018, 6, 170. [Google Scholar] [CrossRef]
- Sardiñas, H.S.; Ponisio, L.C.; Kremen, C. Hedgerow Presence Does Not Enhance Indicators of Nest-site Habitat Quality or Nesting Rates of Ground-nesting Bees. Restor. Ecol. 2016, 24, 499–505. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Roberts, S.; O’Toole, C.; Dafni, A.; Ne’eman, G.; Willmer, P. Role of Nesting Resources in Organising Diverse Bee Communities in a Mediterranean Landscape. Ecol. Entomol. 2005, 30, 78–85. [Google Scholar] [CrossRef]
- Fussell, M.; Corbet, S.A. The Nesting Places of Some British Bumble Bees. J. Apic. Res. 1992, 31, 32–41. [Google Scholar] [CrossRef]
- Kells, A.R.; Goulson, D. Preferred Nesting Sites of Bumblebee Queens (Hymenoptera: Apidae) in Agroecosystems in the UK. Biol. Conserv. 2003, 109, 165–174. [Google Scholar] [CrossRef]
- Svensson, B.; Lagerlöf, J.; Svensson, B.G. Habitat Preferences of Nest-Seeking Bumble Bees (Hymenoptera: Apidae) in an Agricultural Landscape. Agric. Ecosyst. Environ. 2000, 77, 247–255. [Google Scholar] [CrossRef]
- Osborne, J.L.; Martin, A.P.; Carreck, N.L.; Swain, J.L.; Knight, M.E.; Goulson, D.; Hale, R.J.; Sanderson, R.A. Bumblebee Flight Distances in Relation to the Forage Landscape. J. Anim. Ecol. 2008, 77, 406–415. [Google Scholar] [CrossRef]
- Filipiak, M.; Weiner, J. Plant–Insect Interactions: The Role of Ecological Stoichiometry. Acta Agrobot. 2017, 70, 1710. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee Nutrition and Floral Resource Restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Filipiak, M. Key Pollen Host Plants Provide Balanced Diets for Wild Bee Larvae: A Lesson for Planting Flower Strips and Hedgerows. J. Appl. Ecol. 2019, 56, 1410–1418. [Google Scholar] [CrossRef]
- Rodney, S.; Purdy, J. Dietary Requirements of Individual Nectar Foragers, and Colony-Level Pollen and Nectar Consumption: A Review to Support Pesticide Exposure Assessment for Honey Bees. Apidologie 2020, 51, 163–179. [Google Scholar] [CrossRef]
- Enkegaard, A.; Kryger, P.; Boelt, B. Determinants of Nectar Production in Heather. J. Apic. Res. 2016, 55, 100–106. [Google Scholar] [CrossRef]
- Mengist, W.; Soromessa, T.; Legese, G. Method for Conducting Systematic Literature Review and Meta-Analysis for Environmental Science Research. MethodsX 2020, 7, 100777. [Google Scholar] [CrossRef] [PubMed]
- Bentrup, G.; Hopwood, J.; Adamson, N.L.; Powers, R.; Vaughan, M. The Role of Temperate Agroforestry Practices in Supporting Pollinators. In Agroforestry and Ecosystem Services; Springer: Berlin/Heidelberg, Germany, 2021; pp. 275–304. [Google Scholar]
- Morandin, L.A.; Kremen, C. Hedgerow Restoration Promotes Pollinator Populations and Exports Native Bees to Adjacent Fields. Ecol. Appl. 2013, 23, 829–839. [Google Scholar] [CrossRef]
- Filipiak, Z.M.; Denisow, B.; Stawiarz, E.; Filipiak, M. Unravelling the Dependence of a Wild Bee on Floral Diversity and Composition Using a Feeding Experiment. Sci. Total Environ. 2022, 820, 153326. [Google Scholar] [CrossRef]
- Faegri, K.; Van Der Pijl, L. Principles of Pollination Ecology; Pergamon Press: Oxford, UK, 2013. [Google Scholar]
- Denisow, B. Pollen Production of Selected Ruderal Plant Species in the Lublin Area; WUP Wydawnictwo Uniwersytetu Przyrodniczego: Lublin, Poland, 2011. [Google Scholar]
- Raine, N.E.; Chittka, L. Nectar Production Rates of 75 Bumblebee-Visited Flower Species in a German Flora (Hymenoptera: Apidae: Bombus Terrestris). Entomol. Gen. 2005, 30, 191. [Google Scholar] [CrossRef]
- Sulborska, A. Rośliny Pożytkowe; Bee & Honey: Klecza Dolna, Poland, 2019; ISBN 83-953017-2-3. [Google Scholar]
- Drescher, N.; Klein, A.-M.; Schmitt, T.; Leonhardt, S.D. A Clue on Bee Glue: New Insight into the Sources and Factors Driving Resin Intake in Honeybees (Apis mellifera). PLoS ONE 2019, 14, e0210594. [Google Scholar] [CrossRef]
- Nicolson, S.W. Bee Food: The Chemistry and Nutritional Value of Nectar, Pollen and Mixtures of the Two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M.; Vesprini, J. Nectar Biodiversity: A Short Review. Plant Syst. Evol. 2003, 238, 7–21. [Google Scholar] [CrossRef]
- Dmitruk, M.; Strzałkowska-Abramek, M.; Bożek, M.; Denisow, B. Plants Enhancing Urban Pollinators: Nectar Rather than Pollen Attracts Pollinators of Cotoneaster Species. Urban For. Urban Green. 2022, 74, 127651. [Google Scholar] [CrossRef]
- Somme, L.; Moquet, L.; Quinet, M.; Vanderplanck, M.; Michez, D.; Lognay, G.; Jacquemart, A.-L. Food in a Row: Urban Trees Offer Valuable Floral Resources to Pollinating Insects. Urban Ecosyst. 2016, 19, 1149–1161. [Google Scholar] [CrossRef]
- Naef, R.; Jaquier, A.; Velluz, A.; Bachofen, B. From the Linden Flower to Linden Honey–Volatile Constituents of Linden Nectar, the Extract of Bee-stomach and Ripe Honey. In Perspectives in Flavor and Fragrance Research; Wiley: Hoboken, NJ, USA, 2005; pp. 31–40. [Google Scholar]
- Pamminger, T.; Becker, R.; Himmelreich, S.; Schneider, C.W.; Bergtold, M. The Nectar Report: Quantitative Review of Nectar Sugar Concentrations Offered by Bee Visited Flowers in Agricultural and Non-Agricultural Landscapes. PeerJ 2019, 7, e6329. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, B.; Koltowski, Z. Nektarowanie i Wydajnosc Miodowa Robinii Akacjowej [Robinia pseudoacacia L.]. Pszczel. Pol. 1992, 3, 9. [Google Scholar]
- Jablonski, B.; Koltowski, Z. Nektarowanie Roznych Gatunkow i Mieszancow Lipy [Tilia L.]. Pszczel. Zesz. Nauk. 1999, 43, 279–290. [Google Scholar]
- Szklanowska, K. Nektarowanie i Wydajnosc Miodowa Maliny Wlasciwej (Rubus idaeus L.) i Jezyn (Rubus fruticosus L.) w Srodowisku Lesnym. Pszczel. Zesz. Nauk 1972, 16, 133–145. [Google Scholar]
- Gyan, K.Y.; Woodell, S. Nectar Production, Sugar Content, Amino Acids and Potassium in Prunus spinosa L., Crataegus Monogyna Jacq. and Rubus fruticosus L. at Wytham, Oxfordshire. Funct. Ecol. 1987, 1, 251–259. [Google Scholar] [CrossRef]
- Szklanowska, K.; Strzałkowska, M. Blooming Biology and Pollen Exposure of Horse Chestnut Trees (Aesculus L.). Ann. Univ. Mariae Curie-Skłodowska Sect. EEE Hortic. 2000, 8, 107–116. [Google Scholar]
- Farkas, Á.; Zajácz, E. Nectar Production for the Hungarian Honey Industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. [Google Scholar]
- Weryszko-Chmielewska, E.; Masierowska, M.; Konarska, A. Characteristics of Floral Nectaries and Nectar in Two Species of Crataegus (Rosaceae). Plant Syst. Evol. 2003, 238, 33–41. [Google Scholar] [CrossRef]
- Dmitruk, M. Flowering, Nectar Secretion, and Structure of the Nectary in the Flowers of Acer Pseudoplatanus L. Acta Agrobot. 2019, 72, 1787. [Google Scholar] [CrossRef]
- Gill, M.C.; Walters, K.F. Potential Use of Floral Nectar Sugar Characteristics in Plant Selection for Pollinator Habitats. J. Apic. Res. 2022, 62, 266–273. [Google Scholar] [CrossRef]
- Bozek, M. Nectar Production and Spectrum of Insect Visitors in Six Varieties of Highbush Blueberry (Vaccinium Corymbosum L.) in SE Poland. Acta Agrobot. 2021, 74, 7410. [Google Scholar] [CrossRef]
- Carruthers, J.M.; Cook, S.M.; Wright, G.A.; Osborne, J.L.; Clark, S.J.; Swain, J.L.; Haughton, A.J. Oilseed Rape (Brassica napus) as a Resource for Farmland Insect Pollinators: Quantifying Floral Traits in Conventional Varieties and Breeding Systems. GCB Bioenergy 2017, 9, 1370–1379. [Google Scholar] [CrossRef]
- Timberlake, T.P.; Vaughan, I.P.; Memmott, J. Phenology of Farmland Floral Resources Reveals Seasonal Gaps in Nectar Availability for Bumblebees. J. Appl. Ecol. 2019, 56, 1585–1596. [Google Scholar] [CrossRef]
- Szklanowska, K.; Teper, D. Wydajnosc Pylkowa Roznych Gatunkow i Mieszancow Lipy [Tilia L.]. Pszczel. Zesz. Nauk. 1999, 43, 291–302. [Google Scholar]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient Ratios in Pollen Shape Bumble Bee (Bombus Impatiens) Foraging Strategies and Floral Preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and Health in Honey Bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Dmitruk, M.; Wrzesień, M.; Strzałkowska-Abramek, M.; Denisow, B. Pollen Food Resources to Help Pollinators. A Study of Five Ranunculaceae Species in Urban Forest. Urban For. Urban Green. 2021, 60, 127051. [Google Scholar] [CrossRef]
- Zuraw, B.; Sulborska, A.; Stawiarz, E.; Weryszko-Chmielewska, E. Flowering Biology and Pollen Production of Four Species of the Genus Rosa L. Acta Agrobot. 2015, 68, 267–278. [Google Scholar] [CrossRef]
- Scott-Brown, A.S.; Arnold, S.E.; Kite, G.C.; Farrell, I.W.; Farman, D.I.; Collins, D.W.; Stevenson, P.C. Mechanisms in Mutualisms: A Chemically Mediated Thrips Pollination Strategy in Common Elder. Planta 2019, 250, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.L.; Wells, J.D.; Dent, L.K. The Nitrogen Content of Pollen Protein. J. Apic. Res. 1983, 22, 119–123. [Google Scholar] [CrossRef]
- Day, S.; Beyer, R.; Mercer, A.; Ogden, S. The Nutrient Composition of Honeybee-Collected Pollen in Otago, New Zealand. J. Apic. Res. 1990, 29, 138–146. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. Pollen Nutritional Content and Digestibility for Animals. Plant Syst. Evol. 2000, 222, 187–209. [Google Scholar] [CrossRef]
- Weiner, C.N.; Hilpert, A.; Werner, M.; Linsenmair, K.E.; Blüthgen, N. Pollen Amino Acids and Flower Specialisation in Solitary Bees. Apidologie 2010, 41, 476–487. [Google Scholar] [CrossRef]
- Liolios, V.; Tananaki, C.; Papaioannou, A.; Kanelis, D.; Rodopoulou, M.-A.; Argena, N. Mineral Content in Monofloral Bee Pollen: Investigation of the Effect of the Botanical and Geographical Origin. J. Food Meas. Charact. 2019, 13, 1674–1682. [Google Scholar] [CrossRef]
- Bukovinszky, T.; Rikken, I.; Evers, S.; Wäckers, F.L.; Biesmeijer, J.C.; Prins, H.H.; Kleijn, D. Effects of Pollen Species Composition on the Foraging Behaviour and Offspring Performance of the Mason Bee Osmia Bicornis (L.). Basic Appl. Ecol. 2017, 18, 21–30. [Google Scholar] [CrossRef]
- Denisow, B.; Strzałkowska-Abramek, M.; Bożek, M.; Jeżak, A. Early Spring Nectar and Pollen and Insect Visitor Behavior in Two Corydalis Species (Papaveraceae). J. Apic. Sci. 2014, 58, 93–102. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Wrzesień, M. Validation of Floral Food Resources for Pollinators in Agricultural Landscape in SE Poland. J. Sci. Food Agric. 2018, 98, 2672–2680. [Google Scholar] [CrossRef]
- Dicks, L.V.; Baude, M.; Roberts, S.P.M.; Phillips, J.; Green, M.; Carvell, C. How Much Flower-Rich Habitat Is Enough for Wild Pollinators? Answering a Key Policy Question with Incomplete Knowledge. Ecol. Entomol. 2015, 40, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Jachuła, J.; Denisow, B.; Strzałkowska-Abramek, M. Does an Invader Have a Bright Side? Floral Reward in Two Solidago Species. J. Apic. Res. 2020, 59, 599–608. [Google Scholar] [CrossRef]
- Piotrowska, K. Ecological Features of Flowers and the Amount of Pollen Released in Corylus Avellana [L.] and Alnus Glutinosa [L.] Gaertn. Acta Agrobotanica 2008, 61, 1. [Google Scholar] [CrossRef]
- M’Gonigle, L.K.; Ponisio, L.C.; Cutler, K.; Kremen, C. Habitat Restoration Promotes Pollinator Persistence and Colonization in Intensively Managed Agriculture. Ecol. Appl. 2015, 25, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental Factors Driving the Effectiveness of European Agri-environmental Measures in Mitigating Pollinator Loss–a Meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Kay, S.; Kühn, E.; Albrecht, M.; Sutter, L.; Szerencsits, E.; Herzog, F. Agroforestry Can Enhance Foraging and Nesting Resources for Pollinators with Focus on Solitary Bees at the Landscape Scale. Agrofor. Syst. 2020, 94, 379–387. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Wrzesień, M. Habitat Heterogeneity Helps to Mitigate Pollinator Nectar Sugar Deficit and Discontinuity in an Agricultural Landscape. Sci. Total Environ. 2021, 782, 146909. [Google Scholar] [CrossRef]
- Wood, T.J.; Kaplan, I.; Szendrei, Z. Wild Bee Pollen Diets Reveal Patterns of Seasonal Foraging Resources for Honey Bees. Front. Ecol. Evol. 2018, 6, 210. [Google Scholar] [CrossRef]
- Stawiarz, E.; Żuraw, R.; Marut, A. Pollen Sources in the Bojanów Forest Complex Identified on Honeybee Pollen Load by Microscopic Analysis. Acta Agrobot. 2017, 70, 1724. [Google Scholar] [CrossRef]
- McLellan, A. Factors Affecting Pollen Harvesting by the Honeybee. J. Appl. Ecol. 1976, 801–811. [Google Scholar] [CrossRef]
- Coffey, M.F.; Breen, J. Seasonal Variation in Pollen and Nectar Sources of Honey Bees in Ireland. J. Apic. Res. 1997, 36, 63–76. [Google Scholar] [CrossRef]
- Kołtowski, Z.; Miśkiewicz, I. Wielki Atlas Roślin Miododajnych; Przedsiębiorstwo Wydawnicze Rzeczpospolita: Warszawa, Poland, 2006; ISBN 83-60192-13-8. [Google Scholar]
- Van Der Kooi, C.J.; Vallejo-Marín, M.; Leonhardt, S.D. Mutualisms and (A) Symmetry in Plant–Pollinator Interactions. Curr. Biol. 2021, 31, R91–R99. [Google Scholar] [CrossRef] [PubMed]
- Horridge, A. What Does an Insect See? J. Exp. Biol. 2009, 212, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M. Pollen Protein: Lipid Macronutrient Ratios May Guide Broad Patterns of Bee Species Floral Preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Pernal, S.F.; Currie, R.W. The Influence of Pollen Quality on Foraging Behavior in Honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2001, 51, 53–68. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N. The Chemical Ecology and Evolution of Bee–Flower Interactions: A Review and Perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Miñarro, M.; Prida, E. Hedgerows Surrounding Organic Apple Orchards in North-west S Pain: Potential to Conserve Beneficial Insects. Agric. For. Entomol. 2013, 15, 382–390. [Google Scholar] [CrossRef]
- Ponisio, L.C.; Gaiarsa, M.P.; Kremen, C. Opportunistic Attachment Assembles Plant–Pollinator Networks. Ecol. Lett. 2017, 20, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Rollin, O.; Bretagnolle, V.; Decourtye, A.; Aptel, J.; Michel, N.; Vaissière, B.E.; Henry, M. Differences of Floral Resource Use between Honey Bees and Wild Bees in an Intensive Farming System. Agric. Ecosyst. Environ. 2013, 179, 78–86. [Google Scholar] [CrossRef]
- Aronne, G.; Giovanetti, M.; Guarracino, M.R.; de Micco, V. Foraging Rules of Flower Selection Applied by Colonies of A Pis Mellifera: Ranking and Associations of Floral Sources. Funct. Ecol. 2012, 26, 1186–1196. [Google Scholar] [CrossRef]
- Teper, D. Food Plants of the Red Mason Bee (Osmia rufa L.) Determined Based on a Palynological Analysis of Faeces. J. Apic. Sci. 2007, 51, 55–62. [Google Scholar]
- Schindler, M.; Peters, B. Mason Bees Osmia Bicornis and Osmia Cornuta as Suitable Orchard Pollinators? Erwerbsobstbau 2011, 52, 111–116. [Google Scholar]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the Interactions between Resource Availability and the Utilisation of Semi-Natural Habitats by Insect Pollinators in an Intensive Agricultural Landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Ssymank, A.; Kearns, C.; Pape, T.; Thompson, F.C. Pollinating Flies (Diptera): A Major Contribution to Plant Diversity and Agricultural Production. Biodiversity 2008, 9, 86–89. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual Ecosystem Services of Syrphid Flies (Diptera: Syrphidae): Pollinators and Biological Control Agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Fijen, T.P.; Read, S.F.; Walker, M.K.; Gee, M.; Nelson, W.R.; Howlett, B.G. Different Landscape Features within a Simplified Agroecosystem Support Diverse Pollinators and Their Service to Crop Plants. Landsc. Ecol. 2022, 37, 1787–1799. [Google Scholar] [CrossRef]
- Saunders, M.E. Insect Pollinators Collect Pollen from Wind-pollinated Plants: Implications for Pollination Ecology and Sustainable Agriculture. Insect Conserv. Divers. 2018, 11, 13–31. [Google Scholar] [CrossRef]
- Ssymank, A.; Gilbert, F. Anemophilous Pollen in the Diet of Syrphid Flies with Special Reference to the Leaf Feeding Strategy Occurring in Xylotini.(Diptera, Syrphidae). Dtsch. Entomol. Z. 1993, 40, 245–258. [Google Scholar] [CrossRef]
- Fowler, J. Specialist Bees of the Northeast: Host Plants and Habitat Conservation. Northeast. Nat. 2016, 23, 305–320. [Google Scholar] [CrossRef]
- Cane, J.H. A Brief Review of Monolecty in Bees and Benefits of a Broadened Definition. Apidologie 2021, 52, 17–22. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Vereecken, N.J.; Grumiau, L.; Esposito, F.; Lognay, G.; Wattiez, R.; Michez, D. The Importance of Pollen Chemistry in Evolutionary Host Shifts of Bees. Sci. Rep. 2017, 7, 43058. [Google Scholar] [CrossRef]
- Jacquemin, F.; Violle, C.; Munoz, F.; Mahy, G.; Rasmont, P.; Roberts, S.P.; Vray, S.; Dufrene, M. Loss of Pollinator Specialization Revealed by Historical Opportunistic Data: Insights from Network-Based Analysis. PLoS ONE 2020, 15, e0235890. [Google Scholar] [CrossRef]
- Kremen, C.; M’Gonigle, L.K. EDITOR’S CHOICE: Small-scale Restoration in Intensive Agricultural Landscapes Supports More Specialized and Less Mobile Pollinator Species. J. Appl. Ecol. 2015, 52, 602–610. [Google Scholar] [CrossRef]
- Kalivodová, M.; Kanka, R.; Miklós, P.; Sládkovičová, V.H.; Žiak, D. Importance of Wetland Refugia in Agricultural Landscape Provided Based on the Community Characteristics of Small Terrestrial Mammals. Ekológia 2018, 37, 358–368. [Google Scholar] [CrossRef]
- Haight, J.; Hammill, E. Protected Areas as Potential Refugia for Biodiversity under Climatic Change. Biol. Conserv. 2020, 241, 108258. [Google Scholar] [CrossRef]
- Cane, J.H.; Griswold, T.; Parker, F.D. Substrates and Materials Used for Nesting by North American Osmia Bees (Hymenoptera: Apiformes: Megachilidae). Ann. Entomol. Soc. Am. 2007, 100, 350–358. [Google Scholar] [CrossRef]
- Westerfelt, P.; Widenfalk, O.; Lindelöw, Å.; Gustafsson, L.; Weslien, J. Nesting of Solitary Wasps and Bees in Natural and Artificial Holes in Dead Wood in Young Boreal Forest Stands. Insect Conserv. Divers. 2015, 8, 493–504. [Google Scholar] [CrossRef]
- Lye, G.; Park, K.; Osborne, J.; Holland, J.; Goulson, D. Assessing the Value of Rural Stewardship Schemes for Providing Foraging Resources and Nesting Habitat for Bumblebee Queens (Hymenoptera: Apidae). Biol. Conserv. 2009, 142, 2023–2032. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Shropshire, K.J. Ranking Lepidopteran Use of Native versus Introduced Plants. Conserv. Biol. 2009, 23, 941–947. [Google Scholar] [CrossRef]
- Staton, T.; Walters, R.J.; Smith, J.; Girling, R.D. Evaluating the Effects of Integrating Trees into Temperate Arable Systems on Pest Control and Pollination. Agric. Syst. 2019, 176, 102676. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Kjær, C.; Bruus, M.; Bossi, R.; Løfstrøm, P.; Andersen, H.V.; Nuyttens, D.; Larsen, S.E. Pesticide Drift Deposition in Hedgerows from Multiple Spray Swaths. J. Pestic. Sci. 2014, 39, 14–21. [Google Scholar] [CrossRef]
- Schmitz, J.; Schäfer, K.; Brühl, C.A. Agrochemicals in Field Margins—Field Evaluation of Plant Reproduction Effects. Agric. Ecosyst. Environ. 2014, 189, 82–91. [Google Scholar] [CrossRef]
- Ward, L.T.; Hladik, M.L.; Guzman, A.; Winsemius, S.; Bautista, A.; Kremen, C.; Mills, N.J. Pesticide Exposure of Wild Bees and Honey Bees Foraging from Field Border Flowers in Intensively Managed Agriculture Areas. Sci. Total Environ. 2022, 831, 154697. [Google Scholar] [CrossRef]
- Douglas, M.R.; Baisley, P.; Soba, S.; Kammerer, M.; Lonsdorf, E.V.; Grozinger, C.M. Putting Pesticides on the Map for Pollinator Research and Conservation. Sci. Data 2022, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Sutter, L.; Albrecht, M.; Jeanneret, P. Landscape Greening and Local Creation of Wildflower Strips and Hedgerows Promote Multiple Ecosystem Services. J. Appl. Ecol. 2018, 55, 612–620. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; et al. Synthesis Report of the IPCC Sixth Assessment Report (AR6): Summary for Policymakers. Intergovernmental Panel on Climate Change. 2023. Available online: https://pubs.giss.nasa.gov/abs/le05900r.html (accessed on 16 May 2023).
- Descamps, C.; Quinet, M.; Jacquemart, A.-L. Climate Change–Induced Stress Reduce Quantity and Alter Composition of Nectar and Pollen from a Bee-Pollinated Species (Borago Officinalis, Boraginaceae). Front. Plant Sci. 2021, 12, 755843. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Burkle, L.A.; Manson, J.S.; Runyon, J.B.; Trowbridge, A.M.; Zientek, J. Global Change Effects on Plant–Insect Interactions: The Role of Phytochemistry. Curr. Opin. Insect Sci. 2017, 23, 70–80. [Google Scholar] [CrossRef]
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global Warming and Plant–Pollinator Mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar]
- Scaven, V.L.; Rafferty, N.E. Physiological Effects of Climate Warming on Flowering Plants and Insect Pollinators and Potential Consequences for Their Interactions. Curr. Zool. 2013, 59, 418–426. [Google Scholar] [CrossRef]
- Takkis, K.; Tscheulin, T.; Petanidou, T. Differential Effects of Climate Warming on the Nectar Secretion of Early-and Late-Flowering Mediterranean Plants. Front. Plant Sci. 2018, 9, 874. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Dolferus, R. Pollen Developmental Arrest: Maintaining Pollen Fertility in a World with a Changing Climate. Front. Plant Sci. 2019, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Runyon, J.B. Drought and Leaf Herbivory Influence Floral Volatiles and Pollinator Attraction. Glob. Change Biol. 2016, 22, 1644–1654. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.-F.; Pioz, M.; Vaissière, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the Availability of Pollen Resources Affect Honey Bee Health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef]
- Hanski, I. Habitat Loss, the Dynamics of Biodiversity, and a Perspective on Conservation. Ambio 2011, 40, 248–255. [Google Scholar] [CrossRef]
- Ponisio, L.C.; de Valpine, P.; M’Gonigle, L.K.; Kremen, C. Proximity of Restored Hedgerows Interacts with Local Floral Diversity and Species’ Traits to Shape Long-term Pollinator Metacommunity Dynamics. Ecol. Lett. 2019, 22, 1048–1060. [Google Scholar] [CrossRef]
- Montgomery, I.; Caruso, T.; Reid, N. Hedgerows as Ecosystems: Service Delivery, Management, and Restoration. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 81–102. [Google Scholar] [CrossRef]
- Wix, N.; Reich, M.; Schaarschmidt, F. Butterfly Richness and Abundance in Flower Strips and Field Margins: The Role of Local Habitat Quality and Landscape Context. Heliyon 2019, 5, e01636. [Google Scholar] [CrossRef]
- Turner, M.G.; Gardner, R.H. Organisms and Landscape Pattern. In Landscape Ecology in Theory and Practice; Springer: New York, NY, USA, 2015; pp. 229–285. [Google Scholar]
- Gannon, D.G. Plant-Pollinator Interactions in a Changing World: Cryptic Specialization, Pollinator Movement, and Landscape Genetics of Pollinator-Dependent Plants. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2022. [Google Scholar]
- Cranmer, L.; McCollin, D.; Ollerton, J. Landscape Structure Influences Pollinator Movements and Directly Affects Plant Reproductive Success. Oikos 2012, 121, 562–568. [Google Scholar] [CrossRef]
- Klaus, F.; Bass, J.; Marholt, L.; Müller, B.; Klatt, B.; Kormann, U. Hedgerows Have a Barrier Effect and Channel Pollinator Movement in the Agricultural Landscape. J. Landsc. Ecol. 2015, 8, 22–31. [Google Scholar] [CrossRef]
- Ouin, A.; Burel, F. Influence of Herbaceous Elements on Butterfly Diversity in Hedgerow Agricultural Landscapes. Agric. Ecosyst. Environ. 2002, 93, 45–53. [Google Scholar] [CrossRef]
- Földesi, R.; Kovács-Hostyánszki, A. Hoverfly (Diptera: Syrphidae) Community of a Cultivated Arable Field and the Adjacent Hedgerow near Debrecen, Hungary. Biologia 2014, 69, 381–388. [Google Scholar] [CrossRef]
- Haenke, S.; Kovács-Hostyánszki, A.; Fründ, J.; Batáry, P.; Jauker, B.; Tscharntke, T.; Holzschuh, A. Landscape Configuration of Crops and Hedgerows Drives Local Syrphid Fly Abundance. J. Appl. Ecol. 2014, 51, 505–513. [Google Scholar] [CrossRef]
- Joyce, K.; Holland, J.; Doncaster, C. Influences of Hedgerow Intersections and Gaps on the Movement of Carabid Beetles. Bull. Entomol. Res. 1999, 89, 523–531. [Google Scholar] [CrossRef]
- Rands, S.A. Landscape Fragmentation and Pollinator Movement within Agricultural Environments: A Modelling Framework for Exploring Foraging and Movement Ecology. PeerJ 2014, 2, e269. [Google Scholar] [CrossRef]
- Carvell, C.; Roy, D.B.; Smart, S.M.; Pywell, R.F.; Preston, C.D.; Goulson, D. Declines in Forage Availability for Bumblebees at a National Scale. Biol. Conserv. 2006, 132, 481–489. [Google Scholar] [CrossRef]
- Requier, F.; Odoux, J.-F.; Tamic, T.; Moreau, N.; Henry, M.; Decourtye, A.; Bretagnolle, V. Honey Bee Diet in Intensive Farmland Habitats Reveals an Unexpectedly High Flower Richness and a Major Role of Weeds. Ecol. Appl. 2015, 25, 881–890. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutiérrez, J.; Ellis, W.N.; Fox, R.; Groom, Q.; Hennekens, S.; Van Landuyt, W.; Maes, D. Species Richness Declines and Biotic Homogenisation Have Slowed down for NW-European Pollinators and Plants. Ecol. Lett. 2013, 16, 870–878. [Google Scholar] [CrossRef]
- Nichols, R.N.; Goulson, D.; Holland, J.M. The Best Wildflowers for Wild Bees. J. Insect Conserv. 2019, 23, 819–830. [Google Scholar] [CrossRef]
- Anderson, J.T.; Wadgymar, S.M. Climate Change Disrupts Local Adaptation and Favours Upslope Migration. Ecol. Lett. 2020, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.C.; Morales, C.L. Ecological Impacts of Invasive Alien Species on Bees. Apidologie 2009, 40, 388–409. [Google Scholar] [CrossRef]
- Junge, X.; Schüpbach, B.; Walter, T.; Schmid, B.; Lindemann-Matthies, P. Aesthetic Quality of Agricultural Landscape Elements in Different Seasonal Stages in Switzerland. Landsc. Urban Plan. 2015, 133, 67–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).