Cashew Nut Shell Liquid as an Anticorrosive Agent in Ceramic Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Clay Extraction, Preparation, and Characterization
2.2. CNSL Infrared
2.3. Specimens—Preparation, Firing, and Immersion in CNSL
2.4. Corrosion Testing—Standards, Test Preparation, and Execution
2.5. Absorption, Apparent Porosity, and Flexural Strength of Specimens
3. Results
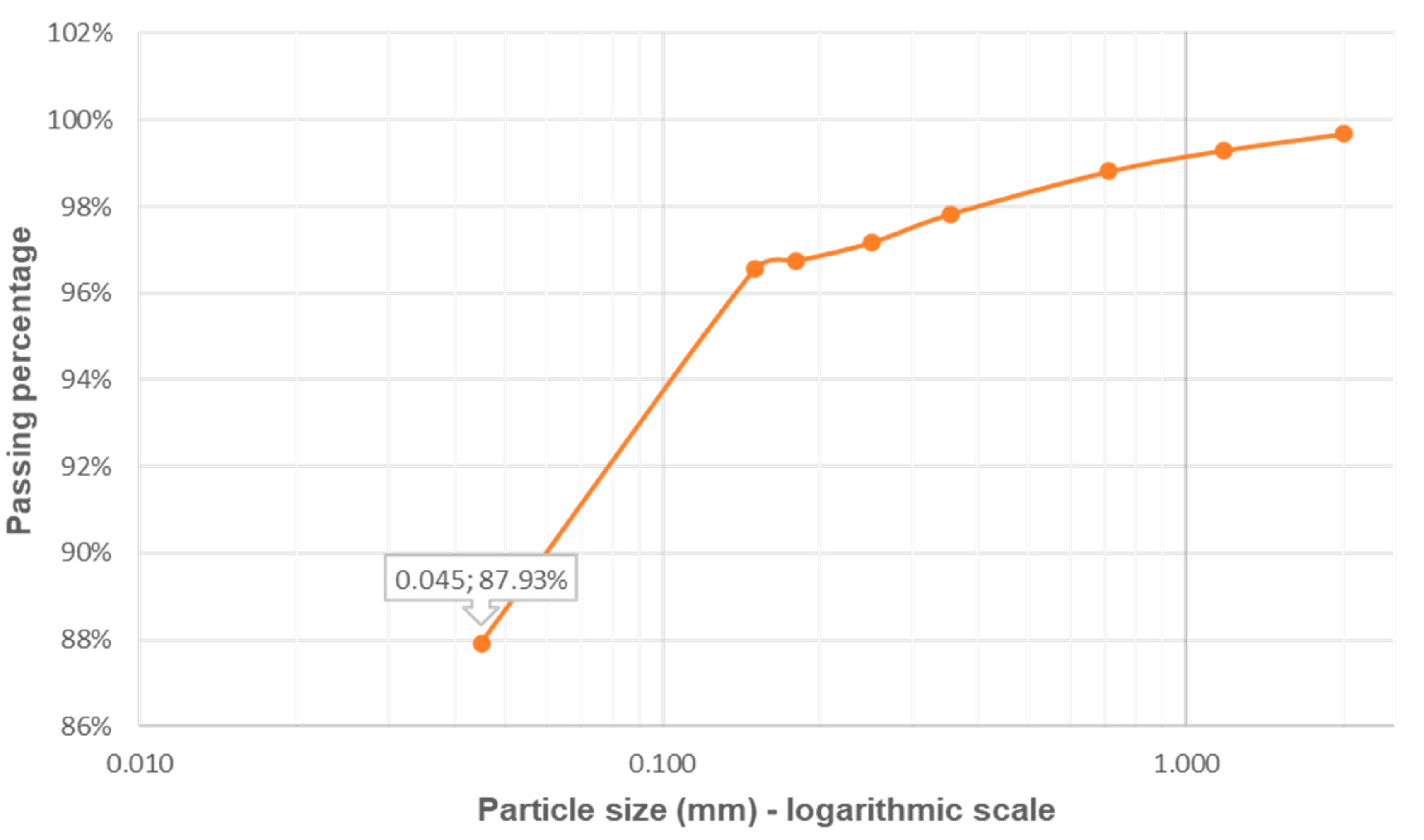
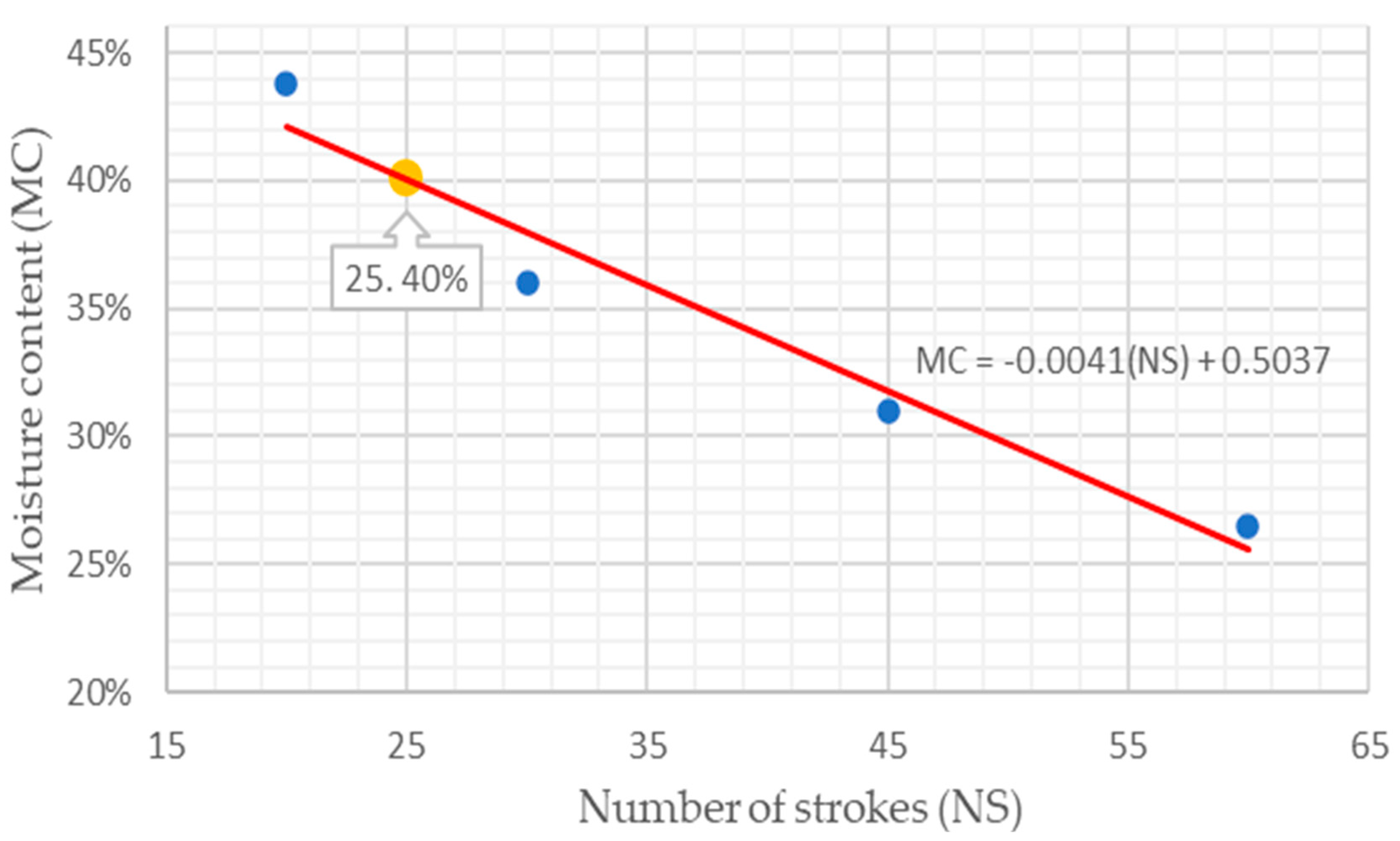
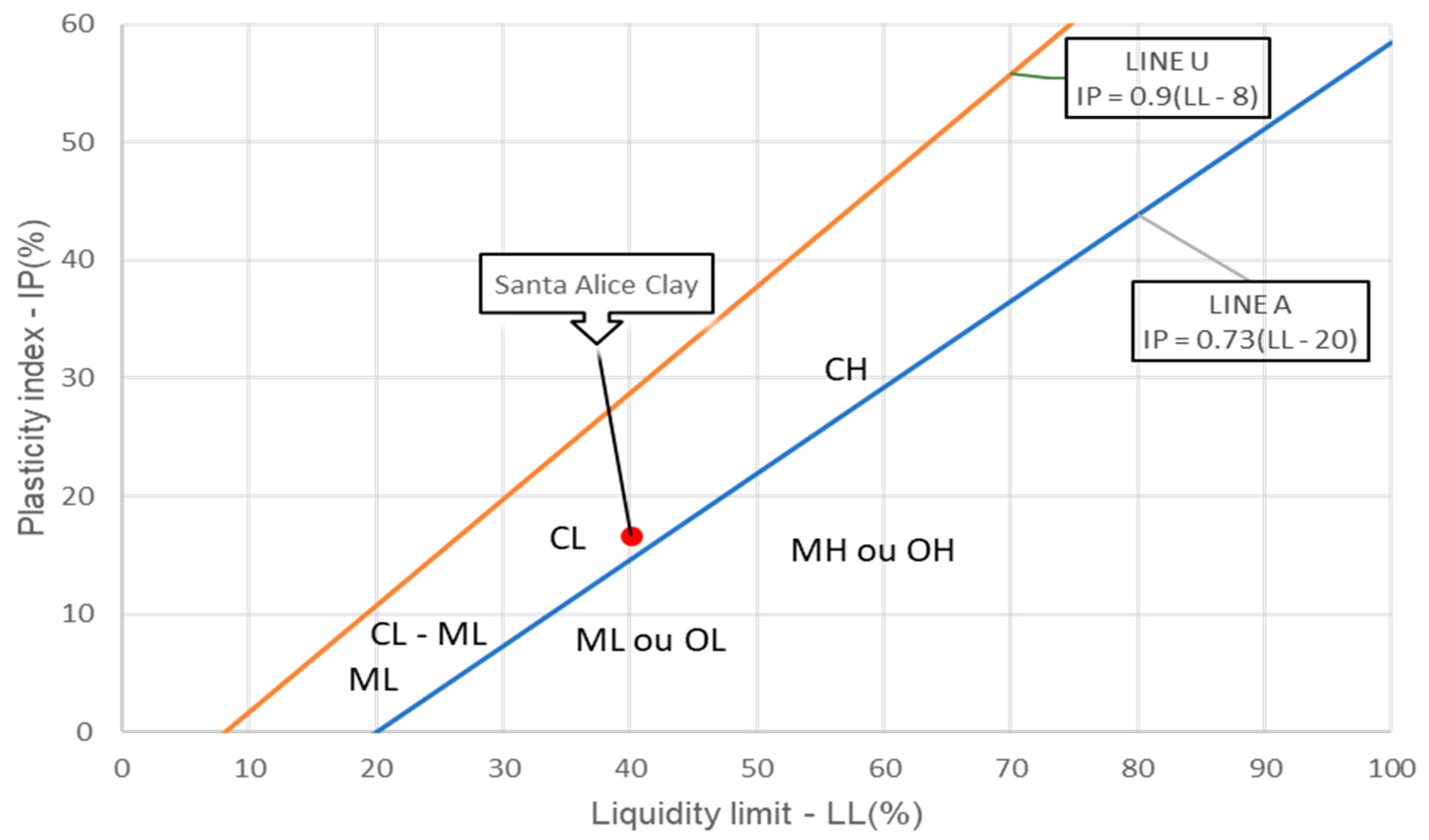
3.1. Clay Granulometry and Plasticity
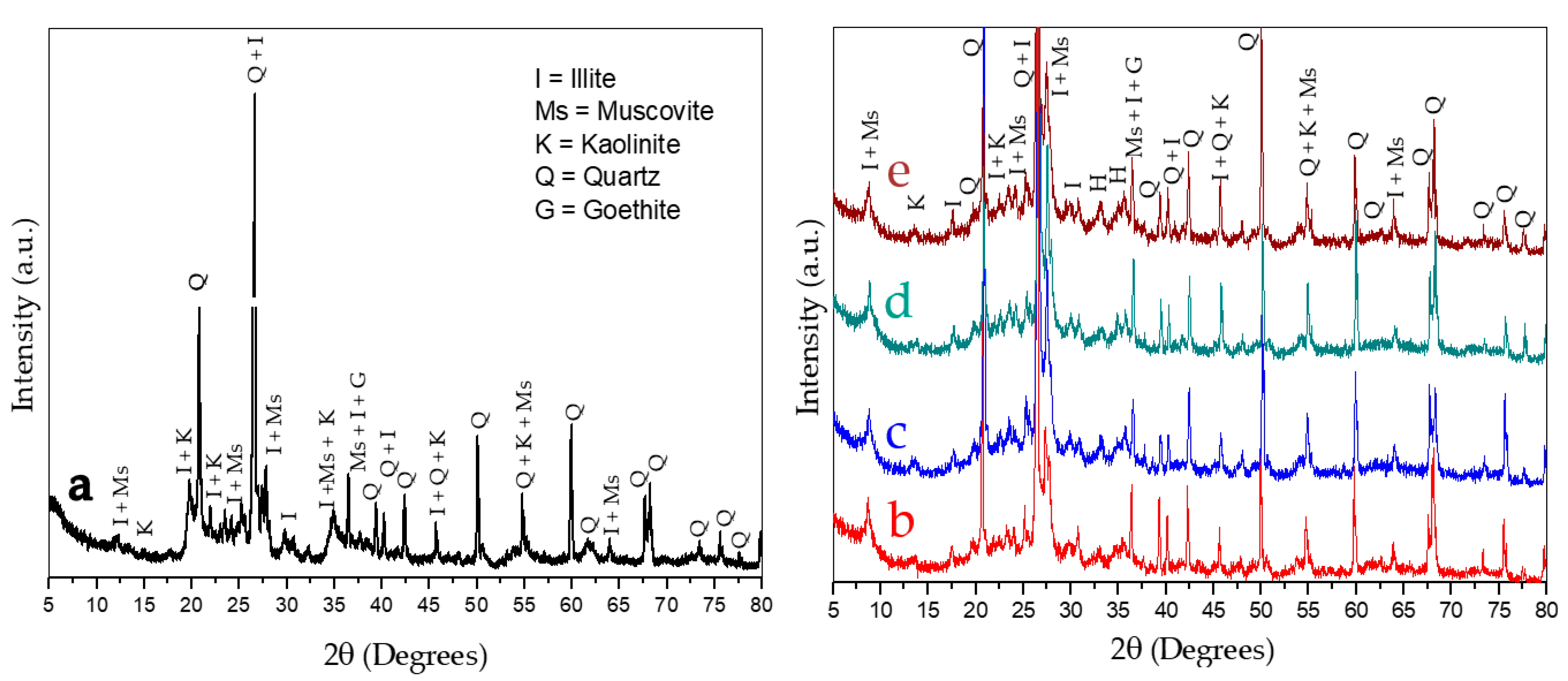
3.2. Clay XRF and XRD
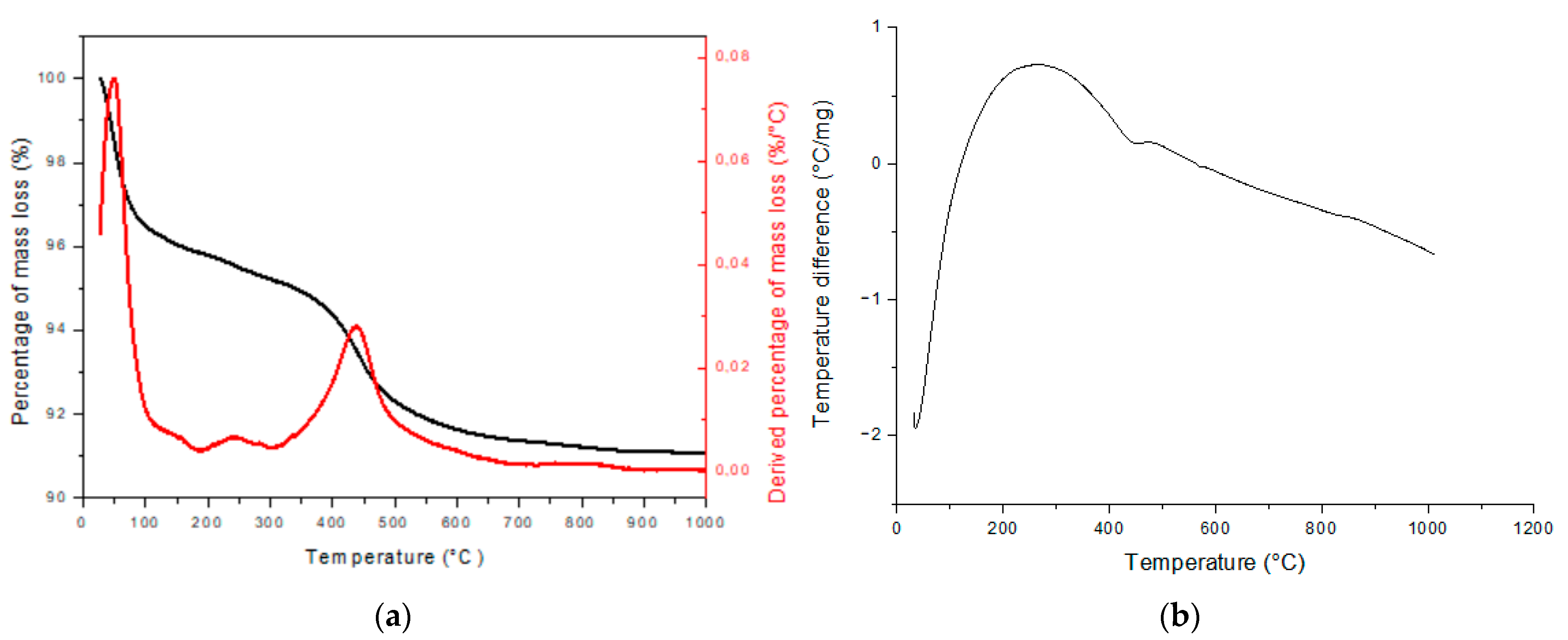
3.3. Clay’s Thermal Analysis
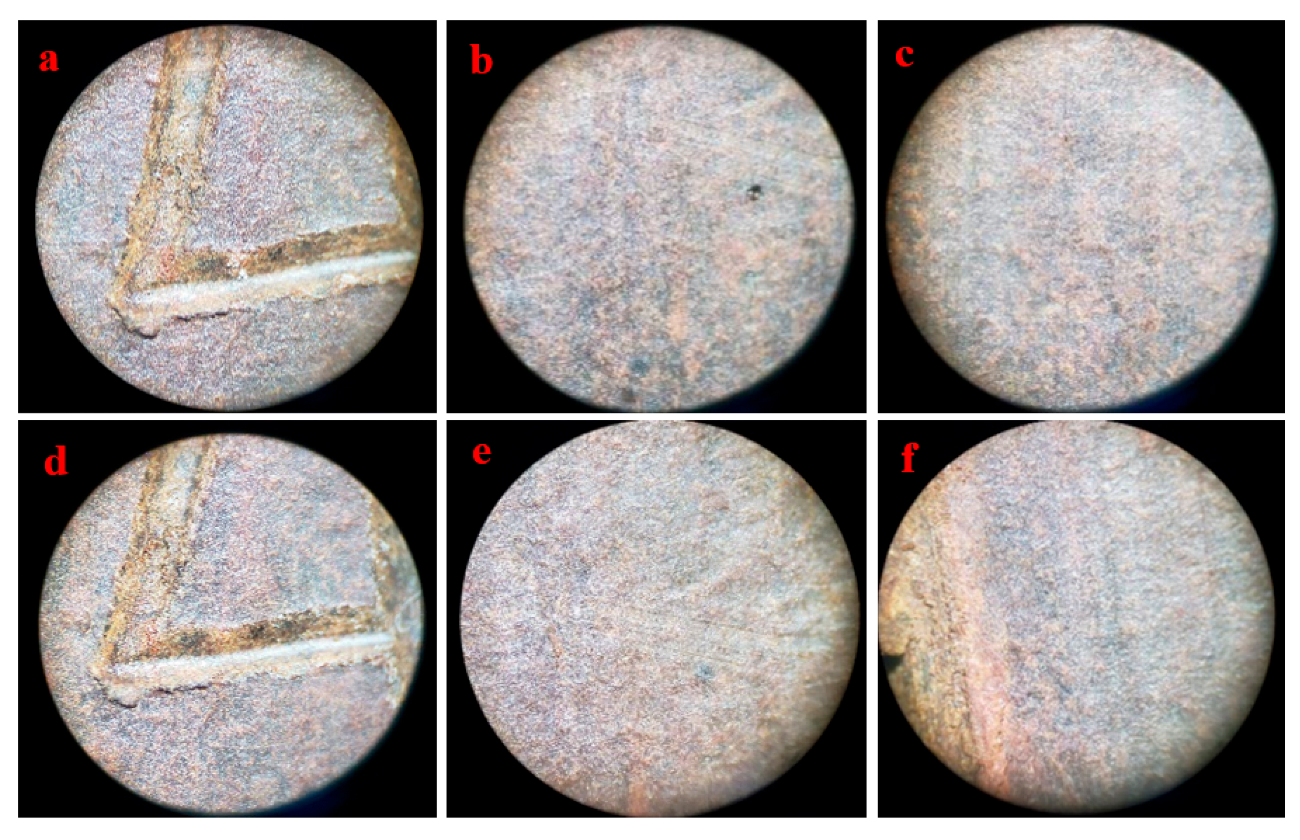
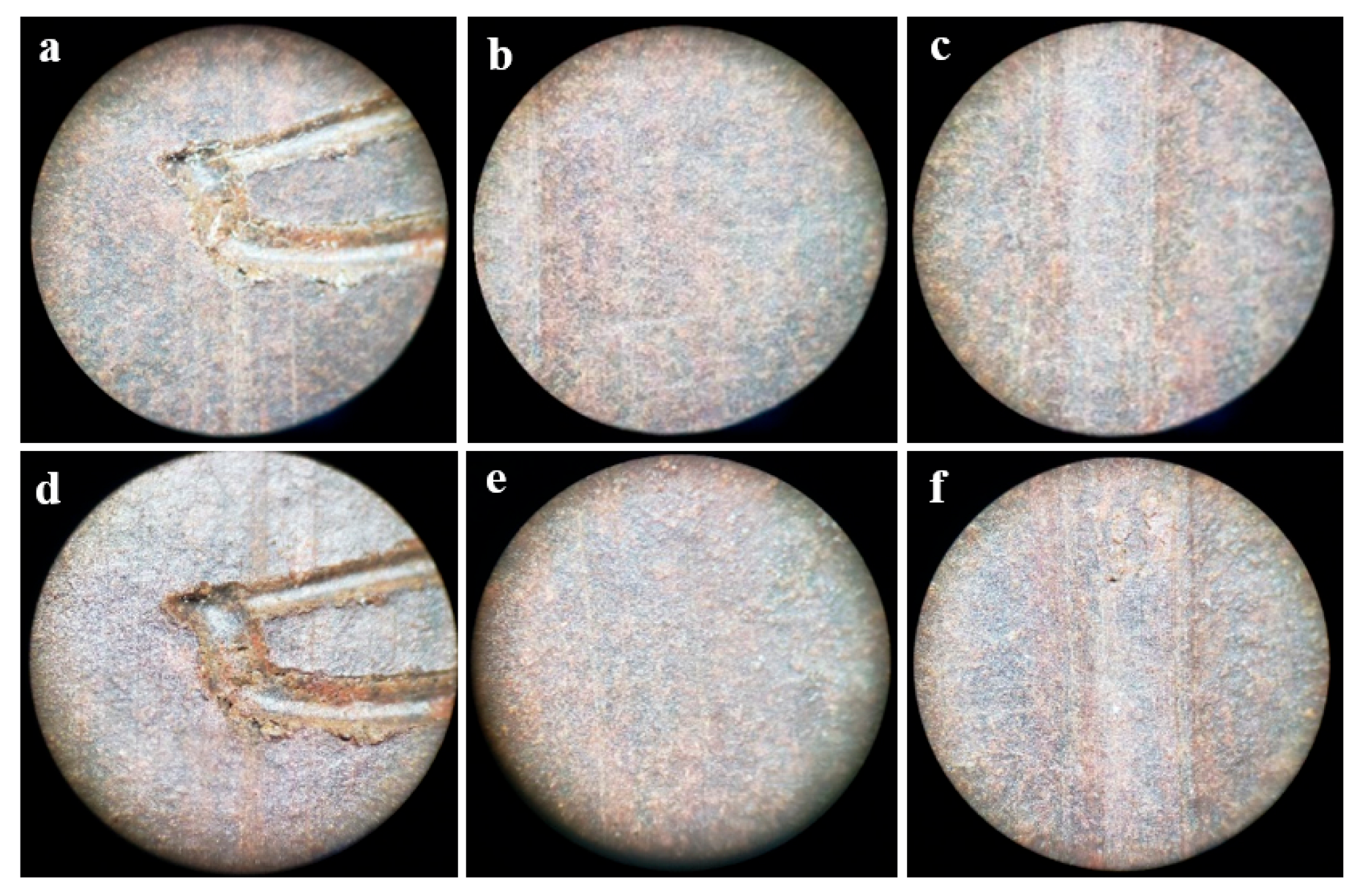
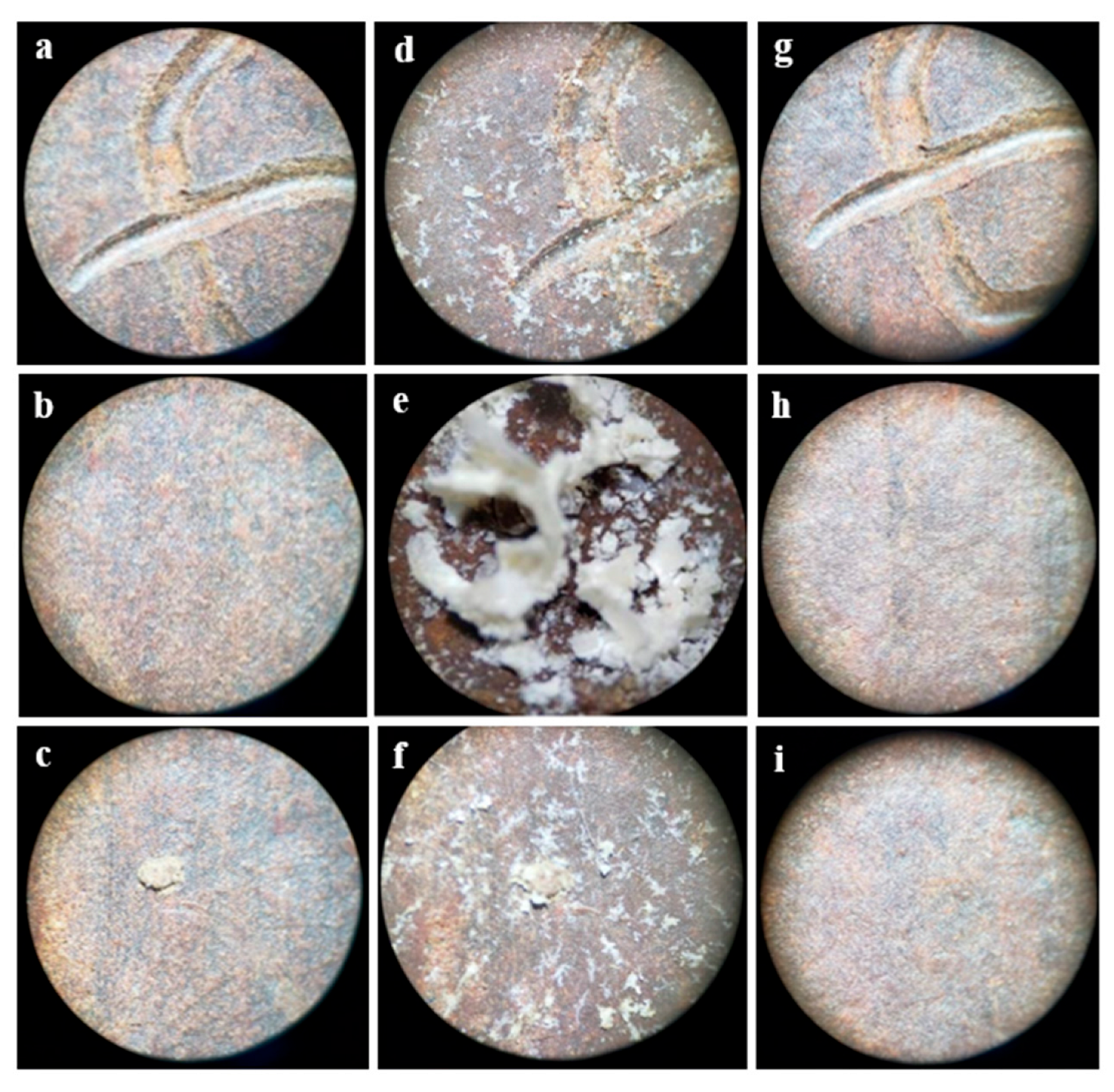
3.4. Immersion Corrosion Test
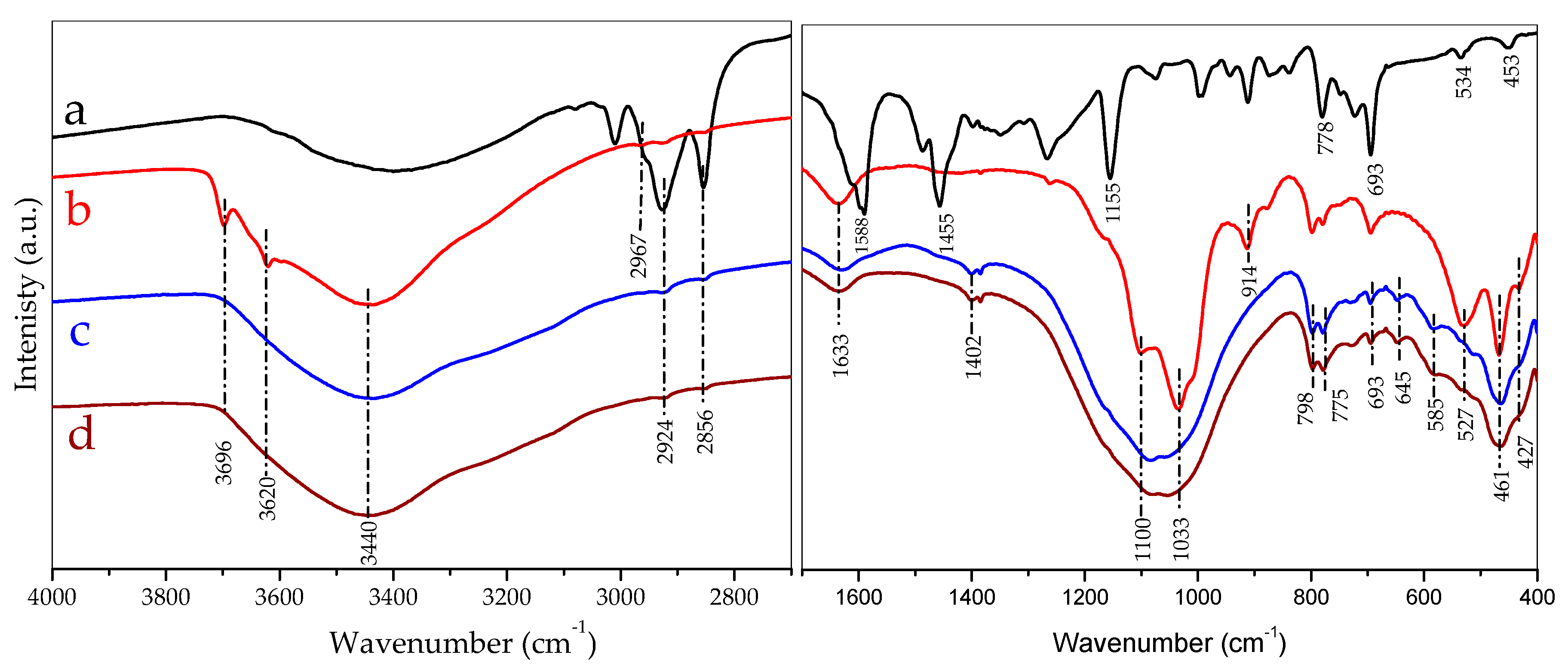
3.5. Infrared Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kano, Y.; Ikeda, I.; Iyasu, T.; Yamaguchi, Y.; Yamada, Y.; Sakurada, O. Relationship between Corrosion Form and Elution Behavior of Copper Tubes Surfaces with Different Residual Carbon Amounts. Mater. Sci. Appl. 2022, 13, 595–602. [Google Scholar] [CrossRef]
- He, J.; Song, M.; Chen, K.; Kan, D.; Zhu, M. Polymer-Derived Ceramics Technology: Characteristics, Procedure, Product Structures, and Properties, and Development of the Technology in High-Entropy Ceramics. Crystals 2022, 12, 1292. [Google Scholar] [CrossRef]
- Bauer, L.A.F. Materiais de Construção; LTC: Rio de Janeiro, Brazil, 2019; ISBN 978-85-216-3235-1. [Google Scholar]
- Jose, S.A.; Merbin, J.; Pradeep, L.M. Cermet Systems: Synthesis, Properties, and Applications. Ceramics 2022, 5, 210–236. [Google Scholar] [CrossRef]
- Coelho, A.C.V.; de Souza Santos, P.; de Souza Santos, H. Argilas especiais: O que são, caracterização e propriedades. Química Nova 2007, 30, 146. [Google Scholar] [CrossRef]
- Lakhdar, Y.; Tuck, C.; Binner, J.; Terry, A.; Goodridge, R. Additive manufacturing of advanced ceramic materials. Prog. Mater. Sci. 2021, 116, 100736. [Google Scholar] [CrossRef]
- Gogotsi, Y.G.; Lavrenko, V.A. Corrosion of High-Performance Ceramics; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- McCaulay, R.A. Corrosion of Ceramic Materials; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Valenzuela-Gutierrez, A.; Lopez-Cuevas, J.; Gonzalez-Angeles, A.; Pilalua-Díaz, N. Addition of ceramics materials to improve the corrosion resistance of alumina refractories. SN Appl. Sci. 2019, 1, 784. [Google Scholar] [CrossRef]
- Krasnyi, B.L.; Tarasovskii, V.P.; Rakhmanova, E.V.; Bondar’, V.V. Resistência Química de Materiais Cerâmicos em Ácidos e Alcalis. Glass Ceram. 2004, 61, 337–339. [Google Scholar] [CrossRef]
- AMPP. Corrosion in the Water and Wastewater Industry. 2021. Available online: https://www.ampp.org/technical-research/what-is-corrosion/corrosion-reference-library/water-wastewater (accessed on 17 March 2023).
- Baboian, R. Corrosion—A National Problem; ASTM Stantardization News: West Conshohocken, PA, USA, 1986. [Google Scholar]
- Chipera, S.J.; Bish, D.L. Fitting full X-ray diffraction patterns for quantitative analysis: A method for readily quantifying crystalline and disordered phases. Adv. Mater. Phys. Chem. 2013, 3, 47–53. [Google Scholar] [CrossRef]
- Avizovas, R.; Baskutis, S.; Navickas, V.; Tamándl, L. Effect of Chemical Composition of Clay on Physical-Mechanical Properties of Clay Paving Blocks. Buildings 2022, 12, 943. [Google Scholar] [CrossRef]
- Golestani, G.; Shahidi, M.; Ghazanfari, D. Evacuação eletroquímica de drogas antibacterianas como inibidores ecológicos para corrosão de aço carbono em solução de HCl. Appl. Surf. Sci. 2014, 308, 347–362. [Google Scholar] [CrossRef]
- Paul, S.; Kar, B. Mitigação da corrosão do aço leve em ácido por inibidores verdes: Fermento, pimenta, alho e café. ISRN Corros. 2012. [Google Scholar] [CrossRef]
- Mohammed, H.; Sobri, S. Corrosion inhibition studies of cashew nut (Anacardium occidentale) on carbon steel in 1.0 M hydrochloric acid environment. Mater. Lett. 2018, 229, 82–84. [Google Scholar] [CrossRef]
- Iloamaeke, I.M.; Onuegbu, T.U.; Umeobika, U.C.; Umedum, N.L. Abordagem verde para inibição de corrosão de aço macio usando Emilia sonchifolia e Vitex doniana em meio HCl 2.5M. Int. J. Sci. Mod. Eng. 2013, 1, 48–52. [Google Scholar]
- Tran, D.T.; Lee, H.S.; Singh, J.K. Influence of phosphate ions on passive film formation in amino acid-containing concrete pore solutions with chloride ions. J. Build. Eng. 2023, 66, 105834. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Wang, Z.M.; Lu, W.Y.; Li, X.Y.; Li, H.J.; Wu, Y.C. Exploration of procyanidin C1 from Uncaria laevigata as a green corrosion inhibitor in industry: Electrochemical assessment, theoretical simulation, and environmental safety. Sep. Purif. Technol. 2023, 318, 123950. [Google Scholar] [CrossRef]
- Ienascu, I.M.C.; Căta, A.; Chis, A.A.; Ştefănut, M.N.; Sfîrloagă, P.; Rusu, G.; Frum, A.; Arseniu, A.M.; Morgovan, C.; Rus, L.L.; et al. Some Brassicaceae Extracts as Potential Antioxidants and Green Corrosion Inhibitors. Materials 2023, 16, 2967. [Google Scholar] [CrossRef]
- Mazzetto, S.E.; Lomonaco, D. Óleo da Castanha de caju: Oportunidades e desafios no contexto do desenvolvimento e sustentabilidade industrial. Química Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Ike, D.C.; Ibezim-Ezeani, M.U.; Akaranta, O. Cashew nutshell liquid and its derivados in oil field applications: An update. Green Chem. Lett. Rev. 2021, 14, 620–633. [Google Scholar] [CrossRef]
- Furtado, L.B.; Nascimento, R.C.; Seidl, P.R.; Guimarães, M.J.O.C.; Costa, L.M.; Rocha, J.C.; Ponciano, J.A.C. Eco-friendly corrosion inhibitors based on Cashew nut shell liquid (CNSL) for acidizing fluids. J. Mol. Liq. 2019, 284, 393–404. [Google Scholar] [CrossRef]
- Nunes, M.S.; Bandeira, R.M.; Figueiredo, F.C.; dos Santos Junior, J.R.; de Matos, J.M.E. Corrosion protection of stainless steel by a new and low-cost organic coating obtained from cashew nutshell liquid. J. Appl. Polym. Sci. 2022, 140, 53420. [Google Scholar] [CrossRef]
- NBR 6457; Amostras de Solo—Preparação Para Ensaios de Compactação e Ensaios de Caracterização. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2016.
- NBR 7180; Solo—Determinação do Limite de Plasticidade. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2016.
- NBR 7181; Solo—Análise Granulométrica. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2018.
- NBR NM-ISO 565; Peneiras de Ensaio—Tela de Tecido Metálico, Chapa Metálica Perfurada e Lâmina Eletroformada—Tamanhos Nominais de Abertura. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 1997.
- NBR 6459; Solo—Determinação do Limite de Liquidez. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2017.
- NBR 10611; Corpos de Prova—Formas e Tipos de Ensaios—Padronização. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2011.
- ASTM G1:03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- Furtado, P. Pintura Anticorrosiva dos Metais; LTC: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- NACE TM0499-2009; Standard Test Method—Immersion Corrosion Testing of Ceramic Materials. National Association of Corrosion Engineers: Houston, TX, USA, 2009.
- ASTM G4:01(2014); Standard Guide for Conducting Corrosion Tests in Field Applications. American Society for Testing and Materials: West Conshohocken, PA, USA, 2014.
- NACE/ASTM TM0169/G31-21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. NACE/ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D1141:98(2021); Standard Practice for the Preparation of Substitute Ocean Water. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- NBR ISO 10545-3; Placas Cerâmicas Parte 3: Determinação da Absorção de Água, Porosidade Aparente, Densidade Relativa Aparente e Densidade Aparente. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2020.
- NBR ISO 10545-4; Placas Cerâmicas Parte 4: Determinação da Carga de Ruptura e Módulo de Resistência à Flexão. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2020.
- ASTM C1161:18(2023); Standard Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- ASTM C674:13(2023); Standard Test Methods for Flexural Properties of Ceramic Whiteware Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Das, B.M.; Sobhan, K. Fundamentos de Engenharia Geotécnica. Tradução da 8ª Edição Norte-Americana: Noveritis do Brasil; Cengage Learning: São Paulo, Brazil, 2017. [Google Scholar]
- Neto, P.M.S. Mecânica dos Solos, 1st ed.; Elsevier: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Bodó, B.; Jones, C. Introdução à Mecânica dos Solos. Tradução Maria Esther Soares Marques, Luiz Antonio Vieira Carneiro; LTC: Rio de Janeiro, Brazil, 2017; ISBN 978-85-216-3269-6. [Google Scholar]
- Santos, P.S. Ciência e Tecnologia de Argilas—Vol. 1; Edgar Blucher: São Paulo, Brazil, 1989. [Google Scholar]
- Gonçalves, W.P.; Silva, V.J.; Lima, L.K.S.; Neves, G.A.; Lira, H.L.; Santana, L.N.L. Efeito da temperatura de queima sobre as propriedades de materiais cerâmicos. In Proceedings of the 22º Congresso Brasileiro de Engenharia e Ciência dos Materiais, Natal, Brazil, 6–10 November 2016. [Google Scholar]
- Reis, A.S.; Oliveira, J.N.; Della-Sagrillo, V.P.; Valenzuela-Diaz, F.R. Caracterização e avaliação das propriedades cerâmicas de argila utilizada em cerâmica estrutural. In Proceedings of the 21º Congresso Brasileiro de Engenharia e Ciência dos Materiais, Cuiabá, Brazil, 9–13 November 2014. [Google Scholar]
- Carvalho, A.A.d., Jr.; Leite, K.d.S.; Elias de Matos, J.M. Viability of Morisca Powder Tailings for Ceramic Applications. Minerals 2023, 13, 459. [Google Scholar] [CrossRef]
- Nazile, U. The relationship between geotechnical index properties and the pore-size distribution of compacted clayey silts. Sci. Eng. Compos. Mater. 2015, 22, 623–632. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Kenichi, S. Fundamentals of Soil Behavior, 3rd ed.; Wiley: New York, NY, USA, 2005. [Google Scholar]
- NBR 15270; Componentes Cerâmicos. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2017.
- Grance, E.G.O.; Souza Jr, F.G.; Varela, A.; Pereira, E.D.; Oliveira, G.E.; Rodrigues, C.H.M. New petroleum absorbers based on lignin-cnsl-formol magnetic nanocomposites. J. Appl. Polym. Sci. 2012, 126, E305–E312. [Google Scholar] [CrossRef]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Economic appraisal of supercritical fluid extraction of refined cashew nut shell liquid. J. Chromatogr. A 2006, 1124, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.C.; Amorim, A.G.N.; Thomasi, S.S.; Figueiredo, F.C.; Carneiro, C.S.; Silva, P.R.P.; Vasconcelos Neto, W.R.; Ferreira, A.G.; Santos Junior, J.R.; Leite, J.R.S.A. Development of an electrolytic method to obtain antioxidant for biodiesel from cashew nut shell liquid. Fuel 2015, 144, 415–422. [Google Scholar] [CrossRef]
- Szabo, Z.G.; Gabor, M.; Varga, N.; Poppl, J.; Najand, J. Multi Method Approach to follow the Changes in Kaolinite Structure. Therm. Anal. 1974, 2, 569–582. [Google Scholar]
- Farmer, V.C. Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974; pp. 1149–1173. [Google Scholar]
- Venkatachalapathy, R.; Sridharan, T.; Dhanapandian, S.; Manoharan, C. Determination of firing temperature of ancient potteries by means of infrared and Mossbauer studies. Spectrosc. Lett. 2002, 35, 769–779. [Google Scholar] [CrossRef]
- Franco, F.; Cecila, J.A.; Perez-Maqueda, L.A.; Perez-Rodriguez, J.L.; Gomes, C.S.F. Particle-size reduction of dickite by ultrasound treatments: Effect on the structure, shape and particle-size distribution. Appl. Clay Sci. 2007, 35, 119–127. [Google Scholar] [CrossRef]
- Kodama, H. Infrared Spectra of Minerals: Reference Guide to Identification and Characterization of Minerals for the Study of Soil; Agriculture Canada, Research Branch: Ottawa, ON, Canada, 1985; p. 198. [Google Scholar]
- Manoharan, C.; Sutharsan, P.; Dhanapandian, S.; Venkatachalapathy, R. Spectroscopic and thermal analysis of red clay for industrial applications from Tamilnadu, India. J. Mol. Struct. 2012, 1027, 99–103. [Google Scholar] [CrossRef]
- Russell, J.D. Infrared Methods—A Hand Book of Determinative Methods in Clay Mineralogy; Wilson, M.J., Ed.; Blackie and Son Ltd.: New York, NY, USA, 1987. [Google Scholar]



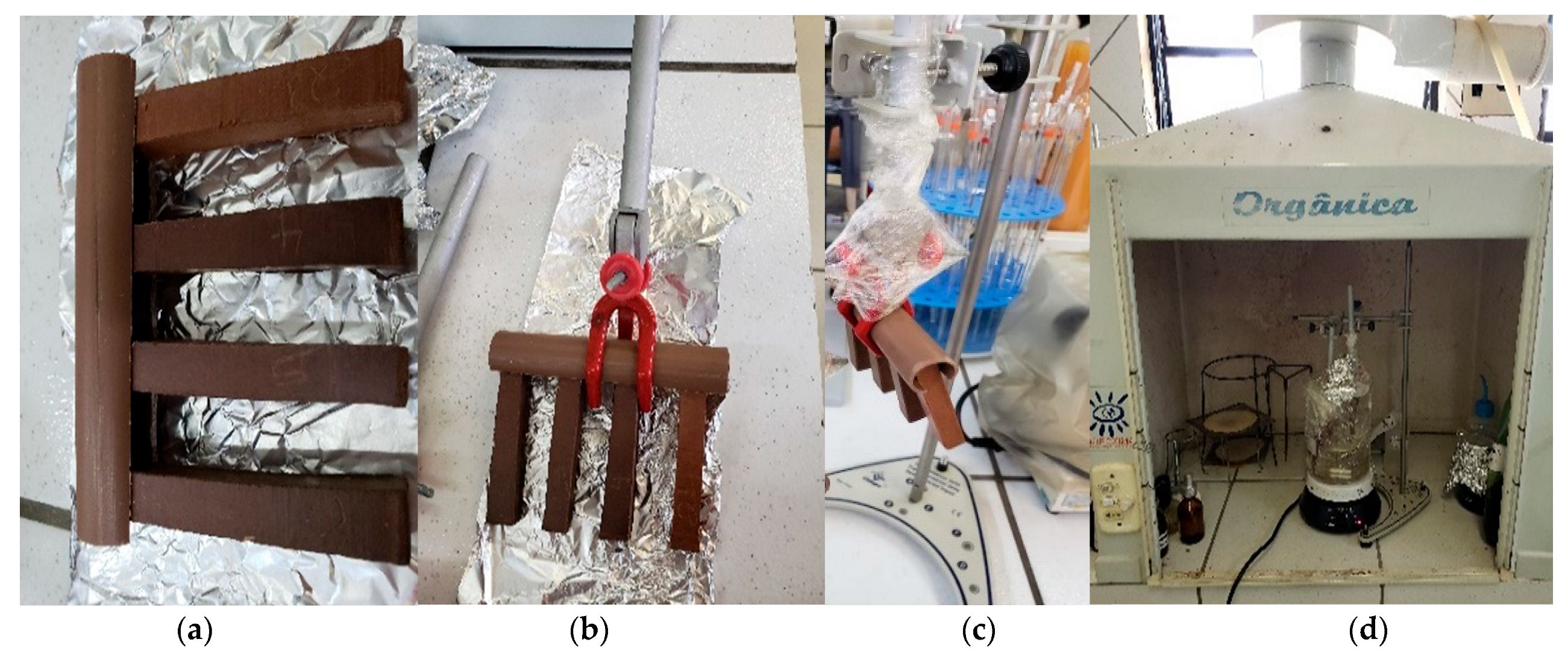


| Ion Type | Cl− | Na+ | SO42− | Mg2+ | K+ | Ca2+ |
|---|---|---|---|---|---|---|
| Simulated Seawater (mg/L) | 17,600 | 9560 | 2430 | 1350 | 380 | 400 |
| LL * | LP ** | IP *** | h † | IC †† |
|---|---|---|---|---|
| 40.08% | 23.45% | 16.63% | 23.10% | 1.02 |
| Oxides | (%) |
|---|---|
| SiO2 | 52.75 |
| Al2O3 | 21.96 |
| Fe3O4 | 9.72 |
| K2O | 3.16 |
| TiO2 | 2.19 |
| MgO | 1.09 |
| Others | 0.44 |
| Fire loss | 8.69 |
| Samples | W1 (g) | W2 (g) | W3 (g) | W (mg) | A (cm2) | T (h) | ρ (g/cm3) | TC (mm/Year) | WSC (g) |
|---|---|---|---|---|---|---|---|---|---|
| CPL18 | 18.76 | 20.52 | 18.81 | - | 37.25 | 432 | 1.71 | - | 1.71 |
| CPL32 | 18.11 | 19.82 | 18.16 | - | 36.83 | 768 | 1.75 | - | 1.66 |
| CPL70 | 18.64 | 20.48 | 18.70 | - | 37.33 | 1680 | 1.77 | - | 1.78 |
| CPWL70 | 19.59 | 21.89 | 19.57 | 20 | 38.43 | 1680 | 1.60 | 0.017 | 2.32 |
| Samples | AA (%) | AP (%) | ρ (g/cm3) | BL (N) | FS (MPa) | Q |
|---|---|---|---|---|---|---|
| CPL18 | 6.08 | 10.40 | 1.71 | 494.66 | 5.89 | 0.70 |
| CPL32 | 4.69 | 8.24 | 1.75 | 495.11 | 6.22 | 0.74 |
| CPL70 | 3.86 | 6.84 | 1.77 | 743.36 | 8.76 | 1.04 |
| CPWL70 | 10.11 | 16.20 | 1.60 | 733.20 | 7.94 | - |
| CPNC | 7.58 | 12.63 | 1.67 | 668.15 | 8.44 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa Leite, K.; de Carvalho, A.A., Jr.; Teixeira, P.R.S.; de Matos, J.M.E. Cashew Nut Shell Liquid as an Anticorrosive Agent in Ceramic Materials. Sustainability 2023, 15, 8743. https://doi.org/10.3390/su15118743
de Sousa Leite K, de Carvalho AA Jr., Teixeira PRS, de Matos JME. Cashew Nut Shell Liquid as an Anticorrosive Agent in Ceramic Materials. Sustainability. 2023; 15(11):8743. https://doi.org/10.3390/su15118743
Chicago/Turabian Stylede Sousa Leite, Kelson, Antônio Alves de Carvalho, Jr., Paulo Ronaldo Sousa Teixeira, and José Milton Elias de Matos. 2023. "Cashew Nut Shell Liquid as an Anticorrosive Agent in Ceramic Materials" Sustainability 15, no. 11: 8743. https://doi.org/10.3390/su15118743
APA Stylede Sousa Leite, K., de Carvalho, A. A., Jr., Teixeira, P. R. S., & de Matos, J. M. E. (2023). Cashew Nut Shell Liquid as an Anticorrosive Agent in Ceramic Materials. Sustainability, 15(11), 8743. https://doi.org/10.3390/su15118743











